Summary
Non-human primates, primarily macaques, have been used to study tuberculosis for decades. However, in the last 15 years, this model has been refined substantially to allow careful investigations of the immune response and host-pathogen interactions in Mycobacterium tuberculosis infection. Low dose challenge with fully virulent strains in cynomolgus macaques result in the full clinical spectrum seen in humans, including latent and active infection. Reagents from humans are usually cross-reactive with macaques, further facilitating the use of this model system to study tuberculosis. Finally, macaques develop the spectrum of granuloma types seen in humans, providing a unique opportunity to investigate bacterial and host factors at the local (lung and lymph node) level. Here we review the past decade of immunology and pathology studies in macaque models of tuberculosis.
Non-human primates as a model of human tuberculosis
The non-human primate (NHP) model of tuberculosis (TB) is an important translational model of human disease that bridges the gap between other animal models and humans. NHPs are the closest genetically to humans of any of the experimental animals used in biological research, with corresponding remarkable immunologic similarities to humans. While there are many challenges to using NHPs in research, including TB research, there are also many advantages. From a practical standpoint, many human reagents cross-react with NHPs and can be used readily, especially in macaques. The immunologic similarities lead one to expect that vaccines and adjuvants will have similar effects in NHPs as in humans, and this has been shown in several studies.
While there are many animal models of TB, the development of the NHP model was primarily focused on understanding the facets of human Mycobacterium tuberculosis (Mtb) infection that could not be addressed in other small animal models. Rabbits, mice, guinea pigs, zebra fish, and mini-pigs have been used to study TB (1,2). The murine model is perhaps the most established model of TB given that it is highly tractable with well-established genetically engineered (transgenic and knockout) strains to easily examine the immunologic components of the host immune response. Mice are also easy to handle and relatively inexpensive to maintain in Biosafety Level 3 containment, which is essential for all TB studies. However, latent infection, the most common manifestation of human Mtb infection, does not occur naturally in the animal models listed above. Modified mouse models have been developed in attempts to mimic human latent infection, either by using antibiotics to reduce the bacterial load or through strain specific infections (3,4). Granulomas, the histopathologic hallmark of TB, are seen in all small animal models but neither the structural architecture of granulomas in these animals(5,6), nor the spectrum of granuloma types are consistent with human lesions (7). Furthermore, in the subset of animals that do produce human-like granulomas (e.g., rabbit, guinea pig, zebrafish), the limited number of immunologic reagents remains an obstacle for studying the pathogenesis of TB.
The epidemic of HIV-TB co-infection is a major worldwide public health concern that is also not well addressed by any of the smaller animal models, although efforts to develop humanized mouse strains that foster HIV replication are being developed (8-10). Cattle are natural hosts for M. bovis, and have been used as a model of TB, with interesting results from these studies, including vaccine studies (reviewed in 11). However, the space needed for performing studies on cattle, especially under BSL3 conditions, and the relatively small number of immunologic reagents available limit the use of this model only to a few specialized facilities. Lastly, some key immunologic features that are important aspects of human anti-mycobacterial immune responses including granulysin expression, CD1-restricted T cells (12), and vitamin D-dependent binding elements (13) near innate response promoters are not present in some animal models but are all found in NHPs. Thus, NHPs play an important role in addressing specific aspects of human-specific Mtb infection that cannot be addressed with other models.
There are two primary macaque species that are commonly used in TB research: rhesus macaques (Macaca mulatta) and cynomolgus macaques (Macaca fascicularis). In addition to immunologic similarities, the range of infection outcomes, clinical presentation, and pathology in these macaques can be remarkably similar to human TB (5,14-18). Although these two macaque species are genetically similar, there are differences in their resistance to Mtb infection, with rhesus macaques being more susceptible to developing active TB than similarly infected cynomolgus macaques (19, 20, authors’ unpublished data). With virulent strains of Mtb (e.g. Erdman), almost all adult rhesus macaques develop active TB, often with extrapulmonary disease, even with low dose infection. Mehra et al. (21) described a model of latent infection in rhesus macaques, but this model relies on the use of the relatively low-virulent Mtb strain CDC1551. In contrast, cohorts of adult cynomolgous macaques infected with a low dose (<25 CFU) of virulent Mtb Erdman strain via bronchoscopic instillation develop equal proportions of animals with active TB and latent infection. The reasons for the increased susceptibility to Mtb infection and development of active TB in rhesus macaques are not currently known, but these genetically similar NHPs provide the opportunity to learn about innate and adaptive mechanisms of Mtb infection control.
The clinical criteria distinguishing active TB from latent infection in NHPs are based on human clinical definitions (15,16,22). Active TB is defined as having clinical signs of disease (e.g. cough, weight loss), an elevated erythrocyte sedimentation rate (ESR), and culture of Mtb from gastric aspirate (GA) or bronchoalveolar lavage (BAL). In contrast, animals with latent infection have evidence of infection indicated by a positive tuberculin skin tests (TST) and Mtb-specific immunologic parameters and asymptomatic, negative for Mtb culture from GA or BAL 2 months post infection, and normal ESR. Most animals with active TB become evident by 4-5 months post infection, whereas latent infection is declared at least 6 months post infection. A small subset of animals (~5%) will develop severe disease within 3 months after infection and are termed ‘rapid progressors’. Pulmonary disease is the most common form of active TB seen in this model; however, all forms of human extrapulmonary TB including brain tuberculomas, uveitis, Pott's disease (TB spondylitis), liver, and splenic TB have been observed (authors’ unpublished data). Just as there is a spectrum of both chronic and severe active TB in humans and cynomolgus macaques, we have shown that cynomolgus macaques also present with a spectrum of latent infection and that this likely contributes to the risk of reactivated disease. We coined the term ‘percolators’ to describe macaques that otherwise appeared to be latently infected (asymptomatic, normal x-rays, and normal ESR) but who intermittently shed Mtb that can be cultured from GA or BAL (16). This spectrum of latent infection has also been described in humans (14, 23), especially in HIV-infected persons where subclinical disease is commonly recognized in the absence of clinical symptoms via a positive interferon-γ release assay (IGRA) or TST, normal chest x-ray but positive Mtb sputum (24). Reactivation of TB from latent infection can occur spontaneously in macaques (<5% incidence in our experience) or can be experimentally induced by immune suppression, further demonstrating the fidelity with which this model can mimic human infection.
Pathology is similar in macaques and humans
The range of pathology seen in macaques, which is remarkably similar to that seen in humans, is a true strength of this model (16). The tuberculous granulomas of both macaques and humans are organized structures of immune cells that form in response to persistent Mtb infection, and are composed largely of macrophages and lymphocytes (16-18, 25) (Fig. 1). One of the primary reasons that we developed a NHP model of TB was to have access to granulomas that are structurally similar to humans to investigate local immune responses in ways that cannot be performed in humans. We have demonstrated that macaques have the full range of granuloma types as seen in humans, with the classical caseous granuloma observed predominantly in the lungs (7, 16, 26). The other types of lung granulomas in macaques include non-necrotizing granulomas, fibrotic or calcified lesions, as well as TB pneumonia, cavities, consolidations, and interstitial fibrosis. Although monkeys characterized to have active TB or latent infection may have a range of granuloma types at any given time, the more complex pathologies (TB pneumonia, consolidations, etc.) are only seen in animals with active TB. The diversity in the type of granulomas within an individual macaque is likely to be an important determinant for its progression to disease, reactivation of latent infection, and response to drug treatment. With respect to the latter point, we demonstrated that the Mtb bacilli that remain after short course drug therapy (in this case, 2 months) are almost all in caseous granulomas (27), suggesting that these granulomas need to be targeted for more effective therapy.
Fig. 1. NHP granulomas exhibit the organizational characteristics of human granulomas.
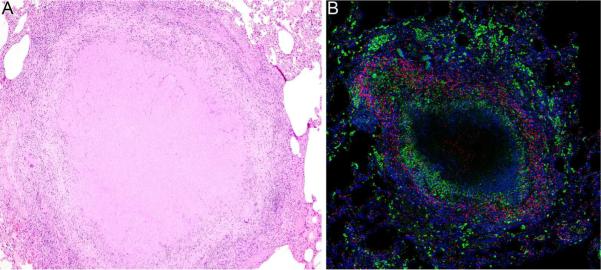
(A). Hematoxylin and eosin stained section of lung tissue containing a necrotic granuloma with a large central region of caseous necrosis (10× magnification). (B). Immunohistochemical staining of a necrotic granuloma showing CD68+ macrophages (green) surrounding the necrotic center (unstained) and CD3+ T cells (red) in the lymphocyte cuff. Scattered clusters of alveolar macrophages, characterized by their morphology and strong CD68 expression, can be seen at the edge of the granuloma and in the adjacent lung tissue. Nuclei are stained in blue (20× magnification).
Course of infection and initiation of an adaptive immune response
Infection by Mtb occurs upon inhalation of bacilli, perhaps even a single bacillus in humans. After Mtb enters the airways, there are likely to be innate immune factors including alveolar macrophages, surfactant, and antimicrobial peptides among others that could prevent establishment of infection, although these are poorly understood. It is estimated that only 10-30% of humans exposed to Mtb become infected, although the risk of becoming infected increases with physical proximity to the index case and the number of repeated exposures (reviewed in 28). For an infection to be established, the bacillus likely enters an alveolar macrophage and begins to replicate. The bacteria then transits to the lung parenchyma, where it continues replicating in macrophages, thus setting off a slow inflammatory response that recruits more macrophages and other innate cells. This is the initiation of a granuloma, although adaptive responses are required to complete granuloma formation.
Priming of T-cell responses to respiratory viruses is initiated in lung draining lymph nodes and responses are seen within 5-8 days in mice (29-34) and humans. However, the initiation of T-cell response to Mtb infection is much slower (35). In humans and macaques (15,16,22), peripheral response to Mtb infection usually take 4-6 weeks before they can be detected by IGRAs, tuberculin skin test, or other immunologic measure (36,37). In mice infected with aerosolized Mtb, culturable Mtb are not present in the single lung-draining mediastinal lymph node until 9-11 days post infection (35, 38, 39), even though bacilli can be cultured from the lungs as early as 1 day post-infection (38). Bhatt et al. (40) showed that bacilli can be transferred to the lymph nodes from the airways via dendritic cells. However, bacilli can also likely be transported from the lungs or granulomas to lung-draining lymph nodes, via inflammatory CCR2+ monocyte/macrophages or dendritic cells (41-43). In mice, infection of the lymph nodes correlated with induction of T-cell responses against Mtb antigens, with responses only observed after the lymph node was infected (11-14 days post-infection). Thus, the relatively slow immune response to Mtb seen in animal models and humans following aerosol exposure may be related to the relatively slow seeding of the lymph node with Mtb and Mtb antigens and the subsequent priming of T-cell responses against these antigens. Other possibilities that include the slow replication rate of the bacillus (compared to respiratory viruses or other bacteria), low initial inoculum, suppression of dendritic cell or macrophage migration to lymph nodes, or inhibition of T-cell priming by Mtb. There are numerous studies on the effects of Mtb on dendritic cells, cell migration, and priming of T cells, but none definitively prove that Mtb is actively interfering with T-cell priming, and studies with higher Mtb inocula in normal (not transgenic) mice did not lead to a more rapid initiation of T-cell responses (38). Nonetheless, the events that occur early in infection in humans remain a black box, and NHPs can be used to begin to dissect these events, with translation to humans.
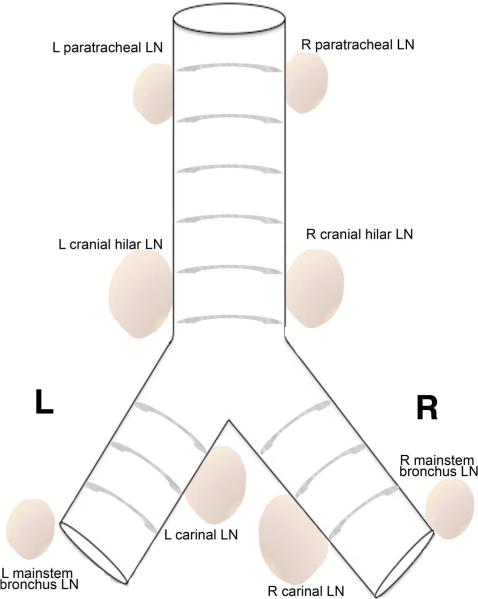
The classic Ghon complex consists of the initial lung granuloma and the lung-associated lymph node with a granuloma. Like humans, macaques have several mediastinal lymph nodes, with the most prominent being the hilar lymph nodes and the carinal lymph nodes on the right and left sides of the airways (Fig. 2). Use of positron emission tomography (PET) and computerized tomography (CT), coupled with the PET-CT probe 2-deoxy-2-(18F)-fluoro-D-glucose (FDG), have revolutionized our ability to examine the early events in the lungs and thoracic lymph nodes. FDG is incorporated into and retained in metabolically active cells, including areas of inflammation and immune cell activity. Thoracic lymph nodes can become infected early in the course of infection (15), and with PET-CT imaging, we find there is increased uptake of FDG in a subset of lymph nodes in most animals by 2-3 weeks after infection. At about the same time, small granulomas can also be reliably identified in the lung lobes (Fig. 3). Since FDG is a relatively non-specific PET probe that labels metabolically active cells, the FDG avidity could signify increased inflammation due to infection of that lymph node or simply the initiation and proliferation of a T-cell response. The trafficking of Mtb between the lungs and lung-draining lymph nodes is not well understood, but data from macaques infected with genetically barcoded Mtb strains indicate that while each granuloma is initiated by a single organism, lung-draining lymph nodes can contain several different barcoded strains (44). This could reflect initial infection from the airways, migration from several different granulomas to the lymph nodes, or both. A more diverse library of genetically barcoded bacteria is currently being used to address these questions.
Fig. 2. Diagrammatic representation of the macaque tracheobronchial tree depicting draining mediastinal lymph nodes observed in NHPs following Mtb infection.
Cranial hilar lymph nodes and the carinal lymph nodes on the right and left side of the airways are most commonly observed. (Abbreviations: LN, lymph node; L, left; R, right).
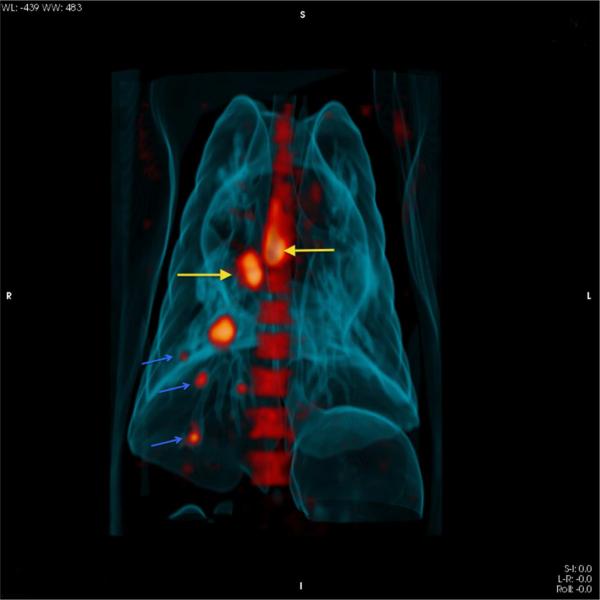
Fig. 3. Three dimensional 18F-FDG PET/CT image showing lymph nodes and granulomas in a macaque three weeks post Mtb infection.
Increased uptake of FDG is observed in cranial hilar and carinal lymph nodes (yellow arrows). Small lung granulomas (blue arrows) with variable FDG uptake are also observed as early as 3 weeks post infection.
Thoracic lymph nodes can be a rich source of Mtb-specific T cells. During early Mtb infection, the number of Mtb-specific T cells making IFN-γ, as assessed by ELISPOT assay, is reliably detectable at 4 weeks post infection and is substantially higher in thoracic lymph nodes compared to granulomatous lung (15). During this time, approximately 34% of CD4+ T cells and 14% of CD8+ T cells have activation phenotypes that are most commonly CD29+ or CD69+. This is in contrast to T cells in the blood and lungs, which differ substantially in their activation marker expression (15). Activated T cells are also found in the thoracic lymph nodes of animals with active and latent TB, although animals with active disease often have fewer T cells in their lymph nodes because the T-cell regions in effaced lymph nodes are displaced by caseous granulomas. However, there are substantially more IFN-γ producing T cells in the lymph nodes of animals with active disease when compared to latently infected animals, a difference that may be driven by bacterial burden. Similarly, greater numbers of Mtb-specific IFN-γ T cells are observed in blood, airway, and granulomatous lung from animals with active disease relative to cells from similar compartments in latently infected animals, although the T cells within each compartment differ in their activation marker and chemokine receptor profiles (16).
PET/CT imaging can be used to track infection in macaques
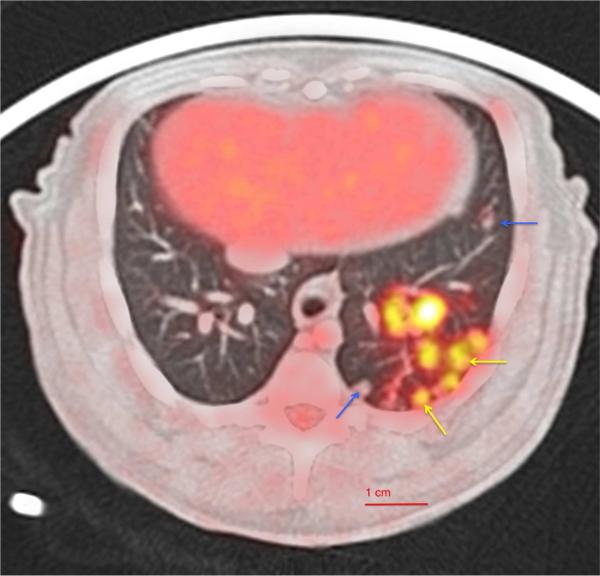
PET-CT imaging has allowed us to detect and follow lesions in vivo over the course of infection in macaques. FDG appears to highlight areas of inflammation associated with metabolically active cells including neutrophils, macrophages, or lymphocytes. Granulomas within the same lung lobe can be strongly FDG-avid or have very little FDG avidity, suggesting a wide range of inflammation in granulomas (Fig. 4). In addition to visualizing metabolic activity, PET-CT imaging reveals the size and spatial orientation of granulomas and can be used to follow their trajectory over the course of infection (Figs 3 and 4), including identification of newly formed granulomas during both primary infection and reactivation of TB. Our findings (unpublished) contradict the long-held assumption that lung disease in the lower lobes is associated with primary Mtb infection and that upper lobe involvement was reactivation of the latent infection (45). This dogma was recently refuted by modern molecular techniques that could detect remote and current circulating strains of Mtb in the community. From these studies, no correlation was found between radiographic pattern and timing of Mtb infection (46,47). Importantly, FDG PET-CT has revealed that individual granulomas within a macaque are dynamic over time and independent from each other (44, 48, 49), corroborating data from PET-CT-imaged humans with TB (Clifton Barry, III, personal communication). We have found that the size, metabolic activity, and spatial and temporal distributions of lung lesions differ in their evolution between animals with active TB and latent infection (49). Even more interesting, early patterns of granuloma number and development of new lesions over time can predict the outcome of infection as early as 6 weeks post infection. We attribute the variability in disease patterns among animals to bacterial heterogeneity [each individual granuloma is generated from a single bacillus, but there is substantial heterogeneity in bacterial burden by as early as 4 weeks post-infection (44)], dissemination of granulomas, and the differences in immunologic response (Gideon et al., manuscript in review) at the granuloma level. Thus, it is the total sum activity of an individual's granuloma burden that ultimately contributes to the clinical outcome.
Fig. 4. Axial section of 18F-FDG PET/CT image showing macaque lung granulomas.
In NHPs, various types and numbers of lung granulomas are observed with variable FDG uptake within a lobe in both active disease and latent infection. Arrows indicate both metabolic active-PET hot (yellow) and PET cold (blue) granulomas seen within a lobe.
Contribution of thoracic lymph nodes to infection outcome
In macaques and humans, granuloma formation and mediastinal lymph node enlargement occurs during Mtb infection. While hilar adenopathy more commonly occurs in children and HIV-infected persons with TB (50), hilar adenopathy by chest x-ray was reported by Poulsen in 56% of adults with primary Mtb infection even before the chemotherapy era (reviewed in 51). Hilar adenopathy reportedly occurs in only 5-10% of reactivation TB cases; however, it is not clear how reactivation of TB was determined (50). Nonetheless, involvement of the mediastinal lymph nodes that drain the lungs clearly occurs in both humans and NHPs, but the extent of their involvement during the course of infection may be variable.
As noted above, migration of Mtb to the lymph nodes, via macrophages or dendritic cells, or even through lymphatics or blood, appears to be a necessary step for priming anti-mycobacterial T-cell responses. However, a consequence of this migration is that the lymph node becomes infected and granuloma formation occurs. Lymph nodes from Mtb-infected macaques can contain a single or multiple small granulomas, or can be completely effaced by caseous granulomas. Thus, the lymph node can be a reservoir for infection. In studies of TB drug treatment in cynomolgus macaques, lymph nodes still retained significant numbers of bacilli after short-term drug therapy, while lung granulomas were more easily sterilized. Our studies on animals with clinically latent infection suggest that lymph nodes can be a significant source of reactivation (52,53, authors’ unpublished data). Animals with latent infection that experience reactivation after TNF neutralization showed histologic signs of reactivated disease in lymph nodes, including the appearance of satellite granulomas next to what appear to be older granulomas (53). In more recent unpublished studies using PET-CT imaging, reactivation in response to TNF neutralization can occur in one or more thoracic lymph nodes, lung granulomas, or both, as measured by increase in size or FDG avidity. When animals with latent infection were depleted of CD4+ T cells, only half of cohort developed reactivation of TB (52). Although CD4+ T cells were depleted peripherally in all macaques, the extent of depletion in thoracic lymph nodes was variable; however, those animals that reactivated had the lowest levels of CD4+ T cells in the lymph nodes. These data support that lymph nodes can be a significant reservoir of Mtb infection during active TB and latent infection and that bacterial replication is held in check by immune pressure. The importance of the lymphatic system in playing a critical role in the establishment of Mtb infection and bacterial dissemination has also been discussed in detail elsewhere (54).
Lymph nodes can also contribute to disease worsening and pathology in macaques. When lymph nodes are infected, they can become greatly enlarged and necrotic. Since the lymph nodes are positioned proximal to the airways, massive enlargement can lead to compression of the airways, possibly resulting in a collapsed or emphysematous lung. In severe cases, the affected lymph nodes can erode into the airways and spread infection throughout the lungs. This situation is far more common in rhesus macaques and rarely seen in cynomolgus macaques. Rhesus macaques, in general, have extensive thoracic lymph node involvement, compared to cynomolgus macaques, with more lymph nodes infected, and with extensive necrotic effacement of the lymph nodes. With respect to the impressive lymph node involvement, the rhesus macaques may perhaps more closely resemble the situation of pediatric TB, which also has significant lymph node involvement. Cynomolgus macaques may represent the adult TB situation; although there may be more lymph node involvement in cynomolgus macaques than in humans, the pathology and progression of infection in cynomolgus macaques is much more similar to that seen in human TB. Cynomolgus macaques with latent infection nearly always have a granuloma, often calcified, in a lymph node, and in many cases a Ghon complex (lung lesion with associated involved lymph node) has been identified.
Immune mediators of Mtb infection control
A real strength of the macaque model of TB is the ability to perform immune modulation to address specific questions about Mtb infection in a human-like system. We showed that the cytokine TNF was important for controlling both early infection and latent infection (53) and that there are several key features shown in this model that are more representative of human biology than the murine models. Mice without functional TNF had disorganized granulomatous inflammation (55-57), whereas cynomolgus macaques treated with anti-TNF neutralizing antibody had normal granuloma structure, although the granulomas were larger in size than granulomas from un-manipulated macaques. Because of the outbred genetic nature of cynomolgus macaques, only 3 of the 4 animals that underwent TNF neutralization at the time of challenge developed severe disease within 5 weeks, suggesting that while TNF is important, some macaques (and likely humans) do not absolutely require TNF to control early infection. Most humanized antibodies can only be delivered to macaques for up to 8 weeks as they tend to develop responses against these antibodies, and thus it is possible that a longer course of TNF neutralization in newly infected macaques would result in all of the animals losing control their ability to control Mtb infection. In studies where animals with latent infection were treated with anti-TNF antibody, only 5 of 9 developed gross evidence of reactivation within 6 weeks of treatment. However, even in macaques that reactivated, the overall architecture of the granulomas remained intact despite TNF neutralization (53). The unusual prominence of extrapulmonary disease during TNF neutralization-induced reactivation (a unique feature given that reactivation most commonly manifests as pulmonary disease in humans) was seen both in macaques and humans treated with TNF-neutralizing agents (58). This observation suggests that while granulomas still appeared to be organized, they were not functioning to prevent dissemination throughout the lungs or to other organs. While there were no differences in IFN-γ production observed between TNF neutralized and control animals, there were alterations in T-cell recruitment and chemokine receptors to both the lung granulomas and lymph nodes. Notably, peripheral responses measured by PBMCs did not show the same immune responses as the lung and lymph node, owing to the compartmentalization of the immune response.
The HIV epidemic has led to a resurgence of TB for which the cynomolgus macaque provides an excellent research model. End stage HIV, by definition, is associated with severely reduced levels of CD4 T cells that were thought to be a primary source of IFN-γ, a critical cytokine in the control of Mtb infection in mice, and likely in humans (59). We and others have shown that CD4+ T cells are critical for controlling early infection in macaques (52, 60). During latent infection, depleting CD4+ T cells with antibody for up to 14 weeks resulted in only half of the animals developing reactivation of TB (52, 60). Despite the loss of CD4+ T cells, control of infection did not correlate with decreased IFN-γ production, suggesting that the loss of CD4+ T cells may have resulted in suboptimal CD8+ T-cell responses (52,60).
Simian immunodeficiency virus (SIV) is used as a model of HIV infection in rhesus and cynomolgus macaques. SIV infection of cynomolgus macaques with latent infection resulted in a significant but short-lived increase in the frequency of mycobacteria-specific T cells (61) followed by reactivation of all animals over a period of 2-11 months (62). The timing of reactivation was dependent on early reductions in peripheral CD4+ T-cell numbers, and although difficult to confirm, the peripheral cytokine perturbations we observed at this time may have correlate with increased bacterial replication in granulomas that were depleted of protective T-cell responses (61, 62). T-cell numbers rebounded in the periphery after this period and granulomas taken from these animals 3-5 weeks post-reactivation had normal or near-normal numbers of T cells. In most cases, granulomas were histologically similar to granulomas from SIV-negative monkeys. Interestingly, a subset of granulomas appeared to have undergone a healing event following reactivation and appeared phenotypically similar to fibrotic granulomas from INH and rifampicin-treated monkeys (27, 62, 63). Subsequent studies suggested that SIV could inhibit antigen-presenting cells and induce production of the cytokine IL-5, thereby inhibiting Mtb-specific T-cell responses (63).
In unpublished studies, we have demonstrated that depletion of CD8+ T cells prior to Mtb infection in macaques prevents control of early infection (Lin et al., in preparation). In another macaque study, BCG-associated protection after Mtb challenge was lost when CD8+ T cells were depleted (64). The effects of CD8+ T-cell depletion were similar to that of CD4+ T-cell depletion in terms of infection, dissemination, and disease, strongly suggesting that both T-cell subsets are essential for control of early infection.
Inflammation can be a positive feature of an immune response, but it can also have negative consequences. From our published and unpublished data, inflammation seems to correlate with worsening disease in macaques. Although there is evidence from murine models suggesting anti-inflammatory mediators, such as regulatory T cells (Tregs) and IL-10, are detrimental to control of Mtb infection (reviewed in 65), there are also several studies suggesting that lack of control of T-cell responses leads to exacerbation of disease. For example, mice deficient in PD-1/PD-L1 signaling, molecules involved in exhaustion of T cells, had rapid onset of lung inflammation and death after Mtb infection (66, 67), suggesting that without downregulation of T-cell responses, lung inflammation results in worsened disease. In humans (23) and in macaques (Gideon et al., manuscript in preparation), an inflammatory neutrophil/Type I IFN response was observed in animals with active TB. In cynomolgus macaques, we found that higher levels of Tregs in blood prior to infection was correlated with improved outcome (i.e. latent infection vs. active TB)(68). Within the first few weeks of infection, the Tregs in the blood of all infected monkeys were rapidly depleted, and there was a concomitant increase in Tregs in the airways. This finding suggests that migration of Tregs to the lung may be important in limiting initial inflammation. Further studies on potential anti-inflammatory factors, such as IL-10, and other host-directed therapies should be undertaken in the human-like macaque model before moving into human trials, since the effects of modulating the inflammatory response on control at the local (lung and lymph node) level are hard to predict.
Immune responses within the granuloma
Most human studies rely primarily on the peripheral blood T-cell responses to understand immune response to vaccine candidates, drug treatment, or immune response during the course of Mtb infection (69,70). However, lung granulomas provide us with better measure of the actual responses induced by Mtb at the site of disease/infection. The relationship between T-cell responses in blood and lung tissues are complex, and the NHP model provides an excellent platform for investigating this system. Limited data from our laboratory suggest that in animals with established active disease or latent infection, the systemic T-cell responses do not accurately reflect the local T-cell responses (Gideon et al, manuscript in review). Even BAL T-cell responses, which are considered to be a closer match to the lung T-cell responses, differ from the responses we see in the granuloma (15). Differences in the T-cell responses might be further complicated due to the existence of a spectrum of infection at both local and systemic levels in non-human primates and humans. Thus, caution should be used in interpreting and extrapolating data from peripheral T-cell responses in humans to the responses in lungs.
Cynomogus macaques provide an excellent model for studying the functional aspects of immunological components of TB granulomas. Recent studies from our lab indicate that animals have substantial variability in the types and numbers of granulomas (Fig. 3), as well as bacterial burden within each individual granuloma, irrespective of whether they develop clinically active TB or latent infection. The spectrum of granulomas in active TB and latent infections is extremely similar to that observed in human TB (7, 15-18, 25, 48). Recent studies from our group find that progressing and healed lesions coexist within the same animal, with nearly all animals being capable of sterilizing at least a subset of individual granulomas (44). However, animals with active TB present with a subset of lesions that do not control infection, which results in dissemination of the infection and the development of complex pathologies, such as TB pneumonia, cavities, and consolidations (44).
The number of viable/culturable Mtb bacilli from individual granulomas ranges, even within individual animals, from 0 (sterilized) to ~106 CFU/granuloma. The CFU/granuloma overlapped substantially among monkeys with active TB or latent infection (44, Gideon et al., manuscript in review). The bacterial burden in individual granulomas was greatest among animal infected for 4 weeks, and we observed a substantial reduction in bacterial burden by 11 weeks post-infection, which coincides with the onset of adaptive immune response (44). However, the substantial range of bacterial burden within each animal suggests that the extent of bacterial containment varies among granulomas. Therefore, the mere presence of granulomas is insufficient to control infection; instead, proper functioning of all granulomas in a host determines the ultimate outcome of infection.
Currently available data support the hypothesis that the outcome of Mtb infection is determined at the granuloma level and the sum total of granulomas, each with its own chance to either inhibit or permit bacterial dissemination, are collectively responsible for disease at the total host level. The complex organization of granulomas, with their populations of macrophages and lymphocytes suggest that there is significant cross-talk between cell types, and that for granulomas to function properly, there needs to be coordination between these cell types. The proper balance of activating and inhibitory signals has not yet been clarified, and many questions remain as to what contributes to the successful containment of bacteria in some granulomas while other progress and disseminate. Here we address some of the major players in granuloma biology as well as their potential functions.
Macrophage mediators in granulomas
Macrophages are major components of macaque and human granulomas and can act as anti-bacterial effector cells, host cells for bacteria, and immunomodulators for T cells. Nitric oxide synthase (NOS) and arginases (arg) are important enzymes in the macrophage response to Mtb infection. Inducible NOS (iNOS) converts L-arginine into nitric oxide (NO), a reactive product that can act in concert with reactive oxygen intermediates to kill microbes (71). Many cell types, including epithelial cells (authors’ unpublished data) and neutrophils (17), can express iNOS in macaque granulomas, but iNOS is most frequently associated with classically activated macrophages (17) or ‘M1-polarized’ macrophages (72). Although macaque macrophages can make NO and granulomas contain active NOS enzymes (17), on a single-cell basis, macaque macrophages express iNOS at a quantitatively lower level than that seen for mouse macrophages. The role of iNOS in human TB has been controversial (73), and there remain questions whether macrophage iNOS enhances killing of Mtb, acts as an immunomodulator, or if NO contributes to pathology and bacterial dissemination. In all likelihood, the consequences of iNOS expression in granulomas encompass all these options and are dependent on a granuloma's inflammatory state. Epithelioid macrophages can also express endothelial NOS (eNOS) (17,73) and although the function of eNOS in these cells remains to be elucidated, co-expression with iNOS suggests it may augment NO production in this population of cells.
NOS activity can be regulated by competition for L-arginine with Arg1 and Arg2 (74). These enzymes convert L-arginine into polyamine precursors and L-proline, an amino acid needed for collagen synthesis (74) and a contributor to fibrotic processes and lesion resolution (75). Other lines of evidence suggest macrophage arginase is not required for fibrosis (76), but that it regulates T-cell responses (76, 77) or engages in other, currently unidentified, antibacterial activities (78). In human (17, 79) and macaque (17) granulomas, neutrophils express more arg2 than arg1, while macrophages express significant quantities of arg1 (17). Arg1-expressing macrophages are described as ‘alternatively activated’ or ‘M2-polarized’ macrophages. While M1 and M2 macrophages are sometimes considered to be distinct cell subsets, Arg1 and iNOS are often co-expressed by the same macrophage in macaque granulomas (17), and the competition between these enzymes or the ratio of iNOS:arg1 expression likely determines a macrophage's functional polarity.
Granulomas need to maximize bacterial killing while limiting immune pathology to adjacent tissues, and the spatial organization of different macrophage subsets may help mediate this balance. In this context, NHP granulomas are organized into specialized microenvironments, with each region's functional character determined by the abundance of M1-like and M2-like macrophages (17). The lymphocyte cuff, where relatively few bacilli are found, contains large populations of M2-like macrophages with low iNOS:arg1 ratios, whereas epithelioid macrophage-dominated regions, which are associated with greater numbers of Mtb, have M1-like macrophages with higher iNOS:arg1 ratios (17). Based on these observations, we hypothesize that the that the M2-like macrophages in the lymphocyte cuff protect nearby tissue from immune pathology and produce the precursors for lesion resolution, while iNOS+eNOS+ M1-like epithelioid macrophages in bacteria-rich microenvironments are specialized for NO-mediated bactericidal activity. Although this appears to be a general property that most granuloma's exhibit, granulomas are dynamic and macrophage populations and phenotypes are likely to undergo significant changes over the course of infection
Granuloma T-cell cytokine expression in macaque models
T lymphocytes are a major cellular component of tuberculosis lung granulomas, and their macrophage-activating and cytolytic activities are considered to be critical to the control of initial and persistent Mtb infection (80-83). Pro-inflammatory cytokines such as IFN-γ and TNF are generally considered necessary for protection, while other pro-inflammatory cytokines, such as IL-2 or IL-17, may stimulate proliferation, activate local cells, or recruit cells from the periphery (55, 80, 83-87). The roles of anti-inflammatory/regulatory cytokines, such as IL-10, in granulomas are controversial (65). These anti-inflammatory cytokines may be important in reducing pathology. Granuloma T cells also express cytolytic and antimicrobial factors including perforin, granzyme B, and granulysin (authors’ unpublished data). Interestingly, granuloma T cells appear to exhibit a segregation of function, with cells either being cytokine producers or CD107+ cytolytic cells but rarely both (61,88). Our recent study investigating the functional dynamics of T cells with respect to the cytokine profile and bacterial burden of individual granulomas has shown that each granuloma is independent with respect to total cell numbers, frequency of T cells, patterns of cytokine response and bacterial burden within an individual host and amongst animals (Gideon et al., manuscript in review). These data further suggest that individual granulomas within a host are unique representations of the infection and cannot be classified as ‘active’ or ‘latent’. Instead, conventional clinical classifications of active disease and latent infection are more appropriate for a global or ‘whole host’ classification reflecting the overall host status of infection and pathology. We observed that T-cell cytokine profiles for individual granulomas within a single animal were distinct, highly variable and overlapped considerably across clinical disease status (Gideon et al., manuscript in review). Due to the recruitment of activated T cells to the site of disease, Mtb-specific T-cell responses are thought to be enriched in the granuloma compared to responses observed the peripheral blood. By contrast, we observed that only a limited proportion (~8%) of T cells in granulomas produce any of the 5 major cytokines we have examined (IFN-γ, IL-2, TNF, IL-17, and IL-10) in response to Mtb antigens, irrespective of a granuloma's bacterial burden or the clinical disease status of animals (Gideon et al., in review). These results, corroborated by IFN-γ ELISpot analysis of granuloma cells giving a comparable or even lower, frequency of cells producing IFN-γ (authors’ unpublished data), beg the question, why are granuloma T-cell responses so low?
There are several possibilities for the unexpected low frequency of functional T cells observed in granulomas. These include the presence of additional regulatory cytokines and cells including regulatory T cells, exhaustion of T cells within the granuloma as a result of chronic antigen exposure, the presence of T cells that migrate to the granuloma in response to chemokine and cytokine gradients but are not specific for mycobacterial antigens, and active downregulation of T-cell responses by mycobacterial factors. A major question raised by these studies is whether enhancing the functional T-cell response, particularly in the early phase of infection (such as in the setting of a vaccine response), would lead to increased killing of bacilli, increased inflammation, or both. In fact, our data suggest that a balance of cytokine responses (pro- and anti-inflammatory) best correlate with bacterial control (Gideon et al., manuscript in review).
B cells in macaque granulomas
The protective benefit of an antibody-mediated humoral response in TB has been controversial. Whether they contribute to protection or not, there are B cells, including plasma cells, in human and NHP granulomas. Activated B cells in germinal center-like clusters are commonly observed in the lymphocyte cuff of cynomolgus macaque granulomas (18, 89). Antibodies specific for Mtb antigens are enriched in granulomas as well (18, 89), although the influence these antibodies exert on control of the infection have not been investigated. Studies in murine models suggest that antibodies could play an immunomodulatory role in Mtb infection, perhaps by interaction with activatory or inhibitory Fcγ receptors on macrophages (reviewed in 90). NHP granuloma B cells also produce cytokines (authors’ unpublished data), but the B-cell contribution to a granuloma's cytokine milieu remains to be determined.
Neutrophils in macaque granulomas
The role of neutrophils in TB remains enigmatic. Neutrophils play a protective role in the early zebrafish granuloma (91), but little is known about their activities during acute Mtb infection of NHPs. At later stages of disease, neutrophils are often associated with poorly controlled TB (92-95). They can be abundant in granulomas, especially at the interface between epithelioid macrophages and caseum, but they are also present in the lymphocyte cuff (17). Neutrophils can be present in large numbers in bacteria-rich areas of necrotic granulomas and are frequently seen in the lymph node granulomas and more complex pathologies of animals with severe disease (17), but ability to control bacterial replication is questionable. Data indicating that neutrophils in human BAL samples contain viable Mtb (93) suggest these cells may not be killing Mtb, and may even provide an additional intracellular niche for Mtb. Though their ability to kill these bacilli in situ is questionable, neutrophils may have other functions that influence the granuloma environment including secretion of proteases and cytokines, nitric oxide production, and immunomodulation by arginase expression.
Modulation of the immune response
Vaccines are the primary strategy for sustained control of infectious disease. Although a vaccine against TB, BCG, exists and is given to children in most countries at birth, it is not effective at preventing infection and subsequent disease. A vaccine with improved efficacy is necessary to stem the tide of TB worldwide. The lack of a surrogate marker of protection is a major obstacle in the development of vaccines against TB. To date, the only well-established indicator is a vaccine-induced reduction in bacterial load using animal models before they go into human trials. An important distinction that must be made is that vaccine-induced immunogenicity has not yet been correlated with protection. The vaccines in clinical trials are primarily aimed at inducing a Th1-type CD4+ T-cell response. Very few, if any, are effective at inducing strong CD8+ T-cell responses, even though it is now believed that such responses are necessary for protection during natural infection. The potential for inducing antibody responses that might be protective has not been fully explored. Thus, a better understanding of how, and where, vaccines might work to prevent infection or disease is necessary.
NHP models are critical to this effort, as the immunologic similarities of humans and non-human primates are likely to lead to results that are translational for human clinical trials. Nonetheless, non-human primate studies are expensive and difficult, and different models give different answers. Previously, vaccines were tested in rhesus or cynomolgus macaques that were challenged with very high doses of Mtb bacilli (500-3000). High dose challenge bears little resemblance to the situation of exposure to Mtb in humans, which likely occurs in relatively small doses over a period of time. For example, a household contact sleeping next to a person with active TB will likely be exposed to small numbers of aerosolized Mtb for several hours nightly and over several weeks before the index case infection is recognized and treatment is initiated. None of our current models accurately recapitulate that situation. However, low dose challenge is likely to be much more relevant than high dose challenge to test vaccines. Low dose challenge (e.g. <25 bacilli) is likely to set off only a moderate inflammatory response, which may result in modest detection of the bacilli by the immune system. In contrast, high dose challenge will induce an inflammatory response, possibly causing a faster T-cell recall response at the site of infection. This could potentially result in findings that supported efficacy of a vaccine, which might be more difficult to observe in response to low dose infection. This has not been conclusively demonstrated, or even tested, in macaques. However, it is clear that a low dose infection more accurately reflects the situation that occurs in humans and therefore is likely to be a more appropriate model for testing vaccines.
Several vaccines have been tested in macaques, including viral-vectored vaccines such as MVA85A, live attenuated vaccines BCG, leuCD-deleted Mtb, and MTBVAC, and the protein-based vaccines H1 (96-99). These have shown modest if any reduction in disease or bacterial burden, and all were done with high dose challenge. We tested the protein-fusion H56 vaccine in a BCG-vaccinated cynomolgus macaques followed by low dose challenge with Mtb (97). Fewer BCG+H56 animals developed active TB, compared to unvaccinated or BCG-only vaccinated animals. This was also seen following high dose challenge, in studies performed in the primate facility in Cebu, The Philippines (97). However, the most interesting aspect of this work was that those monkeys vaccinated with BCG+H56 who presented with latent infection could not be reactivated with TNF neutralization. In contrast, unvaccinated or BCG-only immunized macaques that developed latent infection showed 75% reactivation with TNF neutralization. Thus, even though the vaccine did not provide sterilizing immunity, it was capable of preventing reactivation of latent infection. The mechanisms behind this protection are currently under investigation. Our published data suggested that the vaccine was associated with earlier recall responses to the H56 antigens post-challenge, compared to unvaccinated or BCG-only vaccinated animals. However, the immunologic responses within the granulomas were not assessed early post-infection. The importance of understanding the effects of vaccination on early events in the lungs post-challenge cannot be overstated.
The future for testing vaccines in the macaque models should take full advantage of technological advances, including the ability to track the infection using genetically bar-coded Mtb strains and PET-CT imaging (44) and sophisticated immunologic analysis not only of blood but in the airways, lung parenchyma, granulomas and lymph nodes. Such studies are likely to illuminate mechanisms by which vaccines can provide some measure of protection, and suggest areas for improvement.
Host-directed therapies, such as small molecules or cytokines that modulate the immune response, are of substantial interest in TB as well. Such immunologic modulators could dampen or enhance the inflammatory or T-cell responses in TB and could limit the course of infection. However, we understand very little about such modulators, including whether some could actually worsen the disease. Host-directed therapies may have a role in improving drug treatment or enhancing vaccine efficacy. Much more focus on host-directed therapies is necessary in NHP models that accurately reflect the complex pathology of Mtb infection before testing such strategies in human TB.
Summary
The NHP models of TB provide unique and exciting opportunities for investigating the immunology of TB. The similarities between humans and macaques genetically, physiologically, and immunologically provide greater confidence of direct translation of key findings to humans. Although the models are always being improved and refined, we have learned much from non-human primates in the TB field, and we expect that future findings will also significantly influence development of preventive and therapeutic strategies against TB.
Acknowledgements
This work was supported by grants from the NIH (JLF: AI50732, HL106804, EB012579, AI094745, HL110811, AI105422; PLL: AI063101), the Bill and Melinda Gates Foundation (JLF, PLL) and the Aeras Foundation (JLF). We are grateful to all former and current members of the Flynn and Lin laboratories for their intellectual contributions to this manuscript. Our special thanks to M. Teresa Coleman for contribution of figures. Finally, Drs. Edwin Klein and Christopher Janssen have been invaluable for pathological and clinical insights into the macaque models.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Gupta UD, Katoch VM. Animal models of tuberculosis. Tuberculosis (Edinb) 2005;85:277–293. doi: 10.1016/j.tube.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 2.McShane H, Williams A. A review of preclinical animal models utilised for TB vaccine evaluation in the context of recent human efficacy data. Tuberculosis (Edinb) 2014;94:105–110. doi: 10.1016/j.tube.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dutta NK, Illei PB, Jain SK, Karakousis PC. Characterization of a novel necrotic granuloma model of latent tuberculosis infection and reactivation in mice. Am J Pathol. 2014;184:2045–2055. doi: 10.1016/j.ajpath.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scanga CA, Mohan VP, Joseph H, Yu K, Chan J, et al. Reactivation of latent tuberculosis: variations on the Cornell murine model. Infect Immun. 1999;67:4531–4538. doi: 10.1128/iai.67.9.4531-4538.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Via LE, Lin PL, Ray SM, Carrillo J, Allen SS, et al. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect Immun. 2008;76:2333–2340. doi: 10.1128/IAI.01515-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silva Miranda M, Breiman A, Allain S, Deknuydt F, Altare F. The tuberculous granuloma: an unsuccessful host defence mechanism providing a safety shelter for the bacteria? Clin Dev Immunol. 2012;2012:139127. doi: 10.1155/2012/139127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canetti G. The Tubercle Bacillus. Springer Publishing Co, Inc.; New York, NY: 1955. [Google Scholar]
- 8.Calderon VE, Valbuena G, Goez Y, Judy BM, Huante MB, et al. A humanized mouse model of tuberculosis. PLoS One. 2013;8:e63331. doi: 10.1371/journal.pone.0063331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denton PW, Garcia JV. Humanized mouse models of HIV infection. AIDS Rev. 2011;13:135–148. [PMC free article] [PubMed] [Google Scholar]
- 10.Denton PW, Olesen R, Choudhary SK, Archin NM, Wahl A, et al. Generation of HIV latency in humanized BLT mice. J Virol. 2012;86:630–634. doi: 10.1128/JVI.06120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waters WR, Palmer MV, Thacker TC, Davis WC, Sreevatsan S, et al. Tuberculosis immunity: opportunities from studies with cattle. Clin Dev Immunol. 2011;2011:768542. doi: 10.1155/2011/768542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2001;193:271–280. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gombart AF, Saito T, Koeffler HP. Exaptation of an ancient Alu short interspersed element provides a highly conserved vitamin D-mediated innate immune response in humans and primates. BMC Genomics. 2009;10:321. doi: 10.1186/1471-2164-10-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barry CE, 3rd, Boshoff HI, Dartois V, Dick T, Ehrt S, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7:845–855. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin PL, Pawar S, Myers A, Pegu A, Fuhrman C, et al. Early events in Mycobacterium tuberculosis infection in cynomolgus macaques. Infect Immun. 2006;74:3790–3803. doi: 10.1128/IAI.00064-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin PL, Rodgers M, Smith L, Bigbee M, Myers A, et al. Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect Immun. 2009;77:4631–4642. doi: 10.1128/IAI.00592-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattila JT, Ojo OO, Kepka-Lenhart D, Marino S, Kim JH, et al. Microenvironments in tuberculous granulomas are delineated by distinct populations of macrophage subsets and expression of nitric oxide synthase and arginase isoforms. J Immunol. 2013;191:773–784. doi: 10.4049/jimmunol.1300113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phuah JY, Mattila JT, Lin PL, Flynn JL. Activated B cells in the granulomas of nonhuman primates infected with Mycobacterium tuberculosis. Am J Pathol. 2012;181:508–514. doi: 10.1016/j.ajpath.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharpe SA, Eschelbach E, Basaraba RJ, Gleeson F, Hall GA, et al. Determination of lesion volume by MRI and stereology in a macaque model of tuberculosis. Tuberculosis (Edinb) 2009;89:405–416. doi: 10.1016/j.tube.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Langermans JA, Andersen P, van Soolingen D, Vervenne RA, Frost PA, et al. Divergent effect of bacillus Calmette-Guerin (BCG) vaccination on Mycobacterium tuberculosis infection in highly related macaque species: implications for primate models in tuberculosis vaccine research. Proc Natl Acad Sci U S A. 2001;98:11497–11502. doi: 10.1073/pnas.201404898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehra S, Golden NA, Dutta NK, Midkiff CC, Alvarez X, et al. Reactivation of latent tuberculosis in rhesus macaques by coinfection with simian immunodeficiency virus. J Med Primatol. 2011;40:233–243. doi: 10.1111/j.1600-0684.2011.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capuano SV, 3rd, Croix DA, Pawar S, Zinovik A, Myers A, et al. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect Immun. 2003;71:5831–5844. doi: 10.1128/IAI.71.10.5831-5844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corbett EL, Bandason T, Cheung YB, Munyati S, Godfrey-Faussett P, et al. Epidemiology of tuberculosis in a high HIV prevalence population provided with enhanced diagnosis of symptomatic disease. PLoS Med. 2007;4:e22. doi: 10.1371/journal.pmed.0040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flynn JL, Chan J, Lin PL. Macrophages and control of granulomatous inflammation in tuberculosis. Mucosal Immunol. 2011;4:271–278. doi: 10.1038/mi.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.JoAnne F, Edwin K. A Color Atlas of Comparative Pathology of Pulmonary Tuberculosis. CRC Press; 2010. Pulmonary Tuberculosis in Monkeys. pp. 83–105. [Google Scholar]
- 27.Lin PL, Dartois V, Johnston PJ, Janssen C, Via L, et al. Metronidazole prevents reactivation of latent Mycobacterium tuberculosis infection in macaques. Proc Natl Acad Sci U S A. 2012;109:14188–14193. doi: 10.1073/pnas.1121497109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rieder HL. Risk of travel-associated tuberculosis. Clin Infect Dis. 2001;33:1393–1396. doi: 10.1086/323127. [DOI] [PubMed] [Google Scholar]
- 29.Zhao J, Zhao J, Van Rooijen N, Perlman S. Evasion by stealth: inefficient immune activation underlies poor T cell response and severe disease in SARS-CoV-infected mice. PLoS Pathog. 2009;5:e1000636. doi: 10.1371/journal.ppat.1000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nayar R, Schutten E, Bautista B, Daniels K, Prince AL, et al. Graded levels of IRF4 regulate CD8+ T cell differentiation and expansion, but not attrition, in response to acute virus infection. J Immunol. 2014;192:5881–5893. doi: 10.4049/jimmunol.1303187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lukens MV, van de Pol AC, Coenjaerts FE, Jansen NJ, Kamp VM, et al. A systemic neutrophil response precedes robust CD8(+) T-cell activation during natural respiratory syncytial virus infection in infants. J Virol. 2010;84:2374–2383. doi: 10.1128/JVI.01807-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Cao S, Peppers G, Kim SH, Graham BS. Clonotype-specific avidity influences the dynamics and hierarchy of virus-specific regulatory and effector CD4(+) T-cell responses. Eur J Immunol. 2014;44:1058–1068. doi: 10.1002/eji.201343766. [DOI] [PubMed] [Google Scholar]
- 33.Roman E, Miller E, Harmsen A, Wiley J, Von Andrian UH, et al. CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. J Exp Med. 2002;196:957–968. doi: 10.1084/jem.20021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawrence CW, Braciale TJ. Activation, differentiation, and migration of naive virus-specific CD8+ T cells during pulmonary influenza virus infection. J Immunol. 2004;173:1209–1218. doi: 10.4049/jimmunol.173.2.1209. [DOI] [PubMed] [Google Scholar]
- 35.Reiley WW, Calayag MD, Wittmer ST, Huntington JL, Pearl JE, et al. ESAT-6-specific CD4 T cell responses to aerosol Mycobacterium tuberculosis infection are initiated in the mediastinal lymph nodes. Proc Natl Acad Sci U S A. 2008;105:10961–10966. doi: 10.1073/pnas.0801496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SW, Oh DK, Lee SH, Kang HY, Lee CT, et al. Time interval to conversion of interferon-gamma release assay after exposure to tuberculosis. Eur Respir J. 2011;37:1447–1452. doi: 10.1183/09031936.00089510. [DOI] [PubMed] [Google Scholar]
- 37.Wasz-Hockert O. On the period of incubation in tuberculosis. Ann Med Intern Fenn. 1947;36:764–772. [PubMed] [Google Scholar]
- 38.Myers AJ, Marino S, Kirschner DE, Flynn JL. Inoculation dose of Mycobacterium tuberculosis does not influence priming of T cell responses in lymph nodes. J Immunol. 2013;190:4707–4716. doi: 10.4049/jimmunol.1203465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chackerian AA, Alt JM, Perera TV, Dascher CC, Behar SM. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect Immun. 2002;70:4501–4509. doi: 10.1128/IAI.70.8.4501-4509.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhatt K, Hickman SP, Salgame P. Cutting edge: a new approach to modeling early lung immunity in murine tuberculosis. J Immunol. 2004;172:2748–2751. doi: 10.4049/jimmunol.172.5.2748. [DOI] [PubMed] [Google Scholar]
- 41.Samstein M, Schreiber HA, Leiner IM, Susac B, Glickman MS, et al. Essential yet limited role for CCR2+ inflammatory monocytes during Mycobacterium tuberculosis-specific T cell priming. Elife. 2013;2:e01086. doi: 10.7554/eLife.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peters W, Scott HM, Chambers HF, Flynn JL, Charo IF, et al. Chemokine receptor 2 serves an early and essential role in resistance to Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2001;98:7958–7963. doi: 10.1073/pnas.131207398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khader SA, Partida-Sanchez S, Bell G, Jelley-Gibbs DM, Swain S, et al. Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. J Exp Med. 2006;203:1805–1815. doi: 10.1084/jem.20052545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin PL, Ford CB, Coleman MT, Myers AJ, Gawande R, et al. Sterilization of granulomas is common in active and latent tuberculosis despite within-host variability in bacterial killing. Nat Med. 2014;20:75–79. doi: 10.1038/nm.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woodring JH, Vandiviere HM, Fried AM, Dillon ML, Williams TD, et al. Update: the radiographic features of pulmonary tuberculosis. AJR Am J Roentgenol. 1986;146:497–506. doi: 10.2214/ajr.146.3.497. [DOI] [PubMed] [Google Scholar]
- 46.Geng E, Kreiswirth B, Burzynski J, Schluger NW. Clinical and radiographic correlates of primary and reactivation tuberculosis: a molecular epidemiology study. JAMA. 2005;293:2740–2745. doi: 10.1001/jama.293.22.2740. [DOI] [PubMed] [Google Scholar]
- 47.Jones BE, Ryu R, Yang Z, Cave MD, Pogoda JM, et al. Chest radiographic findings in patients with tuberculosis with recent or remote infection. Am J Respir Crit Care Med. 1997;156:1270–1273. doi: 10.1164/ajrccm.156.4.9609143. [DOI] [PubMed] [Google Scholar]
- 48.Lin PL, Coleman T, Carney JP, Lopresti BJ, Tomko J, et al. Radiologic responses in cynomolgous macaques for assessing tuberculosis chemotherapy regimens. Antimicrob Agents Chemother. 2013 doi: 10.1128/AAC.00277-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coleman MT, Maiello P, Tomko J, Frye LJ, Fillmore D, et al. Early Changes by (18)Fluorodeoxyglucose positron emission tomography coregistered with computed tomography predict outcome after Mycobacterium tuberculosis infection in cynomolgus macaques. Infect Immun. 2014;82:2400–2404. doi: 10.1128/IAI.01599-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeong YJ, Lee KS. Pulmonary tuberculosis: up-to-date imaging and management. AJR Am J Roentgenol. 2008;191:834–844. doi: 10.2214/AJR.07.3896. [DOI] [PubMed] [Google Scholar]
- 51.Jagirdar J, Zagzag D. In: Tuberculosis. Rom WN, Garay SM, editors. Lippincott, Williams & Wilkins.; Philadelphia, PA: 2004. [Google Scholar]
- 52.Lin PL, Rutledge T, Green AM, Bigbee M, Fuhrman C, et al. CD4 T cell depletion exacerbates acute Mycobacterium tuberculosis while reactivation of latent infection is dependent on severity of tissue depletion in cynomolgus macaques. AIDS Res Hum Retroviruses. 2012;28:1693–1702. doi: 10.1089/aid.2012.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin PL, Myers A, Smith L, Bigbee C, Bigbee M, et al. Tumor necrosis factor neutralization results in disseminated disease in acute and latent Mycobacterium tuberculosis infection with normal granuloma structure in a cynomolgus macaque model. Arthritis Rheum. 2010;62:340–350. doi: 10.1002/art.27271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Behr MA, Waters WR. Is tuberculosis a lymphatic disease with a pulmonary portal? Lancet Infect Dis. 2014;14:250–255. doi: 10.1016/S1473-3099(13)70253-6. [DOI] [PubMed] [Google Scholar]
- 55.Algood HM, Lin PL, Flynn JL. Tumor necrosis factor and chemokine interactions in the formation and maintenance of granulomas in tuberculosis. Clin Infect Dis 41 Suppl. 2005;3:S189–193. doi: 10.1086/429994. [DOI] [PubMed] [Google Scholar]
- 56.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 57.Mohan VP, Scanga CA, Yu K, Scott HM, Tanaka KE, et al. Effects of tumor necrosis factor alpha on host immune response in chronic persistent tuberculosis: possible role for limiting pathology. Infect Immun. 2001;69:1847–1855. doi: 10.1128/IAI.69.3.1847-1855.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 59.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, et al. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yao S, Huang D, Chen CY, Halliday L, Wang RC, et al. CD4+ T cells contain early extrapulmonary tuberculosis (TB) dissemination and rapid TB progression and sustain multieffector functions of CD8+ T and CD3-lymphocytes: mechanisms of CD4+ T cell immunity. J Immunol. 2014;192:2120–2132. doi: 10.4049/jimmunol.1301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mattila JT, Diedrich CR, Lin PL, Phuah J, Flynn JL. Simian immunodeficiency virus-induced changes in T cell cytokine responses in cynomolgus macaques with latent Mycobacterium tuberculosis infection are associated with timing of reactivation. J Immunol. 2011;186:3527–3537. doi: 10.4049/jimmunol.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diedrich CR, Mattila JT, Klein E, Janssen C, Phuah J, et al. Reactivation of latent tuberculosis in cynomolgus macaques infected with SIV is associated with early peripheral T cell depletion and not virus load. PLoS One. 2010;5:e9611. doi: 10.1371/journal.pone.0009611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Diedrich CR, Flynn JL. HIV-1/mycobacterium tuberculosis coinfection immunology: how does HIV-1 exacerbate tuberculosis? Infect Immun. 2011;79:1407–1417. doi: 10.1128/IAI.01126-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen CY, Huang D, Wang RC, Shen L, Zeng G, et al. A critical role for CD8 T cells in a nonhuman primate model of tuberculosis. PLoS Pathog. 2009;5:e1000392. doi: 10.1371/journal.ppat.1000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Redford PS, Murray PJ, O'Garra A. The role of IL-10 in immune regulation during M. tuberculosis infection. Mucosal Immunol. 2011;4:261–270. doi: 10.1038/mi.2011.7. [DOI] [PubMed] [Google Scholar]
- 66.Lazar-Molnar E, Chen B, Sweeney KA, Wang EJ, Liu W, et al. Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proc Natl Acad Sci U S A. 2010;107:13402–13407. doi: 10.1073/pnas.1007394107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barber DL, Mayer-Barber KD, Feng CG, Sharpe AH, Sher A. CD4 T cells promote rather than control tuberculosis in the absence of PD-1-mediated inhibition. J Immunol. 2011;186:1598–1607. doi: 10.4049/jimmunol.1003304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Green AM, Mattila JT, Bigbee CL, Bongers KS, Lin PL, et al. CD4(+) regulatory T cells in a cynomolgus macaque model of Mycobacterium tuberculosis infection. J Infect Dis. 2010;202:533–541. doi: 10.1086/654896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tameris M, McShane H, McClain JB, Landry B, Lockhart S, et al. Lessons learnt from the first efficacy trial of a new infant tuberculosis vaccine since BCG. Tuberculosis (Edinb) 2013;93:143–149. doi: 10.1016/j.tube.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wallis RS, Kim P, Cole S, Hanna D, Andrade BB, et al. Tuberculosis biomarkers discovery: developments, needs, and challenges. Lancet Infect Dis. 2013;13:362–372. doi: 10.1016/S1473-3099(13)70034-3. [DOI] [PubMed] [Google Scholar]
- 71.Kleinert H, Schwarz PM, Forstermann U. Regulation of the expression of inducible nitric oxide synthase. Biol Chem. 2003;384:1343–1364. doi: 10.1515/BC.2003.152. [DOI] [PubMed] [Google Scholar]
- 72.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733–3739. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 73.Choi HS, Rai PR, Chu HW, Cool C, Chan ED. Analysis of nitric oxide synthase and nitrotyrosine expression in human pulmonary tuberculosis. Am J Respir Crit Care Med. 2002;166:178–186. doi: 10.1164/rccm.2201023. [DOI] [PubMed] [Google Scholar]
- 74.Munder M. Arginase: an emerging key player in the mammalian immune system. Br J Pharmacol. 2009;158:638–651. doi: 10.1111/j.1476-5381.2009.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 76.Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, El Kasmi KC, et al. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009;5:e1000371. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rodriguez PC, Zea AH, Culotta KS, Zabaleta J, Ochoa JB, et al. Regulation of T cell receptor CD3zeta chain expression by L-arginine. J Biol Chem. 2002;277:21123–21129. doi: 10.1074/jbc.M110675200. [DOI] [PubMed] [Google Scholar]
- 78.Duque-Correa MA, Kuhl AA, Rodriguez PC, Zedler U, Schommer-Leitner S, et al. Macrophage arginase-1 controls bacterial growth and pathology in hypoxic tuberculosis granulomas. Proc Natl Acad Sci U S A. 2014;111:E4024–4032. doi: 10.1073/pnas.1408839111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pessanha AP, Martins RA, Mattos-Guaraldi AL, Vianna A, Moreira LO. Arginase-1 expression in granulomas of tuberculosis patients. FEMS Immunol Med Microbiol. 2012;66:265–268. doi: 10.1111/j.1574-695X.2012.01012.x. [DOI] [PubMed] [Google Scholar]
- 80.Cooper AM, Khader SA. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol Rev. 2008;226:191–204. doi: 10.1111/j.1600-065X.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 82.Flynn JL, Ernst JD. Immune responses in tuberculosis. Curr Opin Immunol. 2000;12:432–436. doi: 10.1016/s0952-7915(00)00116-3. [DOI] [PubMed] [Google Scholar]
- 83.O'Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, et al. The immune response in tuberculosis. Annu Rev Immunol. 2013;31:475–527. doi: 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- 84.Brighenti S, Andersson J. Local immune responses in human tuberculosis: learning from the site of infection. J Infect Dis 205 Suppl. 2012;2:S316–324. doi: 10.1093/infdis/jis043. [DOI] [PubMed] [Google Scholar]
- 85.Khader SA, Gopal R. IL-17 in protective immunity to intracellular pathogens. Virulence. 2010;1:423–427. doi: 10.4161/viru.1.5.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lin PL, Plessner HL, Voitenok NN, Flynn JL. Tumor necrosis factor and tuberculosis. J Investig Dermatol Symp Proc. 2007;12:22–25. doi: 10.1038/sj.jidsymp.5650027. [DOI] [PubMed] [Google Scholar]
- 87.Millington KA, Innes JA, Hackforth S, Hinks TS, Deeks JJ, et al. Dynamic relationship between IFN-gamma and IL-2 profile of Mycobacterium tuberculosis-specific T cells and antigen load. J Immunol. 2007;178:5217–5226. doi: 10.4049/jimmunol.178.8.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Einarsdottir T, Lockhart E, Flynn JL. Cytotoxicity and secretion of gamma interferon are carried out by distinct CD8 T cells during Mycobacterium tuberculosis infection. Infect Immun. 2009;77:4621–4630. doi: 10.1128/IAI.00415-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Slight SR, Rangel-Moreno J, Gopal R, Lin Y, Fallert Junecko BA, et al. CXCR5(+) T helper cells mediate protective immunity against tuberculosis. J Clin Invest. 2013;123:712–726. doi: 10.1172/JCI65728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kozakiewicz L, Phuah J, Flynn J, Chan J. The role of B cells and humoral immunity in Mycobacterium tuberculosis infection. Adv Exp Med Biol. 2013;783:225–250. doi: 10.1007/978-1-4614-6111-1_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang CT, Cambier CJ, Davis JM, Hall CJ, Crosier PS, et al. Neutrophils exert protection in the early tuberculous granuloma by oxidative killing of mycobacteria phagocytosed from infected macrophages. Cell Host Microbe. 2012;12:301–312. doi: 10.1016/j.chom.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ozaki T, Nakahira S, Tani K, Ogushi F, Yasuoka S, et al. Differential cell analysis in bronchoalveolar lavage fluid from pulmonary lesions of patients with tuberculosis. Chest. 1992;102:54–59. doi: 10.1378/chest.102.1.54. [DOI] [PubMed] [Google Scholar]
- 93.Eum SY, Kong JH, Hong MS, Lee YJ, Kim JH, et al. Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest. 2010;137:122–128. doi: 10.1378/chest.09-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brahmbhatt S, Black GF, Carroll NM, Beyers N, Salker F, et al. Immune markers measured before treatment predict outcome of intensive phase tuberculosis therapy. Clin Exp Immunol. 2006;146:243–252. doi: 10.1111/j.1365-2249.2006.03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ashitani J, Mukae H, Hiratsuka T, Nakazato M, Kumamoto K, et al. Elevated levels of alpha-defensins in plasma and BAL fluid of patients with active pulmonary tuberculosis. Chest. 2002;121:519–526. doi: 10.1378/chest.121.2.519. [DOI] [PubMed] [Google Scholar]
- 96.McShane H. Tuberculosis vaccines: beyond bacille Calmette-Guerin. Philos Trans R Soc Lond B Biol Sci. 2011;366:2782–2789. doi: 10.1098/rstb.2011.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lin PL, Dietrich J, Tan E, Abalos RM, Burgos J, et al. The multistage vaccine H56 boosts the effects of BCG to protect cynomolgus macaques against active tuberculosis and reactivation of latent Mycobacterium tuberculosis infection. J Clin Invest. 2012;122:303–314. doi: 10.1172/JCI46252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arbues A, Aguilo JI, Gonzalo-Asensio J, Marinova D, Uranga S, et al. Construction, characterization and preclinical evaluation of MTBVAC, the first live-attenuated M. tuberculosis-based vaccine to enter clinical trials. Vaccine. 2013;31:4867–4873. doi: 10.1016/j.vaccine.2013.07.051. [DOI] [PubMed] [Google Scholar]
- 99.Jensen K, Ranganathan UD, Van Rompay KK, Canfield DR, Khan I, et al. A recombinant attenuated Mycobacterium tuberculosis vaccine strain is safe in immunosuppressed simian immunodeficiency virusinfected infant macaques. Clin Vaccine Immunol. 2012;19:1170–1181. doi: 10.1128/CVI.00184-12. [DOI] [PMC free article] [PubMed] [Google Scholar]