Abstract
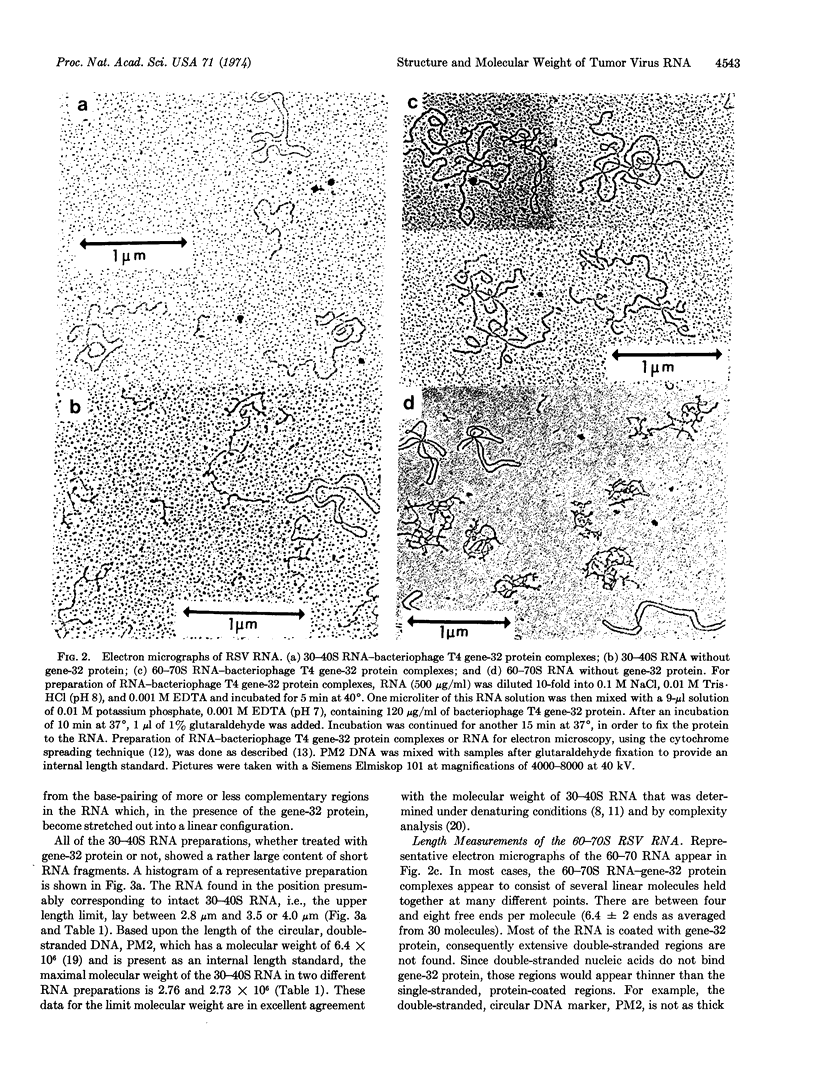
The structure and molecular weight of the 60-70S RNA complex and the 30-40S RNA species of Rous sarcoma virus were analyzed in an electron microscope after treatment of the RNAs with the bacteriophage T4 gene-32 protein to stretch out the RNA strands. Although all RNA preparations treated with gene-32 protein showed considerable heterogeneity in length, a significant fraction of the RNA retained its original sedimentation coefficient after treatment to allow the following conclusions to be made: The 30-40S RNA was confirmed to be a linear polynucleotide with a molecular weight of about 3 × 106. The 60-70S RNA exhibited a network structure with a molecular weight predominantly of about 6 × 106. Therefore, the subunit hypothesis for the 60-70S RNA is confirmed. A model for the structure and molecular weight of the 60-70S RNA postulates that the complex consists of two 30-40S RNA subunits held together at many points. This model elucidates the biological observation that the infectivity of RNA tumor viruses is proportional to the amount of 30-40S RNA in a virus preparation and not to the amount of 60-70S RNA.
Keywords: RNA subunits, bacteriophage T4 gene-32 protein, RNA secondary structure, RNA tumor virus infectivity, electron microscopy of RNA
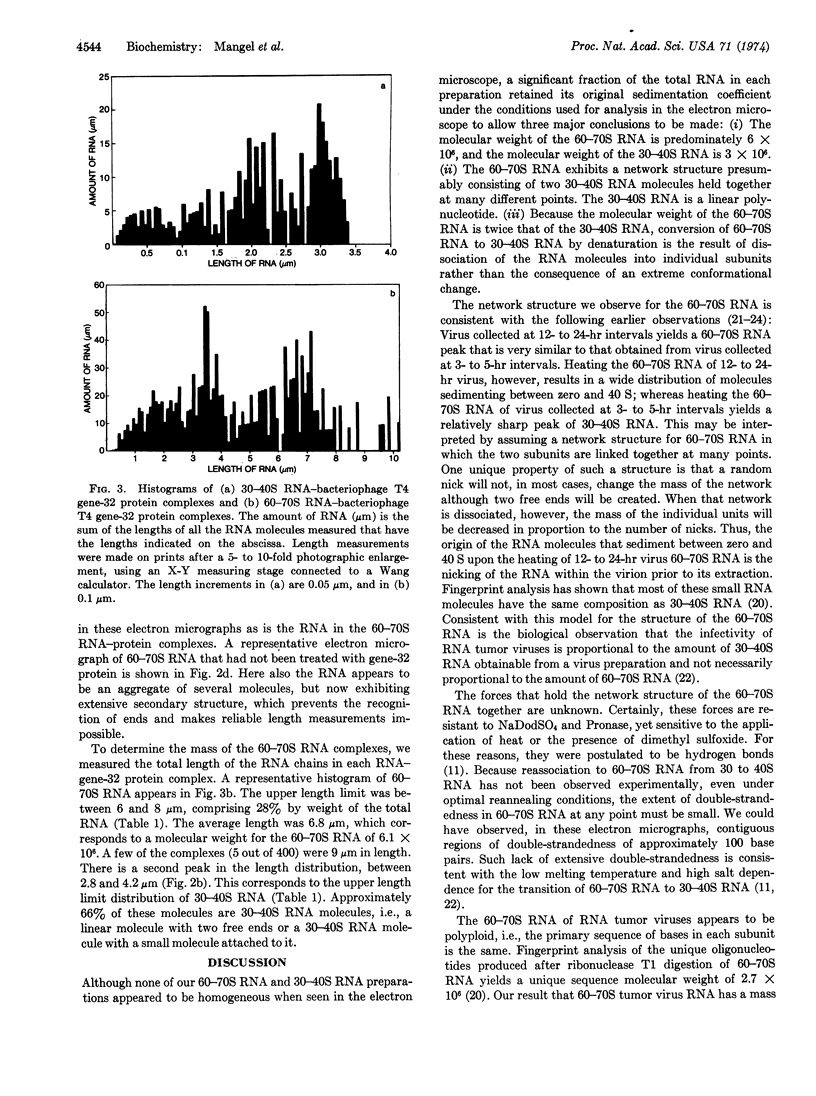
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader J. P., Steck T. L. Analysis of the ribonucleic acid of murine leukemia virus. J Virol. 1969 Oct;4(4):454–459. doi: 10.1128/jvi.4.4.454-459.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boedtker H. Dependence of the sedimentation coefficient on molecular weight of RNA after reaction with formaldehyde. J Mol Biol. 1968 Jul 14;35(1):61–70. doi: 10.1016/s0022-2836(68)80036-1. [DOI] [PubMed] [Google Scholar]
- Canaani E., Duesberg P. Role of subunits of 60 to 70S avian tumor virus ribonucleic acid in its template activity for the viral deoxyribonucleic acid polymerase. J Virol. 1972 Jul;10(1):23–31. doi: 10.1128/jvi.10.1.23-31.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani E., Helm K. V., Duesberg P. Evidence for 30-40S RNA as precursor of the 60-70S RNA of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1973 Feb;70(2):401–405. doi: 10.1073/pnas.70.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius H., Westphal H., Axelrod N. Length measurements of RNA synthesized in vitro by Escherichia coli RNA polymerase. J Mol Biol. 1973 Mar 15;74(4):677–687. doi: 10.1016/0022-2836(73)90056-9. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Cardiff R. D. Structural relationships between the RNA of mammary tumor virus and those of other RNA tumor viruses. Virology. 1968 Dec;36(4):696–700. doi: 10.1016/0042-6822(68)90206-7. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H. Physical properties of Rous Sarcoma Virus RNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1511–1518. doi: 10.1073/pnas.60.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Gel electrophoresis of avian leukosis and sarcoma viral RNA in formamide: comparison with other viral and cellular RNA species. J Virol. 1973 Sep;12(3):594–599. doi: 10.1128/jvi.12.3.594-599.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. RNA species obtained from clonal lines of avian sarcoma and from avian leukosis virus. Virology. 1973 Jul;54(1):207–219. doi: 10.1016/0042-6822(73)90130-x. [DOI] [PubMed] [Google Scholar]
- Erikson R. L. Studies on the RNA from avian myeloblastosis virus. Virology. 1969 Jan;37(1):124–131. doi: 10.1016/0042-6822(69)90313-4. [DOI] [PubMed] [Google Scholar]
- Granboulan N., Huppert J., Lacour F. Examen au microscope electronique du RNA du virus de la myeloblastose aviaire. J Mol Biol. 1966 Apr;16(2):571–575. doi: 10.1016/s0022-2836(66)80196-1. [DOI] [PubMed] [Google Scholar]
- Kakefuda T., Bader J. P. Electron microscopic observations on the ribonucleic acid of murine leukemia virus. J Virol. 1969 Oct;4(4):460–474. doi: 10.1128/jvi.4.4.460-474.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson U., Mulder C., Deluis H., Sharp P. A. Cleavage of adenovirus type 2 DNA into six unique fragments by endonuclease R-RI. Proc Natl Acad Sci U S A. 1973 Jan;70(1):200–204. doi: 10.1073/pnas.70.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. S., Pitkanen A., Rubin H. The nucleic acid of the Bryan strain of Rous sarcoma virus: purification of the virus and isolation of the nucleic acid. Proc Natl Acad Sci U S A. 1965 Jul;54(1):137–144. doi: 10.1073/pnas.54.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar N. H., Moore D. H. Electron microscopy of the nucleic acid of mouse mammary tumor virus. J Virol. 1970 Feb;5(2):230–236. doi: 10.1128/jvi.5.2.230-236.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. Secondary structure maps of RNA: processing of HeLa ribosomal RNA. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2827–2831. doi: 10.1073/pnas.70.10.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]