Summary
A major approach for immunologic intervention in tuberculosis involves redirecting the outcome of the host immune response from the induction of disease to pathogen control. Cytokines and lipid mediators known as eicosanoids play key roles in regulating this balance and as such represent important targets for immunologic intervention. While the evidence for cytokine/eicosanoid function derives largely from the investigation of murine and zebra fish experimental infection models, clinical studies have confirmed the existence of many of the same pathways in tuberculosis patients. Here we summarize new data that reveal important intersections between the cytokine and eicosanoid networks in the host response to mycobacteria and discuss how targeting this crosstalk can promote resistance to lethal Mycobacterium tuberculosis infection. This approach could lead to new host-directed therapies to be used either as an adjunct for improving the efficacy of standard antibiotic treatment or for the management of drug-resistant infections.
Keywords: tuberculosis, cytokines, eicosanoids, lipoxins, prostaglandins, host-directed therapy
Introduction
The immune response to mycobacteria is a proverbial double-edged sword with one edge battling infection and the other triggering tissue pathology. Two classes of mediators, cytokine proteins and eicosanoid lipids, are known to play critical roles as effectors and/or regulators of this swinging immunologic blade. The cytokines (and their associated chemokines) are direct gene products synthesized largely by cells of the immune system in response to mycobacterial infection (1, 2). Eicosanoids, in contrast, are generated enzymatically via oxygenation of the polyunsaturated omega-6 fatty acid, arachidonic acid, by both cyclooxygenases and lipoxygenases (3, 4).
The role of cytokines in host resistance to Mycobacterium tuberculosis (Mtb) is well established and was first demonstrated in elegant gene knockout studies in the murine infection model documenting major functions for interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and interleukin-12 (IL-12) in bacterial control (5–8). Importantly, these early findings were later validated by the observation of exacerbated mycobacterial disease in humans with mutations in the IFN-γ and IL-12 signaling pathways (9, 10) and in patients given TNF blockade to treat rheumatoid arthritis or Crohn’s disease (11, 12). Such concordance between the role of cytokines in a rodent experimental model and its corresponding human disease is not universally seen in other infections and provides an important justification for the use of the mouse in studies of cytokine function and regulation in tuberculosis (TB).
Later research connected these cytokines into an ‘IL-12/IFN-γ axis’ in which IL-12, produced early in infection by antigen-presenting cells (APCs), stimulates the synthesis of IFN-γ by natural killer (NK) cells and T cells which in turn activates macrophages to produce TNF (2, 13, 14). TNF then promotes intracellular killing of the pathogen through the production of reactive oxygen and nitrogen species and contains the pathogen by contributing to granuloma maintenance (13–15) (Fig. 1). Similar TNF-associated, IFN-γ-dependent pathways have been implicated in the mechanism of host resistance to a wide variety of intracellular pathogens (16).
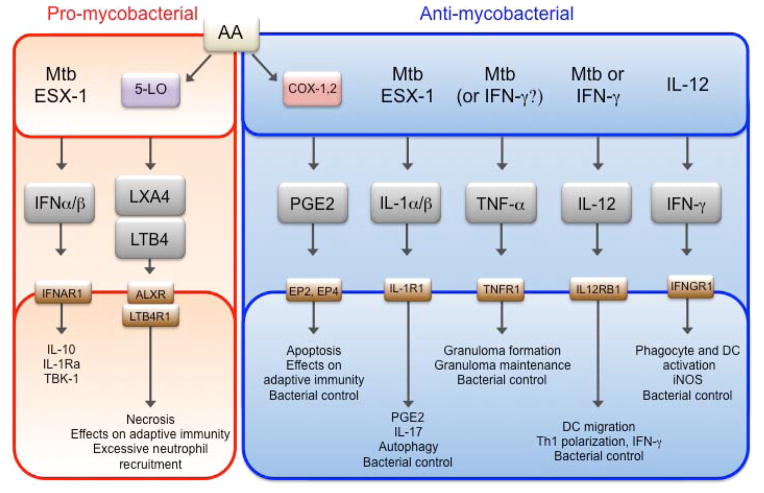
Fig. 1. Overview of eicosanoid and cytokine-driven effector pathways.
Pathways were grouped based on their effector functions (bottom boxes) into pro-(red) or anti-mycobacterial (blue). Upstream inducers of the pathways are depicted in the top box and corresponding receptors signaling triggering downstream functions are designated on the bottom box.
While research on effector cytokines in Mtb progressed, a parallel interest emerged in regulatory cytokines that might promote infection or on the other hand protect the host against pathology mediated by an excessive immune response. IL-10 had been described as a major regulator of IL-12-dependent immune responses, and indeed studies in IL-10-deficient mice confirmed its role in governing the T-helper 1 (Th1) cytokine pathway mediating host resistance to Mtb (17, 18). Evidence for the existence of a second family of cytokines regulating the response to Mtb infection arose from the demonstrations that virulent bacterial isolates stimulate the production of Type I IFNs in human and mouse macrophages in vitro (19–22) and in mice in vivo (23, 24) and that mice deficient in the Type I IFN receptor (IFNAR1) show enhanced resistance to infection (20, 21, 24–26) (Fig. 1). The human disease relevance of these observations has been supported by four independent studies involving different patient cohorts, documenting an association of active TB with an IFN-induced gene signature (27–30).
Although distinct from cytokines in their chemical structure, receptors, and mechanisms of action, lipid mediators of the eicosanoid family have been demonstrated in recent years to have a nearly equivalent influence on the outcome of experimental Mtb infection. The enzyme 5-lipoxygense (5-LO, Alox5) is required for the generation of lipoxin (LXA) and leukotriene (LT) mediators derived from arachidonic acid (AA) (Fig. 1). The finding that Alox5-deficient mice show increased resistance to Mtb infection and that normal susceptibility can be restored by supplementation with a stable lipoxin analog provided the first demonstration of a functional role of eicosanoids in regulating control of the pathogen (31). The importance of lipid mediators was re-enforced by elegant studies establishing a role for eicosanoids in the cell-death modalities of Mtb-infected macrophages, bacterial virulence, and adaptive immunity (32–34). This work also revealed a major function for the prostaglandin arm of arachadonic metabolism, in promoting mycobacterial control, a concept supported by the increased susceptibility of Ptges−/− mice lacking PGE2 synthase (32, 33). Further studies in a zebra fish model of Mycobacterium marinum infection confirmed the association of increased lipoxin production with decreased mycobacterial resistance (35, 36).
A third set of effector cytokines that play important roles in Mtb infection are the IL-1 family members, IL-1α and IL-1β (Fig. 1). While traditionally thought of as the prototypical mediators of acute inflammation, early studies in knockout mice suggested a protective function for the cytokines in virulent Mtb infections (37–39). These findings were later confirmed in work demonstrating that the acute susceptibility to Mtb of mice deficient in the Toll-like receptor (TLR)/IL-1R adaptor MyD88 could be explained largely by their defect in IL-1R signaling (40, 41). In these studies, Il1r1−/− and Myd88−/− mice were directly compared and indistinguishable necrotic lung pathology as well as susceptibility to infection was observed. These findings imply that the early signaling by IL-1 is more critical in the innate response to the pathogen than TLR recognition. It is now understood that both IL-1 species, which signal through the same IL-1R, are independently required for host resistance against Mtb infection and deficiency in either IL-1α or IL-1β renders mice more susceptible to Mtb infection (21). Recent advances in our understanding of the cellular source, protective downstream effector mechanisms and how this potentially dangerous inflammatory cytokine is both held in check and regulated by other cytokine pathways will be discussed in more detail below.
While the above observations revealed a set of inflammatory pathways by which the innate response to Mtb regulates the outcome of infection, both the anti- and pro-bacterial mechanisms they trigger and their potential interactions still remain poorly understood. The following article focuses on recent advances in the integration of these Mtb-induced regulatory pathways as well as on the use of this knowledge in the design of candidate host directed therapies (HDT).
The IL-12/IFN-γ axis: the backbone of host resistance?
The IL-12-induced production of IFN-γ is the most studied and perhaps best validated cytokine pathway regulating Mtb infection (Fig. 1). In addition to being implicated in both human disease and in the murine infection model, this pathway is attractive in that it links the innate with the Th1-dominated adaptive response to Mtb. At its core is the induction of IL-12/23 p40 from different myeloid lineages in infected lung, most notably CD103+CD11b−CD11c+ lung-resident dendritic cells (DC) and CD11b+CD11c+ DCs (21, 42–44) (Fig. 2). One of the IL-12 family cytokines, a homodimer of IL-12 p40 (p402) stimulates chemokine responsiveness in DCs promoting their migration from the lung to the draining lymph nodes (45). The second, IL-12p70 (the p40, p35 heterodimer) is required for the optimal polarization of CD4+ T cells into IFN-γ-producing Th1 cells (8). Finally, IL-23 (the p40, P19 heterodimer) supports the IL-17 response to Mtb within the lung, which although not required for early control of Mtb in the lung can be play a role in vaccine-induced protection (46).
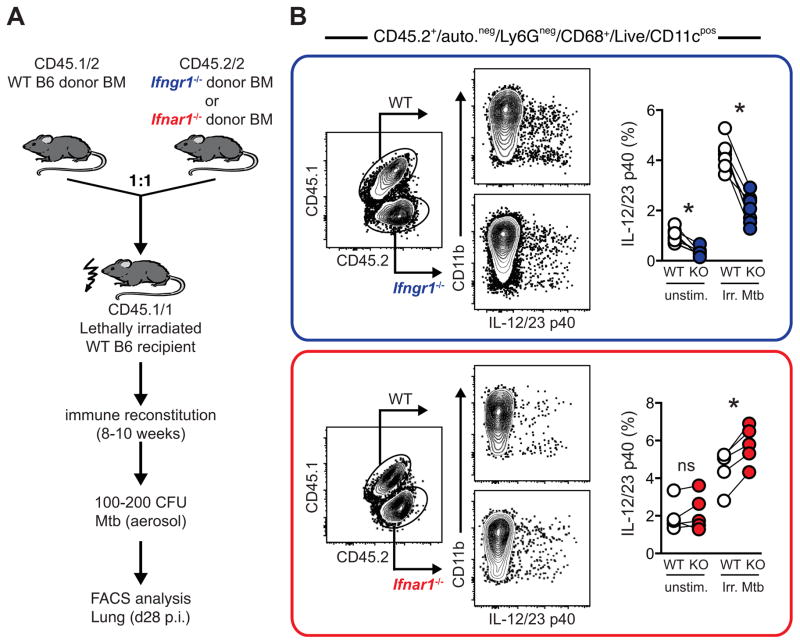
Fig. 2. IL-12 p40 expression is differentially regulated by Type I and Type II IFNs in the lungs of Mtb-infected mice.
(A). WT CD45.1/1 mice were lethally irradiated and reconstituted with equal ratios of WT (CD45.1/2) or Ifngr1−/− or Ifnar1−/− (CD45.2/2) BM cells and infected with Mtb, as described previously (21, 24). After 8–10 weeks of immune reconstitution, mice were infected with 100–200 CFU via aerosol exposure. This experimental approach allows for direct assessment of WT and gene-deficient cells in the same animal, thereby bypassing potential caveats of WT vs. KO comparisons such as vastly different bacterial loads, inflammatory profiles or pathology. (B). Blue Box; Analysis of donor BM derived CD11cpos myeloid cells 4 weeks post infection in isolated lung cells marked by CD45.1 and CD45.2 expression and frequency of IL-12/23p40 expression by WT (white circles) or Ifngr1−/− (KO, blue circles) cells after re-stimulation for 5 h with irradiated Mtb (Irr. Mtb) or without (unstim.). Red Box: Analysis of donor BM-derived CD11cpos myeloid cells 4 weeks post infection in isolated lung cells marked by CD45.1 and CD45.2 expression and frequency of IL-12/23p40 expression by WT (white circles) or Ifnar1−/− (KO, red circles) cells after re-stimulation for 5 h with irradiated Mtb (Irr. Mtb) or without (unstim.). Data are representative of two individual experiments with a minimum of three mice each. Each connecting line depicts an individual animal and asterisk (*) denotes significant differences as assessed by a paired student’s t test.
During Mtb infection in mice, the IL-12/IFN-γ axis represents a closed circuit, in that IL-12 induced IFN-γ in turn amplifies IL-12/23 p40 production by both CD11b+ and CD11b−CD11c+ pulmonary DC subsets (Fig 2A, B, blue box). This cytokine feedforward loop (Fig. 3) is likely critical in triggering optimal anti-mycobacterial effector functions of pulmonary myeloid cells, in particular IFN-γ dependent inducible nitric oxide synthase (iNOS) (2, 21).
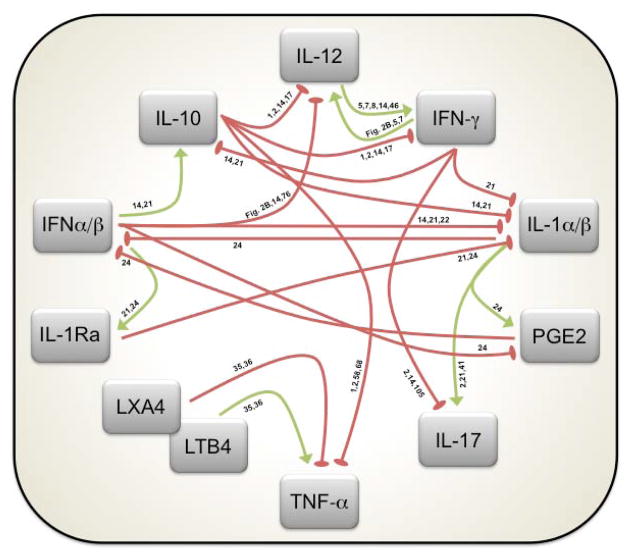
Fig. 3. Cytokine and eicosanoid regulatory networks during Mtb infection.
Conceptualization of the regulatory circuits governing the outcome of Mtb infection as discussed in this review. Connections with green arrows denote positive regulation, while red oval-shaped arrowheads reflect negative regulation. References describing the regulatory pathways involved are associated with each line.
While the Th1 lymphocytes generated as a result of IL-12 polarization are clearly important for control of infection, their effector function is not completely understood. In particular, IFN-γ production by CD4+ T cells, albeit generally important, appears play additional roles that are separate from promoting bacterial control, and evidence has been accumulating that other effector functions of Th1 cells contribute equally to host resistance. For example, in Bacille Calumet Guerrin (BCG)-vaccinated infants, protection against Mtb infection does not positively correlate with the levels of IFN-γ-producing circulating CD4+ T cells (47). Moreover, in mice, Th1 cells can transfer protection even when derived from donors genetically deficient in IFN-γ (21, 48). These data suggest the existence of additional factors besides IFN-γ production associated with Th1 polarization that are critical for bacterial control. For example, Tim3 expression on Th1 cells is known to play a major role in stimulating infected macrophages to restrict intracellular growth of Mtb (49). In addition, it was recently found that the Mtb-specific CD4+ T cells are comprised of two major subsets that either rapidly migrate into the lung parenchyma or are preferentially retained in the lung blood vessels (44, 50). Interestingly, the CD4+ T cells recruited to the lung parenchyma are highly protective against Mtb infection in vivo yet produce lower amounts of IFN-γ than the non-protective cells retained in the vasculature (51). Finally, it is also clear that IFN-γ-producing Th1 cells can play a pathogenic role in Mtb infection (52) and that they can exert an immunosuppressive effect on macrophage IL-1 production (21). Interestingly, while in vitro IFN-γ broad inhibits IL-1α and IL-1β from both macrophage and DCs, in the lungs of Mtb-infected mice, CD4+ T-cell-derived IFN-γ specifically suppresses IL-1 production by inflammatory monocytes but not DCs (24). Thus, immunotherapeutic interventions targeting the IL-12/IFN-γ axis may have unpredictable outcomes that will be highly context and cell type-dependent.
Despite the potential complications noted above, in several clinical trials patients with TB or non-tuberculous mycobacteria (NTM) given IFN-γ in addition to standard chemotherapy showed improved disease outcomes (53–55), and in a murine study, administration of IL-12p70 was found to enhance drug induced clearance of Mycobacterium avium infection (56). Thus, the IL-12/IFN-γ axis remains a candidate target for HDT of mycobacterial disease, although because of their high cost in treatment, the use of recombinant IL-12 and IFN-γ would likely be limited to drug-resistant patients. As noted by Hawn (57), this should not deter the search for other downstream effectors of the pathway that could serve as more practical immunotherapeutics.
TNF-α: the archetypal double-edged sword
Because of its positive regulation by IFN-γ in mononuclear phagocytes and autocrine role in IFN-γ-dependent macrophage activation and microbicidal activity, TNF is often considered a component of the IL-12/IFN-γ Th1 axis (16). In common with IFN-γ, TNF has well-documented roles in both human and murine resistance to Mtb (2, 14, 58). Although there is general agreement concerning its function in intracellular restriction of mycobacterial growth in macrophages, its purported secondary function in the granulomatous response is complex and has been the subject of considerable debate (15, 57, 59, 60). Based on studies in TB-infected patients and multiple mycobacteria-host experimental infection models, it now appears that TNF plays a much greater role in the maintenance than in the formation of granulomas. In this capacity, the cytokine could have probacterial effects by sustaining the mycobacterial niche of the granuloma and as suggested by experiments in the zebrafish model, by promoting reactive oxygen species (ROS)-mediated macrophage necrosis leading to pathogen dissemination (15, 61, 62).
TNF was one of the first cytokines to be targeted in experimental HDT of TB. This was prior to the demonstration of the reactivation of latent TB by antibody mediated TNF blockade and involved the use of thalidomide, a phosphodiesterase (PDE) inhibitor that suppresses TNF and vascular endothelial growth factor (VEGF) production (63). Although the initial clinical trial was stopped because of the drug’s side effects, this stimulated the development of less toxic PDE inhibitors that have been assessed in a variety of subsequent studies with varying degrees of success in animal models (57, 64). The concept that TNF inhibition might benefit the infected host was directly tested by Wallis et al. (65) in a trial on human immunodeficiency virus (HIV)-infected patients with pulmonary TB who were given the TNF blocking drug etanercept at the initiation of antibiotic therapy. The patients showed more rapid sputum clearance than historical controls. The authors had hypothesized that adjunctive TNF blockade by restoring latent TB into an actively replicating form might make bacilli more susceptible to standard chemotherapy, and these findings were consistent with that concept as were the results of later studies in animal models using PDE inhibitors to suppress TNF production concurrent with antibiotic therapy (66, 67). Together, these observations emphasize that TNF plays a series of complex roles in the host-mycobacterium interaction and needs to be targeted in a context specific manner.
A further complication is our limited knowledge of the upstream signals and cellular regulation of TNF during Mtb infection as well as its interplay with other cytokine and lipid mediator networks (Figs 2 and 3). Interestingly, while IL-12-deficient Mtb infected mice display defective TNF production (8), the same does not appear to hold true for IFN-γ-deficient animals (5), thus calling into question the extent of its regulation by the Th1 cytokine pathway. As discussed below, Mtb-induced TNF in some but not all situations is suppressed by IL-10, and recent studies in the zebra fish model have linked the regulation of TNF expression with 5-LO products (68, 69). Because of TNF’s mixed beneficial and harmful consequences during TB infection, defining the effects of these other pathways on TNF production will be important if they are to be deployed as effective and safe host directed therapies.
IL-10: key regulator of the Th1 response to Mtb
As a major downmodulator of both Th1 effector function and inflammation, IL-10 has always been assumed to play a role in the control of anti-mycobacterial immune responses and clearly does so in vitro with cells from both infected mice and humans. Moreover, Mtb-infected IL-10-deficient mice display enhanced control of infection, although this outcome is highly variable between labs and between different mouse strains. In contrast to what has been observed with other pathogens such as Toxoplasma gondii (70), IL-10-deficient mice infected with Mtb do not display overt increases in pathology. Nevertheless, in the well-studied Mtb Il10−/− BALB/c model, enhanced levels and earlier production of IFN-γ, granulocyte-macrophage colony-stimulating factor (GM-CSF), G-CSF, TNF, and IL-17A are observed along with increased T-cell recruitment and Th1 differentiation in lung (17). Along with its known effects in inhibiting macrophage microbicidal activity, IL-10 has also been shown to inhibit DC trafficking to lymph nodes (71), therefore opposing the proposed function of IL-12 in the same process (13, 45). Thus, it is possible that IL-10 may serve the pathogen by delaying innate inflammatory cytokine production, myeloid cell recruitment, and subsequent induction of the T-cell response, thereby allowing Mtb to disseminate before being subjected to immune control.
As discussed below, IL-10 is also a downstream mediator triggered by Type I IFN and perhaps other pathways that promote Mtb infection. While its effects may be limited to certain settings (e.g. progressive disease, as discussed below), IL-10 blockade deserves consideration as strategy for HDT of TB as unlike other candidate interventions (e.g. TNF blockade), it has no reported host-detrimental effects on the immune response to this pathogen.
Type I IFN: master regulator of Mtb disease?
Although well known for their antiviral effects, the Type I IFNs have in recent years been revealed to promote infection by certain bacterial pathogens. In the case of Mtb, early studies revealed an association of bacterial hypervirulence with the enhanced production of Type I IFN in the murine infection model (19). A mechanistic link was provided by the observation (obtained in most but not all laboratories) that Ifnar1−/− mice are more resistant to infection and that administration of IFNα/β or the Type I IFN inducer (poly ICLC) promotes acute disease (19, 21, 23, 24, 72).
A role for Type I IFN as a potential determinant of human tuberculosis was revealed by the seminal observation of a prominent whole blood IFNγ transcriptional signature that correlated with the extent of radiographic disease and that was lessened upon successful chemotherapy (27). This finding originally obtained with patients from the UK and South Africa has been independently validated in studies of additional African and Indonesian cohorts (28–30). In vitro studies in murine and human cells showed that Type I IFN induction is preferentially induced by virulent Mtb and that this property is ESX-1 and therefore RD-1 dependent (20, 22). RD-1 encodes the ESX-1 specialized secretion system that mediates translocation of bacterial effectors from the phagolysosome into the cytoplasm where they can interact with cytosolic receptors. Two candidate mycobacterial products have been identified as cytoplasmic inducers of type I IFN. The first is an unusual peptidoglycan species which interacts with the Nod2 receptor leading to Type I IFN induction through a TBK-1, Irf-5-dependent pathway (73). The second candidate is bacterial DNA, which is postulated to trigger IFN through the Sting/TBK-1/Irf-3 cytosolic surveillance pathway (74). Together these observations provide a molecular basis for the central role played by Type I IFN in mycobacterial virulence determination.
The mechanism(s) by which Type I IFNs promote bacterial infections and disease are a major topic of investigation for the field (75). Much of the early progress in our understanding of how IFNs regulate bacterial infections derived from studies in the mouse model of Listeria monocytogenes infection (14, 76). A common point of intersection for both infections is Type I IFN mediated counter regulation of the host-protective IL-12/IFN-γ axis (77). In vitro and in response to Listeria monocytogenes infection both IFNα and IFNβ potently inhibit IL-12 production (78), and after Mtb infection in vitro and in vivo, IFNγ-induced IL-12 p40 production is impaired by IFNAR1 signals acting directly on IL-12-producing DCs subsets, even when bacterial loads and inflammation are normalized (14) (Fig.2A, B, red box). In the early work with hypervirulent Mtb strains, Type I IFN induction was found to be associated with the suppression of Th1 responses (19) and IFNAR1-mediated inhibition of IFN-γ-driven inducible nitric oxide synthase (iNOS) has been documented in vivo in pulmonary myeloid cells (21). However, in contrast to what has been described in Listeria monocytogenes (79), during Mtb infection in vivo Type I IFN does not significantly suppress myeloid cell responsiveness to IFN-γ via downregulation of CD119 (IFNGR1) (21).
Although able to exert a partial protective effect in the absence of IFNR1 signaling (26) and in some settings be triggered in vitro by avirulent or less virulent mycobacterial strains (80), Type I IFN’s role in Mtb infections in vivo appears to be largely probacterial. Its targeted inhibition may therefore offer an important avenue for host-directed therapy of TB. As discussed in detail below, both the IL-1 and eicosanoid pathways have important inhibitory intersections with the Mtb-triggered Type I IFN response that could be utilized as targets in this type of intervention.
Eicosanoids: lipid mediators in the inflammatory balancing act
As introduced above, eicosanoids are AA derived lipid mediators that trigger a variety of pro-and anti-inflammatory responses and include prostaglandins, resolvins, lipoxins, and leukotrienes (4, 81). Two classes of enzymes, the cyclooxygenases (COX) and lipoxygenases (LO), compete for the AA substrate to generate COX-1 and-2 (ptgs1, ptgs2) dependent prostaglandins or in an opposing pathway 5-lipoxygenase (5-LO, alox5) or 12/15-lipoxygenase (12/15-LO) dependent lipoxins and leukotrienes (Fig. 1). Based on susceptibility studies in the murine infection model, the concept has emerged that prostaglandin E2 (PGE2) confers resistance against Mtb infection, while products of the 5-LO axis, in particularly Lipoxin A4 (LXA4), promote bacterial growth and hinder bacterial control. Thus, Alox5−/− mice are more resistant to Mtb infection, while prostaglandin E synthase (ptges)-deficient mice or PGE2 receptor (EP2)-deficient mice display elevated mycobacterial loads (31–33, 82).
One mechanism of eicosanoid-mediated protection against and susceptibility to Mtb is the ability of these lipids to either directly or indirectly regulate cell death outcomes of infected macrophages (69, 83). The different cell death modalities of Mtb-infected macrophages have been assigned pivotal roles in Mtb pathogenesis due to release of bacteria from infected cells, which allows for infection of neighboring cells and dissemination of Mtb infection (86, 89). It is thought that when cells undergo apoptosis, their cell membrane integrity remains preserved resulting in containment of bacilli, enhanced antigen cross-presentation and efferocytosis, all processes important for controlling infection (34, 83–88). In contrast, more virulent Mtb strains have developed strategies to inhibit apoptosis and instead are associated with lung pathology and necrotic-like cell death, which is thought to foster bacterial escape, extracellular spread, and dissemination (32, 89–92).
In mycobacterial infected macrophages, PGE2 has been shown to inhibit necrotic cell death by protecting against mitochondrial damage and facilitating plasma membrane repair (32, 33, 89, 93), while both LXA4 and leukotriene B4 (LTB4) induction have been associated with necrotic macrophage death (32, 33, 35, 36). This death modality also has long lasting consequences on adaptive immunity against Mtb. Elegant studies utilizing adoptive transfer of Mtb infected pro-apoptotic Alox5−/− alveolar macrophages uncovered an important early role for apoptotic versus necrotic cell death in influencing initiation and priming of both CD4+ and CD8+ T-cell responses 2–3 weeks later. Further studies in the zebrafish model of Mycobacterium marinum infection found a conserved role for leukotriene A4 hydrolase (Lta4h) in both zebrafish and humans in regulating the balance between pro- and anti-inflammatory eicosanoids during mycobacterial infection (35, 69). LTA4H is a zinc-dependent epoxide hydrolase that catalyzes the final steps downstream of 5-LO in the biosynthesis of the pro-inflammatory mediator LTB4 thus balancing LXA4 and LTB4 synthesis. Of note, in the low dose aerosol model of Mtb in B6 mice, absence of the LTB4 receptor did not affect early survival (24). During Mycobacterium marinum infection LTA4H deficiency results in increased LXA4, while excess activity leads to high levels of LTB4 and both of these scenarios ultimately lead to macrophage necrosis thus impairing the host’s ability to control bacterial replication (35, 36). Importantly, as further discussed below, these studies demonstrated for the first time a tight functional link between the pro-inflammatory cytokine response, in this case TNF, and the eicosanoid LTB4 in shaping the outcome of mycobacterial infection.
Another eicosanoid based pathway has been elucidated recently that links PGE2 with the type I IFN response, thereby influencing the outcome of both Mtb and influenza virus infection (24, 94). In both settings, PGE2 potently inhibited the type I IFN response, which, in the case of influenza, resulted in compromised immunity to this virus while augmenting host resistance against Mtb. As discussed further below, these findings have revealed PGE2 and type I IFNs as novel targets for host-directed immunotherapies.
Interleukin 1: a little bit goes a long way in host protection
As noted above, the growing evidence for a central function for IL-1 in the innate response to Mtb has led to a surge in interest in its role in tuberculosis. While its essential role in host resistance against Mtb in the mouse model is now well established (21, 24, 37–41), experimental scenarios also exist where exuberant IL-1 production, driven by bacterial loads, can impair host control by driving tissue damage and pathology (95). Indeed, the potent inflammatory effects of IL-1 signaling must be tightly controlled to prevent host tissue damage, and this is achieved on multiple levels through tight regulation of gene expression, translation, post-translational activation of the immature cytokine through protein cleavage, and through inhibition of receptor binding by interaction with both membrane bound and soluble decoy receptors (96). In addition, the two IL-1 species, IL-1α and IL-1β, which mediate these potent signals through the IL-1R1 are antagonized by the endogenous IL-1 receptor antagonist (IL-1RA) through competition for receptor-binding.
A further biological checkpoint for IL-1β is the cleavage of pro-IL-1β into its 17KD mature form by the inflammasome (97). While clearly required for mature IL-1β production by Mtb infected macrophages in vitro, both the caspase1 and 11-dependent inflammasomes are dispensable in vivo after pulmonary infection with Mtb, suggesting the existence of redundant mechanisms for pro-IL-1β processing in the intact host [we now know that the mice used in the 2010 studies were Caspase1/11 double deficient (97)] (41, 98–100). In addition, there is evidence that inflammasome activity may be inhibited in vivo as a result of Mtb-induced nitric oxide induction (95).
Because IL-1β-deficient mice are highly susceptible to low dose aerosol infection and casapse-1/11, ASC, and NLRP3-deficient mice are not (98, 99), it seems likely that IL-1β can be cleaved in vivo by an inflammasome-independent mechanism and candidate cleavage enzymes include other caspases, chymases, cathepsins, and elastases (96). The latter two proteases are expressed in high levels by neutrophils that have been described to produce and process IL-1β through a caspase-1 independent mechanisms (101). However, while bone marrow-derived neutrophils produce large amounts of IL-1β in response to Mtb infection in vitro and pulmonary neutrophils express high levels of IL-1β mRNA after Mtb infection in vivo (KDMB, unpublished data), they fail to express IL-1β protein at the single cell level in the lungs of Mtb-infected mice (21). Instead two distinct pulmonary myeloid subsets, inflammatory monocytes and DCs, co-produce large amounts of both IL-1α and IL-1in the lungs of Mtb-infected mice based on intracellular cytokine staining (21). The ability to examine IL-1 production at the single cell level enabled the studies discussed below on how IL-1 expression is regulated by interferons in vivo.
IL-1 mediates control of acute bacterial infections with Staphylococcus aureus or Streptococcus pneumonia through the induction of IL-17 and chemokines leading to the rapid recruitment of neutrophils (102, 103). In contrast, Mtb is slow growing and does not trigger a rapid and strong inflammatory response, yet the IL-1 pathway is critical for host control (37–39). While we have detected a major defect in the IL-17 response in the absence of IL-1 in vivo, it is unclear how, in the low dose H37Rv aerosol model on the B6 background, the observed IL-17 deficiency could account for the strong susceptibility phenotype of the IL-1-deficient mice (2, 21). Despite being critical for IL-17 production during Mtb infection, no defects in pulmonary neutrophil recruitment have been observed in Il1r1−/− animals (39, KDMB, unpublished data). In fact, when this acute-like IL-1-IL-17-neutrophil axis is experimentally induced during Mtb infection in mice, host resistance is greatly compromised (104–106).
How then does IL-1 mediate host resistance to Mtb infection in vivo? Interestingly, no major defects have been described in the TNF, IL-12/IFN-γ, or iNOS pathways in IL-1-deficient animals, arguing that IL-1 confers host resistance to Mtb in vivo by distinct mechanisms not involving these effectors already known to be important for control of infection (21, 24, 37–40). A number of other mechanisms have been suggested from in vitro experiments with infected macrophages. These include IL-1β–mediated induction of microbicidal TNF (107) or autophagy (108) in macrophages. A different mechanism of IL-1-dependent TB control has recently emerged from combined in vivo and in vitro experiments (24). This involves the IL-1-dependent induction of COX-2 and subsequent biosynthesis of prostaglandins. As introduced above, prostaglandins have been shown to contribute to mycobacterial control through their influence on the cell death outcome of infected macrophages. Consistent with such a mechanism, in vitro in murine and human cells as well as in vivo in Mtb-infected mice, PGE2 levels were markedly decreased and markers of necrotic cell death, including LXA4, were highly elevated in the absence of IL-1 (24). Functional add-back experiments with PGE2 both in vitro, and more importantly in vivo, supported the conclusion that IL-1 contributes to host protection against Mtb through the induction of PGE2 synthesis. In addition to its role in governing host cell death modalities, recent work has suggested that IL-1-induced PGE2 also inhibits the host-detrimental type I IFN response in Mtb infection, and as discussed further below, this regulatory influence on cytokine production provides a second major pathway explaining the protective effects of IL-1 driven prostaglandins.
The close ties between cytokines and eicosanoids: regulatory networks that determine the outcome of infection
The first description of the close relationship between cytokines and eicosanoid lipid mediators in host resistance to mycobacteria was reported in the zebrafish model of Mycobacterium marinum infection. Here the pro-inflammatory lipid mediator LTB4 lead to rapid induction of TNF mRNA (35). Excessive LTB4 production resulted in increased TNF dependent macrophage cell death, while reduced levels of LTA4H and LTB4 resulted in a relative increase of LXA4, which in turn decreased TNF and led to cell death due to loss of bacterial control (36). Both of these scenarios diminished host resistance, highlighting the importance of the precise regulation of the inflammatory response to Mtb and the necessity for both regulatory cytokine-cytokine and cytokine-lipid mediator networks.
Mtb actively triggers both IL-1 and type I IFNs in macrophages via its ESX-1 type VII secretion system (20, 109), yet as discussed above these two cytokines have opposing roles in host resistance to infection. Indeed, perhaps the best characterized target of Type I IFN regulation of protective host factors in Mtb is the IL-1 response. Production of both IL-1α and IL-1β is inhibited by Type I IFNs in human and mouse macrophages and DCs (21, 22). A competitive mixed bone marrow chimera analysis (as shown in Fig. 2A) demonstrated that the negative regulation of IL-1 production by type I IFN regulation was also operative in vivo in the lungs of Mtb-infected mice (21). Expression of IL-1α and IL-1β was increased in both inflammatory monocytes as well as DCs in IFNAR1-deficient cells when compared with WT cells in the same animal. In addition, Type I IFN induction was accompanied by the synthesis of both the IL-1 receptor antagonist (IL-1RA) and IL-10 with both cytokines likely contributing to Type I IFN-mediated suppression of IL-1-mediated host resistance (21).
Recent work has demonstrated in turn that IL-1 inhibits Type I IFN expression as well as it downstream pro-bacterial effects (24). The latter counter-regulatory pathway appears to be critical in IL-1’s role in host resistance, as mice deficient in both IL-1R and IFNAR1 survived longer than the highly susceptible IL-1R1 mice. Moreover, as introduced above, this cross regulation (Fig. 3) was found to be mediated by IL-1-driven PGE2 biosynthesis and operative in both human and murine cells infected with Mtb, and PGE2 itself was shown to reverse Type I IFN-driven loss of bacterial control both in vitro and in vivo (24).
In a setting of Type I IFN-driven progressive pulmonary disease, manipulation of the eicosanoid balance toward elevated PGE2, by either direct administration of PGE2 or blocking 5-LO activity, resulted in protection against disease (24). While 5-LO blockade increased protective PGE2 levels likely through shunting of AA substrate towards COX1 and COX2, it is possible that both the increase in PGE2 as well as blockade of potentially detrimental 5-LO products contributed to the observed protection. Studies in TB patients support the existence of the same pathway in human tuberculosis. Relative plasma levels of PGE2 to LXA4 and 15-Epi-LXA4 (a stereoisomer of LXA4) were reduced in patients with higher sputum grades, and combined measurement of these lipid markers closely reflected disease severity (24). Thus, manipulation of PGE2 and/or 5-LO-dependent eicosanoids could serve as an approach for countering the Type I IFN signature of active TB patients (27) to alleviate active TB disease. It is of interest that two of the drugs, PGE2 and the 5 LO inhibitor zileuton, used experimentally in the mouse to alter the lipid mediator balance and improve disease outcome are already approved for clinical use. Thus, a clinical trial using one or a combination of these drugs on top of conventional chemotherapy could be attempted with minimal additional pre-clinical testing. An obvious target population would be patients carrying multi-drug resistant Mtb infections. Eicosanoid pharmacology is a rapidly evolving field (3) and drugs targeting the generation of these mediators and/or their biologic activities are continuously being developed that may in the future offer improved treatment options for Mtb beyond the PGE2 and zileuton candidates already studied experimentally.
Concluding comments
As immunologists delve further into the host factors that influence the outcome of Mtb infection, they continue to discover new complexities in the pathways and mediators involved and develop an even greater sense of appreciation of the exquisitely sharp edge dividing host resistance and disease. Clearly, the contextual basis of each response plays a major role in determining its beneficial versus detrimental outcome, and this must be considered and explored carefully in the design of successful immunologic interventions. In the case of TB, this implies that host-directed therapies might have restricted applicability to distinct stages of infection/disease. As we learn to appreciate the spectrum of human TB disease from both cutting edge clinical research as well as novel murine and non-human primate models (110, 111), an intriguing scenario emerges where TB treatment could be optimized and tailored based on the patients individual inflammatory disease and biomarker profile. Such a personalized medicine approach would allow for individualized therapies that combat disease and mortality caused by both drug sensitive and drug resistant Mtb strains.
It has now become clear that the host response to Mtb is governed by a series of overlapping regulatory networks that can interact both cooperatively and antagonistically in influencing disease outcome (Fig. 3). While IFN-γ, TNF, and IL-1 are required for host protection, left unregulated they have the potential to contribute to disease and pathology. The interplay of the inflammatory eicosanoid and cytokine networks provides an increasingly important example of this concept. Although seemingly just another added layer of complexity, the study of the eicosanoid-cytokine interaction in Mtb infection has already yielded new targets for immunotherapeutic intervention and the opportunity to test clinically approved drugs to achieve this end. These developments barely scratch the surface of what hopefully will become a fertile area for further investigation in experimental infection models and in TB infected patients.
Acknowledgments
We thank Anne O’Garra, Andrea Cooper, Charles Serhan, Maziar Divangahi and Heinz Remold for their stimulating discussions and Daniel Barber and Bruno Andrade for their helpful comments on the manuscript. KDM-B and AS are supported by the Intramural Research Program of the NIAID.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Cooper AM, Khader SA. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunological Reviews. 2008;226:191–204. doi: 10.1111/j.1600-065X.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper AM, Mayer-Barber KD, Sher A. Role of innate cytokines in mycobacterial infection. Mucosal Immunol. 2011;4(3):252–60. doi: 10.1038/mi.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8(5):349–61. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flynn JL, et al. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. Journal of Experimental Medicine. 1993;178(6):2249–54. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flynn JL, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2(6):561–72. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 7.Cooper AM, et al. Disseminated tuberculosis in interferon gamma gene-disrupted mice. Journal of Experimental Medicine. 1993;178(6):2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper AM, et al. IL-12 is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. Journal of Experimental Medicine. 1997;186:39–46. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altare F, et al. Interleukin-12 receptor beta1 deficiency in a patient with abdominal tuberculosis. J Infect Dis. 2001;184(2):231–6. doi: 10.1086/321999. [DOI] [PubMed] [Google Scholar]
- 10.Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20:581–620. doi: 10.1146/annurev.immunol.20.081501.125851. [DOI] [PubMed] [Google Scholar]
- 11.Harris J, Keane J. How tumour necrosis factor blockers interfere with tuberculosis immunity. Clin Exp Immunol. 2010;161(1):1–9. doi: 10.1111/j.1365-2249.2010.04146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keane J, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345(15):1098–104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 13.Cooper A. Cell mediated immune responses in tuberculosis. Annual Review of Immunology. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Garra A, et al. The immune response in tuberculosis. Annu Rev Immunol. 2013;31:475–527. doi: 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- 15.Ramakrishnan L. Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol. 2012;12(5):352–66. doi: 10.1038/nri3211. [DOI] [PubMed] [Google Scholar]
- 16.Green SJ, Nacy CA, Meltzer MS. Cytokine-induced synthesis of nitrogen oxides in macrophages: a protective host response to Leishmania and other intracellular pathogens. J Leukoc Biol. 1991;50(1):93–103. doi: 10.1002/jlb.50.1.93. [DOI] [PubMed] [Google Scholar]
- 17.Redford P, et al. Enhanced protection to Mycobacterium tuberculosis infection in IL-10-deficient mice is accompanied by early and enhanced Th1 responses in the lung. European Journal of Immunology. 2010;40(8):2200–2210. doi: 10.1002/eji.201040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roach D, et al. Endogenous inhibition of antimycobacterial immunity by IL-10 varies between mycobacterial species. Scandanavian Journal of Immunology. 2001;54:163. doi: 10.1046/j.1365-3083.2001.00952.x. [DOI] [PubMed] [Google Scholar]
- 19.Manca C, et al. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha /beta. Proc Natl Acad Sci U S A. 2001;98(10):5752–7. doi: 10.1073/pnas.091096998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanley SA, et al. The Type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. Journal of Immunology. 2007;178(5):3143–52. doi: 10.4049/jimmunol.178.5.3143. [DOI] [PubMed] [Google Scholar]
- 21.Mayer-Barber KD, et al. Innate and adaptive interferons suppress IL-1alpha and IL-1beta production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity. 2011;35(6):1023–34. doi: 10.1016/j.immuni.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novikov A, et al. Mycobacterium tuberculosis triggers host type I IFN signaling to regulate IL-1beta production in human macrophages. J Immunol. 2011;187(5):2540–7. doi: 10.4049/jimmunol.1100926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manca C, et al. Hypervirulent M. tuberculosis W/Beijing strains upregulate type I IFNs and increase expression of negative regulators of the Jak-Stat pathway. J Interferon Cytokine Res. 2005;25(11):694–701. doi: 10.1089/jir.2005.25.694. [DOI] [PubMed] [Google Scholar]
- 24.Mayer-Barber KD, et al. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature. 2014;511(7507):99–103. doi: 10.1038/nature13489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ordway D, et al. The hypervirulent Mycobacterium tuberculosis strain HN878 induces a potent TH1 response followed by rapid down-regulation. Journal of Immunology. 2007;179(1):522–31. doi: 10.4049/jimmunol.179.1.522. [DOI] [PubMed] [Google Scholar]
- 26.Desvignes L, Wolf AJ, Ernst JD. Dynamic roles of type I and type II IFNs in early infection with Mycobacterium tuberculosis. J Immunol. 2012;188(12):6205–15. doi: 10.4049/jimmunol.1200255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berry MP, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466(7309):973–7. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cliff JM, et al. Distinct phases of blood gene expression pattern through tuberculosis treatment reflect modulation of the humoral immune response. J Infect Dis. 2013;207(1):18–29. doi: 10.1093/infdis/jis499. [DOI] [PubMed] [Google Scholar]
- 29.Maertzdorf J, et al. Human gene expression profiles of susceptibility and resistance in tuberculosis. Genes Immun. 2011;12(1):15–22. doi: 10.1038/gene.2010.51. [DOI] [PubMed] [Google Scholar]
- 30.Ottenhoff TH, et al. Genome-wide expression profiling identifies type 1 interferon response pathways in active tuberculosis. PLoS One. 2012;7(9):e45839. doi: 10.1371/journal.pone.0045839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bafica A, et al. Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase-dependent lipoxin production. Journal of Clinical Investigations. 2005;115:1601–1606. doi: 10.1172/JCI23949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen M, et al. Lipid mediators in innate immunity against tuberculosis: opposing roles of PGE2 and LXA4 in the induction of macrophage death. Journal of Experimental Medicine. 2008;205(12):2791–801. doi: 10.1084/jem.20080767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Divangahi M, et al. Mycobacterium tuberculosis evades macrophage defenses by inhibiting plasma membrane repair. Nature Immunology. 2009;10(8):899–906. doi: 10.1038/ni.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Divangahi M, et al. Eicosanoid pathways regulate adaptive immunity to Mycobacterium tuberculosis. Nature Immunology. 2010;11(8):751–758. doi: 10.1038/ni.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tobin DM, et al. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell. 2010;140(5):717–730. doi: 10.1016/j.cell.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tobin DM, et al. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell. 2012;148(3):434–46. doi: 10.1016/j.cell.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamada H, et al. Protective role of interleukin-1 in mycobacterial infection in IL-1 alpha/beta double-knockout mice. Lab Invest. 2000;80(5):759–67. doi: 10.1038/labinvest.3780079. [DOI] [PubMed] [Google Scholar]
- 38.Juffermans NP, et al. Interleukin-1 signaling is essential for host defense during murine pulmonary tuberculosis. J Infect Dis. 2000;182(3):902–8. doi: 10.1086/315771. [DOI] [PubMed] [Google Scholar]
- 39.Sugawara I, et al. Role of interleukin (IL)-1 type 1 receptor in mycobacterial infection. Microbiol Immunol. 2001;45(11):743–50. doi: 10.1111/j.1348-0421.2001.tb01310.x. [DOI] [PubMed] [Google Scholar]
- 40.Fremond CM, et al. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. Journal of Immunology. 2007;179(2):1178–89. doi: 10.4049/jimmunol.179.2.1178. [DOI] [PubMed] [Google Scholar]
- 41.Mayer-Barber KD, et al. Caspase-1 independent IL-1beta production is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. J Immunol. 2010;184(7):3326–30. doi: 10.4049/jimmunol.0904189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leepiyasakulchai C, et al. Infection rate and tissue localization of murine IL-12p40-producing monocyte-derived CD103(+) lung dendritic cells during pulmonary tuberculosis. PLoS One. 2013;8(7):e69287. doi: 10.1371/journal.pone.0069287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reeme AE, Miller HE, Robinson RT. IL12B expression is sustained by a heterogenous population of myeloid lineages during tuberculosis. Tuberculosis (Edinb) 2013;93(3):343–56. doi: 10.1016/j.tube.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson KG, et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc. 2014;9(1):209–22. doi: 10.1038/nprot.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khader S, et al. Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. Journal of Experimental Medicine. 2006;203:1805–1815. doi: 10.1084/jem.20052545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khader S, et al. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. Journal of Immunology. 2005;175:788–795. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- 47.Kagina BM, et al. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette-Guerin vaccination of newborns. Am J Respir Crit Care Med. 2010;182(8):1073–9. doi: 10.1164/rccm.201003-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gallegos AM, et al. A gamma interferon independent mechanism of CD4 T cell mediated control of M. tuberculosis infection in vivo. PLoS Pathog. 2011;7(5):e1002052. doi: 10.1371/journal.ppat.1002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jayaraman P, et al. Tim3 binding to galectin-9 stimulates antimicrobial immunity. J Exp Med. 2010;207(11):2343–54. doi: 10.1084/jem.20100687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakai S, Mayer-Barber KD, Barber DL. Defining features of protective CD4 T cell responses to Mycobacterium tuberculosis. Curr Opin Immunol. 2014;29C:137–142. doi: 10.1016/j.coi.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sakai S, et al. Cutting edge: control of Mycobacterium tuberculosis infection by a subset of lung parenchyma-homing CD4 T cells. J Immunol. 2014;192(7):2965–9. doi: 10.4049/jimmunol.1400019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barber DL, et al. CD4 T cells promote rather than control tuberculosis in the absence of PD-1-mediated inhibition. J Immunol. 2011;186(3):1598–607. doi: 10.4049/jimmunol.1003304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Condos R, Rom WN, Schluger NW. Treatment of multidrug-resistant pulmonary tuberculosis with interferon-gamma via aerosol. Lancet. 1997;349(9064):1513–5. doi: 10.1016/S0140-6736(96)12273-X. [DOI] [PubMed] [Google Scholar]
- 54.Dawson R, et al. Immunomodulation with recombinant interferon-gamma1b in pulmonary tuberculosis. PLoS One. 2009;4(9):e6984. doi: 10.1371/journal.pone.0006984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Milanes-Virelles MT, et al. Adjuvant interferon gamma in patients with pulmonary atypical Mycobacteriosis: a randomized, double-blind, placebo-controlled study. BMC Infect Dis. 2008;8:17. doi: 10.1186/1471-2334-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doherty TM, Sher A. IL-12 promotes drug-induced clearance of Mycobacterium avium infection in mice. J Immunol. 1998;160(11):5428–35. [PubMed] [Google Scholar]
- 57.Hawn TR, et al. Host-directed therapeutics for tuberculosis: can we harness the host? Microbiol Mol Biol Rev. 2013;77(4):608–27. doi: 10.1128/MMBR.00032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin PL, et al. Tumor necrosis factor and tuberculosis. J Investig Dermatol Symp Proc. 2007;12(1):22–5. doi: 10.1038/sj.jidsymp.5650027. [DOI] [PubMed] [Google Scholar]
- 59.Lin PL, et al. Tumor necrosis factor neutralization results in disseminated disease in acute and latent Mycobacterium tuberculosis infection with normal granuloma structure in a cynomolgus macaque model. Arthritis Rheum. 2010;62(2):340–50. doi: 10.1002/art.27271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Flynn JL, Chan J, Lin PL. Macrophages and control of granulomatous inflammation in tuberculosis. Mucosal Immunol. 2011;4(3):271–8. doi: 10.1038/mi.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davis J, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136:37–49. doi: 10.1016/j.cell.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clay H, Volkman HE, Ramakrishnan L. Tumor necrosis factor signaling mediates resistance to mycobacteria by inhibiting bacterial growth and macrophage death. Immunity. 2008;29(2):283–94. doi: 10.1016/j.immuni.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schoeman JF, et al. Adjunctive thalidomide therapy for childhood tuberculous meningitis: results of a randomized study. J Child Neurol. 2004;19(4):250–7. doi: 10.1177/088307380401900402. [DOI] [PubMed] [Google Scholar]
- 64.Kaufmann SH, et al. Progress in tuberculosis vaccine development and host-directed therapies--a state of the art review. Lancet Respir Med. 2014;2(4):301–20. doi: 10.1016/S2213-2600(14)70033-5. [DOI] [PubMed] [Google Scholar]
- 65.Wallis RS, et al. A study of the safety, immunology, virology, and microbiology of adjunctive etanercept in HIV-1-associated tuberculosis. AIDS. 2004;18(2):257–64. doi: 10.1097/00002030-200401230-00015. [DOI] [PubMed] [Google Scholar]
- 66.Maiga M, et al. Successful shortening of tuberculosis treatment using adjuvant host-directed therapy with FDA-approved phosphodiesterase inhibitors in the mouse model. PLoS One. 2012;7(2):e30749. doi: 10.1371/journal.pone.0030749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Subbian S, et al. Phosphodiesterase-4 inhibition alters gene expression and improves isoniazid-mediated clearance of Mycobacterium tuberculosis in rabbit lungs. PLoS Pathog. 2011;7(9):e1002262. doi: 10.1371/journal.ppat.1002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Turner J, et al. In vivo IL-10 production reactivates chronic pulmonary tuberculosis in C57BL/6 mice. J Immunol. 2002;169(11):6343–51. doi: 10.4049/jimmunol.169.11.6343. [DOI] [PubMed] [Google Scholar]
- 69.Tobin DM, Ramakrishnan L. TB: the Yin and Yang of lipid mediators. Curr Opin Pharmacol. 2013;13(4):641–5. doi: 10.1016/j.coph.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gazzinelli RT, et al. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996;157(2):798–805. [PubMed] [Google Scholar]
- 71.Demangel C, Bertolino P, Britton WJ. Autocrine IL-10 impairs dendritic cell(DC)-derived immune responses to mycobacterial infection by suppressing DC trafficking to draining lymph nodes and local IL-12 production. European Journal of Immunology. 2002;32:994–1002. doi: 10.1002/1521-4141(200204)32:4<994::AID-IMMU994>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 72.Antonelli LR, et al. Intranasal Poly-IC treatment exacerbates tuberculosis in mice through the pulmonary recruitment of a pathogen-permissive monocyte/macrophage population. J Clin Invest. 2010;120(5):1674–82. doi: 10.1172/JCI40817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pandey AK, et al. NOD2, RIP2 and IRF5 play a critical role in the type I interferon response to Mycobacterium tuberculosis. PLoS Pathog. 2009;5(7):e1000500. doi: 10.1371/journal.ppat.1000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Manzanillo PS, et al. Mycobacterium tuberculosis activates the DNA-dependent cytosolic surveillance pathway within macrophages. Cell Host Microbe. 2012;11(5):469–80. doi: 10.1016/j.chom.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McNab FW, Mayer-Barber KD, Sher A, Wack A, O’Garra A. Type I interferons in infectious disease. Nature Reviews Immunology. 2014 doi: 10.1038/nri3787. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kearney S, Delgado C, Lenz LL. Differential effects of type I and II interferons on myeloid cells and resistance to intracellular bacterial infections. Immunol Res. 2013;55(1–3):187–200. doi: 10.1007/s12026-012-8362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McRae BL, et al. Type I IFNs inhibit human dendritic cell IL-12 production and Th1 cell development. Journal of Immunology. 1998;160:4298–4304. [PubMed] [Google Scholar]
- 78.McNab FW, et al. TPL-2-ERK1/2 signaling promotes host resistance against intracellular bacterial infection by negative regulation of type I IFN production. J Immunol. 2013;191(4):1732–43. doi: 10.4049/jimmunol.1300146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rayamajhi M, et al. Induction of IFN-alphabeta enables Listeria monocytogenes to suppress macrophage activation by IFN-gamma. J Exp Med. 2010;207(2):327–37. doi: 10.1084/jem.20091746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shah S, et al. Cutting edge: Mycobacterium tuberculosis but not nonvirulent mycobacteria inhibits IFN-beta and AIM2 inflammasome-dependent IL-1beta production via its ESX-1 secretion system. J Immunol. 2013;191(7):3514–8. doi: 10.4049/jimmunol.1301331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Buckley CD, Gilroy DW, Serhan CN. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity. 2014;40(3):315–27. doi: 10.1016/j.immuni.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kaul V, et al. An important role of prostanoid receptor EP2 in host resistance to Mycobacterium tuberculosis infection in mice. J Infect Dis. 2012;206(12):1816–25. doi: 10.1093/infdis/jis609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Behar SM, Divangahi M, Remold HG. Evasion of innate immunity by Mycobacterium tuberculosis: is death an exit strategy? Nature Reviews Microbiology. 2010;8(9):668–674. doi: 10.1038/nrmicro2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martin CJ, et al. Efferocytosis is an innate antibacterial mechanism. Cell Host Microbe. 2012;12(3):289–300. doi: 10.1016/j.chom.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martin CJ, Peters KN, Behar SM. Macrophages clean up: efferocytosis and microbial control. Curr Opin Microbiol. 2014;17C:17–23. doi: 10.1016/j.mib.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee J, Hartman M, Kornfeld H. Macrophage apoptosis in tuberculosis. Yonsei Med J. 2009;50(1):1–11. doi: 10.3349/ymj.2009.50.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee J, et al. Mycobacterium tuberculosis induces an atypical cell death mode to escape from infected macrophages. PLoS One. 2011;6(3):e18367. doi: 10.1371/journal.pone.0018367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Porcelli SA, Jacobs WR., Jr Tuberculosis: unsealing the apoptotic envelope. Nat Immunol. 2008;9(10):1101–2. doi: 10.1038/ni1008-1101. [DOI] [PubMed] [Google Scholar]
- 89.Chen M, Gan H, Remold HG. A mechanism of virulence: virulent Mycobacterium tuberculosis strain H37Rv, but not attenuated H37Ra, causes significant mitochondrial inner membrane disruption in macrophages leading to necrosis. Journal of Immunology. 2006;176(6):3707–3716. doi: 10.4049/jimmunol.176.6.3707. [DOI] [PubMed] [Google Scholar]
- 90.Fratazzi C, et al. Macrophage apoptosis in mycobacterial infection. Journal of Leukocyte Biology. 1999;66:763–764. doi: 10.1002/jlb.66.5.763. [DOI] [PubMed] [Google Scholar]
- 91.Keane J, Remold HG, Kornfeld H. Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J Immunol. 2000;164(4):2016–20. doi: 10.4049/jimmunol.164.4.2016. [DOI] [PubMed] [Google Scholar]
- 92.Park JS, et al. Virulent clinical isolates of Mycobacterium tuberculosis grow rapidly and induce cellular necrosis but minimal apoptosis in murine macrophages. J Leukoc Biol. 2006;79(1):80–6. doi: 10.1189/jlb.0505250. [DOI] [PubMed] [Google Scholar]
- 93.Assis PA, et al. Mycobacterium tuberculosis expressing phospholipase C subverts PGE2 synthesis and induces necrosis in alveolar macrophages. BMC Microbiol. 2014;14:128. doi: 10.1186/1471-2180-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Coulombe F, et al. Targeted prostaglandin E2 inhibition enhances antiviral immunity through induction of type I interferon and apoptosis in macrophages. Immunity. 2014;40(4):554–68. doi: 10.1016/j.immuni.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 95.Mishra BB, et al. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1beta. Nat Immunol. 2013;14(1):52–60. doi: 10.1038/ni.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–50. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 97.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157(5):1013–22. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 98.McElvania Tekippe E, et al. Granuloma formation and host defense in chronic Mycobacterium tuberculosis infection requires PYCARD/ASC but not NLRP3 or caspase-1. PLoS One. 2010;5(8):e12320. doi: 10.1371/journal.pone.0012320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Walter K, et al. NALP3 is not necessary for early protection against experimental tuberculosis. Immunobiology. 2010;215(9–10):804–11. doi: 10.1016/j.imbio.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 100.Dorhoi A, et al. Activation of the NLRP3 inflammasome by Mycobacterium tuberculosis is uncoupled from susceptibility to active tuberculosis. Eur J Immunol. 2012;42(2):374–84. doi: 10.1002/eji.201141548. [DOI] [PubMed] [Google Scholar]
- 101.Greten FR, et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 2007;130(5):918–31. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.van de Veerdonk FL, et al. Inflammasome activation and IL-1beta and IL-18 processing during infection. Trends Immunol. 2011;32(3):110–6. doi: 10.1016/j.it.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 103.Krishna S, Miller LS. Host-pathogen interactions between the skin and Staphylococcus aureus. Curr Opin Microbiol. 2012;15(1):28–35. doi: 10.1016/j.mib.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Castillo EF, et al. Autophagy protects against active tuberculosis by suppressing bacterial burden and inflammation. Proc Natl Acad Sci U S A. 2012;109(46):E3168–76. doi: 10.1073/pnas.1210500109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cruz A, et al. Pathological role of Interleukin 17 in mice subjected to repeated BCG vaccination after infection with Mycobacterium tuberculosis. Journal of Experimental Medicine. 2010;207(8):1609–1616. doi: 10.1084/jem.20100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nandi B, Behar SM. Regulation of neutrophils by interferon-gamma limits lung inflammation during tuberculosis infection. J Exp Med. 2011;208(11):2251–62. doi: 10.1084/jem.20110919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jayaraman P, et al. IL-1beta promotes antimicrobial immunity in macrophages by regulating TNFR signaling and caspase-3 activation. J Immunol. 2013;190(8):4196–204. doi: 10.4049/jimmunol.1202688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pilli M, et al. TBK-1 promotes autophagy-mediated antimicrobial defense by controlling autophagosome maturation. Immunity. 2012;37(2):223–34. doi: 10.1016/j.immuni.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Koo IC, et al. ESX-1-dependent cytolysis in lysosome secretion and inflammasome activation during mycobacterial infection. Cell Microbiol. 2008;10(9):1866–78. doi: 10.1111/j.1462-5822.2008.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Barry CE, 3rd, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7(12):845–55. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Flynn JL. Lessons from experimental Mycobacterium tuberculosis infections. Microbes Infect. 2006;8(4):1179–88. doi: 10.1016/j.micinf.2005.10.033. [DOI] [PubMed] [Google Scholar]