Abstract
Background
Juvenile myelomonocytic leukemia (JMML) is not durably responsive to chemotherapy, and approximately 50% of patients relapse after hematopoietic stem cell transplant (HSCT). Here we report the activity and acute toxicity of the farnesyl transferase inhibitor tipifarnib, the response rate to 13-cis retinoic acid (CRA) in combination with cytoreductive chemotherapy, and survival following HSCT in children with JMML.
Procedure
Eighty-five patients with newly diagnosed JMML were enrolled on AAML0122 between 2001 and 2006. Forty-seven consented to receive tipifarnib in a phase II window before proceeding to a phase III trial of CRA in combination with fludarabine and cytarabine followed by HSCT and maintenance CRA. Thirty-eight patients enrolled only in the phase III trial.
Results
Overall response rate was 51% after tipifarnib and 68% after fludarabine/cytarabine/CRA. Tipifarnib did not increase pre-transplant toxicities. Forty-six percent of the 44 patients who received protocol compliant HSCT relapsed. Five-year overall survival was 55±11% and event-free survival was 41±11%, with no significant difference between patients who did or did not receive tipifarnib.
Conclusions
Administration of tipifarnib in the window setting followed by HSCT in patients with newly diagnosed JMML was safe and yielded a 51% initial response rate as a single agent, but failed to reduce relapse rates or improve long-term overall survival.
Keywords: JMML, hematopoietic stem cell transplant, farnesyl transferase inhibitor, 13-cis retinoic acid, tipifarnib
INTRODUCTION
Juvenile myelomonocytic leukemia (JMML) is an aggressive, rare, clonal malignancy of early childhood [1]. The median age at presentation is 1.8 years, and 96% of patients present by age 5 [2]. Left untreated, death typically occurs within 12 months from infection or organ failure secondary to progressive infiltration by monocytes and macrophages [3]. Recent studies have demonstrated the critical role of mutations that promote hyperactive Ras signaling in JMML development. Mutually exclusive loss of function mutations in the tumor suppressor genes NF1 and CBL (the latter with concomitant acquired isodisomy of a mutant CBL allele hypothesized to confer oncogenic activity), and gain of function lesions in the oncogenes NRAS, KRAS and PTPN11 have been identified in 80–90% of JMML patients [4, 5]. New diagnostic criteria thus include both clinical parameters and JMML-related genetic mutations [6].
Responses to conventional chemotherapy are generally transient, and durable remissions rare [7–11]. HSCT may be curative, but the 5-year event-free survival (EFS) is ~50%, with relapse the primary cause of death [12]. While up to 30% of patients with JMML who relapse after HSCT may be curable with a second transplant, there is high mortality associated with a second conditioning regimen [13]. There is no demonstrable survival benefit of pre-transplant cytotoxic chemotherapy. Patients receiving either low dose or no pre-HSCT chemotherapy had identical EFS (52% vs 50%), relapse rate (35% vs 38%) and treatment-related mortality (13% vs 13%) as patients receiving intensive pre-transplant chemotherapy [12]. One alternative approach is to include 13-cis retinoic acid (CRA), a vitamin A analog that induces terminal granulocytic differentiation and inhibits spontaneous proliferation of human JMML cells in culture [14, 15]. CRA reduces organomegaly and normalizes white blood cell count (WBC) in 40–50% of JMML patients with tolerable toxicity, but <10% achieve durable remissions [16, 17]. CRA has not been tested in combination with cytotoxic chemotherapy. Another approach is to target the activated Ras pathway. Ras must undergo post-translational farnesylation by the enzyme farnesyl transferase to be fully functional [18]. Tipifarnib is a selective farnesyl transferase inhibitor which blocks proliferation of Ras-transformed tumors in murine models [19]. Analogs of tipifarnib effectively inhibited in vitro spontaneous growth of JMML samples [20]. A Phase I trial of tipifarnib in pediatric patients with relapsed or refractory hematologic malignancies demonstrated that the drug was well-tolerated at 300 mg/m2/dose twice daily, resulting in a mean 82% inhibition of farnesyl transferase activity in leukemic blasts [21].
Here we describe the findings of Children’s Oncology Group Phase II/III study AAML0122 in patients with de novo JMML. The objectives of the study were to (1) define the acute toxicity of tipifarnib and estimate rate of response in patients with previously untreated JMML in a Phase II window, (2) determine response rate to CRA in combination with cytarabine and fludarabine, and (3) establish the 5-year EFS in JMML patients following this regimen and HSCT.
METHODS
Eligibility
AAML0122 (registered at www.clinicaltrials.gov as NCT00070174) was activated in June, 2001. Patients with newly diagnosed JMML with normal hepatic and renal function were eligible. JMML diagnosis was based on international criteria [22]. The phase II and III portions of the study closed to enrollment in February, 2005 and October, 2006, respectively, after meeting target accrual. Institutional review boards at participating centers approved the study, and legal guardians signed written informed consent. Patients had the option of participating in the phase II window without affecting eligibility for enrollment in the phase III portion of the study.
Chemotherapy and Dose Adjustments
The phase II window was designed to assess the activity of tipifarnib administered orally twice daily for 21 days, followed by a 7-day rest. Tipifarnib was supplied by the Cancer Therapy Evaluation Program (NCI). Patients with stable or responding disease (see response criteria) could receive a second course. After completing 1 or 2 cycles of tipifarnib, patients proceeded to phase III therapy. Previous studies had suggested that 300 mg/m2 would be required for sufficient inhibition of farnesyl transferase activity but no safety data was available for children at this dose [23]. The dosage of tipifarnib was therefore 200 mg/m2/dose twice daily for the first 11 patients, and escalated to 300 mg/m2/dose twice daily for all subsequent patients after tolerability was demonstrated at the lower dose.
Phase III therapy consisted of two courses of fludarabine (30 mg/m2 IV) and cytarabine (2 g/m2 IV) given daily for 5 days. The second course of fludarabine/cytarabine started when the post-nadir absolute neutrophil count (ANC) was >1 000/μL and unsupported platelet count was >75 000/μL. CRA (100 mg/m2, or 3 mg/kg for children <1 year of age) PO once daily was started on day 1 of fludarabine/cytarabine and continued until the start of HSCT conditioning. All clinically stable patients were recommended for splenectomy after response assessment to fludarabine/cytarabine/CRA.
Conditioning and Transplant
All patients who met eligibility criteria (adequate organ function and absence of active viral or fungal infection) proceeded to transplant within 6 months of diagnosis and following fludarabine/cytarabine/CRA. Treating physicians could postpone HSCT in favor of continuing CRA monotherapy in clinically stable infants <12 months of age. Donor selection was institution-dependent, but the hematopoietic stem cell source was T-cell replete bone marrow or unrelated cord blood unless a haploidentical donor was used. The prescribed preparative regimen included 1200 cGy total body irradiation in 150 cGy fractions from day −7 to day −4 and cyclophosphamide 60 mg/kg/day IV on days −3 and −2. Recipients of unrelated donor hematopoietic stem cells also received anti-thymocyte globulin 15 mg/kg/dose IV every 12 hours on days −3 to −1. Cyclosporine or tacrolimus plus methotrexate or prednisone were recommended for GVHD prophylaxis. Approximately 60 days after transplant, patients were restarted on daily CRA for one year to test whether CRA would enhance long-term disease control.
Response Criteria and Definitions
Standard clinical and hematologic response criteria were used to assess the antileukemic effect of tipifarnib and pre-HSCT chemotherapy plus CRA [6]. Complete Response (CR): normalization of WBC count and organomegaly on physical exam; Partial Response (PR): >50% reduction in WBC and organomegaly; Marginal Response (MR): >25% but ≤50% reduction in WBC and organomegaly or PR in WBC but no change in organomegaly or PR in organomegaly but no change in WBC; Stable Disease (SD): ≤25% reduction and <25% increase in WBC and organomegaly; Progressive Disease (PD): > 25% increase in WBC or organomegaly.
Primary graft failure was defined as failure after two months to achieve an ANC of 500/mm3 for three consecutive measurements on different days by day 60 post-HSCT. Consensus guidelines were used to grade the severity of acute and chronic GVHD [24]. Toxicity to tipifarnib, CRA and chemotherapy was graded according to the NCI common toxicity criteria, version 2.0. Remission was defined as no evidence of leukemia on marrow biopsy post-HSCT. Residual disease was defined as failure to eradicate leukemia on biopsy post-HSCT with no prior documentation of remission. Relapse was diagnosed upon reappearance of clinical or hematologic features of JMML. Patients were taken off protocol therapy if they had progressive disease during chemotherapy, if they were not candidates for HSCT, had recurrence of disease post-HSCT, died, refused further study treatment, or withdrew consent for further data submission.
Correlative Biology Studies
GM-CSF Hypersensitivity Assay
Mononuclear cells were isolated from peripheral blood or marrow using density gradient centrifugation and colony assays performed as previously described [3].
Mutation Detection
The coding regions of NRAS, KRAS, PTPN11 and CBL were sequenced from DNA harvested from marrow or peripheral blood using previously published primers [5, 25, 26]. Clinical NF1 status was determined by the treating physician according to consensus diagnostic criteria [27].
Farnesyl Transferase Activity
Marrow or peripheral blood (if WBC ≥20 000/μL) was collected pre-treatment and between days 15–21 of course 1 of tipifarnib. Mononuclear cells were isolated as above. Farnesyl transferase activity was measured with a scintillation proximity assay (Amersham Biosciences, Piscataway, NJ) as previously described [21, 28]. Serial measurements of farnesylated and unfarnesylated HDJ-2 were performed to indicate depth of farnesyl transferase inhibition [23]. Log phase THP1 and UOCM1 cells (American Type Culture Collection, Manassas, VA) were treated with 2 day regimens of 400 nM tipifarnib or 100 μM Compactin or DMSO as positive and negative controls, respectively. Paired pre-treatment and steady state samples for analysis of farnesyl transferase activity were available for 16 patients (n=3 at 200 mg/m2/dose and n=13 at 300 mg/m2/dose dosing levels).
Statistical Analysis
The study was powered to detect whether patients treated with tipifarnib in an upfront window prior to HSCT had a superior EFS compared to patients who were not treated with tipifarnib. Using a two-stage design, accrual of up to 46 patients in the phase II window was estimated to provide a reasonable (83%) power to reject the null hypothesis of 20% CR/PR rate for a true CR/PR rate of 40% with one-sided type I error of 0.041. Accrual of up to 54 patients in the phase III portion of the study was estimated to provide 98% power to reject the null hypothesis of 20% CR/PR rate for a true CR/PR rate of 40% with one-sided type I error of 0.040.
Primary toxicity endpoints were death related to tipifarnib administration and the frequency of all toxicities from tipifarnib and fludarabine/cytarabine/CRA. Primary response measures were rates of CR or PR after two courses of tipifarnib and fludarabine/cytarabine/CRA, overall survival (OS), EFS, relapse risk (RR) and transplant-related mortality (TRM) as of June 8, 2010. The significance of observed differences in proportions was tested using the chi-squared test and Fisher’s exact test when data were sparse. The Mann-Whitney test was used to determine the differences in medians. The Kaplan-Meier method was used to estimate probabilities of OS and EFS. OS was defined as time from study entry to death. EFS was defined as time from study entry to relapse, death, or graft failure. The probabilities of RR and TRM for patients who received HSCT were estimated using the method of cumulative incidence that accounts for competing events. RR was defined as time from transplant to relapse or death due to disease, where deaths from non-relapse causes were competing events. TRM was defined as time from transplant to death due to non-relapse causes, where relapses and deaths due to disease were competing events. All analyses were performed based on initial assignment (± tipifarnib) at study entry, and all patients were included in the survival analyses up to the time of death or were censored at last contact, including patients who withdrew from the treatment protocol.
RESULTS
Patient Characteristics
Eighty-nine patients were enrolled on AAML0122. Four were ineligible due to incorrect diagnosis or registration violations. Patient characteristics for 85 eligible patients are listed in Table I. Forty-seven patients chose to enroll in the tipifarnib phase II window (11 at 200 mg/m2/dose and 36 at 300 mg/m2/dose); the remaining 38 did not receive tipifarnib, with no significant differences in clinical characteristics between the two groups (Table I). Thirty-seven of the patients who enrolled in the phase II window received two courses of tipifarnib, the remaining ten received one course (Table II). Twenty-nine patients withdrew from the study (Supplemental Figure 1), the majority between completion of pre-transplant chemotherapy and transplant (Table II). Fifty-two patients underwent splenectomy, and 44 patients received HSCT according to AAML0122. Twenty-three patients developed progressive disease, and 9 died on-study (Supplemental Figure 1).
Table I.
Patient Demographics and Baseline Clinical Characteristics.
| Characteristic | FTI 200mg/m2 | FTI 300mg/m2 | No FTI |
|---|---|---|---|
|
| |||
| No. Patients | 11 | 36 | 38 |
|
| |||
| Median Age, Months (range) | 13.1 (3.3–21.7) | 18.9 (1.8–76.6) | 18.6 (1–104) |
|
| |||
| Gender | |||
| Male (%) | 5 (45) | 23 (64) | 26 (68) |
| Female (%) | 6 (55) | 13 (36) | 12 (32) |
|
| |||
| Race | |||
| White (%) | 9 (82) | 27 (75) | 31 (82) |
| Black (%) | 0 (0) | 2 (6) | 1 (3) |
| Asian (%) | 1 (9) | 4 (11) | 2 (5) |
| Other (%) | 1 (9) | 3 (8) | 4 (11) |
|
| |||
| Blood counts | |||
| Median WBC Count ×109/L | 24.9 | 33.2 | 34.1 |
| Median Monocyte % ϕ | 11 | 16.5 | 21 |
| Median Platelet Count ×109/L | 45 | 52.5 | 57 |
|
| |||
| Organomegaly* | |||
| Median Spleen, cm (range) | 4 (0–8) | 5 (0–16.7) | 4 (0–8) |
| Median Liver, cm (range) | 7 (4–18) | 7 (0–20.3) | 8 (0–12) |
|
| |||
| Fetal hemoglobin | |||
| Elevated (%) | 10 (91) | 28 (78) | 21 (60) |
| Not Elevated (%) | 1 (9) | 8 (22) | 14 (40) |
| Unknown | 0 | 0 | 3 |
| Median Elevation (range) | 21.5 (0.4–64.2) | 23.1 (0.9–68.9) | 30.3 (3–87.2) |
|
| |||
| GM-CSF Hypersensitivity | |||
| Yes (%) | 8 (100) | 12 (92) | 8 (67) |
| No (%) | 0 (0) | 1 (8) | 4 (33) |
| Not Done | 3 | 23 | 26 |
|
| |||
| Monosomy 7 | |||
| Yes (%) | 0 (0) | 6 (17) | 3 (8) |
| No (%) | 11 (100) | 29 (83) | 34 (92) |
| Not Done | 0 | 1 | 1 |
|
| |||
| Mutation Status† | |||
| KRAS (%) | 1 (10) | 4 (12) | 7 (23) |
| NRAS (%) | 1 (11) | 7 (22) | 4 (13) |
| PTPN11 (%) | 3 (33) | 16 (47) | 12 (39) |
| CBL (%) | 1 (13) | 0 (0) | 2 (6) |
|
| |||
| Neurofibromatosis Type 1 (%)† | 0 (0) | 2 (6) | 1 (3) |
Abbreviations: FTI, farnesyl transferase inhibitor tipifarnib
Organomegaly is measured as centimeters (cm) below the costal margin.
Percentage is calculated based on the number of patients with known mutation status.
Median monocyte count was the only baseline characteristic/demographics that had a statistically significant difference between the 3 groups.
Table II.
Reasons patients came off protocol therapy during each course.
| FTI 200 mg/m2 | FTI 300 mg/m2 | No FTI | |
|---|---|---|---|
|
| |||
| No. Patients Enrolled | 11 | 36 | 38 |
|
| |||
| FTI course 1 | |||
| Completed | 11 | 36 | n/a |
|
| |||
| FTI course 2 | |||
| Completed | 10 | 27 | n/a |
| Skipped course 2 | 1 | 8 | |
| Withdrew | 0 | 1 | |
|
| |||
| Fludarabine/cytarabine/ C-RA Courses 1&2 | |||
| Completed | 11 | 29 | 27 |
| PD | 0 | 1 | 4 |
| Death | 0 | 3 | 2 |
| Complicating disease | 0 | 1 | 0 |
| Withdrew | 0 | 1 | 4 |
| Lost to F/U | 0 | 0 | 1 |
|
| |||
| Splenectomy/Pre-Tx | |||
| Completed/Withdrew | 8/3 | 21/8 | 17/10 |
|
| |||
| Transplant | |||
| Completed | 6 | 15 | 14 |
| PD | 1 | 2 | 2 |
| Death | 1 | 1 | 1 |
| Complicating disease | 0 | 1 | 0 |
| Withdrew | 0 | 2 | 0 |
|
| |||
| 3 months of C-RA | |||
| Completed | 4 | 10 | 10 |
| PD | 2 | 4 | 3 |
| Death | 0 | 1 | 0 |
| Complicating disease | 0 | 0 | 1 |
|
| |||
| 6 months of C-RA | |||
| Completed | 3 | 9 | 9 |
| PD | 1 | 1 | 1 |
|
| |||
| 9 months of C-RA | |||
| Completed | 3 | 8 | 9 |
| PD | 0 | 1 | 0 |
|
| |||
| 12 months of C-RA | |||
| Completed | 3 | 7 | 8 |
| Complicating disease | 0 | 0 | 1 |
| Withdrew | 0 | 1 | 0 |
FTI, farnesyl transferase inhibitor tipifarnib; C-RA, cis-retinoic acid; PD, progressive disease.
Response to Pre-transplant Therapy
Fifty-one percent of patients who received two cycles of tipifarnib were in CR or PR prior to starting cytoreductive therapy; of the patients in PR, most had normalized WBC counts but incomplete resolution of organomegaly (Table III). As it did not affect statistical significance, patients who received both dose levels of tipifarnib were combined for all analyses. Sixty-eight percent of all patients were in CR or PR at the end of the second cycle of fludarabine/cytarabine/CRA, irrespective of whether or not they received tipifarnib (Table III, p=0.674). Of the 44 patients who underwent a HSCT on study, 5/27 who also received tipifarnib had progression of disease prior to transplant, whereas 1/17 patients who did not receive tipifarnib had progression prior to transplant (p=0.38). Response rates for tipifarnib alone and pre-transplant cytoreductive chemotherapy each exceeded 20%, allowing rejection of the null hypotheses.
Table III.
Hematologic and clinical responses to tipifarnib and pre-transplant cytoreductive chemotherapy.
| WBC Only | WBC and Organomegaly Combined | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| CR n (%) |
CR n (%) |
PR n (%) |
MR n (%) |
SD n (%) |
PD n (%) |
|
|
| ||||||
| Tipifarnib Course 1 | ||||||
| FTI: 200mg/m2 (n=11) | 6 (55) | 0 (0) | 4 (36) | 4 (36) | 2 (18) | 1 (9) |
| FTI: 300mg/m2 (n=36) | 18 (50) | 1 (3) | 17 (47) | 9 (25) | 4 (11) | 5 (14) |
| Any FTI (n=47) | 24 (51) | 1 (2) | 21 (45) | 13 (28) | 6 (13) | 6 (13) |
|
| ||||||
| Tipifarnib Course 2 | ||||||
| FTI: 200mg/m2 (n=10) | 6 (60) | 0 (0) | 5 (50) | 2 (20) | 2 (20) | 1 (10) |
| FTI: 300mg/m2 (n=27) | 17 (63) | 2 (7) | 12 (44) | 6 (22) | 3 (11) | 4 (15) |
| Any FTI (n=37) | 23 (62) | 2 (5) | 17 (46) | 8 (22) | 5 (14) | 5 (14) |
|
| ||||||
| Flu/Cytarabine/C-RA Courses 1&2 | ||||||
| FTI: 200mg/m2 (n=11) | 9 (82) | 1 (9) | 5 (45) | 4 (36) | 1 (9) | 0 (0) |
| FTI: 300mg/m2 (n=32) | 27 (84) | 10 (31) | 12 (38) | 7 (22) | 1 (3) | 2 (6) |
| Any FTI (n=43) | 36 (84) | 11 (26) | 17 (40) | 11 (26) | 2 (5) | 2 (5) |
| No FTI (n=34) | 27 (79) | 12 (35) | 12 (35) | 8 (24) | 0 (0) | 2 (6) |
Abbreviations: WBC, white blood cell count; CR, complete response; PR, partial response; MR, minimal response; SD, stable disease; PD, progressive disease; FTI, farnesyl transferase inhibitor tipifarnib.
Toxicity of Pre-transplant Therapy
The first 11 patients tolerated tipifarnib at 200 mg/m2/dose, so the dosage was increased to 300 mg/m2/dose for subsequent patients. Tipifarnib was well tolerated at both dose levels with the most common grade 3/4 adverse events being thrombocytopenia (40%), anemia (40%), neutropenia (15%) and diarrhea (6%). 11% of patients experienced infection with grade 3/4 neutropenia during tipifarnib therapy. Other adverse events documented in <10% of subjects receiving tipifarnib included dyspnea, melena, skin rash and irritability. No toxicity required discontinuation of tipifarnib or resulted in a patient death. The most frequent toxicities associated with fludarabine/cytarabine/CRA were marrow suppression, febrile neutropenia, and hypokalemia. There was no difference in infection risk or neutropenia between those who received tipifarnib (37%) and those who did not (41%).
Response to Transplant
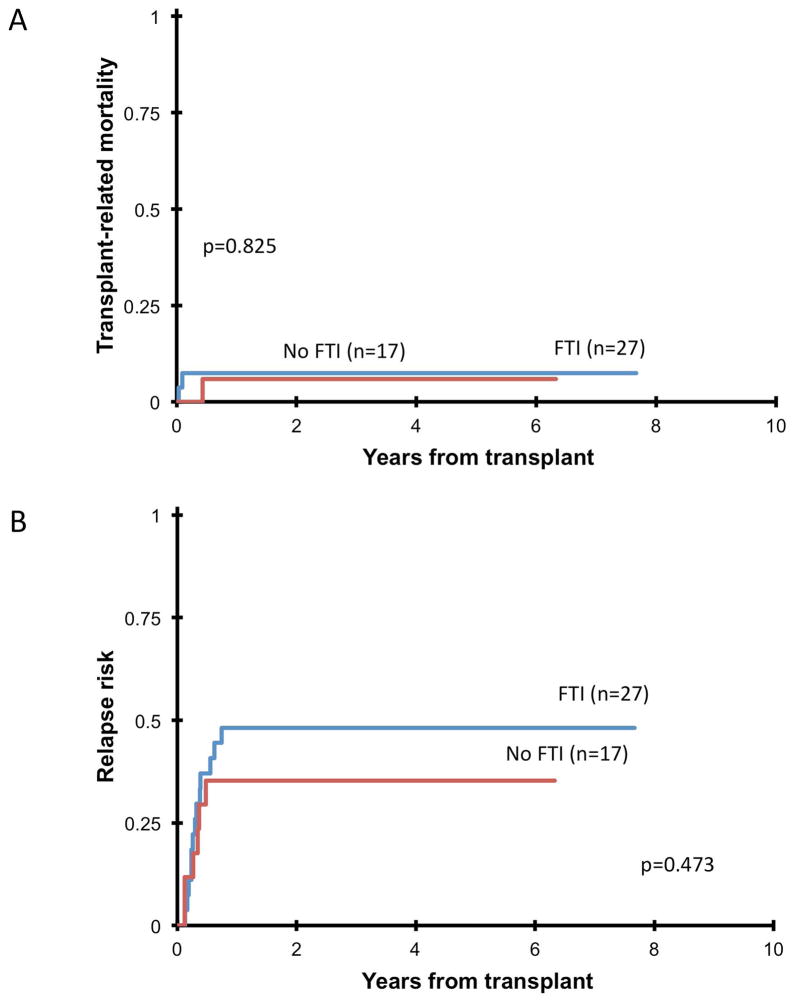
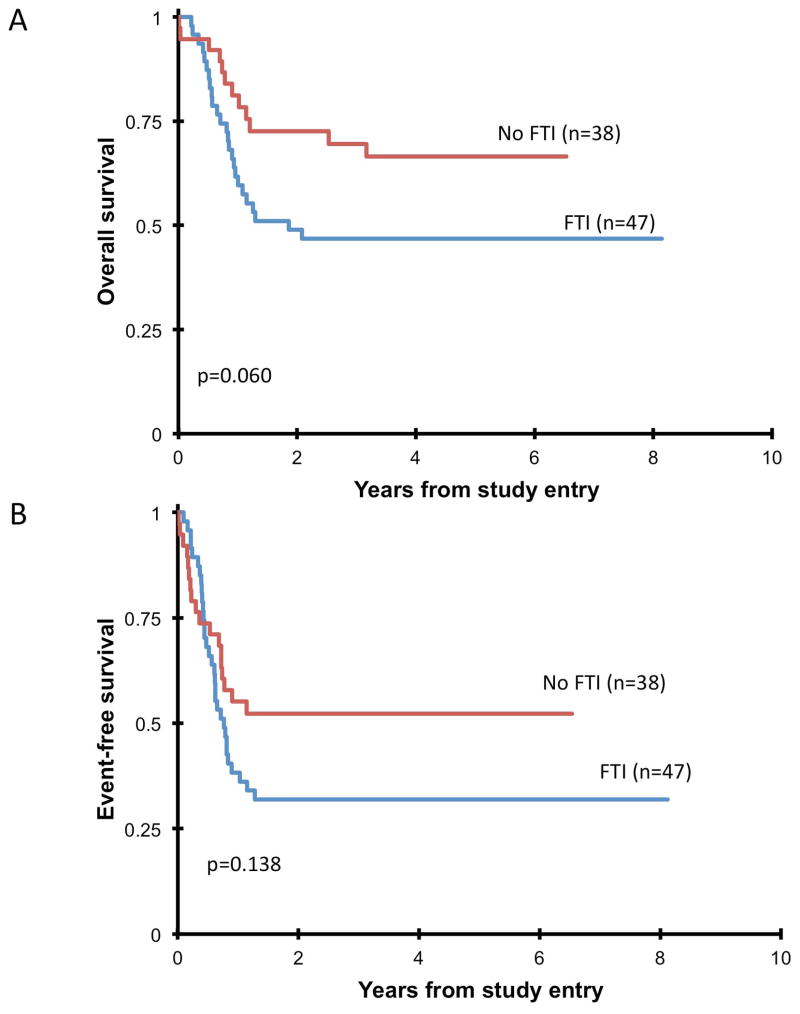
Forty-four patients received a study compliant HSCT on AAML0122. Median time from diagnosis to transplant was 55 days longer (p<0.001) in patients who received tipifarnib (Table IV). Two patients experienced primary graft failure: one had a mismatched unrelated cord donor, the other a matched unrelated donor, and both had received tipifarnib at 300 mg/m2/dose (Table IV). Acute GVHD occurred in 61%, and was limited to grades I–II in 80%. No grade IV acute GVHD was seen. Five-year TRM was 7±8%, with no difference (p=0.825) between patients who did/did not receive tipifarnib (Figure 1a). Forty-one percent (n=18) of patients who received HSCT on-study relapsed within twelve months, with no difference (p=0.473) in RR between patients who received tipifarnib and those who did not (Figure 1b). Five-year OS of all patients enrolled was 55±11% (Figure 2a), and 5-year EFS was 41±11% (Figure 2b), with no statistically significant differences (OS: p=0.06; EFS: p=0.138) between patients who received tipifarnib and those who did not. Thirteen of the patients transplanted on AAML0122 were in CR prior to transplant, the remaining 30 were not in CR (15 PR, 12 MR, 1 SD, 1 PD) and 1 was not evaluated. Patients in CR prior to transplant did not have significantly longer 5-year EFS (54% vs. 33%, p=0.144), 5-year OS (62% vs. 57%, p=0.755) or lower 5-year RR (38% vs. 57%, p=0.154) compared to patients not in CR, although the study was not powered to test this comparison.
Table IV.
Results of Transplant on AAML0122.
| FTI 200mg/m2 | FTI 300mg/m2 | No FTI | |
|---|---|---|---|
|
| |||
| No. Patients Receiving Transplant | 7 | 20 | 17 |
|
| |||
| Donor type* | |||
| Matched related (%) | 1 (14) | 2 (10) | 2 (12) |
| Matched unrelated (%) | 4 (57) | 7 (35) | 6 (35) |
| Mismatched related (%) | 0 (0) | 5 (25) | 3 (18) |
| Mismatched unrelated (%) | 2 (29) | 6 (30) | 6 (35) |
|
| |||
| Median time in days to transplant | |||
| From diagnosis (range) | 231 (158–300) | 179 (111–776) | 124 (88–238) |
| From study entry (range) | 166 (146–235) | 155.5 (105–229) | 110 (71–231) |
|
| |||
| Primary graft failure (%) | 0 (0) | 2 (10) | 0 (0) |
|
| |||
| Acute GVHD, all | 5 (71) | 14 (70) | 8 (47) |
| Grade III (%)** | 1 (14) | 1 (5) | 3 (18) |
|
| |||
| 5-Year TRMϕ | 14±26% | 5±10% | 6±11% |
|
| |||
| 5-Year OS† | 43±37% | 50±22% | 71±22% |
Abbreviations: FTI, farnesyl transferase inhibitor tipifarnib; GVHD, graft-versus-host disease; TRM, transplant related mortality.
Stem cell source information was not collected in most cases.
No grade IV acute GVHD was reported.
p value comparing TRM between the three groups is 0.68.
p value comparing OS between the three groups is 0.30.
Fig 1.
(A) Transplant-related mortality in patients who completed HSCT on-study and received (FTI) or did not receive (no FTI) tipifarnib. Three patients died from transplant-related causes: one patient who received 200 mg/m2/dose of tipifarnib and a matched unrelated stem cell source died at 13 days post-transplant due to sinusoidal obstructive syndrome, one who received 300 mg/m2/dose of tipifarnib and a matched unrelated stem cell source died at 35 days post-transplant due to infection, and one who did not receive tipifarnib and was transplanted using mismatched unrelated cord blood died at 158 days post-transplant due to infection. (B) Relapse risk after transplant in patients who completed HSCT on-study and received (FTI) or did not receive (no FTI) tipifarnib.
Fig 2.
(A) Five-year overall survival of all eligible patients who received (FTI) or did not receive (no FTI) tipifarnib. (B) Five-year event-free survival of all eligible patients who received (FTI) or did not receive (no FTI) tipifarnib.
Off Study Transplant
There were 41 patients who withdrew from the study, of which 24 patients went on to receive a non-protocol transplant (Supplemental Table I). Of the 24 patients who received a non-protocol transplant, 10 of these patients received tipifarnib prior to transplant while 14 did not. At last contact, 8/10 (80%) of those who received tipifarnib and 12/14 (86%) who did not, were still alive. In total, 20/24 (83%) patients in the non-protocol transplant group were alive at last contact compared to 25/44 (57%) in the on study transplant group (p=0.034). Patients who received non-protocol transplant have significantly better overall survival from study entry (5-year 82% vs 57%, p=0.045) and non-significantly better EFS from study entry (5-year 62% vs 39%, p=0.092) compared with patients who received protocol compliant transplant.
Biology Studies
In vitro assays for GM-CSF hypersensitivity were successful in 33 children and positive in 28 (85%) of these. Mutation analyses for PTPN11, NRAS, KRAS or CBL were performed in 88% of patients (Table I). Three patients (4%) were diagnosed with neurofibromatosis type 1. Mutations were evenly distributed across treatment groups. HDJ2 prenylation was inhibited in 14/16 patients tested, and the average inhibition of farnesyl transferase activity at 300 mg/m2/dose was 54% (range 38–90%). There was no relationship between gene mutation, degree of farnesyl transferase inhibition or HDJ2 prenylation, and clinical response to tipifarnib [29]. There was no difference in EFS between patients who did and did not respond to tipifarnib, and EFS was not influenced by elevated fetal hemoglobin, clinical neurofibromatosis type 1, monosomy 7 or specific gene mutation (data not shown), although the study was not powered to test these hypotheses.
DISCUSSION
JMML is a rare childhood neoplasm characterized by proliferation of clonal monocytic cells and GM-CSF hypersensitivity. HSCT, the only known curative therapy for JMML, is associated with a 64% 5-year overall survival rate [30], and no standard chemotherapy regimen used pre-transplant has decreased the high relapse rate [11]. Given that JMML is almost always the result of Ras pathway mutations, incorporation of Ras pathway inhibitors is a rational therapeutic strategy. Unfortunately, no small molecule inhibitors directly target the myriad defects in Neurofibromin, Ras, Shp-2 or Cbl proteins. We tested the farnesyl transferase inhibitor tipifarnib as pre-transplant therapy, hypothesizing that disrupting post-translational modification of Ras would decrease leukemic burden pre-HSCT and improve EFS.
Tipifarnib was well-tolerated, with toxicities primarily limited to myelosuppression, febrile neutropenia, and diarrhea, consistent with other trials [31]. Seventy-three percent of patients demonstrated decreased WBC counts and organomegaly (MR/PR/CR) in response to tipifarnib. These responses did not translate into improved event-free or overall survival. The tipifarnib dose used in AAML0122 resulted in a mean 54% inhibition of farnesyl transferase activity, lower than reported in children previously [21]. While a higher dose might result in more complete enzyme inhibition and greater clinical response, adult trials using higher tipifarnib doses resulted in unacceptable renal and neurologic toxicities [32]. Lack of improvement in EFS or OS may also be related to alternative lipid modification of K-Ras and N-Ras by the enzyme geranylgeranyl transferase when farnesyl transferase is inhibited, leading to retention of biologic activity [33]. More effective inhibition of K-Ras and N-Ras signaling (e.g. via inhibition of MEK) could yield greater clinical efficacy.
Recent findings suggest that specific patient subsets may benefit from tipifarnib: gene expression profiling can identify patients more likely to respond to tipifarnib [34, 35], while leukemia cells from a patient with early T-cell precursor-acute lymphoblastic leukemia with homozygous Nf1 inactivation were unusually sensitive to tipifarnib in vitro [36]. We saw no correlation between specific gene mutation and clinical outcome.
The 5-year OS and EFS for all eligible patients in our study were 55±11% and 41±11%, respectively. The relapse rate for patients receiving protocol compliant HSCT was 43 ±15%. These are similar to historic survival and post-transplant relapse rates in JMML [12]. Importantly, achievement of CR prior to HSCT did not result in significant differences of OS, EFS or RR compared to patients not in CR. TRM was slightly lower than previous reports, with similar rates of acute GVHD [12, 37]. An important limitation of the study is that 41 patients came off study prior to HSCT. Some died or had progressive disease, but many withdrew to receive an alternative HSCT regimen. We report contrasting results when comparing those that received HSCT on protocol and those that received non-protocol HSCT. Patients who received non-protocol transplant have significantly better overall survival from study entry (5-year 82% vs 57%, p=0.045) and non-significantly better EFS from study entry (5-year 62% vs 39%, p=0.092) compared with patients who received protocol compliant transplant. Although it is not possible to precisely determine the cause of the discrepancy, there are factors that could help to explain the different outcomes. First, the median follow up for those alive at last contact in the non-protocol transplant group was 1594.5 days (245 – 2,974) compared to 1954 days (1,246 – 2,966) in the protocol transplant group (p = 0.087). This trend most likely reflects the expected contrast in off-study reporting vigilance between patients completing protocol therapy and those that did not. However, considering that most relapses in JMML occur within 6–12 months after transplant, this difference in follow up time does not fully explain the discrepancy in outcomes between the on study and off study transplant groups. As mentioned, some individual investigators chose not to include TBI as part of the preparatory regimen for HSCT as prescribed on this clinical trial (personal communications). Reasons given were the potential of neuropsychiatric sequelae of TBI and its lack of superiority over other conditioning regimens [12, 30]. Incorporation of TBI as part of the conditioning regimen on this study may have led to the higher OS noted in the non-protocol transplants. However, this single arm transplant study was not designed to evaluate this hypothesis. In general, TBI is no longer suggested for patients undergoing first transplantation of JMML.
An interesting observation was the trend toward increased 5-year overall survival in patients who did not receive tipifarnib compared to those who did (71% vs 48%, p=0.06). As the only statistically significant difference between patients that did and did not receive tipifarnib was a median 55 day delay in time from diagnosis to transplant, the delay in time to HSCT caused by the phase II window may have resulted in an adverse effect on overall and event-free survival. While there is no definitive published data demonstrating that time-to-HSCT clearly impacts prognosis, our results do suggest that initiating HSCT as soon as possible may be a beneficial clinical practice.
In summary, farnesyl transferase inhibition and the addition of CRA to cytotoxic chemotherapy and HSCT in patients with newly diagnosed JMML was safe and produced initial clinical responses, but did not improve upon historic rates of relapse or long-term survival. Very few patients experienced progressive disease while on tipifarnib, suggesting that the agent may be an effective method of controlling disease while awaiting HSCT. However, determination of response in this study was limited to clinical variables. While this is the current standard, and WBC count and splenomegaly are considered clinically meaningful response measures in JMML, WBC count is non-specific and assessment of splenomegaly is somewhat subjective. By definition, patients with JMML have bone marrow blast percentages of less than 20% at diagnosis [38], and this variable is not a predictor of outcome [39], making serial bone marrow analyses of limited utility in measuring response in JMML. This highlights a key challenge of following patients afflicted with JMML who are treated with either conventional or novel therapies. Recent advances in unraveling the molecular lesions that contribute to 85% of JMML and our ability to sensitively follow allele burden will greatly enhance the precision of response assessment in future studies [40].
Finally, the trend toward worse long-term survival rates in patients with delayed time to transplant suggests that the strategy of testing novel therapies in a pre-transplant window for patients with JMML will require rigorous biomarkers and biologic endpoints to predict response. Ideally, creation of biologically-based risk stratifiers to predict those patients who will fail therapy quickly will facilitate rapid allocation to HSCT or identify those who will benefit from specific interventions before or after HSCT. With many novel strategies in development, it will be critical to identify important biologic markers such as mutation burden or assessment of dynamic signal transduction cascades that will predict response rates and ultimately improve overall survival.
Supplementary Material
Supplemental Figure 1. Reasons for Off-Protocol Therapy. The percent of patients who received (FTI) and did not receive (no FTI) tipifarnib who came off protocol therapy due to completion of study therapy, disease progression, death, complicating disease, or withdrawal. For each off-therapy reason, there was no difference in the percentage of patients who did and did not receive tipifarnib (N.S. = not significant). The absolute number of patients included is indicated above each column; one patient who did not receive tipifarnib was lost to follow-up and not included in this figure (see Supplemental Table I).
Supplemental Table I. Reasons for patients not receiving transplant on AAML0122 (n=41).
Acknowledgments
The authors would like to thank Chris Dvorak for helpful comments regarding the manuscript.
Footnotes
CONFLICT OF INTEREST STATEMENT
Peter D. Emanuel has research funding from Johnson and Johnson. The remaining authors declare no conflict of interest.
References
- 1.Busque L, Gilliland DG, Prchal JT, et al. Clonality in juvenile chronic myelogenous leukemia. Blood. 1995;85:21–30. [PubMed] [Google Scholar]
- 2.Niemeyer CM, Arico M, Basso G, et al. Chronic myelomonocytic leukemia in childhood: a retrospective analysis of 110 cases. European Working Group on Myelodysplastic Syndromes in Childhood (EWOG-MDS) Blood. 1997;89:3534–3543. [PubMed] [Google Scholar]
- 3.Emanuel PD, Bates LJ, Castleberry RP, et al. Selective hypersensitivity to granulocyte-macrophage colony-stimulating factor by juvenile chronic myeloid leukemia hematopoietic progenitors. Blood. 1991;77:925–929. [PubMed] [Google Scholar]
- 4.Emanuel PD. Juvenile myelomonocytic leukemia. Curr Hematol Rep. 2004;3:203–209. [PubMed] [Google Scholar]
- 5.Loh ML, Sakai DS, Flotho C, et al. Mutations in CBL occur frequently in juvenile myelomonocytic leukemia. Blood. 2009;114:1859–1863. doi: 10.1182/blood-2009-01-198416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan RJ, Cooper T, Kratz CP, et al. Juvenile myelomonocytic leukemia: a report. 2nd International JMML Symposium; 2009; pp. 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan HS, Estrov Z, Weitzman SS, et al. The value of intensive combination chemotherapy for juvenile chronic myelogenous leukemia. J Clin Oncol. 1987;5:1960–1967. doi: 10.1200/JCO.1987.5.12.1960. [DOI] [PubMed] [Google Scholar]
- 8.Festa RS, Shende A, Lanzkowsky P. Juvenile chronic myelocytic leukemia: experience with intensive combination chemotherapy. Med Pediatr Oncol. 1990;18:311–316. doi: 10.1002/mpo.2950180411. [DOI] [PubMed] [Google Scholar]
- 9.Hasle H, Kerndrup G, Yssing M, et al. Intensive chemotherapy in childhood myelodysplastic syndrome. A comparison with results in acute myeloid leukemia. Leukemia. 1996;10:1269–1273. [PubMed] [Google Scholar]
- 10.Lilleyman JS, Harrison JF, Black JA. Treatment of juvenile chronic myeloid leukemia with sequential subcutaneous cytarabine and oral mercaptopurine. Blood. 1977;49:559–562. [PubMed] [Google Scholar]
- 11.Bergstraesser E, Zimmermann M, Hasle H, et al. Non-hematopoietic stem cell transplantation treatment of juvenile myelomonocytic leukemia: A retrospective analysis and definition of response criteria. Pediatr Blood Cancer. 2007;49:629–633. doi: 10.1002/pbc.21038. [DOI] [PubMed] [Google Scholar]
- 12.Locatelli F. Hematopoietic stem cell transplantation (HSCT) in children with juvenile myelomonocytic leukemia (JMML): results of the EWOG-MDS/EBMT trial. Blood. 2005;105:410–419. doi: 10.1182/blood-2004-05-1944. [DOI] [PubMed] [Google Scholar]
- 13.Yoshimi A, Mohamed M, Bierings M, et al. Second allogeneic hematopoietic stem cell transplantation (HSCT) results in outcome similar to that of first HSCT for patients with juvenile myelomonocytic leukemia. Leukemia. 2007;21:556–560. doi: 10.1038/sj.leu.2404537. [DOI] [PubMed] [Google Scholar]
- 14.Huang ME, Ye YC, Chen SR, et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988;72:567–572. doi: 10.1182/blood-2016-11-750182. [DOI] [PubMed] [Google Scholar]
- 15.Emanuel PD, Zuckerman KS, Wimmer R, et al. In vivo 13-cis retinoic acid therapy decreases the in vitro GM-CSF hypersensitivity in juvenile chronic myelogenous leukemia (JCML) [abstract] Blood. 1991;78:170a. [Google Scholar]
- 16.Castleberry RP, Emanuel PD, Zuckerman KS, et al. A pilot study of isotretinoin in the treatment of juvenile chronic myelogenous leukemia. N Engl J Med. 1994;331:1680–1684. doi: 10.1056/NEJM199412223312503. [DOI] [PubMed] [Google Scholar]
- 17.Castleberry RP, Chang M, Maybee D, et al. A phase II study of 13-cis retinoic acid (CRA) in juvenile myelomonocytic leukemia (JMML): a Pediatric Oncology Group (POG) study [abstract] Blood. 1997;90:346a. [Google Scholar]
- 18.Casey PJ, Solski PA, Der CJ, et al. p21ras is modified by a farnesyl isoprenoid. Proc Nati Acad Sci. 1989;86:8323–8327. doi: 10.1073/pnas.86.21.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.End DW, Smets G, Todd AV, et al. Characterization of the antitumor effects of the selective farnesyl protein transferase inhibitor R115777 in vivo and in vitro. Cancer Res. 2001;61:131–137. [PubMed] [Google Scholar]
- 20.Emanuel PD, Snyder RC, Wiley T, et al. Inhibition of juvenile myelomonocytic leukemia cell growth in vitro by farnesyltransferase inhibitors. Blood. 2000;95:639–645. [PubMed] [Google Scholar]
- 21.Widemann BC, Arceci RJ, Jayaprakash N, et al. Phase 1 trial and pharmacokinetic study of the farnesyl transferase inhibitor tipifarnib in children and adolescents with refractory leukemias: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2011;56:226–233. doi: 10.1002/pbc.22775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niemeyer C, Fenu S, Hasle H, et al. Differentiating juvenile myelomonocytic leukemia from infectious disease. Blood. 1998;91:365–367. [PubMed] [Google Scholar]
- 23.Karp JE, Lancet JE, Kaufmann SH, et al. Clinical and biologic activity of the farnesyltransferase inhibitor R115777 in adults with refractory and relapsed acute leukemias: a phase 1 clinical-laboratory correlative trial. Blood. 2001;97:3361–3369. doi: 10.1182/blood.v97.11.3361. [DOI] [PubMed] [Google Scholar]
- 24.Thomas ED, Storb R, Clift RA, et al. Bone-marrow transplantation (second of two parts) N Engl J Med. 1975;292:895–902. doi: 10.1056/NEJM197504242921706. [DOI] [PubMed] [Google Scholar]
- 25.Kalra R, Paderanga DC, Olson K, et al. Genetic analysis is consistent with the hypothesis that NF1 limits myeloid cell growth through p21ras. Blood. 1994;84:3435–3439. [PubMed] [Google Scholar]
- 26.Loh ML, Vattikuti S, Schubbert S, et al. Mutations in PTPN11 implicate the SHP-2 phosphatase in leukemogenesis. Blood. 2004;103:2325–2331. doi: 10.1182/blood-2003-09-3287. [DOI] [PubMed] [Google Scholar]
- 27.Gutmann DH, Aylsworth A, Carey JC, et al. The diagnostic evaluation and multidisciplinary management of neurofibromatosis 1 and neurofibromatosis 2. J Am Med Assoc. 1997;278:51–57. [PubMed] [Google Scholar]
- 28.Widemann BC, Salzer WL, Arceci RJ, et al. Phase I trial and pharmacokinetic study of the farnesyltransferase inhibitor tipifarnib in children with refractory solid tumors or neurofibromatosis type I and plexiform neurofibromas. J Clin Oncol. 2006;24:507–516. doi: 10.1200/JCO.2005.03.8638. [DOI] [PubMed] [Google Scholar]
- 29.Castleberry RP, Loh ML, Jayaprakash N, et al. Phase II Window Study of the Farnesyltransferase Inhibitor R115777 (Zarnestra) In Untreated Juvenile Myelomonocytic Leukemia (JMML): A Children’s Oncology Group Study. Blood. 2005;106:727a–728a. [Google Scholar]
- 30.Locatelli F, Niemeyer C, Angelucci E, et al. Allogeneic bone marrow transplantation for chronic myelomonocytic leukemia in childhood: a report from the European Working Group on Myelodysplastic Syndrome in Childhood. J Clin Oncol. 1997;15:566–573. doi: 10.1200/JCO.1997.15.2.566. [DOI] [PubMed] [Google Scholar]
- 31.Harousseau J-L. Farnesyltransferase inihibitors in hematologic malignancies. Blood reviews. 2007;21:173–182. doi: 10.1016/j.blre.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Braun T, Fenaux P. Farnesyltransferase inhibitors and their potential role in therapy for myelodysplastic syndromes and acute myeloid leukaemia. Br J Haematol. 2008;141:576–586. doi: 10.1111/j.1365-2141.2008.07099.x. [DOI] [PubMed] [Google Scholar]
- 33.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 34.Raponi M, Lancet JE, Fan H, et al. A 2-gene classifier for predicting response to the farnesyltransferase inhibitor tipifarnib in acute myeloid leukemia. Blood. 2008;111:2589–2596. doi: 10.1182/blood-2007-09-112730. [DOI] [PubMed] [Google Scholar]
- 35.Raponi M, Harousseau J-L, Lancet JE, et al. Identification of molecular predictors of response in a study of tipifarnib treatment in relapsed and refractory acute myelogenous leukemia. Clin Cancer Res. 2007;13:2254–2260. doi: 10.1158/1078-0432.CCR-06-2609. [DOI] [PubMed] [Google Scholar]
- 36.Biagi C, Astolfi A, Masetti R, et al. Pediatric early T-cell precursor leukemia with NF1 deletion and high-sensitivity in vitro to tipifarnib. Leukemia. 2010;24:1230–1233. doi: 10.1038/leu.2010.81. [DOI] [PubMed] [Google Scholar]
- 37.Manabe A, Okamura J, Yumura-Yagi K, et al. Allogeneic hematopoietic stem cell transplantation for 27 children with juvenile myelomonocytic leukemia diagnosed based on the criteria of the International JMML Working Group. Leukemia. 2002;16:645–649. doi: 10.1038/sj.leu.2402407. [DOI] [PubMed] [Google Scholar]
- 38.Emanuel PD. Juvenile myelomonocytic leukemia and chronic myelomonocytic leukemia. Leukemia. 2008;22:1335–1342. doi: 10.1038/leu.2008.162. [DOI] [PubMed] [Google Scholar]
- 39.Hasle H, Baumann I, Bergsträsser E, et al. The International Prognostic Scoring System (IPSS) for childhood myelodysplastic syndrome (MDS) and juvenile myelomonocytic leukemia (JMML) Leukemia. 2004;18:2008–2014. doi: 10.1038/sj.leu.2403489. [DOI] [PubMed] [Google Scholar]
- 40.Archambeault S, Flores NJ, Yoshimi A, et al. Development of an allele-specific minimal residual disease assay for patients with juvenile myelomonocytic leukemia. Blood. 2008;111:1124–1127. doi: 10.1182/blood-2007-06-093302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Reasons for Off-Protocol Therapy. The percent of patients who received (FTI) and did not receive (no FTI) tipifarnib who came off protocol therapy due to completion of study therapy, disease progression, death, complicating disease, or withdrawal. For each off-therapy reason, there was no difference in the percentage of patients who did and did not receive tipifarnib (N.S. = not significant). The absolute number of patients included is indicated above each column; one patient who did not receive tipifarnib was lost to follow-up and not included in this figure (see Supplemental Table I).
Supplemental Table I. Reasons for patients not receiving transplant on AAML0122 (n=41).