Abstract
In recent years, to circumvent the interpretive limitations associated with intake tests commonly used to assess taste function in rodents, investigators have developed devices called gustometers to deliver small volumes of taste samples and measure immediate responses, thereby increasing confidence that the behavior of the animal is under orosensory control. Most of these gustometers can be used to measure unconditioned licking behavior to stimuli presented for short durations and/or can be used to train the animal to respond to various fluid stimuli differentially so as to obtain a reward and/or avoid punishment. Psychometric sensitivity and discrimination functions can thus be derived. Here, we describe a new gustometer design, successfully used in behavioral experiments, that was guided by our experience with an older version used for over 2 decades. The new computer-controlled gustometer features no dead space in stimulus delivery lines, effective cleaning of the licking substrate, and the ability to measure licking without passing electrical current through the animal. The parts and dimensions are detailed, and the benefits and limitations of certain design features are discussed. Schematics for key circuits are provided as supplemental information. Accordingly, it should be possible to fabricate this device in a fashion customized for one’s needs.
Key words: gustatory system, licking, operant responding, psychophysics, rodents, taste testing
Introduction
Rodents have been the model of choice for research on the neural bases of taste function and ingestive behavior. The most common behavioral assay of taste responsiveness remains the 2-bottle preference test in which 2 bottles of fluid are placed on an animal’s cage for a prescribed period of time, usually 24h, with 1 bottle containing a taste solution (or water) and the other containing a different taste solution; the relative intake from the 2 bottles is then measured. This test has the significant attribute of simplicity. It does not demand complex equipment and is relatively straightforward to administer. However, such simplicity comes at the price of interpretive power. Although it is uncontested that taste plays a large role in guiding the amount and type of foods and fluids ingested as in a 2-bottle test, other factors such as postingestive events can influence such behavior.
In recent years, many taste researchers using rats and mice as subjects have adopted behavioral techniques that involve the delivery of small volumes of taste solutions and the measurement of immediate responses (see Spector 2003). Such methodological features increase the confidence that the behavior is guided by orosensory stimulation. Rodents have well-developed oromotor control of the tongue and are gifted lickers (see Halpern 1977; Travers et al. 1997). Thus, the tongue lick has effectively served as the fundamental unit of response in a variety of experimental paradigms. Some tasks involve the measurement of unconditioned licking responses driven by the natural appeal or aversiveness of taste compounds. In some cases, the taste compounds are previously conditioned to be preferred or aversive using Pavlovian stimulus pairings. Other tasks involve the use of licking as an operant to obtain a distinctly flavored fluid reinforcer, such as a sucrose solution. Finally, some tasks involve the use of taste as a discriminative stimulus to guide a response that leads to a reinforcer or a punishment. The latter techniques are effective in psychophysical analyses because the responding is only guided, but not driven, by the taste stimulus. Thus, the behavior measured reflects more purely sensory, rather than hedonic, evaluations. In all of these tasks, regardless of their functional orientation, some type of device must be used to deliver the fluid solutions and to measure the licking responses. Different apparatuses, some commercially available and some custom-built, have been used for this purpose and are sometimes referred to as gustometers (e.g., Shaber et al. 1970; Brosvic and Slotnick 1986; Spector and Grill 1988; Spector et al. 1990; Thaw and Smith 1992; Reilly et al. 1994; Smith 2001).
The goal of this article is to describe a new design for a gustometer, based on the experience with older designs used by the Spector laboratory for over 2 decades (e.g., Spector et al. 1990; Spector et al. 1995; Spector et al. 1996; St John et al. 1997; St John and Spector 1998; Geran and Spector 2000; Eylam and Spector 2002; Spector and Kopka 2002; Eylam and Spector 2004; Blonde et al. 2006; Dotson and Spector 2007; Grobe and Spector 2008). In particular, unlike other designs, this apparatus uses force transducers instead of electrical circuits in the measurement of licks, thus minimizing electrical artifacts that could potentially occur during simultaneous electrophysiological recording. The transducers also allow the measurement of lick force as a dependent variable. Additionally, there is no dead space in the stimulus delivery system, increasing investigator confidence that fluid volumes are consistent across trials. Finally, the entire surface of the stimulus delivery substrate that the tongue contacts is cleaned between trials. The following sections discuss the different components of the device in sufficient detail to allow others to basically fabricate it, along with any modifications deemed necessary. The description is accompanied by some reflection on the pros and cons of various design decisions. Table 1 provides information on commercially available components used in this design. All procedures involving animals reported here were approved by the Florida State University Animal Care and Use Committee.
Table 1.
Commercially available components
| Component | Description |
|---|---|
| Sound attenuation cubicle | 100-qt plastic cooler |
| Air intake fan | Box fan: DC 12V 0.8 A |
| Air outtake fan | Squirrel cage fan: DC 12V 0.72 A |
| House light | Interior dome light |
| Air outtake tubing | 0.75ʺ hose assembly |
| Background noise speaker | 8-Ohm PUI audio |
| Camera | USB day and night |
| Taste sample ball | Borosilicate |
| Reinforcement ball | Polyoxymethylene |
| Force transducer | 25 N/5.5-lb capacity |
| Plunger o-ring | Rubber; 0.5cm thick; OD: 2.59 cm |
| Lever arm motor (sample) | 1.8° step size |
| Rinse water motor | Delrin peristaltic low-volume pump |
| Stimulus stepping motor | 1.8° step size |
| Pressure pump | 1-hp quiet air compressor |
| Vacuum pump | 1/8 hp, 115V, 1 Ph |
| Pressure and vacuum tanks | 20-lb empty propane tank |
| Controller board 1 | PCI-DIO48H; 48 digital I/O lines |
| Controller board 2 | PCI-DAS08; A/D; digital I/O |
| Power supply | 12-V DC |
Apparatus design
Housing
The housing is divided into 2 compartments: the outer sound attenuation enclosure and the inner test chamber (Figure 1, left). The sound attenuation enclosure serves to isolate the animal from external events. We have found that a common plastic cooler is relatively inexpensive, readily available, and serves the purpose well. The cooler is oriented on its side with the lid serving as the enclosure door, opening towards the researcher. Holes are drilled in necessary positions to accommodate an intake fan, located in the top right of the back wall (which is actually the bottom of the cooler), and an output fan located in the top left portion of the back wall. One end of a piece of plastic ball-and-socket flexible tubing (outer diameter [OD] = 1.9cm) is attached to polyvinylchloride plumbing, which in turn is connected to the output fan; the other end has a plastic nozzle that is positioned ~10cm above the stimulus sample ball (described below). The tubing is anchored to the ceiling by cable clamps. The purpose of this tubing is to help draw vapors away from the animal in an attempt to minimize olfactory cues. The house light is attached to the inside ceiling of the cubicle and is positioned slightly to the right of center and half the distance between the back and front walls, to orient it directly above the inner test chamber. A small speaker is mounted on the left wall and provides background noise to mask potential auditory cues associated with taste stimulus delivery. A small infrared-illuminated video camera can be placed near the stimulus sample ball to monitor the animal as it licks. In fact, this camera can be positioned in other locations as well, as desired by the user. A single hole is drilled in the lower left back wall to allow passage of cables connecting the load cells, motors, and camera with the computer. Tubing from the rinse water and reinforcement pumps to their respective outputs also enters through this hole. These components and the systems to which they contribute are described below.
Figure 1.
Gustometer images. Left: a photograph of the entire apparatus. The sound attenuation chamber contains the manipulanda and motors associated with stimulus and reinforcer delivery. The stimulus delivery pumps, rinse and waste water carboys, vacuum reservoir, and purge cylinder are located underneath. Top right: magnified view of components within the sound attenuation chamber. Bottom right: the mounting of the sample ball to a bracket that is attached to a load cell is shown as is the position of the ball relative to turret tubing and cage. The right response ball assembly and the stainless steel shield have been removed in this image to allow better visualization.
The sound attenuation enclosure is supported by a frame made from 4 legs (137cm high) with horizontal cross bracing (see Figure 1, left). A 1-mm thick stainless steel sheet sits on top of the 4 legs above the cubicle. This supports the computer, monitor, keyboard, and lick sensitivity adjustment interface box. A baseplate anchors the legs and supports the rinse and waste water reservoirs, the ball wash system, and the 12-V DC power supply.
The inner test chamber is composed of polycarbonate side and back walls and a stainless steel front wall. The stainless steel wire mesh floor is suspended over a stainless steel droppings tray, to which the legs are attached. The lid is made of polycarbonate and has a handle on the outside to facilitate removal. The entire chamber can be removed from the sound attenuation cubicle for cleaning as necessary. There are feet on the outer bottom of the droppings tray that fit into specially machined holes in the base to assure proper orientation of the cage relative to the stimulus delivery system. The relative dimensions of the inner chamber vary depending on whether it is designed for rats or mice, and are reported in Table 2. In the front wall there are 3 vertical slots aligned horizontally to one another. The central slot provides the animal with access to the stimulus sample ball. The 2 side slots provide access to the response balls. All of these slots can be covered by manual shutters, each rotating around its respective thumbscrew attached to the outside surface of the front wall. One cue light (an LED set in a polyoxymethylene housing), independently controllable with multiple illumination levels, is positioned above each side slot. A small stainless steel shelf protrudes from the front wall, with an adjustable height from the cage floor, to support the forelimbs of the animal as it licks through the slots.
Table 2.
Dimensionsa of inner test chamber
| Rat | Mouse | |
|---|---|---|
| Cage | ||
| Length (cm) | 35.5 | 30.2 |
| Width (cm) | 24 | 17.9 |
| Height (cm) | 24 | 21.2 |
| Access slots | ||
| Width | 7.5 | 4.5 |
| Height | 25 | 20 |
| Separation (cm) | 4.0 | 3.8 |
| Distance from cage floor (cm)b | 3.8 | 2 |
| Paw shelf | ||
| Width | 18 | 10 |
| Distance from cage floorb | 10 | 20 |
| Cue light | ||
| Diameter | 18 | 18 |
| Distance from access slot (cm)b | 2.3 | 3.2 |
| Sample ball diameter | 14 | 9.5 |
| Response ball diameter | 13 | 10 |
| Ball distance from baseplate (cm)b | 11.5 | 11 |
aMeasurements are reported in millimeters unless otherwise specified.
bCan be varied.
Stimulus delivery system
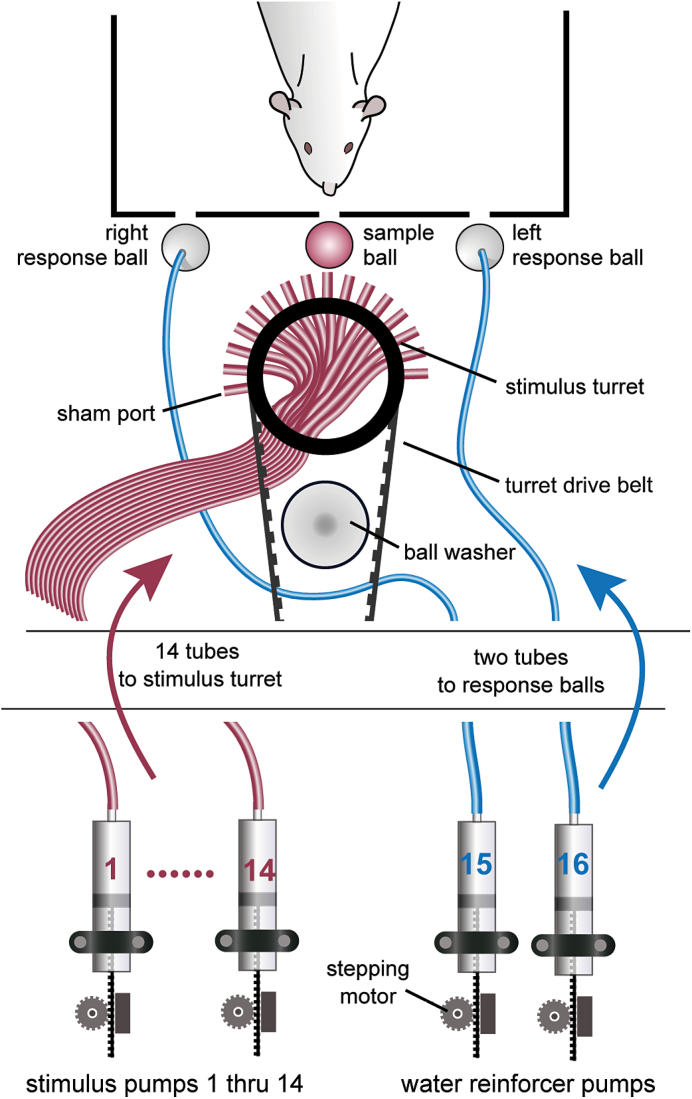
Sixteen stepping motors are anchored to the legs below the sound attenuation chamber (see Figure 1, left) and drive the custom-made plungers of their respective commercially available 60-mL syringes, 14 of which are the reservoirs for taste stimuli and 2 of which serve as reservoirs for reinforcer solutions (usually water; see Figure 2). The circuit schematic for the stepping motor driver can be found in the supplemental information (see Supplementary Figure S1). The outflow of the syringes is connected via a blunted 17-G needle and Teflon tubing to a belt-driven turret (machined from polyoxymethylene; see Figure 1, top and bottom right) that can position any sample stimulus tube behind a small glass stimulus delivery ball (referred to as the sample ball; see Table 2 for specific dimensions), allowing a transfer of solution from a single selected syringe without mixing. The animal is trained to lick the sample ball, which spins around its horizontal axis. This ball is connected to a sensitive force transducer (i.e., load cell) that can accurately measure tongue contact duration and force profile without passing any electrical current through the animal (see Figure 1, bottom right). The sample ball is recessed (adjustable; typically by ~2mm) behind the centrally positioned slot in the front wall of the test chamber that is just large enough to allow tongue protrusion, thus preventing inadvertent contact with other parts of the body (e.g., paws) during licking. Two similar balls, also mounted on force transducers, serve as both response manipulanda and water reinforcer delivery devices. They are positioned behind access slots (adjustable; typically ~2mm) on either side of the stimulus sample ball and are connected directly through Tygon tubing to their respective water-filled syringe pumps so that reward water can be made available on a lick-by-lick basis. The turret has 14 tubes mounted in a single row, with an additional sham port (“dummy” tube from which no stimulus is delivered) at each end of the row to serve as visual distractors. The sample ball position is adjustable but typically aligned to be ~1mm from the end of the tubes to avoid direct contact while still allowing adhesion to deposit fluid on the ball. A stainless steel sheet (1mm thick) that is slightly taller than the turret and shaped into an arc to match the turret is anchored to the base of the turret. It has a notch cut out of the top that is slightly wider than the stimulus ball and serves to block all but the ball and the closest tubes from view during a trial.
Figure 2.
Schematic of fluid delivery system. Stimuli and reinforcement solutions are placed in reservoirs sealed with plungers that are mounted onto stepper motors. Stimuli are delivered via tubing (colored in red) threaded into a turret that rotates to position the necessary tube behind the sample ball. Reinforcement solutions are delivered via tubing (colored in blue) directly through the response balls to the bottom. The ball washer positioned behind the turret is where the sample ball is cleaned between trials.
Fluid is pushed through the tubing of any given line by movement of the appropriate stepping motor. The system is designed for commercially available 60-mL syringes, resulting in 0.32 µL of fluid dispensed per step based on the degree per step of the motor and the diameter of the syringes. The arm of the plunger is made from a stainless steel gear rack, with its teeth being pushed by the gear on the stepper motor, resulting in the plunger being depressed to expel fluid. The head of the plunger is machined from polyoxymethylene and has a groove around its circumference in which a rubber O-ring sits to create a tight seal within the reservoir. It is important that a good seal be maintained for proper syringe pump operation, so these O-rings need to be replaced occasionally because of wear. It is also important to expel the air out of the syringe when filled before connecting it, via a Luer-lock blunt 17-G needle, to its fluid line. Before the start of a session, fluid is pushed all the way through each line. If a filled line sits idle for more than about 10min, air starts to be drawn into the outflow orifice as there is some negative pressure generated. This, however, can be counteracted by having the software activate the pump for a brief period of time to push the air out.
In our initial design, we were hoping that small drops of fluid could be deposited on a stationary sample ball and they would naturally flow to the bottom where the animal could lick them. Unfortunately, this did not occur reliably, requiring redesign. Ultimately, we allowed the ball to spin freely along its horizontal axis. In addition, high-speed filming revealed that a portion of fluid adhered to the surface of the ball, so we deliver a 10-µL preload of the stimulus to “wet” the ball, whereas subsequent licks deliver a rationed amount of fluid per lick (e.g., ~1 µL for mice, ~5 µL for rats). As used with previous designs, this preload typically occurs after 2 licks within 250ms to ensure that the animal is engaged in licking when the fluid is delivered. It should be noted that if the preload is delivered before licking begins, there is the chance of evaporation affecting subsequent lick volumes should there be a delay in the onset of behavior, although we have not explicitly tested this possibility. Perhaps more importantly, if the preload were delivered before the onset of licking, the stimulus would be available for the animal to sniff without tongue contact. As a result of this design, although the amount delivered from the stimulus line per lick is relatively precise, the amount of fluid delivered to the tongue is partially under the control of the animal due to the initial larger volume of the preload. Nevertheless, we have found that this design and the parameters chosen work exceptionally well in assessing the taste-related behavioral responses of rats (manuscripts in preparation) and mice (Smith et al. 2012; Treesukosol and Spector 2012).
The mechanism for delivering fluid from the stationary response balls is simpler because only 1 solution (usually water) is ever delivered from it during a session. The fluid line from the syringe is connected to stainless steel hypodermic tubing that is friction fit into a hole that is drilled through the vertical axis of a polyoxymethylene ball. The tube rests on an inner shelf very close to the bottom orifice of the ball so that the tube will never slip through and make a rough surface that could potentially damage the tongue when the animal is licking. A precise volume of fluid from a stepper motor is delivered at the bottom of the ball upon each lick.
Sample ball cleaning system
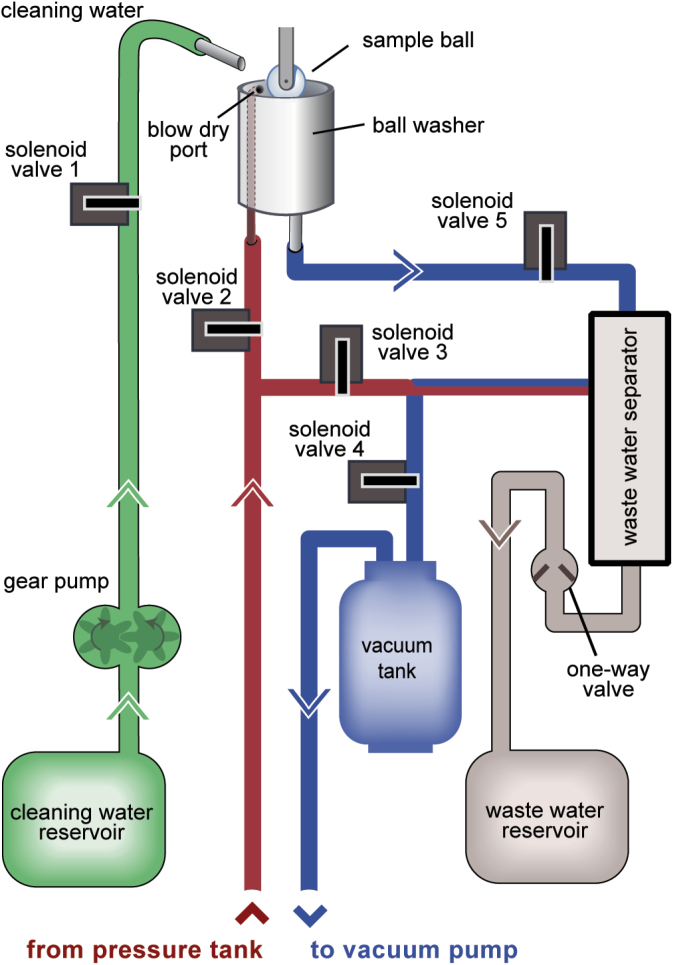
One of the principal merits of the design of the stimulus delivery system is that there is little opportunity for cross-contamination of fluid. There is virtually no dead space in the stimulus lines and they are easily cleaned by simply flushing water through them and occasionally performing an ethyl alcohol rinse. Software can be used to automatically flush the lines. The surface of the glass taste sample ball can be cleaned in between trials. A good portion of the gustometer is devoted to just that process. A ball washer is positioned behind the turret. Upon termination of a taste trial, a motor rotates a cam that retracts a lever arm, to which the load beam and sample ball are connected, and positions the ball in the well of the washer (see Figure 1, top right). The sample ball cleaning system is shown in Figure 3. Once in the well, the ball is sprayed with deionized water from a stainless steel tube connected through polyvinylchloride tubing to a solenoid valve (solenoid valve 1, Figure 3) and gear pump that draws from a 10-L cleaning-water reservoir. The stainless steel cleaning-water delivery tube (9 G) is anchored above the well and aimed just off of center. As rinse water is delivered, it causes the ball to spin, distributing fluid to the entire surface. The fluid is evacuated through a stainless steel tube (6 G) centered at the bottom of the well, serving as a drain out of the sound attenuation chamber. This process is facilitated by the opening of the vacuum line solenoid valve (solenoid 5, Figure 3), which draws water into the waste water separator compartment. This wash/evacuation cycle occurs 3 times, during which time vacuum is constantly being maintained within the waste water separator compartment by solenoid 4 (Figure 3). A third stainless steel tube (7/0) travels inside the side wall of the ball washer to connect to a small hole (0.94mm diameter) positioned 5mm from the top and delivers pressurized air, which dries the ball once a solenoid valve is activated (solenoid valve 2, Figure 3) after all water is evacuated from the washer. At the end of the wash cycle, water is drained from the waste water separator compartment into a 10-L waste water reservoir. The latter is aided by the opening of solenoid valve 3 (Figure 3), which directs pressurized air into the top of the waste water separator and pushes collected fluid out of the bottom through a 1-way valve that prevents both back-flow from the waste reservoir and loss of vacuum. Figure 4 depicts the relative timing of the relevant events of the cleaning cycle in our behavioral studies. The total cleaning process takes about 5 s, including time to move the sample ball to and from the ball washer, but it can be modified to be shorter or longer depending on the needs of the user as the operation of the solenoids and gear pump are all under software control. With the exception of the ball washer (and its internal pressurized air port) and the cleaning-water delivery tube, components are stationed below the sound attenuation chamber to keep noise levels (i.e., from valves) low for the animals (see Figure 1, left).
Figure 3.
Sample ball cleaning system. After the sample ball is brought into the ball washer, deionized water (shown in green) is delivered via the gear pump motor and solenoid valve 1. Pressure (shown in red) is used to dry the sample ball (solenoid valve 2) and to remove water from the waste water separator (solenoid valve 3) into a waste water reservoir to be discarded. Vacuum (shown in blue) is maintained in the waste water separator by solenoid valve 4 through a vacuum tank, which facilitates the evacuation of water from the ball washer (solenoid valve 5) and into the waste water separator.
Figure 4.
Relative timing of solenoid valves used in the sample ball cleaning system. These solenoids correspond to those depicted in Figure 2. The valve controlling the flow of deionized water for rinses is shown in green. Valves controlling pressure are shown in red. Valves controlling vacuum are shown in blue.
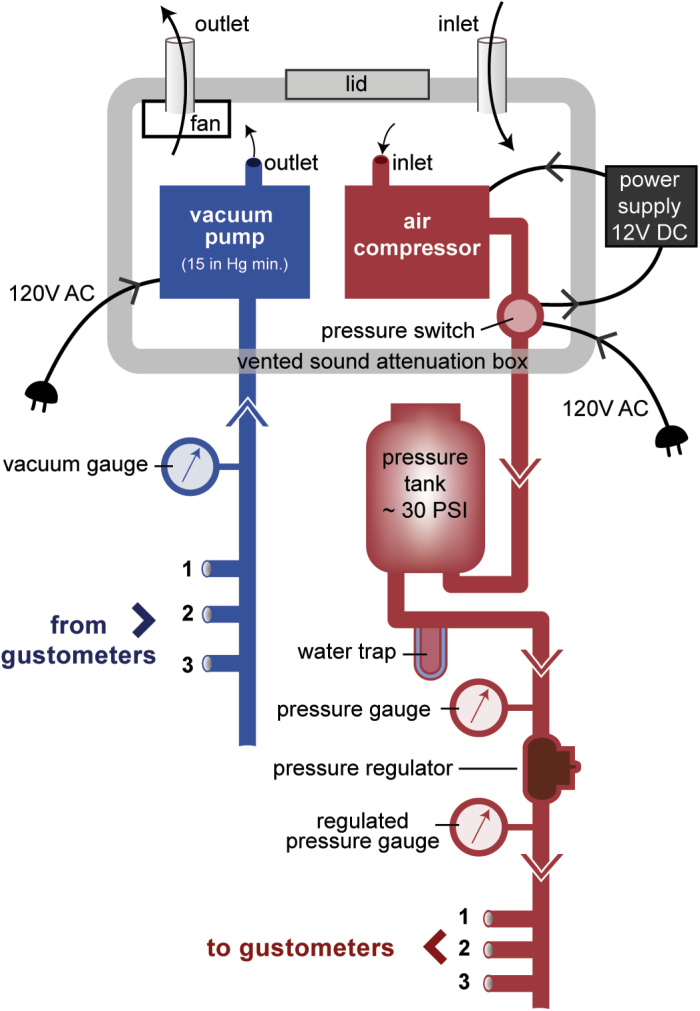
The vacuum and pressure needed for the ball washing process are provided by pumps (Figure 5). A single air compressor pressurizes a tank to 30 PSI and the pressure-regulated output can service up to 6 gustometers. Likewise, a single vacuum pump that is capable of producing at least 15 mm Hg in negative pressure is connected to up to 6 vacuum tanks, each dedicated to a single gustometer. The operation of these pumps is loud and so they are placed in a custom-fabricated sound attenuation box that significantly dampens the noise but has sufficient ventilation to avoid overheating. If sources of central air pressure and vacuum were available in the room housing the gustometer, as is not uncommon in laboratories, they could potentially circumvent the need for the pumps.
Figure 5.
Pressure and vacuum system. To maintain the necessary vacuum and pressure for the sample ball wash system, 1 AC vacuum pump (shown in blue) and 1 DC air compressor (shown in red) are installed in a vented sound attenuation box and connected to multiple machines. The vacuum pump draws air from each machine’s vacuum tank to individually maintain enough vacuum for each wash. The air compressor maintains ~30 psi in a pressure tank reservoir with a water trap to collect condensation. A pressure switch turns the air compressor on and off as the tank needs to be refilled with use by the connected machines. Gauges for both pressure and vacuum are also installed for the users.
Lick measurement
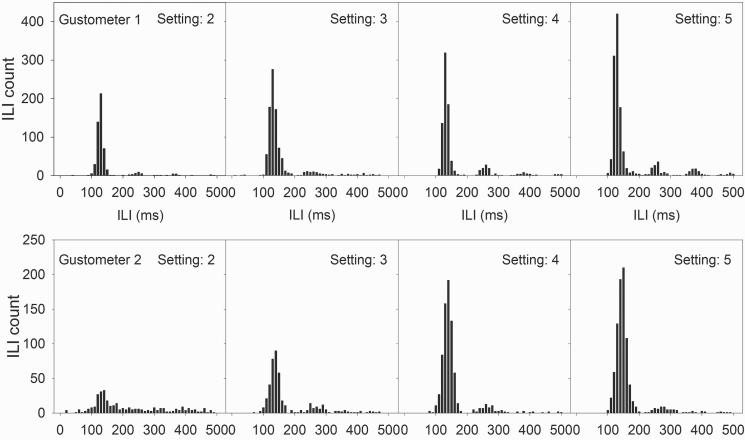
Licking is measured by a load cell that transduces force into an electrical signal and reliably reports tongue contact without passing electrical current through the animal (see Supplementary Figure S1 for circuit schematic). This method of tongue contact measurement was chosen to preclude the possibility of electrical current interfering with electrophysiological assays of neuronal responsiveness from chronically implanted electrodes in the brain as the animal is engaged in licking. Although we have yet to fully exploit this capability, the load cell also offers the chance to use lick force as a dependent measure (e.g., Moss et al. 2001). Figure 6 illustrates the ability of the load cells to register the temporal distribution of force that corresponds to each lick. Two exemplar lick force profiles are presented for lick bursts by water-deprived rats trained to lick the same response ball connected to a load cell to receive water. Despite significantly different lick force profiles (with the first rat generating much less force per lick), the load cell shows clear force signals that rise and decay quickly between licks. The load cell itself is capable of handling changes in force at a KHz frequency, (i.e., >1000 lick onset times per second), which is much faster than an animal is capable of producing. Indeed, the typical interlick interval (ILI) distributions generated by this lick circuit (Figure 7) attest to the fact that these load cells are sufficient for the application.
Figure 6.
Exemplar lick force profiles by rats. Each plot depicts the force registered by the same load cell in bins of ~2ms for 2 water-deprived rats trained to lick for water. Note the difference in maximal force registered by each rat, yet the load cell is capable of providing distinct force signals with quick rise and decay times associated with each lick.
Figure 7.
ILI distribution exemplars in relation to sample load cell sensitivity setting. Optimal placement for a load cell sensitivity provides a clear and normal ILI distribution (as seen in the left-most panel, top row; right-most panel, bottom row). When the setting is increased beyond the optimal placement for a load cell (top row), sensitivity is decreased and some licks are not registered. This results in multiple peaks in the distribution (far right, top row). Conversely, if the setting is too low (bottom row), then true licks are masked by noise. As the setting is increased, these “false licks” are filtered out and the distribution becomes more normal (far right, bottom row). Note that the setting numbers are arbitrary in this figure.
One drawback is that the load cells are expensive and at times difficult to acquire, but they have worked quite well. Figure 7 illustrates the ILI distributions of 2 mice tested with different user-adjustable circuit sensitivity values. Different gustometers (and thus, load cells) were used to test each mouse, with the animal licking the same load cell set to different sensitivities across days. When increasing the setting (decreasing sensitivity) above optimal placement for that load cell, subharmonics begin to appear in the distribution indicating missed licks, as illustrated in the top row of graphs. In other cases, when the setting is too low, the distribution is more dispersed as a result of noise due to the increased sensitivity of the load cell circuit; increasing the setting reduces these “false licks” and the ILI distribution becomes clear (Figure 7, lower panel).
One alternative to cut down on the cost, especially if neural recording is not part of the experiment, would be to use conductive material on the response balls and couple this with an electrical contact circuit. In this strategy, the sample ball would still be used with the load cell but a ground wire would need to be connected to the wire mesh floor or paw shelf in order to complete the reinforcement lick circuit through the animal.
We found that the orientation of the sample ball load cell was critical in the reliability of measuring licks. The initial design had the load cell horizontal and perpendicular to the vertical stem of the saddle that holds the spinning sample ball. This worked well for the stationary response balls, but for the spinning sample ball, we found that with this topography licks could be missed regardless of the sensitivity setting. This was easily corrected by angling the load cell–saddle assembly by −45° (see Figure 1, bottom right). Consequently, the transducer could take advantage of both horizontal and vertical force vectors during licking. As shown in Figure 7, with the proper circuit sensitivity, this design modification solved the problem. This requires some machining of the load cell bracket that attaches to the rotating lever (see Figure 1, bottom right) and it is important that care be exercised in mounting the delicate load cell in a mill so as not to damage its internal components.
Computer software
Customized software has been written in Visual BASIC 6.0 and Visual BASIC.NET to control the drivers for the solenoid valves and stepping motors as well as to operate the cue and house lights and noise generator, and to measure licking. Different programs have been written for different types of operations. A single program can be used to present 1 or more solutions in short or long durations, as set by the user. A separate program controls the contingencies used in 2-response operant tasks, which requires not only recording lick information from the stimulus ball load cell, but registering licks on the response balls and executing contingencies (e.g., reinforcement delivery or time-out). Further, any relevant output (e.g., licks taken, onset times, correct or incorrect responses) can be saved in a format readily processed by commercial spreadsheet software for later analysis. Because programming for any apparatus is heavily dependent on precise information such as interface board addresses and because there are more contemporary programming languages, that would likely be more efficient with computer resources that users might wish to apply, it would be pointless to describe the software in detail here. Suffice it to say that custom designing software to control the gustometer and manage data acquisition should not be a problem for any skilled computer programmer who has experience with machine-computer interfaces.
Final remarks
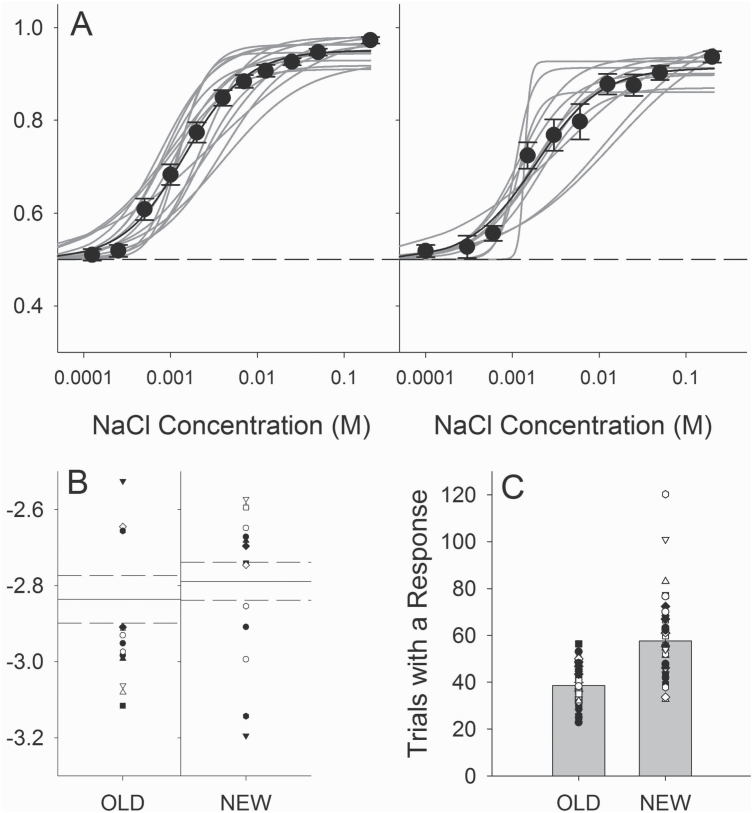
We have already used this device to test mice and rats in a variety of taste-related behavioral paradigms with great success. As can be seen in Figure 7, the gustometer can be used in experiments in which licking to a single stimulus is assessed and very detailed information can be obtained making it excellent for microstructural studies of ingestive behavior. If a single solution is presented ad libitum, then either of the response balls can be used providing precise control of lick volume. The gustometer can also be used in a brief-access taste test experiment in which licking in response to various taste solutions presented during short trials (e.g., 5–10 s) is assessed (Smith et al. 2012). However, perhaps the greatest benefit of the apparatus is its amenability to psychophysical experiments aimed at assessing taste sensitivity and discriminability in rodents. We have used this gustometer along with a 2-response operant taste discrimination task (Treesukosol and Spector 2012; Smith and Spector, 2014) to measure taste sensitivity in wild type and T1R1, T1R2, T1R3, and T1R2 + 3 taste receptor knock-out mice as well as in rats. A family of curves and the associated EC50 values (taken as an operational definition of threshold) from 2 sets of rats trained and tested similarly with NaCl in our old (Blonde et al. 2006) and new gustometer (unpublished), respectively, are displayed in Figure 8. Although such comparisons of measures of NaCl sensitivity between these studies must be regarded with great caution, the data derived from rats tested in the new gustometer are remarkably comparable to those collected from rats tested in the old, as attested to by the similar EC50 values derived from the curves (Figure 8b). We have also tested the rats for their ability to discriminate between NaCl and KCl on the basis of taste in the new gustometer.
Figure 8.
Comparisons of data generated by old versus new gustometer designs. (A) Logistic curves fit to individual rat performance on a NaCl sensitivity task in our previous (left panel; from Blonde et al. 2006) and current gustometer designs (right panel), showing a concentration dependency that is very similar across designs. (B) EC50 values from the curves plotted in A, showing that the 2 data sets are fairly consistent. (C) Average number of trials with a response initiated per session by individual mice during similar experimental phases in the old and new gustometers. The reliable delivery of smaller volumes by the new design allows for the generation of more data per session for mouse experiments.
Interestingly, with mice, the most notable difference between the old and new gustometers in our psychophysical operant tasks is that the new design tends to yield more trials with a response during a single session. Figure 8c displays average numbers of trials with a response by mice across multiple studies employing our operant taste detection/discrimination task (Dotson and Spector 2007; Treesukosol et al. 2011; Treesukosol and Spector 2012; Smith and Spector 2014) in each machine (lower panel). These data are taken from similar experimental phases across each experiment and show that the new design allows the generation of many more observations per session. This is likely due to the ability of the new gustometer to deliver smaller fluid volumes (~1 µL vs. ~1.8–2.0 µL) with each lick, thus delaying satiation. In all of our uses, the data generated in control animals have been quite orderly and the tasks employed have been able to reveal interesting and often robust effects of the genetic or anatomical manipulation of the gustatory system on taste function. Thus, the gustometer presented here has great experimental versatility. Future users can more specifically modify the basic design in ways to better match their methodological needs.
Supplementary material
Supplementary material can be found at http://www.chemse.oxfordjournals.org/
Funding
This work was supported, in part, by the National Institutes of Health (R01-DC004574, R01-DC009821, and P30-DC010364).
R.P.H. is President of DiLog Instruments, Inc. This did not conflict with the design or construction of the gustometer, which was conducted at the Florida State University Department of Psychology Instrumentation Shops.
Supplementary Material
Acknowledgements
We would like to thank Tom Shaker and Kimberly Smith for helping collect some of data evaluating the performance of the device, Charles Badland for generating system schematics and taking the provided photographs, and Shawn Whitman for his help machining certain parts.
References
- Blonde GD, Garcea M, Spector AC. 2006. The relative effects of transection of the gustatory branches of the seventh and ninth cranial nerves on NaCl taste detection in rats. Behav Neurosci. 120(3):580–589. [DOI] [PubMed] [Google Scholar]
- Brosvic GM, Slotnick BM. 1986. Absolute and intensity-difference taste thresholds in the rat: evaluation of an automated multi-channel gustometer. Physiol Behav. 38(5):711–717. [DOI] [PubMed] [Google Scholar]
- Dotson CD, Spector AC. 2007. Behavioral discrimination between sucrose and other natural sweeteners in mice: implications for the neural coding of T1R ligands. J Neurosci. 27(42):11242–11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eylam S, Spector AC. 2004. Stimulus processing of glycine is dissociable from that of sucrose and glucose based on behaviorally measured taste signal detection in Sac ‘taster’ and ‘non-taster’ mice. Chem Senses. 29(7):639–649. [DOI] [PubMed] [Google Scholar]
- Eylam S, Spector AC. 2002. The effect of amiloride on operantly conditioned performance in an NaCl taste detection task and NaCl preference in C57BL/6J mice. Behav Neurosci. 116(1):149–159. [PubMed] [Google Scholar]
- Geran LC, Spector AC. 2000. Sodium taste detectability in rats is independent of anion size: the psychophysical characteristics of the transcellular sodium taste transduction pathway. Behav Neurosci. 114(6):1229–1238. [PubMed] [Google Scholar]
- Grobe CL, Spector AC. 2008. Constructing quality profiles for taste compounds in rats: a novel paradigm. Physiol Behav. 95(3):413–424. [DOI] [PubMed] [Google Scholar]
- Halpern BP. 1977. Functional anatomy of the tongue and mouth of mammals. In: Weijnen JAWM, Mendelson J, editors. Drinking behavior. New York: Plenum Press; p. 1–92. [Google Scholar]
- Moss SJ, Wang G, Chen R, Pal R, Fowler SC. 2001. 3-Acetylpyridine reduces tongue protrusion force but does not abolish lick rhythm in the rat. Brain Res. 920(1-2):1–9. [DOI] [PubMed] [Google Scholar]
- Reilly S, Norgren R, Pritchard TC. 1994. A new gustometer for testing taste discrimination in the monkey. Physiol Behav. 55(3):401–406. [DOI] [PubMed] [Google Scholar]
- Shaber GS, Brent RL, Rumsey JA. 1970. Conditioned suppression taste thresholds in the rat. J Comp Physiol Psychol. 73(2):193–201. [DOI] [PubMed] [Google Scholar]
- Smith JC. 2001. The history of the “Davis Rig”. Appetite. 36(1):93–98. [DOI] [PubMed] [Google Scholar]
- Smith KR, Spector AC. 2014. The importance of the presence of a 5’-ribonucleotide and the contribution of the T1R1 + T1R3 heterodimer and an additional low-affinity receptor in the taste detection of L-glutamate as assessed psychophysically. J Neurosci. 34(39):13234–13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Treesukosol Y, Paedae AB, Contreras RJ, Spector AC. 2012. Contribution of the TRPV1 channel to salt taste quality in mice as assessed by conditioned taste aversion generalization and chorda tympani nerve responses. Am J Physiol Regul Integr Comp Physiol. 303(11):R1195–R1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AC. 2003. Psychophysical evaluation of taste function in non-human mammals. In: Doty RL, editor. Handbook of olfaction and gestation. New York: Marcel Dekker; p. 861–879. [Google Scholar]
- Spector AC, Andrews-Labenski J, Letterio FC. 1990. A new gustometer for psychophysical taste testing in the rat. Physiol Behav. 47(4):795–803. [DOI] [PubMed] [Google Scholar]
- Spector AC, Grill HJ. 1988. Differences in the taste quality of maltose and sucrose in rats: issues involving the generalization of conditioned taste aversions. Chem Senses. 13:95–113. [Google Scholar]
- Spector AC, Guagliardo NA, St John SJ. 1996. Amiloride disrupts NaCl versus KCl discrimination performance: implications for salt taste coding in rats. J Neurosci. 16(24):8115–8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AC, Kopka SL. 2002. Rats fail to discriminate quinine from denatonium: implications for the neural coding of bitter-tasting compounds. J Neurosci. 22(5):1937–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AC, Scalera G, Grill HJ, Norgren R. 1995. Gustatory detection thresholds after parabrachial nuclei lesions in rats. Behav Neurosci. 109(5):939–954. [PubMed] [Google Scholar]
- St John SJ, Markison S, Guagliardo NA, Hackenberg TD, Spector AC. 1997. Chorda tympani transection and selective desalivation differentially disrupt two-lever salt discrimination performance in rats. Behav Neurosci. 111(2):450–459. [PubMed] [Google Scholar]
- St John SJ, Spector AC. 1998. Behavioral discrimination between quinine and KCl is dependent on input from the seventh cranial nerve: implications for the functional roles of the gustatory nerves in rats. J Neurosci. 18(11):4353–4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaw AK, Smith JC. 1992. Conditioned suppression as a method of detecting taste thresholds in the rat. Chem Senses 17:211–223. [Google Scholar]
- Travers JB, Dinardo LA, Karimnamazi H. 1997. Motor and premotor mechanisms of licking. Neurosci Biobehav Rev. 21(5):631–647. [DOI] [PubMed] [Google Scholar]
- Treesukosol Y, Mathes CM, Spector AC. 2011. Citric acid and quinine share perceived chemosensory features making oral discrimination difficult in C57BL/6J mice. Chem Senses. 36(5):477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treesukosol Y, Spector AC. 2012. Orosensory detection of sucrose, maltose, and glucose is severely impaired in mice lacking T1R2 or T1R3, but polycose sensitivity remains relatively normal. Am J Physiol Regul Integr Comp Physiol. 303(2):R218–R235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.