Abstract
Spinal cord injury (SCI) can be defined as a loss of communication between the brain and the body due to disrupted pathways within the spinal cord. While many promising molecular strategies have emerged to reduce secondary injury and promote axonal regrowth, there is still no effective cure and recovery of function remains limited. Functional electrical stimulation (FES) represents a strategy developed to restore motor function without the need for regenerating severed spinal pathways. Despite its technological success, however, FES has not been widely integrated into the lives of spinal cord injury survivors. In this review, we briefly discuss the limitations of existing FES technologies. Additionally, we discuss how optogenetics, a rapidly evolving technique used primarily to investigate select neuronal populations within the brain, may eventually be used to replace FES as a form of therapy for functional restoration following SCI.
Keywords: Optogenetics, spinal cord injury, functional electrical stimulation, sensorimotor, FES, SCI
Spinal cord injury
Despite efforts to elucidate the pathophysiology of spinal cord injury (SCI) in the last few decades, the search for a cure continues 1-3. Currently, the gold standard of care is to provide intense physical rehabilitation following the acute injury phase, in an attempt to maximize any spontaneous recovery of respiratory, hand, arm, leg, bowel, bladder and sexual function 4. While this paradigm increases the possibility of some degree of recovery, particularly in patients with incomplete injuries, most patients do not experience a full recovery and have only limited gains with current rehabilitation therapy 4-7.
The poor chance of recovery following SCI has inspired a significant amount of research aimed at restoring lost function in SCI survivors. From a biological standpoint, these efforts have primarily focused on molecular manipulations to lessen the degree of secondary injury that occurs via ischemia and excitotoxicity 5,8-14, replacement of lost neurons and glia via stem cell transplantation 15,16, and remyelination or axonal regeneration by either reducing glial scar formation 17 or by inserting biomaterial substrates 18 that promote neural regrowth 19-23. Unfortunately, these approaches have been met with limited success due to the complexity involved with degrading glial scarring while regenerating neural tissue and directing appropriate neural connections required to restore severed spinal pathways 24.
An alternative to molecular manipulations is to activate remaining neuromuscular components, which, despite the loss of descending input, can still be activated via external stimuli. Historically, the most common form of stimuli has been electricity. Namely, functional electrical stimulation (FES) has been successfully used to restore breathing 25,26, lower 27-29 and upper extremity function 30,31, and bladder and bowel control 32-35.
Presently, FES systems can restore lost function, however, they have a narrow scope of application and generally only restore one previously lost function at a time. For example, phrenic pacing has allowed for individuals with high cervical injuries and intact phrenic nerves to successfully wean from mechanical ventilation, leading to increased survival rates and improved quality of life 36,37. Additionally, Parastep ®, a commercially available device that relies on surface stimulation of the quadriceps, gluteal muscles, and peroneal nerves, permits individuals with lower SCI to ambulate for distances over a quarter of a mile 38. Furthermore, Vocare ® utilizes anterior sacral root stimulation to restore micturition 39,40.
Despite the proven effectiveness of the systems described above, technological shortcomings and practical limitations such as inadequate activation control strategies 41, electrical current spillover 42-44, and muscle fatigue 45 have led to a limited integration of FES systems into the daily lives of SCI survivors 41. Optogenetics, a novel stimulation modality that uses light to either excite or inhibit genetically modified neurons, has the potential to overcome some of the limitations facing current FES strategies 1,46,47.
Optogenetics
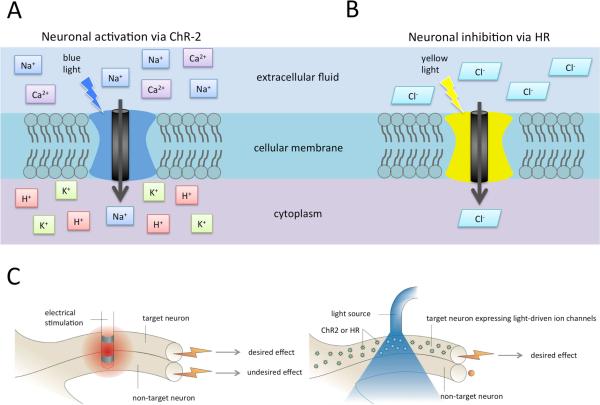
Optogenetics is a rapidly evolving technique originally developed to study neural activity in select neuronal populations 48. The genetic material of specific cell populations is modified via viral vectors to express a trans-membrane protein reactive to light (opsins). These trans-membrane proteins undergo a conformational change when light of a specific wavelength (390–700 nm) is directly applied to the cells, resulting in selective ionic current flow across the cell membrane. In turn, positively-charged (cations) or negatively-charged (anions) ionic movement will lead to cell depolarization or hyperpolarization, respectively. Therefore, specific viral vectors can be chosen and modified to transduce specific neuronal populations allowing for selective modulation with light. Excitatory responses can be achieved by activating Channelrhodopsin-2 (ChR-2) cation channels (responsive to 470 nm wavelength blue light), which allow entry of positively-charged sodium and calcium ions into the cell (Figure 1A) 50,51. In contrast, inhibitory responses can be evoked by activating Halorhodopsin (HR), a trans membrane ion-pump, using 580 nm yellow light, which facilitates the movement of negatively-charged chloride ions (Figure 1B) 5,47.
Figure 1. Mechanisms of neuromodulation via optogenetics.
A) Application of blue light (470 nm wavelength) leads to a conformational change of the trans membrane ion channel protein, channelrhodopsin, allowing a flow of positively charged ions into the cytoplasm, ultimately leading to neuron depolarization.
B) Yellow light application (580 nm wavelength) changes the conformation of the trans membrane ion pump protein, halorhodopsin, allowing negatively charged ions to move into the cytoplasm, leading to neuron hyperpolarization.
C) Schematic comparing the non-specific activation characteristic of electrical activation, leading to both desired and undesired effects, and optical activation of only targeted neurons, leading to only desired effects.
Ca2+ = calcium ion; ChR-2 = channelrhodopsin; Cl- = chloride ion; H+ = hydrogen ion; HR = halorhodopsin; K+ = potassium ion; Na+ = sodium ion
Adapted with permission from Macmillan Publishers Ltd: [Nature Reviews Neuroscience], 49 copyright 2007.
The use of optogenetics has previously focused on characterization of neuronal mechanisms of excitation and inhibition within the brain 5,8,10,12-14,52. However, increased interest in translational applications of optogenetics technology has resulted in the pursuit of novel clinical avenues for restoration of vision, seizure control, and treatment of cardiac arrhythmias 19,53,54. Light offers clear advantages for modulating neuronal behavior. Specifically, optical stimulation can provide real-time, selective control of cellular activity 51. Additionally, efforts to expand the toolbox for controlling neurons via light have led to an increased variety of ChR-2s that are altered to respond to various light wavelengths with enhanced ion channel kinetics and selectivity 55. More recent efforts have led to the first light-gated chloride channel, engineered from the ChR-2 trans membrane family of proteins, which is designed to decrease the latency between light activation and cell inhibiton that is observed with HR ion pumps 56,57. Furthermore, optical control of muscle function has been achieved by controlling murine stem cells, previously engineered to express ChR-2, followed by implantation distal to a nerve ligation in an attempt to establish a possible regenerative medicine therapeutic intervention 58. Moreover, the combination of genes that express ChR-2 with genes that express the light-generating protein luciferase demonstrated that it is possible to activate neurons by exogenous application of the luciferase substrate, leading to cell luminescence and light-driven auto-activation in vitro 59. Finally, computational modeling evidence has shown that optogenetic activation of axons follows a physiologic, small-to-large diameter axon recruitment order 60, which could prove invaluable for restoring motor function following SCI.
Restoration of motor function following SCI via optical stimulation
Applications of optogenetic technology for restoring function following SCI are already underway in small animal models. In fact, optogenetics has recently been used to dissect select spinal cord circuitry responsible for evoking both rhythmic, and stimulation-triggered limb movements 46,47,61. Specifically, Towne and colleagues demonstrated the ability of using optical stimulation to selectively activate hind limb muscles in a rodent model of SCI using retrograde transduction of motor neurons with ChR-2 via intramuscular inoculation with an adeno-associated virus (AAV) 50. Similarly, Alilain and colleagues showed that it is possible to restore motor activity in the diaphragm muscle of rodents that sustained a cervical SCI using optical stimulation of the spinal cord at cervical vertebral levels 3-6 62. Additionally, Hagglund and colleagues showed rhythmic activation of selective muscles necessary for locomotion using optical stimulation in a transgenic mouse line expressing ChR-2 channels in spinal interneurons 47.
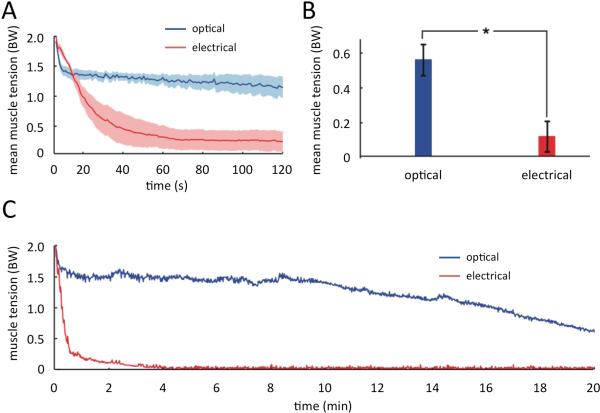
The continued development of optogenetic technology promises to overcome several limitations of electrical stimulation techniques for restoring motor function following SCI. First, optical stimulation allows selective muscle activation and fine motor control due to increased specificity associated with viral transduction of select motor neurons 61, as well as the theoretical possibility of direct transduction and control of skeletal itself 63. Second, optogenetics may restore function in a more physiologic manner particularly for functions that involve complex patterns of excitation and inhibition of different neuronal populations. An example is micturition, which requires activation of parasympathetic neural circuitry to initiate bladder emptying and simultaneous inhibition of sphincter contraction. While electrical stimulation can be used to achieve bladder emptying, 53,64,65 it occurs in a suboptimal tetanic fashion. Third, muscle fatigue associated with existing electrical stimulation technologies 66-68 can be delayed by activating slow twitch, fatigue-resistant fibers before any fast fatigable fibers are activated (Figure 2) 61,69,70. Despite the significant advantages of optical stimulation over electrical stimulation, multiple limitations have to be addressed before optogenetics can be clinically used to restore function in SCI survivors.
Figure 2. Fatigue resistance comparison between optical and electrical stimulation.
A) Average tetanic muscle tension during 2 min stimulation with 250 ms trains of stimulation at 1 Hz using electrical and optical stimulation (n=7, shaded region is standard error of the mean, average body weight = 0.258 ± 0.01 N).
B) Average fatigue index measured as decline in tetanic muscle tension over 2 min (n=7).
C) Tetanic tension from a single mouse during optical and electrical stimulation in the hind limbs over 20 minutes.
BW = average body weight
Adapted with permission from Macmillan Publishers Ltd: [Nature Medicine], 61 copyright 2010.
Limitations of optogenetic applications
Numerous studies using direct administration of AAV vectors with different serotypes in small animal models have demonstrated robust transduction rates 71-74. Additionally, gene therapy using viral vectors has been successfully translated to clinical practice, however, its use has uncovered multiple issues that need to be addressed before viral delivery of optogenetics can be used clinically in humans. First, efforts to reproduce efficient transduction in large animal models have been largely unsuccessful in the past. More recently, improvements in transduction efficiency have been reported in both the brain and spinal cord in swine 75-77 and nonhuman primate models 76,78,79. Second, integration of foreign genomic material can also result in numerous adverse events including expression of proto-oncogenes 80, silencing of tumor-suppressor genes 81,82 which could lead to neoplastic transformation or protein mutations, leading to undesired changes in downstream cellular functions 81,82. Third, peripherally-administered vectors can initiate immune responses leading to inhibition of vector function, decreased expression duration, and cytotoxic effects 83-85. Current strategies to lessen immune responses include altering the capsid of the viral vector, modifying the vector delivery route, or applying techniques to inhibitor or modulate immune system activity 86,87. Alternatively, non-viral techniques could be used along with biomaterial and molecular strategies to systemically deliver genetic material into target locations 20,88,89.
Further work is also needed to identify optimal vectors (viral or nonviral) and specific administration routes for targeting specific neuronal populations. For example, efficient and selective transduction of alpha motor neurons within the ventral spinal cord will likely require intraparenchymal or intrathecal vector injection into the spinal gray matter. Alternatively, this could be achieved by retrograde transport from intraneural or intramuscular injection sites.
Finally, multiple barriers must be overcome before chronically implantable optical systems can be developed. Some of these barriers include 1) minimizing glial responses to the implanted light guides, similar to the glial scarring observed with other chronic neural interface systems such as deep brain stimulation and intracortical recording systems; 2) optimizing light delivery paradigms to enhance temporal and spatial activation of target neurons while improving light penetration through tissue surrounding the light source 90; and 3) reducing heating effects on tissue surrounding the light source.
Future directions
Small animal studies suggest that optogenetics offers multiple advantages over electrical stimulation techniques. However, multiple steps need to be taken before optogenetics can be clinically used to restore function in SCI survivors. First, it is necessary to devise appropriate strategies for safe transgene delivery to target cell types in vivo. These strategies will require controlled transduction (via appropriate vectors and serotypes) and expression (via appropriate gene regulation promoters). Second, it is paramount to extend the expression lifetime to allow for single (or minimally repeated) administration of viral vectors and promoters. Third, stimulation systems need to be developed that optimize light delivery paradigms in a tissue specific manner while reducing glial responses to light delivery devices. Finally, stimulation will need to be controlled in a natural manner by the user while also allowing for real-time adjustment to account for perturbations within the user's environment 41,91.
Conclusions
While there is still no cure for SCI, advances in stimulation and neural interfacing technology show promise for restoring neurologic function. Optogenetics offers to improve upon existing FES technology by better following physiologic muscle activation, increasing selectivity, and providing simultaneous control of excitatory and inhibitory responses. In turn, advances in optogenetics technology could provide an avenue for optimal restoration of function following SCI, thereby improving the quality of life for those living with paralysis.
Acknowledgements
This work was supported by The Grainger Foundation (to KHL) and NIH R21 NS087320 (to JLL).
Abbreviations
- AAV
adeno-associated virus
- ChR-2
channelrhodopsin
- FES
functional electrical stimulation
- HR
halorhodopsin
- SCI
spinal cord injury
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure:
J.L.L. has intellectual property licensed to Boston Scientific. The authors declare that this review was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Smedemark-Margulies N, Trapani JG. Tools, methods, and applications for optophysiology in neuroscience. Front Mol Neurosci. 2013;6:18. doi: 10.3389/fnmol.2013.00018. doi:10.3389/fnmol.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sekhon LHS, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine. 2001;26(24 Suppl):S2–12. doi: 10.1097/00007632-200112151-00002. [DOI] [PubMed] [Google Scholar]
- 3.DeVivo MJ. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord. 2012;50(5):365–372. doi: 10.1038/sc.2011.178. doi:10.1038/sc.2011.178. [DOI] [PubMed] [Google Scholar]
- 4.Burns AS, Ditunno JF. Establishing Prognosis and Maximizing Functional Outcomes After Spinal Cord Injury: A Review of Current and Future Directions in Rehabilitation Management. Spine. 2001;26(24S):S137. doi: 10.1097/00007632-200112151-00023. [DOI] [PubMed] [Google Scholar]
- 5.Zhang F, Wang L-P, Brauner M, et al. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446(7136):633–639. doi: 10.1038/nature05744. doi:10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- 6.Kim HS, Jeong HJ, Kim MO. Changes of functional outcomes according to the degree of completeness of spinal cord injury. Ann Rehabil Med. 2014;38(3):335–341. doi: 10.5535/arm.2014.38.3.335. doi:10.5535/arm.2014.38.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scivoletto G, Tamburella F, Laurenza L, Torre M, Molinari M. Who is going to walk? A review of the factors influencing walking recovery after spinal cord injury. Front Hum Neurosci. 2014;8:141. doi: 10.3389/fnhum.2014.00141. doi:10.3389/fnhum.2014.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng MY, Wang EH, Woodson WJ, et al. Optogenetic neuronal stimulation promotes functional recovery after stroke. Proc Natl Acad Sci USA. 2014 doi: 10.1073/pnas.1404109111. doi:10.1073/pnas.1404109111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tzen Y-T, Brienza DM, Karg PE, Loughlin PJ. Effectiveness of local cooling for enhancing tissue ischemia tolerance in people with spinal cord injury. j spinal cord med. 2013;36(4):357–364. doi: 10.1179/2045772312Y.0000000085. doi:10.1179/2045772312Y.0000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tyan L, Chamberland S, Magnin E, et al. Dendritic inhibition provided by interneuron- specific cells controls the firing rate and timing of the hippocampal feedback inhibitory circuitry. J Neurosci. 2014;34(13):4534–4547. doi: 10.1523/JNEUROSCI.3813-13.2014. doi:10.1523/JNEUROSCI.3813-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tufan K, Oztanir N, Ofluoglu E, et al. Ultrastructure protection and attenuation of lipid peroxidation after blockade of presynaptic release of glutamate by lamotrigine in experimental spinal cord injury. Neurosurg Focus. 2008;25(5):E6. doi: 10.3171/FOC.2008.25.11.E6. doi:10.3171/FOC.2008.25.11.E6. [DOI] [PubMed] [Google Scholar]
- 12.Cho J-H, Deisseroth K, Bolshakov VY. Synaptic encoding of fear extinction in mPFC- amygdala circuits. Neuron. 2013;80(6):1491–1507. doi: 10.1016/j.neuron.2013.09.025. doi:10.1016/j.neuron.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton LS, Sohl-Dickstein J, Huth AG, Carels VM, Deisseroth K, Bao S. Optogenetic activation of an inhibitory network enhances feedforward functional connectivity in auditory cortex. Neuron. 2013;80(4):1066–1076. doi: 10.1016/j.neuron.2013.08.017. doi:10.1016/j.neuron.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paz JT, Davidson TJ, Frechette ES, et al. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nature Neuroscience. 2012;16(1):64–70. doi: 10.1038/nn.3269. doi:10.1038/nn.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMahon SS, Albermann S, Rooney GE, et al. Engraftment, migration and differentiation of neural stem cells in the rat spinal cord following contusion injury. Cytotherapy. 2010;12(3):313–325. doi: 10.3109/14653241003695018. doi:10.3109/14653241003695018. [DOI] [PubMed] [Google Scholar]
- 16.Olson HE, Rooney GE, Gross L, et al. Neural stem cell- and Schwann cell-loaded biodegradable polymer scaffolds support axonal regeneration in the transected spinal cord. Tissue Eng Part A. 2009;15(7):1797–1805. doi: 10.1089/ten.tea.2008.0364. doi:10.1089/ten.tea.2008.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Starkey ML, Bartus K, Barritt AW, Bradbury EJ. Chondroitinase ABC promotes compensatory sprouting of the intact corticospinal tract and recovery of forelimb function following unilateral pyramidotomy in adult mice. Eur J Neurosci. 2012;36(12):3665–3678. doi: 10.1111/ejn.12017. doi:10.1111/ejn.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen BK, Knight AM, Madigan NN, et al. Biomaterials. Biomaterials. 2011;32(32):8077–8086. doi: 10.1016/j.biomaterials.2011.07.029. doi:10.1016/j.biomaterials.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zarbin MA, Arlow T, Ritch R. Regenerative Nanomedicine for Vision Restoration. Mayo Clinic Proceedings. 2013 doi: 10.1016/j.mayocp.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 20.Yao L, Daly W, Newland B, et al. Improved axonal regeneration of transected spinal cord mediated by multichannel collagen conduits functionalized with neurotrophin-3 gene. Gene Ther. 2013;20(12):1149–1157. doi: 10.1038/gt.2013.42. doi:10.1038/gt.2013.42. [DOI] [PubMed] [Google Scholar]
- 21.Tom VJ, Sandrow-Feinberg HR, Miller K, et al. Exogenous BDNF enhances the integration of chronically injured axons that regenerate through a peripheral nerve grafted into a chondroitinase-treated spinal cord injury site. Experimental Neurology. 2013;239:91–100. doi: 10.1016/j.expneurol.2012.09.011. doi:10.1016/j.expneurol.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren H, Han M, Zhou J, et al. Repair of spinal cord injury by inhibition of astrocyte growth and inflammatory factor synthesis through local delivery of flavopiridol in PLGA nanoparticles. Biomaterials. 2014;35(24):6585–6594. doi: 10.1016/j.biomaterials.2014.04.042. doi:10.1016/j.biomaterials.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 23.Deng L-X, Hu J, Liu N, et al. GDNF modifies reactive astrogliosis allowing robust axonal regeneration through Schwann cell-seeded guidance channels after spinal cord injury. Experimental Neurology. 2011;229(2):238–250. doi: 10.1016/j.expneurol.2011.02.001. doi:10.1016/j.expneurol.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81(2):229–248. doi: 10.1016/j.neuron.2013.12.034. doi:10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glenn WW, Hogan JF, Phelps ML. Ventilatory support of the quadriplegic patient with respiratory paralysis by diaphragm pacing. Surg Clin North Am. 1980;60(5):1055–1078. doi: 10.1016/s0039-6109(16)42233-4. [DOI] [PubMed] [Google Scholar]
- 26.Glenn WW, Holcomb WG, Shaw RK, Hogan JF, Holschuh KR. Long-term ventilatory support by diaphragm pacing in quadriplegia. Ann Surg. 1976;183(5):566–577. doi: 10.1097/00000658-197605000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kantor C, Andrews BJ, Marsolais EB, Solomonow M, Lew RD, Ragnarsson KT. Report on a conference on motor prostheses for workplace mobility of paraplegic patients in North America. 311993:439–456. doi: 10.1038/sc.1993.73. doi:10.1038/sc.1993.73. [DOI] [PubMed] [Google Scholar]
- 28.Glaser RM. Physiology of Functional Electrical Stimulation-Induced Exercise: Basic Science Perspective. Neurorehabil Neural Repair. 1991;5(1-2):49–61. doi:10.1177/136140969100500106. [Google Scholar]
- 29.Ragnarsson KT, Pollack SF, Twist D. Lower Limb Endurance Exercise After Spinal Cord Injury: Implications for Health and Functional Ambulation. Neurorehabil Neural Repair. 1991;5(1-2):37–48. doi:10.1177/136140969100500105. [Google Scholar]
- 30.Crago PE, Nakai RJ, Chizeck HJ. Feedback regulation of hand grasp opening and contact force during stimulation of paralyzed muscle. IEEE Trans Biomed Eng. 1991;38(1):17–28. doi: 10.1109/10.68205. doi:10.1109/10.68205. [DOI] [PubMed] [Google Scholar]
- 31.Peckham PH, Kilgore KL, Keith MW, Bryden AM. An advanced neuroprosthesis for restoration of hand and upper arm control using an implantable controller. The Journal of Hand Surgery. 2002 doi: 10.1053/jhsu.2002.30919. [DOI] [PubMed] [Google Scholar]
- 32.Brindley GS. The first 500 patients with sacral anterior root stimulator implants: general description. Paraplegia. 1994;32(12):795–805. doi: 10.1038/sc.1994.126. doi:10.1038/sc.1994.126. [DOI] [PubMed] [Google Scholar]
- 33.Brindley GS, Rushton DN. Spinal Cord - Abstract of article: Long-term follow-up of patients with sacral anterior root stimulator implants. Spinal Cord. 1990 doi: 10.1038/sc.1990.63. [DOI] [PubMed] [Google Scholar]
- 34.Creasey GH, Craggs MD. Functional electrical stimulation for bladder, bowel, and sexual function. Handb Clin Neurol. 2012;109:247–257. doi: 10.1016/B978-0-444-52137-8.00015-2. doi:10.1016/B978-0-444-52137-800015-2. [DOI] [PubMed] [Google Scholar]
- 35.Peckham PH. Functional electrical stimulation: current status and future prospects of applications to the neuromuscular system in spinal cord injury. Paraplegia. 1987;25(3):279–288. doi: 10.1038/sc.1987.52. doi:10.1038/sc.1987.52. [DOI] [PubMed] [Google Scholar]
- 36.Romero FJ, Gambarrutta C, Garcia-Forcada A, et al. Long-term evaluation of phrenic nerve pacing for respiratory failure due to high cervical spinal cord injury. Spinal Cord. 2012;50(12):895–898. doi: 10.1038/sc.2012.74. doi:10.1038/sc.2012.74. [DOI] [PubMed] [Google Scholar]
- 37.Hirschfeld S, Exner G, Luukkaala T, Baer GA. Mechanical ventilation or phrenic nerve stimulation for treatment of spinal cord injury-induced respiratory insufficiency. Spinal Cord. 2008;46(11):738–742. doi: 10.1038/sc.2008.43. doi:10.1038/sc.2008.43. [DOI] [PubMed] [Google Scholar]
- 38.Graupe D, Cerrel-Bazo H, Kern H, Carraro U. Walking performance, medical outcomes and patient training in FES of innervated muscles for ambulation by thoracic-level complete paraplegics. Neurol Res. 2008;30(2):123–130. doi: 10.1179/174313208X281136. doi:10.1179/174313208X281136. [DOI] [PubMed] [Google Scholar]
- 39.Martens FMJ, Heesakkers JPFA. Clinical results of a brindley procedure: sacral anterior root stimulation in combination with a rhizotomy of the dorsal roots. Adv Urol. 2011;2011:709708. doi: 10.1155/2011/709708. doi:10.1155/2011/709708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martens FMJ, Hollander den PP, Snoek GJ, Koldewijn EL, van Kerrebroeck PEVA, Heesakkers JPFA. Quality of life in complete spinal cord injury patients with a Brindley bladder stimulator compared to a matched control group. Neurourol Urodyn. 2011;30(4):551–555. doi: 10.1002/nau.21012. doi:10.1002/nau.21012. [DOI] [PubMed] [Google Scholar]
- 41.Grahn PJ, Mallory GW, Berry BM, Hachmann JT, Lobel DA, Lujan JL. hayashibe M, editor. Restoration of motor function following spinal cord injury via optimal control of intraspinal microstimulation: toward a next generation closed-loop neural prosthesis. Front Neurosci. 2014:1–37. doi: 10.3389/fnins.2014.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niddam DM, Graven-Nielsen T, Arendt-Nielsen L, Chen AC. Non-painful and painful surface and intramuscular electrical stimulation at the thenar and hypothenar sites: differential cerebral dynamics of early to late latency SEPs. Brain Topogr. 2001;13(4):283–292. doi: 10.1023/a:1011180713285. [DOI] [PubMed] [Google Scholar]
- 43.Lujan JL, Crago PE. Automated optimal coordination of multiple-DOF neuromuscular actions in feedforward neuroprostheses. IEEE Trans Biomed Eng. 2009;56(1):179–187. doi: 10.1109/TBME.2008.2002159. doi:10.1109/TBME.2008.2002159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Triolo RJ, Liu MQ, Kobetic R, Uhlir JP. Selectivity of intramuscular stimulating electrodes in the lower limbs. Journal of Rehabilitation Research and Development. 2001;38(5):533–544. [PubMed] [Google Scholar]
- 45.Popovic MR, Popović DB, Keller T. Neuroprostheses for grasping. Neurol Res. 2002;24(5):443–452. doi: 10.1179/016164102101200311. doi:10.1179/016164102101200311. [DOI] [PubMed] [Google Scholar]
- 46.Hägglund M, Borgius L, Dougherty KJ, Kiehn O. Activation of groups of excitatory neurons in the mammalian spinal cord or hindbrain evokes locomotion. Nature Neuroscience. 2010;13(2):246–252. doi: 10.1038/nn.2482. doi:10.1038/nn.2482. [DOI] [PubMed] [Google Scholar]
- 47.Hägglund M, Dougherty KJ, Borgius L, Itohara S, Iwasato T, Kiehn O. Optogenetic dissection reveals multiple rhythmogenic modules underlying locomotion. Proc Natl Acad Sci USA. 2013;110(28):11589–11594. doi: 10.1073/pnas.1304365110. doi:10.1073/pnas.1304365110/-/DCSupplemental. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deisseroth K. Optogenetics. Nat Meth. 2011;8(1):26–29. doi: 10.1038/nmeth.f.324. doi:doi:10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: optical technologies for probing neural signals and systems. Nat Rev Neurosci. 2007;8(8):577–581. doi: 10.1038/nrn2192. doi:10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- 50.Towne C, Montgomery KL, Iyer SM, Deisseroth K, Delp SL. Kremer EJ, editor. Optogenetic Control of Targeted Peripheral Axons in Freely Moving Animals. PLoS ONE. 2013;8(8):e72691. doi: 10.1371/journal.pone.0072691. doi:10.1371/journal.pone.0072691.s006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nature Neuroscience. 2005;8(9):1263–1268. doi: 10.1038/nn1525. doi:10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 52.Nashold BS, Friedman H, Grimes J. Electrical stimulation of the conus medullaris to control the bladder in the paraplegic patient. A 10-year review. Appl Neurophysiol. 1981;44(4):225–232. doi: 10.1159/000102205. [DOI] [PubMed] [Google Scholar]
- 53.Grill WM, Bhadra N, Wang B. Bladder and urethral pressures evoked by microstimulation of the sacral spinal cord in cats. Brain Research. 1999;836(1-2):19–30. doi: 10.1016/s0006-8993(99)01581-4. [DOI] [PubMed] [Google Scholar]
- 54.Bentley JN, Chestek C, Stacey WC, Patil PG. Optogenetics in epilepsy. Neurosurg Focus. 2013;34(6):E4. doi: 10.3171/2013.3.FOCUS1364. doi:10.3171/2013.3.FOCUS1364. [DOI] [PubMed] [Google Scholar]
- 55.Prigge M, Schneider F, Tsunoda SP, et al. Color-tuned Channelrhodopsins for Multiwavelength Optogenetics. Journal of Biological Chemistry. 2012;287(38):31804–31812. doi: 10.1074/jbc.M112.391185. doi:10.1074/jbc.M112.391185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berndt A, Lee SY, Ramakrishnan C, Deisseroth K. Structure-guided transformation of channelrhodopsin into a light-activated chloride channel. Science. 2014;344(6182):420–424. doi: 10.1126/science.1252367. doi:10.1126/science.1252367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wietek J, Wiegert JS, Adeishvili N, et al. Conversion of channelrhodopsin into a light- gated chloride channel. Science. 2014;344(6182):409–412. doi: 10.1126/science.1249375. doi:10.1126/science.1249375. [DOI] [PubMed] [Google Scholar]
- 58.Bryson JB, Machado CB, Crossley M, et al. Optical control of muscle function by transplantation of stem cell-derived motor neurons in mice. Science. 2014;344(6179):94–97. doi: 10.1126/science.1248523. doi:10.1126/science.1248523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berglund K, Birkner E, Augustine GJ, Hochgeschwender U. Light-emitting channelrhodopsins for combined optogenetic and chemical-genetic control of neurons. PLoS ONE. 2013;8(3):e59759. doi: 10.1371/journal.pone.0059759. doi:10.1371/journal.pone.0059759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arlow RL, Foutz TJ, McIntyre CC. Theoretical principles underlying optical stimulation of myelinated axons expressing channelrhodopsin-2. Neuroscience. 2013;248(C):541–551. doi: 10.1016/j.neuroscience.2013.06.031. doi:10.1016/j.neuroscience.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Llewellyn ME, Thompson KR, Deisseroth K, Delp SL. Orderly recruitment of motor units under optical control in vivo. Nature Publishing Group. 2010;16(10):1161–1165. doi: 10.1038/nm.2228. doi:10.1038/nm.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alilain WJ, Li X, Horn KP, et al. Light-Induced Rescue of Breathing after Spinal Cord Injury. J Neurosci. 2008;28(46):11862–11870. doi: 10.1523/JNEUROSCI.3378-08.2008. doi:10.1523/JNEUROSCI.3378-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bruegmann T, Malan D, Hesse M, et al. Optogenetic control of heart muscle in vitro and in vivo. Nat Meth. 2010;7(11):897–900. doi: 10.1038/nmeth.1512. doi:10.1038/nmeth.1512. [DOI] [PubMed] [Google Scholar]
- 64.Yoo PB, Klein SM, Grafstein NH, et al. Pudendal nerve stimulation evokes reflex bladder contractions in persons with chronic spinal cord injury. Neurourol Urodyn. 2007;26(7):1020–1023. doi: 10.1002/nau.20441. doi:10.1002/nau.20441. [DOI] [PubMed] [Google Scholar]
- 65.Liske H, Towne C, Anikeeva P, et al. Optical inhibition of motor nerve and muscle activity in vivo. Muscle Nerve. 2013;47(6):916–921. doi: 10.1002/mus.23696. doi:10.1002/mus.23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fisher LE, Miller ME, Nogan SJ, et al. Preliminary evaluation of a neural prosthesis for standing after spinal cord injury with four contact nerve-cuff electrodes for quadriceps stimulation. Conf Proc IEEE Eng Med Biol Soc. 2006;1:3592–3595. doi: 10.1109/IEMBS.2006.260833. doi:10.1109/IEMBS.2006.260833. [DOI] [PubMed] [Google Scholar]
- 67.Singh K, Richmond FJ, Loeb GE. Recruitment properties of intramuscular and nerve- trunk stimulating electrodes. IEEE Trans Rehabil Eng. 2000;8(3):276–285. [PubMed] [Google Scholar]
- 68.Lertmanorat Z, Durand DM. Extracellular voltage profile for reversing the recruitment order of peripheral nerve stimulation: a simulation study. J Neural Eng. 2004;1(4):202–211. doi: 10.1088/1741-2560/1/4/003. doi:10.1088/1741-2560/1/4/003. [DOI] [PubMed] [Google Scholar]
- 69.Gordon T, Thomas CK, Munson JB, Stein RB. The resilience of the size principle in the organization of motor unit properties in normal and reinnervated adult skeletal muscles. Can J Physiol Pharmacol. 2004;82(8-9):645–661. doi: 10.1139/y04-081. doi:10.1139/y04-081. [DOI] [PubMed] [Google Scholar]
- 70.Mendell LM. The size principle: a rule describing the recruitment of motoneurons. Journal of Neurophysiology. 2005;93(6):3024–3026. doi: 10.1152/classicessays.00025.2005. doi:10.1152/classicessays.00025.2005. [DOI] [PubMed] [Google Scholar]
- 71.Cederfjäll E, Nilsson N, Sahin G, et al. Continuous DOPA synthesis from a single AAV: dosing and efficacy in models of Parkinson's disease. Sci Rep. 2013;3 doi: 10.1038/srep02157. doi:10.1038/srep02157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hippert C, Ibanes S, Serratrice N, et al. Corneal Transduction by Intra-Stromal Injection of AAV Vectors In Vivo in the Mouse and Ex Vivo in Human Explants. PLoS ONE. 2012;7(4):e35318. doi: 10.1371/journal.pone.0035318. doi:10.1371/journal.pone.0035318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Palfi A, Chadderton N, McKee AG, et al. Efficacy of Codelivery of Dual AAV2/5 Vectors in the Murine Retina and Hippocampus. Human Gene Therapy. 2012;23(8):847–858. doi: 10.1089/hum.2011.142. doi:10.1089/hum.2011.142. [DOI] [PubMed] [Google Scholar]
- 74.Ito T, Okada T, Mimuro J, et al. Adenoassociated Virus–Mediated Prostacyclin Synthase Expression Prevents Pulmonary Arterial Hypertension in Rats. Hypertension - Journal of the American Heart Association. 2007 doi: 10.1161/HYPERTENSIONAHA.107.091348. [DOI] [PubMed] [Google Scholar]
- 75.Federici T, Taub JS, Baum GR, et al. Robust spinal motor neuron transduction following intrathecal delivery of AAV9 in pigs. Gene Ther. 2012;19(8):852–859. doi: 10.1038/gt.2011.130. doi:10.1038/gt.2011.130. [DOI] [PubMed] [Google Scholar]
- 76.Passini MA, Bu J, Richards AM, et al. Translational fidelity of intrathecal delivery of self- complementary AAV9-survival motor neuron 1 for spinal muscular atrophy. Human Gene Therapy. 2014;25(7):619–630. doi: 10.1089/hum.2014.011. doi:10.1089/hum.2014.011. [DOI] [PubMed] [Google Scholar]
- 77.Kornum BR, Stott SRW, Mattsson B, et al. Adeno-associated viral vector serotypes 1 and 5 targeted to the neonatal rat and pig striatum induce widespread transgene expression in the forebrain. Experimental Neurology. 2010;222(1):70–85. doi: 10.1016/j.expneurol.2009.12.009. doi:10.1016/j.expneurol.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 78.Samaranch L, Salegio EA, San Sebastian W, et al. Strong cortical and spinal cord transduction after AAV7 and AAV9 delivery into the cerebrospinal fluid of nonhuman primates. Human Gene Therapy. 2013;24(5):526–532. doi: 10.1089/hum.2013.005. doi:10.1089/hum.2013.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meyer K, Ferraiuolo L, Schmelzer L, et al. Improving single injection CSF delivery of AAV9-mediated gene therapy for SMA - a dose response study in mice and nonhuman primates. Mol Ther. 2014 doi: 10.1038/mt.2014.210. doi:10.1038/mt.2014.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vannucci L, Lai M, Chiuppesi F, Ceccherini-Nelli L, Pistello M. Viral vectors: a look back and ahead on gene transfer technology. New Microbiol. 2013;36(1):1–22. [PubMed] [Google Scholar]
- 81.High KA. The gene therapy journey for hemophilia: are we there yet? Blood. 2012;120(23):4482–4487. doi: 10.1182/blood-2012-05-423210. doi:10.1182/blood-2012-05-423210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mukherjee S, Thrasher AJ. Gene therapy for PIDs: progress, pitfalls and prospects. Gene. 2013;525(2):174–181. doi: 10.1016/j.gene.2013.03.098. doi:10.1016/j.gene.2013.03.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mingozzi F, High KA. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013;122(1):23–36. doi: 10.1182/blood-2013-01-306647. doi:10.1182/blood-2013-01-306647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pachter JS, De Vries HE, Fabry Z. The Blood-Brain Barrier and Its Role in Immune Privilege in the Central Nervous System. J Neuropathol Exp Neurol. 2003;62(6):593. doi: 10.1093/jnen/62.6.593. [DOI] [PubMed] [Google Scholar]
- 85.Xiao B-G, Link H. Immune regulation within the central nervous system. Journal of the Neurological Sciences. 1998;157(1):1–12. doi: 10.1016/s0022-510x(98)00049-5. doi:10.1016/S0022-510X(98)00049-5. [DOI] [PubMed] [Google Scholar]
- 86.Mikals K, Nam H-J, Van Vliet K, et al. The structure of AAVrh32.33, a novel gene delivery vector. J Struct Biol. 2014;186(2):308–317. doi: 10.1016/j.jsb.2014.03.020. doi:10.1016/j.jsb.2014.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Basner-Tschakarjan E, Bijjiga E, Martino AT. Pre-Clinical Assessment of Immune Responses to Adeno-Associated Virus (AAV) Vectors. Front Immunol. 2014;5:28. doi: 10.3389/fimmu.2014.00028. doi:10.3389/fimmu.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bergen JM, Park I-K, Horner PJ, Pun SH. Nonviral approaches for neuronal delivery of nucleic acids. Pharm Res. 2008;25(5):983–998. doi: 10.1007/s11095-007-9439-5. doi:10.1007/s11095-007-9439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grahn PJ, Vaishya S, Knight A, et al. Implantation of cauda equina nerve roots through a biodegradable scaffold at the conus medullaris in rat. Spine J. 2014 doi: 10.1016/j.spinee.2014.01.059. doi:10.1016/j.spinee.2014.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vaziri A, Emiliani V. Reshaping the optical dimension in optogenetics. Current Opinion in Neurobiology. 2012;22(1):128–137. doi: 10.1016/j.conb.2011.11.011. doi:10.1016/j.conb.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 91.Lobel DA, Lee KH. Brain machine interface and limb reanimation technologies: restoring function after spinal cord injury through development of a bypass system. Mayo Clinic Proceedings. 2014;89(5):708–714. doi: 10.1016/j.mayocp.2014.02.003. doi:10.1016/j.mayocp.2014.02.003. [DOI] [PubMed] [Google Scholar]