Abstract
Diethylenetriaminepentaacetic acid (DTPA) is an FDA approved chelating agent for enhancing the elimination of transuranic elements such as americium from the body. Early access to therapy minimizes deposition of these radionuclides in tissues such as the bone. Due to its poor oral bioavailability, DTPA is administered as an IV injection, delaying access. Therefore a diethyl-ester analog of DTPA, named C2E2, was synthesized as a means to increase oral absorption. As a hexadentate ligand, it was hypothesized that C2E2 was capable of binding americium directly. Therefore, the protonation constants and americium stability constant for C2E2 were determined by potentiometric titration and a solvent extraction method, respectively. C2E2 was shown to bind americium with a log K of 19.6. The concentrations of C2E2, its metabolite C2E1, and DTPA required to achieve effective binding in rat, beagle, and human plasma were studied in vitro. Dose response curves for each ligand were established and the 50% maximal effective concentrations were determined for each species. As expected, higher concentrations of C2E2 were required to achieve the same degree of binding as DTPA. The results indicated that chelation in beagle plasma is more representative of the human response than rats. Finally, the pharmacokinetics of C2E2 were investigated in beagles and the data was fit to a two-compartment model with elimination from the central compartment, along with first-order absorption. Based on the in vitro data, a 100 mg kg−1 dose of C2E2 can be expected to have an effective duration of action of 3.8 hours in beagles.
Keywords: chelation, decorporation, 241Am, DTPA
Introduction
Recent nuclear accidents such as the incident at the Fukushima nuclear power plant in 2011 and the increasing threat of terrorism has spurred research interest in medical evaluation and treatment of contaminated individuals (Cassatt et al., 2008). In the event of a public health emergency the US maintains a Strategic National Stockpile containing medical supplies used protect the public. Of particular interest is diethylenetriamine pentaacetic acid (DTPA), one of only three drugs with FDA approval for treatment of internal radionuclide contamination. DTPA is an aminopolycarboxylic acid chelating agent with eight potential metal coordination sites that allow binding to a wide range of metals with high affinity (Durham and Ryskiewich, 1958). Two DTPA products, namely the pentetate calcium trisodium injection (Ca-DTPA) and pentetate zinc trisodium injection (Zn-DTPA), received FDA approval for treatment of internal contamination with plutonium, americium or curium. However, due to its poor bioavailability (~3%) (Volf, 1984), DTPA requires intravenous administration by medical professionals, which would prevent timely distribution in a mass casualty scenario. Americium rapidly clears from the plasma to the tissues with > 90% cleared 60 min after an IV injection; only a small fraction is excreted in the urine(Turner and Taylor, 1968). Early access to treatment therefore improves total body decorporation by minimizing tissue deposition of the radioactive material. The loss of efficacy caused by treatment delay cannot be compensated for by increasing the cumulative dose(Seidel, 1975), thus, there is a need to develop patient-friendly formulations that can be easily distributed and self-administered.
In order to improve the oral bioavailability of DTPA a number of novel formulation approaches have been investigated, such as nanoDTPA capsules (Reddy et al., 2012) and permeation enhancers (Shankar, 2014). Alternatively, in our lab we have focused on the development of DTPA pro-drugs (Sueda et al., 2012), (Sadgrove et al., 2012). The penta-ethyl ester of DTPA, although highly absorbed, is limited by its toxicity and the potential to form up to ten metabolites, thus complicating its regulatory approval. Therefore, in order to avoid these difficulties, the di-ethyl ester of DTPA, named C2E2, was selected for further development due to its simple metabolic profile and lower toxicity (Huckle, 2013).
There is an insufficient population of contaminated individuals to conduct well-controlled clinical efficacy trials of radionuclide decorporation agents. Furthermore, it is unethical to contaminate healthy individuals in order to determine efficacy. In these circumstances, the FDA ‘Animal Efficacy Rule’ 21CFR(314.600) provides a regulatory pathway that allows for approval of a drug based on animal efficacy, usually demonstrated in more than one species, in conjunction with safety data from animals and healthy human volunteers (Abersold, 2012). For this pathway, the mechanism of action needs to be understood and the animal models selected to establish efficacy must be demonstrated as appropriate. In order to fully understand the mechanism of action of these agents their binding affinities should be determined. Additionally, previous research with DTPA revealed that there are species differences in the plasma concentrations of DTPA required for chelation of 241Am (Sueda et al., 2013). Consequently, in order to justify the animal models used for efficacy studies, the binding of novel agents should also be evaluated in animal plasma to confirm the appropriateness of the species.
C2E2 was initially designed to be a pro-drug of DTPA, but with three free acids and three tertiary amines acting as metal coordination sites, it is also likely to act as a chelator itself. Similarly, its primary metabolite, the mono-ethyl ester of DTPA, named C2E1, is also expected to bind metals with affinities between those of DTPA and C2E2. The binding affinities of C2E2 and C2E1 for Am3+ are of significance, although they are expected to be lower than DTPA, as the presence of these ligands in plasma could contribute to the overall decorporation efficacy of C2E2 therapy. Furthermore, even if the binding affinities are lower than those of DTPA, they may still be several orders of magnitude greater than those of the plasma proteins to which 241Am is bound.
The ability of C2E2 and its metabolite C2E1 to chelate 241Am was investigated. The differences in plasma concentrations required for efficacy in rat, beagle and human plasma were also determined. The in vitro plasma concentrations were then combined with pharmacokinetic data from dogs to determine the dose required to achieve efficacy and the likely duration of action. These data can be used to support justification of animal model selection in future efficacy studies and aid in the development of a pharmacokinetic-pharmacodynamic model to help establish the required human efficacious dose.
Methods
Materials
DTPA diethyl ester (C2E2) was synthesized by preparing DTPA bis-anhydride and subsequently refluxing the bis-anhydride with ethanol as previously described(Guilmette et al., 1979). The monoethyl ester of DTPA (C2E1), an impurity formed during the synthesis of C2E2, was obtained by chromatographic separation. The 241Am(NO3)3 stock solution was obtained from Eckert & Ziegler Isotope Products (Valencia, CA). Rat, beagle and human plasma were obtained from Innovative Research Inc. (Novi, MI). Pierce Strong Cation Exchange Spin Columns were purchased from Thermo Fisher Scientific (Rockford, IL). HDEHP and solvents were purchased from Sigma Aldrich.
Ionization Constants
The protonation constants for C2E2/C2E1 were determined by preparing a 5 mM solution of free ligand in 0.15 M KCl. The constants were calculated from three replicates, with each experiment consisting of a titration with acid, followed by a titration with base. After each addition of titrant, a 30 second equilibration time passed before pH measurement with a Seven Easy pH meter (Ag/AgCl reference) and glass electrode (Metler Tolledo, Columbus, OH) was obtained. At the end of the titrations the presence of C2E2 or C2E1 was confirmed by HPLC using a Corona Ultra Charged Aerosol Detector (CAD) (ESA, Chelmsford, MA).
Solvent Extraction Studies
The formation constant for the 241Am-C2E2 complex was determined by a solvent extraction method similar to that described previously (Leguay et al., 2012). The experiments conducted with tracer amounts of 241Am (16.7 MBq. L−1) using an organic phase of 0.1 M HDEHP in dodecane and an aqueous phase consisting of 0.1 M NaCl at pH 1.3. The concentration of ligand in the aqueous phase ranged from 0.05 to 100 µM. Upon addition of the aqueous phase, the samples were vortexed and then maintained at 25°C for 60 minutes by means of Eppendorf automatic shaker (Hamburg, Germany). Following incubation the two phases were separated by centrifugation (10,000 × g, 10 min) and a 100 µL sample of each phase was collected. The 241Am content in each sample was measured by gamma scintillation counting (Perkin Elmer Model 2470 Gamma Counter, Waltham, MA) and the distribution between the two phases calculated. Background-corrected radioactivity was quantified over 30 min using a 40–80 keV energy window to measure the 59.7 keV photon emission associated with 241Am decay. Experiments were performed in triplicate. In addition to determining the distribution of 241Am between the two phases in the presence of C2E2, C2E1 or DTPA, the HDEHP solvent loaded with 241Am was combined with a 0.1 M NaCl solution at pH 1.3, free of chelating ligand to determine Do (the distribution value in the absence of ligand).
Competitive Binding Studies
The ability of C2E2 and its primary metabolite C2E1 to chelate protein-bound 241Am in blood was investigated by in vitro experimentation using a method developed in our lab for DTPA (Sueda et al., 2013). As previously described, various concentrations of chelating ligand were combined with 241Am in rat, beagle or human plasma before separation and evaluation. All samples utilized 385 µL of plasma, 8 µL of ligand and 7 µL of 241Am solution. The ligand stock solutions were prepared in 10 mM sodium phosphate buffer at pH 7.4. The final concentrations of ligand ranged from 0.5 to 750 µM in rat, beagle and human plasma for both C2E2 and C2E1. The 241Am stock solution was prepared by diluting 241Am(NO3) 3 in 1 M hydrochloric acid. The resulting concentration of 241Am nitrate after addition to the plasma samples containing the ligands was 3 nM.
The samples were subsequently incubated for 60 minutes in a heating block at 37°C with mild agitation before being transferred to an Amicon Ultra centrifugal filter with an Ultracel 3K membrane (Millipore, Billerica, MA) and centrifuged at 14,000×g for 30 min to extract the protein-bound fraction. The filtrates were subsequently transferred to Pierce Strong Cation Exchange Spin Columns that had been conditioned with 0.8 M ammonium hydroxide and water to separate positively charged 241Am species from ligand-bound 241Am. Elution of the ligand-bound 241Am species was achieved by centrifuging at 2000×g for 5 min. The positively charged 241Am species were collected by further centrifugation at 2000 ×g with 1 M nitric acid. The 241Am content of each fraction was determined by gamma scintillation counting. Background corrected radioactivity was quantified over 30 min using a 40–80 keV energy window to measure the 59.7 keV photon emission associated with 241Am decay.
The dose-response curves were produced by calculating the fraction of total 241Am that was bound to C2E1, C2E2 or plasma proteins. The sigmoidal regression profiles were generated by Gauss-Newton non-linear least squares regression analysis using SAS 9.3 (SAS, Cary, NC). The regression applied the logistic equation (Hoehler, 1995);
where a describes the slope as the curve approaches the asymptote, b is the inflection point, c is the asymptote, and x is the log transformed concentration of C2E1 or C2E2 that corresponds to y, the fraction of bound 241Am. The EC50 values were determined by setting y equal to 50% of the c term and calculating for x. The same regression analysis was applied to the data for 241Am-protein binding; however, a transformation was applied to yprotein such that,
where y’ was used for the regression analysis. Subsequent reversal of the transformation produced the final binding curves.
Pharmacokinetics of C2E2 in Beagle Dogs
Two male and two female beagle dogs were administered a single 100 mg kg−1 dose of C2E2 via oral gavage (12.05 mg mL−1). The dogs were 8 to 10 months old, and their body weights were 11.4 and 12.0 kg for the males, and 10.1 and 10.9 kg for the females. During the study they were housed individually and fasted overnight prior to dosing with food returned approximately 2 hours after dose administration. Blood (0.7 mL) was collected via a jugular vein pre-dose and at 0.5, 1, 2 4, 6, 8, 12 and 24 hours post dose in pre-chilled vacutainer tubes containing 5 mg sodium fluoride and 4 mg potassium oxalate (BD, Franklin Lakes, NJ). Samples were inverted several times prior to centrifugation at 4°C for 10 minutes (1300 × g) within 15 minutes of collection. Plasma aliquots (125 µL) were transferred to a micro centrifuge tube containing 125 µl of 20% formic acid, vortexed and stored on dry ice prior to being stored in a freezer (−80 °C) until analysis was conducted.
The plasma concentrations of C2E2 and its metabolites, C2E1 and DTPA, were quantified using two LC/MS/MS methods. One method was used for C2E2 and C2E1 and another for DTPA. For C2E2 and C2E1 analysis, 100 µL of the acidified samples were treated with 400 µL of acetonitrile containing an internal standard (13C5-C2E5) followed by mixing and centrifugation. A 400 µL aliquot of the supernatant was subsequently removed, evaporated and reconstituted with 500 µL of water/acetonitrile/formic acid (85:15:0.1). The separation of the components was performed on a YMC ODS-AM (100 × 2.1 mm, 3.5 µm) column (Allentown, PA) with a mobile phase gradient starting with 0.1% formic acid in water and ending with 0.1% formic acid in acetonitrile. The compounds were detected on a triple quadrapole mass spectrometer using heated electrospray ionization in the positive-ion mode (Thermo Fisher Scientific, Waltham, MA). For DTPA, an excess of Fe(III) was utilized to ensure formation of an Fe(III)-DTPA complex for quantification. A 13C5-DTPA internal standard was used. One hundred µl of the acidified samples was treated with 50 µL of 2 mM Fe(III)Cl2 and allowed to incubate for 90 minutes. Acetonitrile (400 µL) containing internal standard was added. After mixing and centrifugation, 350 µL of the supernatant was removed, evaporated and reconstituted with 100 µL of mobile phase. Separation was performed using a Phenomenex Luna phenyl-hexyl (150 × 2mm, 3 µm) column (Torrance, CA) with a mobile phase gradient starting with water/methanol (90:10) with 1 mM acetic acid and 1 mM tributylamine, and ending with acetonitrile/methanol (50:50) with 1 mM acetic acid and 1 mM tributylamine. The DTPA was detected on a triple quadrapole mass spectrometer using heated electrospray ionization in the negative-ion mode.
Quantification of C2E2 and DTPA was based on the peak areas of analyte to their respective internal standards. C2E1 concentrations are estimated values as the system response was not validated due to lack of material. The range of quantification was 100 to 100,000 ng mL−1 for C2E2, 5 to 1,000 ng mL−1 for C2E1 and 10 to 1,000 ng mL−1 for DTPA. The approximate precursor-product ion transitions and approximate retention times are described in Table 1.
Table 1.
The precursor → product ion transitions and retention times for C2E2, C2E1 and DTPA used during LC/MS/MS quantification
| Analyte | Precursor → product | Approx. retention time (min) |
|---|---|---|
| Fe-DTPA | m/z 445 → m/z 313 | 3.7 |
| Fe-DTPA-IS | m/z 450 → m/z 350 | 3.7 |
| C2E1 | m/z 442 → m/z 160,188 | 1.0 |
| C2E2 | m/z 450 → m/z 188, 216 | 3.1 |
| C2E5-IS | m/z 539 → m/z 218 | 6.3 |
The general procedures for animal care and housing were conducted in accordance with the National Research Council for the Care and Use of Laboratory Animals and the Animal Welfare Standards. All procedures and protocols used in animal studies were reviewed and approved by the Institutional Animal Care and Use Committee of Covance Laboratories Inc. (Raleigh, NC) and were performed in AAALAC accredited facilities.
Results
Ionization Constants
Evaluation of the titration curve for C2E2 (Figure 1) identified 6 pKa values that correspond to the three tertiary amines and three carboxylic acids. Solutions of C2E2 exhibit resistance to pH change over the region of 1.8 – 2.7 where the molecules overlapping pKa’s lie. In order to determine these overlapping values, a Bjerrum plot was used where the average number of protons (nH) bound to the ligand at each pH was calculated, and the pKa values occur at half integer values of nH. These plots have previously been utilized to establish the 6 pKa values for vancomycin and even the 30 pKa values for apo-metallothionein (Avdeef, 2001). The values determined from the Bjerrum plots were used as seed values for refinement in HYPERQUAD (Gans et al., 1996). The calculated ionization constants are shown in Table 2. The pKa’s determined for C2E2 are consistent with DTPA analogues previously investigated for use as MRI contrast agents in which two carboxylic acids are functionalized such as DTPA-BMA (Rizkalla et al., 1993) and DTPA-BBA (Geraldes et al., 1995).
Figure 1.
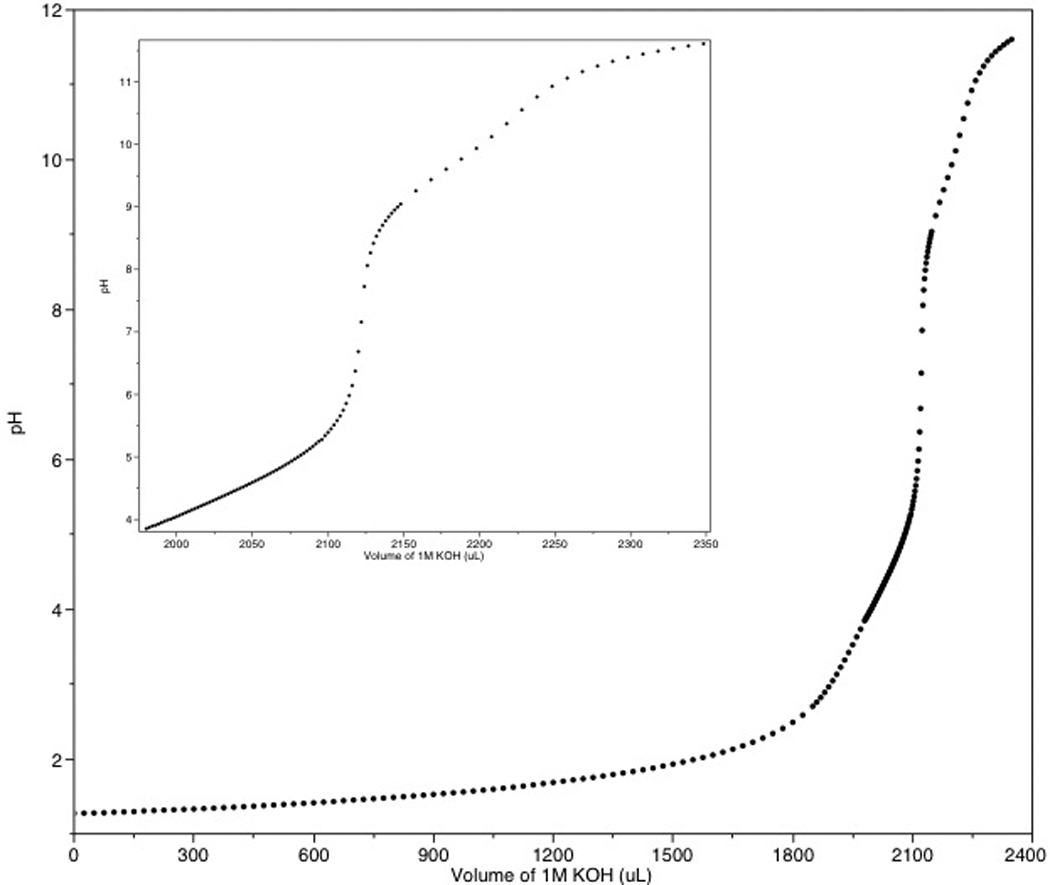
Titration of DTPA di-ethyl ester (C2E2) with 1 N potassium hydroxide
Table 2.
Acid dissociation constants determined for C2E2 and C2E1 compared to those reported for DTPA.
| Ligand | pKa7 | pKa6 | pKa5 | pKa4 | pKa3 | pKa2 | pKa1 |
|---|---|---|---|---|---|---|---|
| DTPA | 1.45 ± 0.15 | 1.60 ± 0.15 | 1.80 ± 0.05 | 2.55 ± 0.05 | 4.31 ± 0.02 | 8.54 ± 0.02 | 10.51 ± 0.01 |
| C2E1 | < 1.5 | < 1.5 | 1.18 ± 0.45 | 1.72 ± 1.5 | 2.99 ± 0.05 | 6.35 ± 0.38 | 9.63 ± 0.03 |
| C2E2 | - | 1.45 ± 0.09 | 1.76 ± 0.05 | 1.87 ± 0.05 | 3.52 ± 0.03 | 4.68 ± 0.02 | 9.40 ± 0.02 |
Solvent Extraction Studies
The formation constants of the Am(III)-ligand complex were determined using a solvent extraction method. Tracer levels of 241Am were combined with HDEHP diluted in the dodecane organic phase. Distribution ratios, D, were determined as a function of ligand concentration at constant pH (1.3). As expected, when the concentration of competing ligand was increased the 241Am was chelated and extracted from the organic phase into the aqueous phase (Figure 2). The results indicate that C2E2 retains the ability to chelate 241Am, albeit at higher concentrations than those of DTPA. In order to achieve the same degree of binding, C2E2 concentrations need to be approximately 20-fold higher than those of DTPA. In comparison C2E1 retains a higher affinity for 241Am with only 5-fold increases in concentration required to match DTPA.
Figure 2.
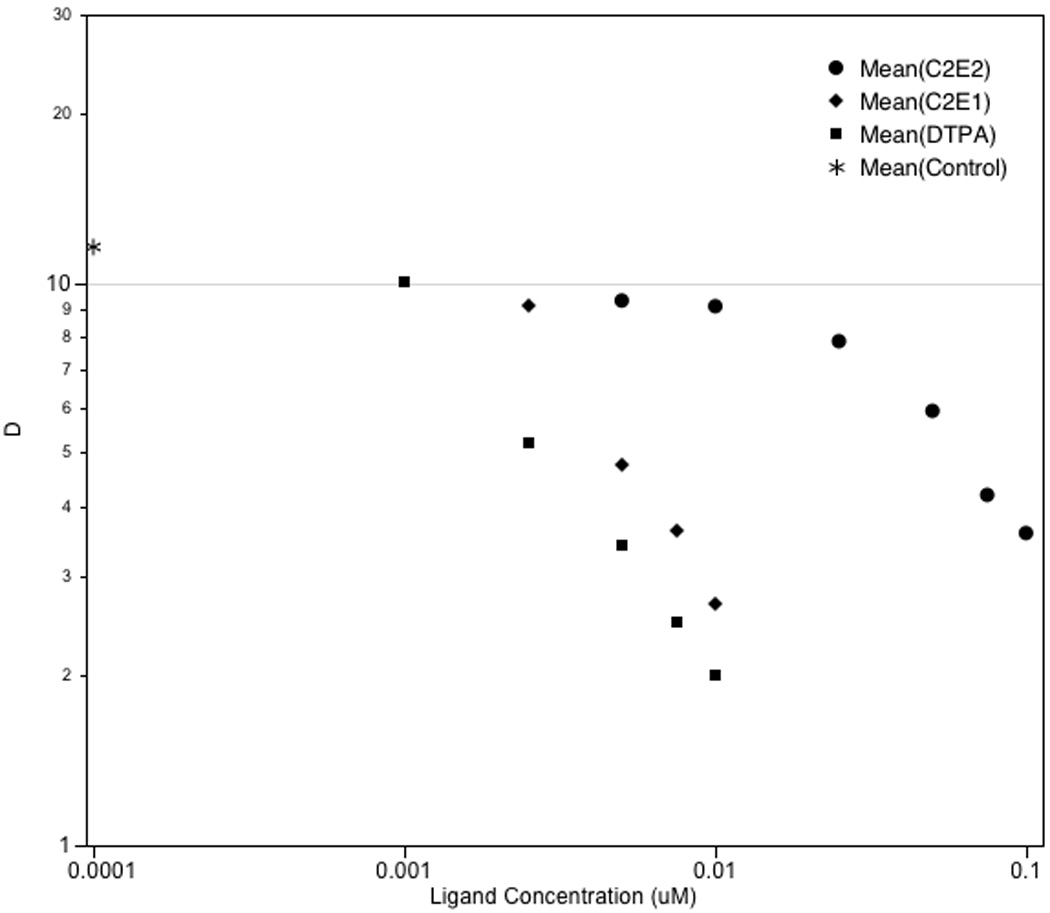
Distribution of 241Am as a function of total ligand concentration at I = 0.1 M (NaCl), T = 25°C, pH = 1.3, and CHDEHP = 0.1 M in dodecane.
In order to quantify these differences in affinity for 241Am, the conditional stability constants (lg βcond) of the complexes were determined by linear fitting of the variations of (D/Do − 1) versus CLigand as described by Leguay et al. (Leguay et al., 2012), where Do represents the D value in the absence of ligand. The lg βcond values for C2E2, C2E1 and DTPA were 1.36, 2.46 and 2.70, respectively. From the pKa values it is known that at pH 1.3, the predominant ligand species is in the protonated form and therefore the calculated formation constants are 19.6 ± 0.2, 21.7 ± 0.5 and 25.4 ± 0.3 for C2E2, C2E1 and DTPA, respectively. These values indicate that the stability of the C2E2-241Am complex is 6 log units lower than that of the DTPA-241Am complex. The solvent extraction data determined for DTPA and the corresponding formation constant is consistent with that previously determined (Leguay et al., 2012).
Competitive Binding Studies
The suitability of the method for separation of both protein-bound 241Am and DTPA-bound 241Am was previously established by Sueda et al. (Sueda et al., 2013b) At physiological pH, 241Am in solution is present as the +3 oxidation state and DTPA exists in the −5 oxidation state. Therefore, DTPA-bound 241Am results in a negatively charged complex, which is able to pass through the cationic filter. If the same logic is followed for C2E1 and C2E2, then the complexes with 241Am are expected to be negatively and neutrally charged. As with DTPA, preliminary studies were conducted to confirm that C2E1- or C2E2-bound 241Am passed through the cationic filter further confirming that the protein, ligand and free fractions could be adequately separated. In the previously performed studies the 241Am stock was a citrate salt; however, for the present work, 241Am nitrate was used to ensure consistency between the in vitro characterization and planned in vivo studies. Re-evaluation of DTPA binding with 241Am nitrate was therefore performed to help further characterize the model and enable comparisons between each ligand.
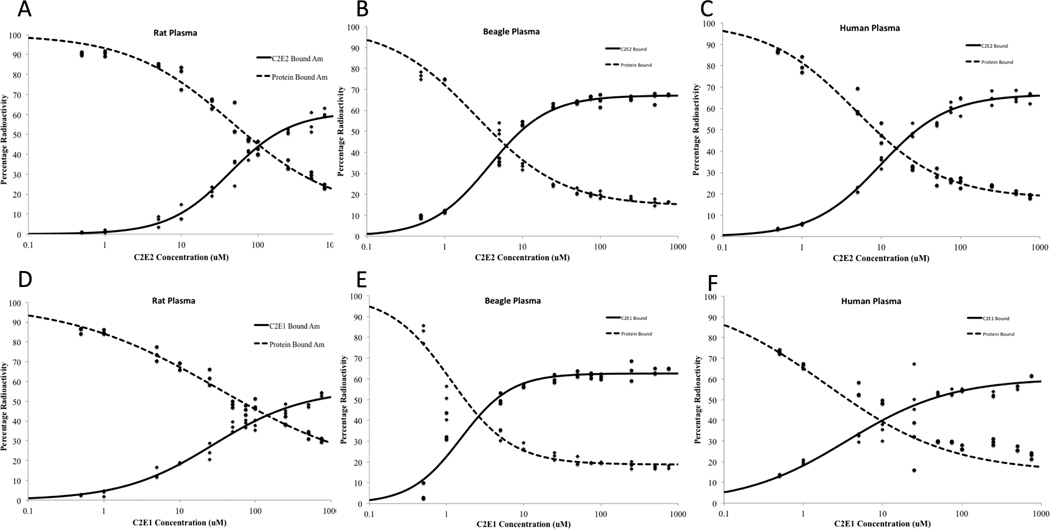
The dose response curves for C2E2, C2E1 in rat, beagle and human plasma are shown in figures 3a–f. The binding of 241Am to either the ligand or plasma proteins follows a sigmoidal profile characteristic of a competitive binding relationship. The parameters obtained from the logistic regression analysis are show in Table 2. There were minor losses of activity during transfer steps. However, there was > 95% recovery for all samples. A minor fraction (< 10%) of each sample was considered to be associated with either small organic or inorganic ligands present in the plasma with affinity for 241Am such as carbonates and citrates.
Figure 3.
The competitive binding of 3 nM 241Am by C2E2 (a–c) and C2E1 (d–f) (solid line) and plasma proteins (dotted line) after 0.5 h incubation at 37°C in rat (a, d), beagle (b, e) and human plasma (c, f).
Similar to the solvent extraction study, C2E2 and C2E1 were able to chelate 241Am from the plasma, albeit at higher concentrations than required with DTPA. The efficiency with which each ligand bound 241Am varied between species. To permit comparisons between the different species and ligands, the EC50 values for the each condition were calculated. The EC50 value represents the concentration of ligand at which 50% of maximal chelation occurs. The concentrations of ligand to achieve similar levels of chelation were highest in rat plasma and lowest in beagle plasma. The concentrations required in human plasma lay between those of each species. This suggests that if used for approval via the animal rule, efficacy in the rat model may underestimate the human response while beagle efficacy studies may result in an overestimation.
The EC50 for each ligand can be used to determine the dose of each ligand required to obtain chelation. In addition, incorporation of the relative binding numbers into a pharmacokinetic model will help to determine the contribution of each ligand to the overall efficacy of C2E2.
Pharmacokinetics
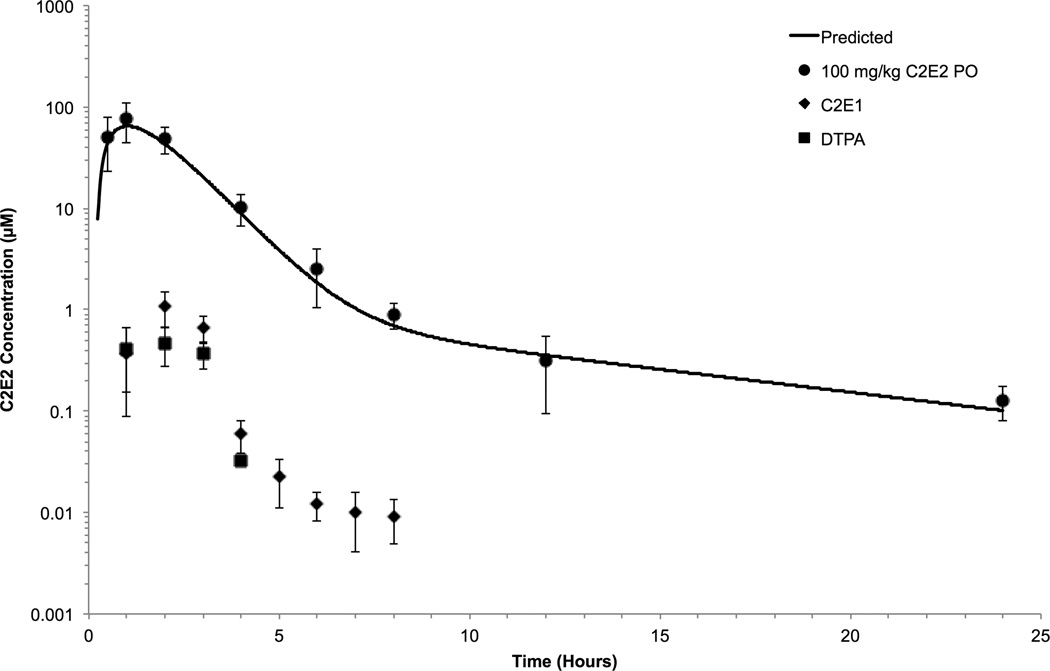
The homogeneity and concentration of the C2E2 dosing solution was confirmed by HPLC by taking two samples from the top, middle and bottom of the prepared solution. The solution was homogeneous and was found to be 97.8 ± 0.5% of the target concentration. The concentration-time profile for C2E2 and its metabolites in beagle dogs is shown in Figure 4. There was no significant difference between the AUC0–24 of the males and females (although the lack of significance may be due to the small the size of each group). Therefore, the data was combined into a single profile and modeled using a two-compartment model with elimination from the central compartment, along with first-order absorption. The use of a two-compartment model is consistent with the elimination of DTPA from dogs (Stevens et al., 1978). C2E2 was rapidly absorbed, reaching its peak concentration 1 hour after administration. Elimination occurred from the first compartment with a half-life of 0.69 hours and from the second compartment with a half-life of 6.66 hrs. Only a small fraction (~2%) of the absorbed dose was metabolized to C2E1 or DTPA, and the concentrations of both of these metabolites were below those required to effectively chelate 241Am. These results suggest that any efficacy observed in dogs at 100 mg kg−1 doses is due to C2E2 and not its metabolites. By combining the in vitro competitive binding concentrations with the pharmacokinetic profile, the time for which plasma concentrations of C2E2 are above those necessary to achieve both 50 and 90% of the maximal binding can be determined. The concentration of C2E2 was above the 90% threshold for 3.8 hrs and above the 50% threshold for 4.7 hrs. In comparison, after a 300 µM IV DTPA dose, the plasma concentration of DTPA is above the 90 and 50 % thresholds for 2.9 and 3.6 hours, respectively, based on beagle pharmacokinetic data (Durbin and Schmidt, 1989).
Figure 4.
The plasma concentrations of C2E2, C2E1 and DTPA after administration of a 100 mg kg−1 oral solution. The effective duration based on EC50 and EC90 values are indicated by dotted lines.
Discussion
Due to the nature of internal radioactive contamination, FDA approval of decorporation agents may proceed under the animal rule whereby animal studies can be used to provide evidence for efficacy. Under this rule the mechanism of action must be known and efficacy must be demonstrated in more than one species, both of which should be shown to react with a response similar to humans, unless a single species represents a sufficiently well-characterized animal model for predicting response in humans (Food and Drug Administration, 2014). Exposure to 241Am is most likely to occur through either inhalation or wound sites from where redistribution to the primary sites of deposition, such as the liver and bone, via the blood will occur. The rate of redistribution is based on many factors including the site of deposition, the chemical form of the radionuclide, and its rate of solubilization and absorption into the systemic circulation (Stradling et al., 2000).
The primary site of action for C2E2 is in the plasma during the redistribution of americium. Once in the plasma, approximately 30% of the total 241Am is bound to transferrin, another 30% is associated with additional plasma proteins such as albumin and globulins, and the remainder is bound to low molecular weight complexes such as citrate (Taylor, 1998). The americium-transferrin complex has a stability constant (log K) of 10.4 and that of the citrate complex is 8.6 (Ansoborlo et al., 2007). Thus, even after removal of two of DTPA’s coordination sites, C2E2 retains the ability to bind americium at a strength nine orders of magnitude higher than that of the strongest ligand in the plasma.
In addition to determining the affinity of C2E2 for 241Am, it is useful to determine the concentration required for effective chelation in plasma to aid justification of a specific dose. The data presented here confirms previous research suggesting that there are differences in the effective concentrations required for chelation of 241Am between species (Sueda et al., 2013b). This has implications for the choice of animal models used to demonstrate efficacy of decorporation agents. For DTPA, in humans the concentration required for chelation falls approximately half way between the concentrations required in rats and dogs. For C2E2, the concentration required for chelation in humans is closer to that of dogs (less than 2-fold difference) rather than rats (greater than 5-fold difference). Based on this plasma binding, the dog is a better model for predicting human efficacy as the rat model may underestimate C2E2 efficacy compared to DTPA. Plasma has a complex composition and the source of the species differences in 241Am binding has not been determined. The concentration of transferrin, albumin and other serum globulins, which are responsible for binding approximately 60% of the 241Am are at similar concentrations across all three species, however the concentration of serum iron is higher in rat than beagle or human plasma. Iron binds to DTPA with a higher stability constant (log K = 28.0 (Martell, 1982)) than 241Am and will compete for its binding sites. The extra iron present in rat plasma could be one cause for the higher concentrations required.
C2E2 and 241Am can also distribute into the interstitial fluid where the plasma protein concentrations are lower (only 42% of the plasma transferrin (Sloop et al., 1987)). In this environment, it is likely that the concentrations of C2E2 required to achieve binding are lower than those determined for plasma, therefore the duration of action may be longer than predicted using effective ligand concentrations derived from plasma.
In most contamination scenarios, americium will be deposited as either the nitrate or oxide salt. Thus, the use of the nitrate was used throughout these studies; this is in contrast to the previous studies with DTPA where the citrate salt was used. Comparison of the concentrations required for effective DTPA chelation identified a difference between each salt. Differences in the plasma clearance of the citrate and nitrate complexes were reported (Turner and Taylor, 1968) with americium citrate clearing the plasma faster than the nitrate complex. This may be due to the low affinity of nitrate for americium (log K = 1.3 (Silva and OECD Nuclear Energy Agency., 1995)) resulting in rapid binding to transferrin as opposed to citrate where the binding to transferrin may occur more slowly.
The administration of chelation therapy via the oral route provides fast, economical and noninvasive access to treatment. In addition, oral administration results in a lower Cmax than I.V. treatments and although the elimination rate remains constant and oral dose maintains an effective plasma concentration for longer due to continued absorption. Although, C2E2 was designed as a pro-drug, limited metabolism to C2E1 and DTPA was observed. Based on our PK and binding data, this should not limit efficacy since after a 100 mg kg−1 oral dose of C2E2 the predicted effective duration in dogs is double that of the standard I.V. treatment with DTPA. The administration of higher doses of C2E2 may result in further extension of the effective duration and increased concentrations of C2E1 and DTPA that may yield further increases in efficacy.
Conclusion
Although the number of coordination sites available is reduced when DTPA is esterified, C2E2 and its metabolite C2E1 maintain high affinity for 241Am. In addition to determining their binding constants relative to DTPA, the concentrations required to achieve binding in plasma were investigated in vitro. As expected, higher concentrations of C2E2 are required to achieve the same effect as DTPA. Species differences were noted in the concentrations of ligand required to achieve chelation with beagle plasma most representative of a human response. Oral administration of a 100 mg kg−1 C2E2 dose to beagles demonstrated that the effective plasma concentrations could be achieved. The binding constants and pharmacokinetic parameters determined as part of these studies can be combined with efficacy data into a pharmacokinetic-pharmacodynamic model which could be used to predict decorporation efficacy from different dosages and dosing regimes.
Table 3.
Logistic equation parameters and calculated EC50 and EC90 values for C2E2, C2E1 and DTPA binding with 241Am.
| Ligand | Plasma | Parameters for logistic equation (Estimate ± 95% Conf. Interval) |
EC50 (µM) | EC90 (µM) | ||
|---|---|---|---|---|---|---|
| a | b | c | ||||
| C2E2 | Rat | 0.04 ± 0.00 | 46.17 ± 3.43 | 54.62 ± 1.58 | 46.17 | 101.10 |
| Beagle | 0.37 ± 0.02 | 4.91 ± 0.18 | 64.79 ± 0.50 | 4.91 | 10.85 | |
| Human | 0.23 ± 0.04 | 8.87 ± 0.80 | 60.29 ± 1.25 | 8.87 | 18.42 | |
| C2E1 | Rat | 0.06 ± 0.01 | 25.50 ± 3.36 | 45.74 ± 1.51 | 25.50 | 60.12 |
| Beagle | 0.57 ± 0.13 | 2.02 ± 0.38 | 61.69 ± 1.4 | 2.02 | 5.87 | |
| Human | 0.16 ± 0.03 | 5.26 ± 0.74 | 55.22 ± 1.06 | 5.26 | 18.99 | |
| DTPA | Rat | 0.31 ± 0.03 | 5.36 ± 0.43 | 35.69 ± 0.85 | 5.36 | 12.44 |
| Beagle | 1.87 ± 0.69 | 1.08 ± 0.18 | 54.75 ± 2.16 | 1.08 | 2.25 | |
| Human | 0.98 ± 0.13 | 2.82 ± 0.30 | 55.88 ± 1.40 | 2.82 | 5.05 | |
Acknowledgments
This work was funded in part by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and U.S. Department of Health and Human Services under contracts HHSN266200500045C and HHSN272201000030C.
Footnotes
The authors declare no conflicts of interest
References
- Abersold P. FDA Experience with Medical Countermeasures under the Animal Rule. Advances in Preventive Medicine. 2012:1–11. doi: 10.1155/2012/507571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansobrolo E, Amekraz B, Moulin C, Moulin V, Taran F, Bailly T, Burfada R, Henge-Napoli MH, Jeanson A, Den Auwer C, Bonin L, Moisy P. Review of actinide decorporation with chelating agents. Comptes Rendus Chimie. 2007;10:1010–1019. [Google Scholar]
- Avdeef A. Physicochemical profiling (solubility, permeability and charge state) Curr Top Med Chem. 2001;1:277–351. doi: 10.2174/1568026013395100. [DOI] [PubMed] [Google Scholar]
- Cassatt DR, Kaminski JM, Hatchett RJ, Dicarlo AL, Benjamin JM, Maidment BW. Medical countermeasures against nuclear threats: Radionuclide decorporation agents. Radiation Research. 2008;170:540–548. doi: 10.1667/rr1485.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin PW, Schmidt CT. Predicting the kinetics of chelating angents in man from animal data. Health Physics. 1989;57(sup.1):165–174. doi: 10.1097/00004032-198907001-00021. [DOI] [PubMed] [Google Scholar]
- Durham EJ, Ryskiewich DP. The Acid Dissociation Constants of Diethylenetriaminepentaacetic Acid and the Stability Constants of Some of Its Metal Chelates. Journal of the American Chemical Society. 1958;80:4812–4817. [Google Scholar]
- Food and Drug Administration. Guidence for Industry: Product Development Under the Animal Rule (draft guidance) Silver Spring, MD: 2014. [Google Scholar]
- Gans P, Sabatini A, Vacca A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta. 1996;43:1739–1753. doi: 10.1016/0039-9140(96)01958-3. [DOI] [PubMed] [Google Scholar]
- Geraldes CFGC, Delgado R, Urbano AM, Costa J, Jasanada F, Nepveu F. Complexes of Ga3+ and In3+ with the N,N"-Bis(Butylamide) Derivative of Diethylenetriaminepentaacetic Acid - Stability-Constants and Nuclear-Magnetic-Resonance Studies in Aqueous-Solution. Journal of the Chemical Society-Dalton Transactions. 1995:327–335. [Google Scholar]
- Guilmette RA, Parks JE, Lindenbaum A. Synthesis and Therapeutic Testing of Mono- and Dialkyl Esters of Pentetic (Diethylenetriaminepentaacetic) Acid for Decorporation of Polymeric Plutonium. Journal of Pharmaceutical Sciences. 1979;68:194–196. doi: 10.1002/jps.2600680219. [DOI] [PubMed] [Google Scholar]
- Hoehler FK. Logistic equations in the analysis of S-shaped curves. Comput Biol Med. 1995a;25:367–371. doi: 10.1016/0010-4825(95)00013-t. 1995. [DOI] [PubMed] [Google Scholar]
- Huckle JE, Sadgrove MP, Pacyniak E, Mumper RJ, Jay M. Efficacy of a di-ethyl ester prodrug of DTPA as an orally bioavailable radionuclide decorporation agent. 11th International Conference on the Health Effects of Incorporated Radionuclides; Berkeley, CA. 2013. [Google Scholar]
- Leguay S, Vercouter T, Topin S, Aupiais J, Guillamont D, Miguirditchian M, Moisy P, Le Naour C. New Insights into Formation of Trivalent Actinides Complexes with DTPA. Inorganic Chemistry. 2012;51:12638–12649. doi: 10.1021/ic3011019. [DOI] [PubMed] [Google Scholar]
- Martell AE, Smith RM. Critical Stability Constants. Springer US: 1982. [Google Scholar]
- Reddy JD, Cobb RR, Dungan NW, Matthews LL, Aiello KV, Ritter G, Eppler B, Kirk JF, Abernethy JA, Tomisaka DM, Talton JD. Preclinical Toxicology, Pharmacology, and Efficacy of a Novel Orally Administered Diethylenetriaminepentaacetic acid (DTPA) Formulation. Drug Development Research. 2012;73:232–242. [Google Scholar]
- Rizkalla EN, Choppin GR, Cacheris W. Thermodynamics, Pmr, Fluorescence Studies for the Complexation of Trivalent Lanthanides, Ca-2+, Cu-2+, and Zn-2+ by Diethylenetriaminepentaacetic Acid Bis(Methylamide) Inorganic Chemistry. 1993;32:582–586. [Google Scholar]
- Sadgrove MP, Leed MGD, Shapariya S, Madhura DB, Jay M. Evaluation of a DTPA Prodrug, C2E5 as an Orally Bioavailable Radionuclide Decorporation Agent. Drug Development Research. 2012;73:243–251. [Google Scholar]
- Seidel A. Removal from Rat of Internally Deposited Am-241 by Long-Term Treatment with Cadtpa and Zndtpa. Radiation Research. 1975;61:478–487. [PubMed] [Google Scholar]
- Shankar GN, Potharaju S, Green CE. Evaluating the Toxicity of Novel Zn-DTPA Tablet Formulation in Dogs and Rats. Drug Development Research. 2014;75(1):37–46. doi: 10.1002/ddr.21165. [DOI] [PubMed] [Google Scholar]
- Silva RJ. OECD Nuclear Energy Agency, Chemical thermodynamics of americium Amsterdam. New York: Elsevier; 1995. [Google Scholar]
- Sloop CH, Dory L, Roheim PS. Interstitial Fluid Lipoproteins. Journal of Lipid Research. 1987;28:225–237. [PubMed] [Google Scholar]
- Stevens W, Bruenger FW, Atherton DR, Buster DS, Howerton G. The retention and distribution of 241Am and 65Zn, given as DTPA chelates in rats and of [14C]DTPA in rats and beagles. Radiat Res. 1978;75:397–409. [PubMed] [Google Scholar]
- Stradling GN, Henge-Napoli MH, Paquet F, Poncy JL, Fritsch P, Taylor DM. Approaches for experimental evaluation of chelating agents. Radiation Protection Dosimetry. 2000;87:19–27. [Google Scholar]
- Sueda K, Sadgrove MP, Fitzsimmons JM, Jay M. Physicochemical characterization of a prodrug of a radionuclide decorporation agent for oral delivery. J Pharm Sci. 2012;101:2844–53. doi: 10.1002/jps.23218. [DOI] [PubMed] [Google Scholar]
- Sueda K, Sadgrove MP, Jay M, Di Pasqua AJ. Species-dependent effective concentration of DTPA in plasma for chelation of 241Am. Health Phys. 2013;105:208–214. doi: 10.1097/HP.0b013e318290ca33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DM. The bioinorganic chemistry of actinides in blood. Journal of Alloys and Compounds. 1998;271:6–10. [Google Scholar]
- Turner GA, Taylor DM. Transport of Plutonium Americium and Curium in Blood of Rats. Physics in Medicine and Biology. 1968;13:535–547. doi: 10.1088/0031-9155/13/4/304. [DOI] [PubMed] [Google Scholar]
- Volf V. Effect of Drinking Zn-Dtpa on Pu-238 and Am-241 in Rat Bones. Radiation and Environmental Biophysics. 1984;23:141–143. doi: 10.1007/BF01213743. [DOI] [PubMed] [Google Scholar]