Abstract
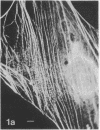
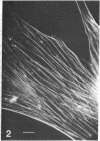
Myosin in human, rat, mouse, and chicken fibroblasts was localized by indirect immunofluorescence microscopy using antibodies prepared in rabbits against highly purified chicken gizzard myosin. Filaments containing myosin span the interior of the cells and are often parallel to each other. The majority of the fibers are concentrated toward the adhesive side of the cell. Most of the myosin-containing filaments show “interruptions” or “striations.” From a comparison of these fibers in fluorescence and phase microscopy and from previous results on actin-containing fibers, we conclude that at least some of the cytoplasmic myosin can be found in the actin-containing fibers, which themselves have been shown to be very similar or identical to the microfilament bundles. The occurrence of both myosin and actin in the microfilament bundles provides a basis for the motility and contractility of the cell.
Keywords: actin, immunofluorescence, microfilaments, cell movement
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behnke O., Kristensen B. I., Nielsen L. E. Electron microscopical observations on actinoid and myosinoid filaments in blood platelets. J Ultrastruct Res. 1971 Nov;37(3):351–369. doi: 10.1016/s0022-5320(71)80129-6. [DOI] [PubMed] [Google Scholar]
- Buckley I. K., Porter K. R. Cytoplasmic fibrils in living cultured cells. A light and electron microscope study. Protoplasma. 1967;64(4):349–380. doi: 10.1007/BF01666538. [DOI] [PubMed] [Google Scholar]
- Cohen I., Kaminski E., De Vries A. Actin-linked regulation of the human platelet contractile system. FEBS Lett. 1973 Aug 15;34(2):315–317. doi: 10.1016/0014-5793(73)80820-8. [DOI] [PubMed] [Google Scholar]
- Gröschel-Stewart U. Comparative studies of human smooth and striated muscle myosins. Biochim Biophys Acta. 1971 Feb 16;229(2):322–334. doi: 10.1016/0005-2795(71)90191-7. [DOI] [PubMed] [Google Scholar]
- Gwynn I., Kemp R. B., Jones B. M., Gröschel-Stewart U. Ultrastructural evidence for myosin of the smooth muscle type at the surface of trypsin-dissociated embryonic chick cells. J Cell Sci. 1974 Jul;15(2):279–289. doi: 10.1242/jcs.15.2.279. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Bischoff R., Holtzer H. Formation of arrowhead complexes with heavy meromyosin in a variety of cell types. J Cell Biol. 1969 Nov;43(2):312–328. [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund R. E., Pastan I., Adelstein R. S. Myosin in cultured fibroblasts. J Biol Chem. 1974 Jun 25;249(12):3903–3907. [PubMed] [Google Scholar]
- Pollard T. D., Weihing R. R. Actin and myosin and cell movement. CRC Crit Rev Biochem. 1974 Jan;2(1):1–65. doi: 10.3109/10409237409105443. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H., Hatano S. Extraction of native tropomyosin-like substances from myxomycete plasmodium and the cross reaction between plasmodium F-actin and muscle native tropomyosin. Biochim Biophys Acta. 1972 Feb 29;257(2):445–451. doi: 10.1016/0005-2795(72)90297-8. [DOI] [PubMed] [Google Scholar]
- Weber A., Murray J. M. Molecular control mechanisms in muscle contraction. Physiol Rev. 1973 Jul;53(3):612–673. doi: 10.1152/physrev.1973.53.3.612. [DOI] [PubMed] [Google Scholar]
- Yang Y. Z., Perdue J. F. Contractile proteins of cultured cells. I. The isolation and characterization of an actin-like protein from cultured chick embryo fibroblasts. J Biol Chem. 1972 Jul 25;247(14):4503–4509. [PubMed] [Google Scholar]