Abstract
Perhaps development is more than just morphogenesis. We now recognize that the conceptus expresses epigenetic marks that heritably affect it phenotypically, indicating that the offspring are to some degree genetically autonomous, and that ontogeny and phylogeny may coordinately determine the fate of such marks. This scenario mechanistically links ecology, ontogeny and phylogeny together as an integrated mechanism for evolution for the first time. As a functional example, the Parathyroid Hormone-related Protein (PTHrP) signaling duplicated during the Phanerozoic water-land transition. The PTHrP signaling pathway was critical for the evolution of the skeleton, skin barrier, and lung function, based on experimental evidence, inferring that physiologic stress can profoundly affect adaptation through internal selection, giving seminal insights to how and why vertebrates were able to evolve from water to land. By viewing evolution from its inception in unicellular organisms, driven by competition between pro- and eukaryotes, the emergence of complex biologic traits from the unicellular cell membrane offers a novel way of thinking about the process of evolution from its beginnings, rather than from its consequences as is traditionally done. And by focusing on the epistatic balancing mechanisms for calcium and lipid homeostasis, the evolution of unicellular organisms, driven by competition between pro- and eukaryotes, gave rise to the emergence of complex biologic traits derived from the unicellular plasma lemma, offering a unique way of thinking about the process of evolution. By exploiting the cellular-molecular mechanisms of lung evolution as ontogeny and phylogeny, the sequence of events for the evolution of the skin, kidney and skeleton become more transparent. This novel approach to the evolution question offers equally novel insights to the primacy of the unicellular state, hologenomics and even a priori bioethical decisions.
Keywords: evolution, cell-cell signaling, homeostasis, Parathyroid Hormone-related Protein, growth factor signaling, unicellular state, paracrine, ontogeny, phylogeny, homology
INTRODUCTION
No matter how many times one goes over the mechanism of cellular evolution, it always comes down to biology as calcium homeostasis counterbalanced by lipid homeostasis [1]. Right from the very triggering of fertilization of the egg by the sperm there is a calcium burst, which sustains us throughout the life cycle until the penultimate moment when we die. Throughout life we have ‘peak experiences’ (Maslow) during the course of the life cycle as sentient beings, creative bursts, procreative bursts, the runner’s high, the near-death experience (the light seen during this phenomenon being the return of the calcium ‘spark’ of life, snatched from the jaws of death), etc. It is hypothetically possible that the zygote is the principle state of evolutionary selection, which is counterintuitive based on descriptive biology, but is the logical conclusion reached from a cellular-molecular approach, working backwards in ontogeny and phylogeny to our origins in unicellular life [2]. Does this view of biology provide more answers than questions? It is certainly worthy of consideration, given the counterintuitive nature of conventional physiology, not to mention its lack of predictive power [3].
Life began as a dynamic equilibrium of balancing selection between lipids and calcium to sustain negentropy within the cell in defiance of the Second Law of Thermodynamics [2]. Since that is the ultimate mechanism for evolution, is it any wonder that we go back to those first principles during the life cycle, or did we never leave them in the first place? That is, the multicellular state is derivative of the first principles, used to monitor the ever-changing environment, gleaning information, subsequently having it filtered by the embryo as a mechanism for stability or change as the case may be [4]. That perspective is a radical departure from that gained by the adult as the principal state of being, in contrast to that which is mechanistically in synch with what actually transpires. We are aware that we are made up of atoms, but we do not think in those terms on a moment-to-moment basis, other than to contemplate our existence from time to time. If we were to embrace the idea that all the biota evolved from the same cellular construct, like Kinship Theory, which says that empathy is a function of how closely related we are, we would be far more universally compassionate as a species!
There have been several breakthrough moments in human history, such as Archimedes’ realization of buoyancy (Eureka!), Magellan demonstrating that the world is round, and Copernicus pointing the way to Heliocentrism. And there were several attempts to construct a predictive Periodic Table of Elements, yet only one was successful. Mendeleyev finally came up with the correct initial condition for periodicity of the elements, that of atomic number as the organizing principle. Similarly, there have been innumerable theories for the existence of life, ranging from Creationism to Darwinism, but all of them are predicated on descriptive, materialistic biology. It is only once it is realized that the initial conditions of life as autonomous, self-organizing cells, defying the Second Law of Thermodynamics that the meaning of life can actually be understood. Like equating mass and energy (E=MC2), that idea is transformative. And because it is predictive of many otherwise counterintuitive phenomena in biology, it seems to be correct.
For example, did you ever wonder why life goes in reiterative cycles from zygote to adult, and back to the zygote?
The evolutionary ‘arc’ of complex physiologic principles can be traced using the calcium ‘spark’, from primitive cells lying at the interface between water and land, like sea foam, to the human brain. Easier said than done, yet all structural-functional links have formed in service to calcium flux, mediated by the lipids that form a continuum from cholesterol in primitive eukaryotes, to the myelination of neurons. And bear in mind that the homeostatic mechanisms involved are highly conserved at every functional level-cell, tissue, organ, and systemic physiology. Undergirding all of that is the foundational principle of Life, fomented by negentropy, generated by chemiosmosis and endomembranes, sustained by homeostasis- a squishy, compliant, organic automaton, conceived through its own devices; like the Greek metaphor of life, the Ouroboros, the snake catching its own tail, self-organizing and self-perpetuating. Viewed from this vantage point, the notion that the unicellular state of the Life Cycle is the primary site for selection pressure becomes tenable. The Life Cycle is not stagnant, it has a vectorial direction and magnitude of change, either moving upward or downward as it evolves to adapt, or devolves towards extinction. If so, what is the initiating event, since this process must be inhomogeneous. Conventionally, it is thought that the adult form is the apotheosis of such a mechanism, but this is a narcissistic, anthropocentric viewpoint, like geocentrism, and we know how that ended up.
Alternatively, consider the unicellular zygote, particularly in view of new evidence that epigenetic marks on the egg and sperm are not exspunged during meiosis, as had long been thought [5]. What is the functional significance of such epigenetic marks, and what determines which ones are retained, and which ones are eliminated? Such considerations are not trivial, since only ~1-3% of inherited human diseases are Mendelian, leaving a huge void in the constellation of heritable diseases that may be filled by epigenetics. And perhaps this is why phylogeny is recapitulated during the process of ontogeny. Haeckel’s Biogenetic Law [6] was rejected long ago for lack of experimental evidence that embryogenesis faithfully recapitulated all the phenotypic milestones of phylogeny, but that was before the discovery of the cellular-molecular signaling mechanisms that underpin such morphogenetic changes. Such data are referred to in the Evolutionary Biology literature as ‘ghost lineages’, but in the realm of molecular embryogenesis, such data are how and why structure and function develop as a continuum. That knowledge also alludes to the possibility that phylogeny must mechanistically recapitulate itself in order to ensure that any newly-acquired epigenetic mutations are in compliance with the homeostatic and allostatic mechanisms that they might affect in introducing them into the organism’s gene pool. In so saying, the unicellular state is ultimately the overall determinant and arbiter of this process- the unicellular state dominates. And if so, Man, as a species needs to reassess our priorities among our cousins, plant and animal alike. Maybe this will lead to bioethical considerations based on first principles, rather than on self-serving actions. (The last portion was deleted to aid better reception of the article. Being mindful of such matters is pragmatic for articles of this nature.) OK Man has thrived on this planet as a species up until now by using resources that were not truly only ours, though this is how he/she has behaved.
So maybe Haeckel’s dictum needs to be reconsidered in formulating a central theory of biology. In so doing, we may also want to reconsider Internal Selection as a mechanistic extension of Natural Selection.
Up until recently there was a widely unacknowledged blind spot created by the absence of cell biology from evolution theory. The only explanation for this seeming oversight has come from Betty Smocovitis, who noted in her book ‘Unifying Biology’ [7] (We need to retain the quotes for book titles. It is just a formatting convention.) OK that there was a parting of the ways between the embryologists and the evolutionists back at the end of the 19th Century, resulting in the absence of a cellular approach to evolution. That has been exacerbated in the interim by evolutionists lacking training in cell biology, and the resultant cultural breakdown between cell biology and evolution theory, exemplified by the comment by SJ Gould that internal selection is tantamount to cancer [8]. And even the more recent advent of Evolutionary Developmental Biology, which merely places these two disciplines in close proximity to one another, but does not take advantage of the mechanistic synergy between them. But recriminations aside, what is the residual of this abyss between cell biology and evolution? A roll-out of a cellular perspective on the process of evolution, and a discussion of its benefits will be presented for consideration.
Embryologic development is the only process known for determining the mechanisms for morphogenesis of tissues and organs. The major breakthrough in this field was the discovery that embryonic development is dependent on cell-cell signaling [9] mediated by soluble growth factors and their cognate receptors [10], signaling for the growth and differentiation of the cells that ultimately determine form, function and homeostasis. But what if Spemann had been able to determine the nature of his ‘Organizer’ back in the 19th century? How would that have affected evolution theory? A way of tracing the evolution of the lung has been conceptualized [11], which can then be traced all the way back to its origins in the unicellular plasma lemma [1, 2], affording a way of then looking in the forward direction to determine how and why the lung evolved from that simple unicellular structure for gas exchange, as follows.
The mammalian lung develops from the foregut starting on the 9th day of embryonic development in the mouse embryo. The trachea forms from the esophagus, and the major conducting airways are subsequently formed, followed by the alveoli, all through reciprocating, sequential interactions between the endodermal and mesodermal germ layers, ultimately giving rise to more than 40 different cell-types [12]. The key to understanding both lung development and evolution is the formation of the alveolar epithelium [1, 2], which produces lung surfactant, a soapy material that prevents the collapse of the alveoli on deflation. By comparing gene regulatory networks both across phyla and during development, the sequence of events by which structures and their functions evolved can be determined [13].
In a recent publication, how the lung may have evolved from the swim bladder of fish based on the parathyroid hormone-related protein (PTHrP) signaling pathway was shown, a pathway necessary for both lung homeostasis and development [1, 2]. PTHrP signaling predicts the magnitude and direction of lung maturation [14], and may also predict the phylogenetic changes in the vertebrate lung, characterized by decreasing alveolar diameter [15-17], accompanied by the thinning [18] and buttressing [19] of the alveolar wall.
PTHrP is expressed throughout vertebrate phylogeny, beginning with its expression in the fish swim bladder as an adaptation to gravity as buoyancy; microgravity down-regulates the expression of PTHrP by alveolar type II epithelial cells, and by the bones of rats exposed to 0 × g [20], suggesting that PTHrP signaling has evolved in adaptation to gravity. PTHrP signaling is up-regulated by stretching alveolar type II cells and interstitial fibroblasts [21], whereas over-distension down-regulates PTHrP and PTHrP receptor expression [22], further suggesting a deep evolutionary adaptation since these genes evolved independenly over biologic time. Both surfactant homeostasis and alveolar capillary perfusion are under PTHrP control [23], indicating that alveolarization and ventilation/perfusion matching, the physiologic principle of the alveolus, may have evolved under the influence of PTHrP signaling.
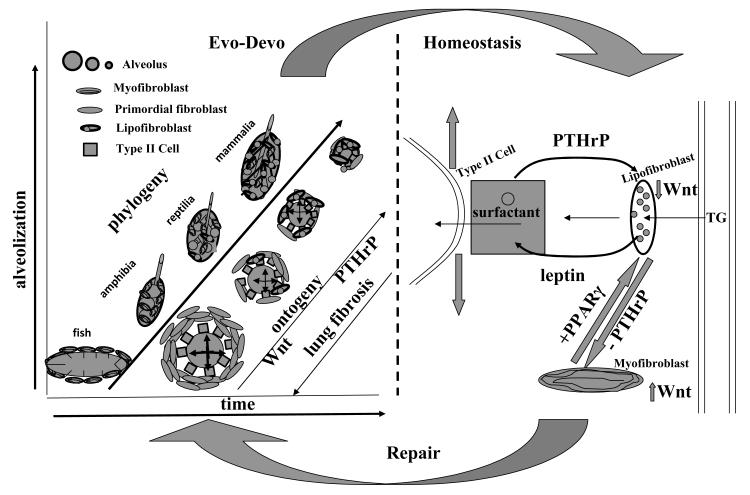
PTHrP is a highly evolutionarily-conserved, stretch-regulated gene that is unusual among the paracrine growth factors that have been identified to mediate lung development because the PTHrP gene deletion is stage-specific, and results in failure to form alveoli, the major lung adaptation for gas-exchange in land vertebrates; unlike other such growth factors that emanate from the mesoderm and bind to the endoderm, PTHrP is unusual in being expressed in the endoderm, binding to the mesoderm, providing a reciprocating mechanism for morphogenesis; only PTHrP has been shown to act pleiotropically to integrate surfactant synthesis and alveolar capillary perfusion, mediating the on-demand surfactant mechanism of alveolar homeostasis. In contrast to this, others have focused on the importance of the epithelial-mesenchymal trophic unit [24], and on the importance of the fibroblasts of the “scaffold” that act as “sentinels” to regulate local inflammatory responses [25]. However, PTHrP signaling from the epithelium to the mesoderm is highly significant. The earliest developmental signals for alveolar development originate from the endoderm [26], and we have demonstrated the dependence of the fibroblast phenotype on epithelially-derived PTHrP for development, homeostasis, and repair. All of these features of PTHrP biology justify its use as an archetype for our proposed model of lung evolution. This integrated approach for lung developmental and comparative biology, homeostasis, and repair has been schematized in Figure 1.
Fig. 1.
Lung biologic continuum from ontogeny–phylogeny to homeostasis and repair. The schematic compares the cellular– molecular progression of lung evolution from the fish swim bladder to the mammalian lung (left portion) with the development of the mammalian lung, or evo-devo, as the alveoli become progressively smaller (see legend in upper left corner), increasing the surface area-blood volume ratio. This is facilitated by the decrease in alveolar myofibroblasts, and the increase in lipofibroblasts, due to the decrease in Wingless/int (Wnt) signaling, and increase in PTHrP signaling, respectively. Lung fibrosis progresses in the reverse direction (lower left corner). Lung homeostasis (right portion) is characterized by PTHrP/leptin signaling between the type II cell and lipofibroblast, coordinately regulating the stretch regulation of surfactant production with alveolar capillary perfusion- PTHrP acts as both a potent vasodilator and stimulates lipofibroblast uptake of the surfactant phospholipid substrate triglyceride (TG), which is actively transferred to the type II cell for surfactant synthesis. Failure of PTHrP signaling causes increased Wnt signaling, decreased PPARγ expression by lipofibroblasts, and transdifferentiation to myofibroblasts, causing lung fibrosis. Repair (arrow from homeostasis back to ontogeny–phylogeny), is the recapitulation of ontogeny–phylogeny, resulting in increased PPARγ expression.
Ontogeny and homeostasis
Stimulation of PTHrP and its receptor by alveolar wall distension coordinates the physiologic increase in surfactant production [27] with alveolar capillary blood flow, maximizing the efficiency of gas exchange across the alveolar wall, referred to conventionally based on descriptive biology as ventilation/perfusion (V/Q) matching. V/Q matching is the net result of the evolutionary integration of cell/molecular interactions by which the lung and pulmonary vasculature have functionally adapted to the progressive increase in metabolic demand for oxygen [28-30] as vertebrates evolved to accommodate land life. The structural adaptation for gas exchange is threefold: the decrease in alveolar diameter [31], the thinning of the alveolar wall [32], and the maximal increase in total surface area [32, 33]. These structural adaptations have resulted from the phylogenetic amplification of the PTHrP signaling pathway. PTHrP signaling through its receptor is coordinately stimulated by stretching the alveolar parenchyma [34]. The binding of PTHrP to its receptor activates the cyclic Adenosine Monophosphate (cAMP)-dependent Protein Kinase A (PKA) signaling pathway [35]. Stimulation of this signaling pathway results in the differentiation of the alveolar interstitial lipofibroblast, characterized by increased expression of adipocyte differentiation related protein (ADRP) and leptin. ADRP is necessary for the trafficking of substrate for surfactant production [36], and leptin stimulates the differentiation of the alveolar type II cell [37]. PTHrP affects the cellular composition of the alveolar interstitium in at least three ways that are synergistic with one another: it inhibits fibroblast growth [38] and stimulates apoptosis [39], causing septal thinning; it stimulates epithelial type II cell differentiation by leptin [40], which can inhibit epithelial cell growth [41]; leptin may up-regulate type IV collagen synthesis [42], reinforcing the alveolar wall [43]. Type IV collagen likely evolved in the water-land transition as a natural water barrier, since its evolved amino acid composition is hydrophobic [44].
Ontogeny and phylogeny
Cell-cell interactions between primordial lung endoderm and mesoderm cause their differentiation into over 40 different cell-types. We know a great deal about the growth factor signaling that determines these processes, and the downstream signals that alter nuclear read-out. And because a great deal of effort has been put into understanding the consequences of preterm birth, we also know how these mechanisms lead to homeostasis, or fail to do so, in which case the phenotype for chronic lung disease informs us of the mechanism of lung fibrosis.
Embryonic lung development is subdivided into branching morphogenesis and alveolarization, the former being ‘hard-wired’, the latter being highly plastic [45]. Deleting the PTHrP gene results in failed alveolarization [46], inferring the relevance of PTHrP to lung evolution, since alveolarization was the primary mechanism for vertebrate lung evolution [47, 48]. Note, for example that the lung specifically evolved from the swim bladder of physostomous fish, which have a pneumatic duct connecting their esophagus to the swim bladder, a homolog of the trachea, but have no alveoli; the swim bladder forms two subdivisions, which may be homologs of alveoli. Because PTHrP and its receptor are highly conserved [49] and stretch-regulated [50, 51], functionally linking the endoderm and mesoderm to the vasculature [52], we are compelled to investigate its overall role in lung phylogeny and evolution.
The combined effects of features cited in the previous section would lead to natural selection for progressive, concomitant decreases in both alveolar diameter and alveolar wall thickness through ontogeny [53] and phylogeny [54-56], optimally increasing the gas-exchange surface area-to-blood volume ratio of the lung. PTHrP shuts off myofibroblast differentiation by inhibiting the glioblastoma gene Gli [57], the first molecular step in the mesodermal Wingless/int (Wnt) pathway, and by inactivating β-catenin [57], followed by the activation of LEF-1/TCP, C/EBPα, and peroxisome proliferator-activated receptor γ (PPARγ). The downstream targets for PPARγ are adipogenic regulatory genes such as ADRP and leptin. PTHrP induces the lipofibroblast phenotype, first described by Vaccaro and Brody [58, 59]. This cell-type is expressed in the lungs of a wide variety of species, including both newborn and adult humans [60]. They are found next to type II cells in the adepithelial interstitium [60], and are characterized by neutral lipid inclusions enwrapped in ADRP, which actively mediates the uptake and trafficking of lipid from the lipofibroblast to the type II cell for surfactant phospholipid synthesis [61-63], protecting the alveolar acinus against oxidant injury [64]. The concomitant inhibitory effects of PTHrP on both fibroblast and type II cell growth, in combination with PTHrP augmentation of surfactant production, would have the net effect of distending and “stenting” the thinning alveolar wall, synergizing with the up-regulation of PTHrP, and physiologically stabilizing what otherwise would be an unstable structure that would tend to collapse [65].
Myofibroblast transdifferentiation as evolution in reverse
Lung development prepares the fetus for birth and physiological homeostasis [66]. Surfactant production in particular is crucial for effective gas exchange [67]. Based on this integrated functional linkage between lung development and homeostasis, we have generated data demonstrating that the underlying mechanisms of repair may recapitulate ontogeny. If lung fibroblasts are deprived of PTHrP, their structure changes [68]. First, the PTHrP receptor is down-regulated, as are its downstream targets ADRP and leptin; the decline in the lipofibroblast phenotype is mirrored by the gain of the myofibroblast phenotype, characteristic of fibrosis.
During the process of fetal lung development, the mesodermal fibroblasts are characterized by Wnt/β-catenin signaling that determines the splanchnic mesodermal fibroblast [69]. During alveolarization, the formation of lung fluid actively up-regulates the PTHrP signaling pathway in the endoderm by distending the alveolar wall, causing the down-regulation of the Wnt/β-catenin pathway [57], leading to the differentiation of the lipofibroblast. These cells dominate the alveolar acinus during fetal lung development, but are highly apoptotic in the postnatal lung [70, 71], giving rise to the alveolar septa [72]. A functional hallmark of this paracrine determination of the mesodermal cell-types is the failure of the fibroblasts to terminally differentiate [68].
Phylogenetically, the swim bladder and frog lung interstitium are characterized by myofibroblasts; lipofibroblasts do not appear during phylogeny until reptiles and mammals [73-77]. The recapitulation of myofibroblasts during lung injury is consistent with the similarities between lung ontogeny and phylogeny, and with the molecular mechanisms of fibroblast transdifferentiation described above, and may, therefore, represent the process of lung evolution in reverse.
A wide variety of factors can inhibit the normal paracrine induction of the lipofibroblast, and promote myofibroblast proliferation and fibrosis, including prematurity, barotrauma, oxotrauma, nicotine, and infection. In all of these instances, injury of the epithelial type II cell can cause down-regulation of PTHrP [78], causing the mesodermal fibroblasts to default to the myofibroblast phenotype [79]. Myofibroblasts cannot promote the growth and differentiation of the alveolar type II cell for alveolarization [68], and produce angiotensin II, which further damages the type II cell population [80, 81].
The PTHrP receptor is present on the surfaces of adepithelial fibroblasts [82]. Stretching of the alveolus by fluid or air coordinately up-regulates both PTHrP ligand [14] and PTHrP receptor activity [14], promoting surfactant production by the type II cell, and lipofibroblast neutral lipid uptake, protecting both of them against oxidant injury [83]. PTHrP receptor binding stimulates cAMP-dependent Protein Kinase A expression, which determines the lipofibroblast phenotype. Treatment of the transdifferentiating myofibroblast, either in vitro [84] or in vivo [84], with PPARγ agonists blocks the transdifferentiation of the myofibroblast, preventing fibrotic injury [84].
The roles of PPARγ in ontogeny and repair
PTHrP induces lipofibroblast differentiation via the protein kinase A pathway, which blocks Wnt signaling by inhibiting both Gli and glycogen synthase kinase (GSK) 3β, and up-regulates the lipofibroblast phenotype, PTHrP receptor, ADRP, leptin and triglyceride uptake by stimulating PPARγ expression [84].
On the basis of the minimalist idea that development culminates in homeostasis, disruption of homeostasis may lead back to earlier developmental and evolutionary motifs [85]. This occurs in various lung diseases [78,79,86], and by focusing on the continuum from development and evolution to homeostasis, select treatments that are more consistent with promoting cellular physiologic reintegration than merely stopping inflammation can be devised. For example, bronchopulmonary dysplasia (BPD) can be induced by over-distending an otherwise healthy but immature newborn baboon lung [87]. Destabilizing the homeostatic balance of the alveolus by knocking out surfactant protein genes B, C, or D leads to alveolar remodeling that is either grossly flawed (B) or less than optimal (C, D) physiologically. Interfering with epithelial-mesenchymal signaling blocks lung development [26], usually resulting in alveolar simplification (or ‘reverse-evolution’). Conversely, replacing missing developmental elements can re-establish lung development [40], homeostasis [56], and structure [66].
Repair recapitulates ontogeny because it is programmed to express the cross-talk between epithelium and mesoderm through evolution [13]. This model is based on three key principles: the cross-talk between epithelium and mesoderm is necessary for homeostasis; damage to the epithelium impedes the cross-talk, leading to loss of homeostasis and re-adaptation through myofibroblast proliferation; normal physiology will either be faithfully re-established, or cell/tissue remodeling/altered lung function may occur, and/or fibrosis will persist, leading to chronic lung disease. The cellular-molecular injury affecting epithelial-mesenchymal cross-talk recapitulates ontogeny and phylogeny (in reverse), providing effective diagnostic and therapeutic targets.
The relevance of lung evolution to physiologic evolution in general
The premise of this essay is that the First Principles of Physiology (FPPs) are knowable. The hypothesis is that such FPPs were co-opted during the transition of vertebrates from water to land, beginning with the acquisition of cholesterol by eukaryotes [1, 2], facilitating unicellular evolution over the course of the first 4.5 billion years of the Earth’s history in service to the reduction in intracellular entropy, far from equilibrium, circumventing the Second Law of Thermodynamics. That mechanism was initiated, supported, and perpetuated by the introduction of cholesterol into the cell membrane of unicellular eukaryotes [1, 2], ultimately giving rise to the metazoan homologs of the gut, lung, kidney, skin, bone, and brain [1, 2]. Central to this working hypothesis is that homeostatic control is “plastic,” allowing for inheritance of a range of set-points, rather than just one genetically fixed state [1, 2]. It is important to note that this perspective is one hundred-and-eighty degrees out of synch with traditional genetic determinism [88], in which evolution is considered to result exclusively from random mutation and natural selection. Yet such plasticity is totally in keeping with ideas such as the Barker Hypothesis for the fetal origins of adult disease [89], the role of epigenetics [90], and what we know of the variation in growth factor determination of morphogenesis and homeostasis [91]. Tiktaalik, the fish-to-tetrapod transitional fossil discovered by Neil Shubin in 2004 [92] provides a heuristic for the vertebrate water-to-land transition. To make that transition, Tiktaalik had to have been “preadapted” for respiration (as the primary selection pressure), kidney, skin, gut, bone, and brain traits amenable to land life. When thought of in the context of fish physiology as the antecedent for such a critical transition, importantly, the swim bladder has been definitively shown to be structurally, functionally (as a gas exchanger), and genomically homologous to the tetrapod lung [93]. Both organs are outpouchings of the gut, and mediate the uptake and release of atmospheric oxygen and carbon dioxide. Furthermore, among the most highly-expressed genes in the zebrafish swim bladder is parathyroid hormone-related protein (PTHrP) [93], whose signaling receptor underwent a gene duplication event during the transition from fish to amphibians [94]. That event made atmospheric gas exchange for the water-to-land transition possible, since PTHrP is necessary for the formation of lung alveoli; if one deletes the PTHrP gene in mice, the offspring die within a few minutes of birth due to the absence of alveoli [46]. PTHrP is expressed in both the epithelial cells that line the swim bladder of fish, and the alveoli of land vertebrates. In alveoli, PTHrP stimulates the production of surfactant, which maintains their structure and function by reducing surface tension; in the absence of surfactant, the alveoli will collapse, rendering them dysfunctional.
PTHrP and lung cell-molecular evolutionary homeostasis
PTHrP is a peptide secreted by alveolar type II cells in response to stretching [53]. PTHrP acts locally (i.e., in a paracrine manner) via cell surface receptors to induce specialized connective tissue fibroblasts to become lipofibroblasts [56] (Fig. 1). The lipofibroblasts appear to be critical in the evolution of the lung for two reasons: first, they protect the alveolus against oxidant injury [64] by actively recruiting and storing neutral lipids from the alveolar microcirculation [61], acting as antioxidants [64]; secondly, the stored neutral lipids are actively “trafficked” from the lipofibroblasts to the alveolar type II cells for surfactant synthesis [64] through the mechanically coordinated biochemical effects of PTHrP [14], leptin [14], and prostaglandin E2 [95], which act via their respective cognate receptors that reside on the apposing surfaces of neighboring epithelial type II cells and lipofibroblasts. Ultimately, PTHrP regulates alveolar epithelial calcium uptake, suggesting its evolutionary history; calcium concentration in the alveolar lining aqueous, protein-containing hypophase regulates the formation and dissolution of tubular myelin, which determines its effect on surface tension [96]; tubular myelin is a lipid-defensin complex homolog of the lipid-defensin barrier formed by the stratum corneum in the skin [97] to prevent fluid leak and protect against microbial infection. Since the skin is the most primitive organ of land vertebrate gas exchange, it may have provided a molecular homeostatic co-option for further evolution of the lung.
It can be calculated that such a mechanism would have taken approximately 9 × 1016 years to have occurred by chance, which is seven orders of magnitude longer than the estimated 5 × 109 year existence of the Earth. An alternative mechanism is “descent with modification”: phylogenetically, PTHrP is a gene involved in fish adaptation for buoyancy, which was co-opted by land vertebrates for stretch-regulated (the biologic homolog of gravity) surfactant production [85].
ADRP as a deep homology that interconnects evolved functional homologies, or “Oh, the Places You’ll Go!”-Dr. Seuss
The key molecule that mediates neutral lipid trafficking [62] between the alveolar microcirculation, lipofibroblast, and epithelial type II cell is adipocyte differentiation-related protein (ADRP) [36]. It is a member of the Perilipin-ADRP-TIP47, or PAT, family of intracellular lipid cargo proteins that mediate lipid uptake, storage, and secretion in a wide variety of cells, tissues, and organs, ranging from fat cells to endothelium, liver, and steroidogenic endocrine organs [98]. PAT proteins are expressed in many organisms, ranging from mammals to slime molds and fungi [98]. ADRP was first discovered to be involved in early adipocyte differentiation [99] and subsequently shown to be necessary for the uptake and storage of intracellular lipid droplets when overexpressed in Chinese hamster ovary cells, which do not naturally express ADRP [100].
In the lung, ADRP (Fig. 1) is physiologically up-regulated by the stretching of the alveolar type II cells, which produce PTHrP; PTHrP then binds to its cognate receptor on the epithelial lipofibroblasts, stimulating PPARγ [101], which then up-regulates ADRP. This mechanism may have initially evolved to protect the alveolar wall against hyperoxia, since the rising atmospheric oxygen tension causes the differentiation of myofibroblasts into lipofibroblasts [102]. This mechanism may subsequently have been co-opted to regulate surfactant synthesis during the vertebrate water-to-land transition [1, 2], consistent with the phylogenetic adaptation of the alveolus from the swim bladder of fish to the highly adapted lungs of mammals and birds. This phenomenon is of particular interest in the context of exploiting such functional molecular homologies when one considers the homologies between the alveolar lipofibroblast and endocrine steroidogenesis. For example, oxygen in the atmosphere did not increase linearly from zero to 21%; rather, it has gone up and down episodically, ranging between 15 and 35% over the past 500 million years [103]. Bearing in mind that hypoxia is the most stressful of all physiologic agonists, it would have put huge physiologic constraints on both the evolving lung and endocrine systems. Perhaps fortuitously, the vertebrate pulmonary and endocrine systems were preadapted for such an adaptation through PAT genes; thus, the well-recognized effects of the adrenocortical system on lung development and homeostasis [1, 2] can be seen (Fig. 3 below) as part of a logical evolutionary progression of external and internal selection mechanisms [104].
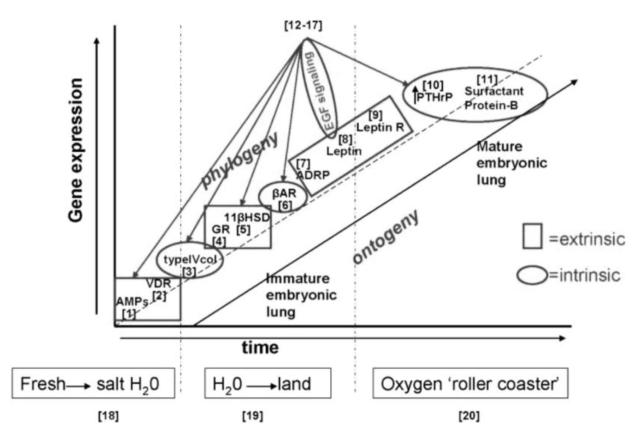
Fig. 3.
Alternating extrinsic and intrinsic selection pressures for the genes of lung phylogeny and ontogeny. The effects of the extrinsic factors (salinity, land nutrients, and oxygen on the x-axis) on genes that determine the phylogeny and ontogeny of the mammalian lung alternate sequentially with the intrinsic genetic factors (y-axis), highlighted by the squares and circles, respectively. Steps 1–11 appear in the sequence they appear during phylogeny and ontogeny: [1] AMPs; [2] VDR; [3] type IV collagen; [4] GR; [5] 11β HSD; [6] βAR; [7] ADRP; [8] leptin; [9] leptin receptor; [10] PTHrP; and [11] SP-B. Steps 12–17 represent the pleiotropic effects of leptin on the EGF in oval signaling pathways integrating steps 1–6, 10, and 11. Steps 18–20 are major geologic epochs that have “driven” intrinsic lung evolution.
This is not a tautology, or a “Just So Story,” since, for example, the same morphogenetic mechanisms occur during both ontogeny and phylogeny [85], and we observe the reversal of this evolutionary process in chronic lung diseases, in which there is “simplification” of the alveolar bed, resulting in a frog-like structure in mammals. Experimentally, Besnard et al. [105] found that when they deleted a gene necessary for the synthesis of cholesterol, the most primitive of lung surfactants [106], specifically from mouse lung alveolar type II cells, the lung developmentally “compensated” by over-expressing the lipofibroblast population in the alveoli, suggesting that these cells have an evolutionary capacity to facilitate surfactant production, both ontogenetically and phylogenetically. This rational cell/molecular approach to understanding how and why the lung evolved can be carried one step further, since catecholamine/β-adrenergic receptor signaling was essential for regulation of blood pressure in the lung independent of the systemic circulation, facilitating a further increase in the surface area of the evolving lung [107]. Our ancestors were organisms able to survive the whipsawing physiologic effects of alternating hyperoxia and hypoxia [103] by structurally and functionally adapting their pulmonary and endocrine systems (see below). Here again, the β-adrenergic receptor underwent a gene duplication during the fish-amphibian transition that allowed for a further increase in lung surface area to support metabolic demand [34]. At this phase in vertebrate evolution, the glucocorticoid receptor is documented to have evolved from the mineralocorticoid receptor, perhaps as a counterbalancing selection for the blood pressure-elevating effect of mineralocorticoids. That epistatic mechanism would have been synergized by concomitant glucocorticoid stimulation of β adrenergic receptor expression, further alleviating the blood pressure constraint. The emergence of the physiologic glucocorticoid mechanism may have been further facilitated by the presence of pentacyclic triterpenoids in land vegetation, a product of rancidification unique to the land environment. These compounds inhibit 11β-hydroxysteroid dehydrogenase type II (11β HSD2), which inactivates cortisol’s blood pressure-stimulating activity, causing positive selection pressure for the tissue-specific expression of 11 β HSD1, 2 in a wide variety of glucocorticoid target organs, including the lung [108], thereby permitting local tissue-specific activation and inactivation of cortisol. Reinforcing this hypothesis, when pituitary adrenocorticotropic hormone (ACTH) stimulates glucocorticoid production by the adrenal cortex, the hormone passes through the intra-adrenal portal vascular system of the medulla, providing it with uniquely high local concentrations of glucocorticoids [109]. These high concentrations are needed to induce the medullary enzyme phenylethanolamine-N-methyltransferase (PNMT) [110], which controls the synthesis of catecholamine, thus coordinately up-regulating both of the primary adrenal stress hormones for a maximally adapted “fight or flight” response. As a further note in proof of this mechanism, in fish the adrenal cortex and medulla are independent structures, further attesting to the active selection pressure for robust adrenaline production in response to physiologic stress.
PTHrP and kidney cell-molecular evolutionary homeostasis
Akin to its role as a stretch-regulated gene product that maintains alveolar homeostasis, PTHrP is also integral to renal physiology (Fig. 2). In the glomerulus, PTHrP is produced by the epithelially-derived podocytes that line them [111], maintaining the function of the mesangium [112], a stretch-sensitive fibroblastic structure that determines systemic fluid volume and electrolyte homeostasis by regulating glomerular filtration. Parenthetically, this molecular homology between the lung and kidney should not be surprising, since both structures produce amniotic fluid during embryonic development [113]. It should be borne in mind that the glomerulus also makes its appearance during the phylogenetic transition from fish to amphibians [114], and subsequently to reptiles, mammals, and birds.
Figure 2. The alveolus and glomerulus are stretch sensors.
In the lung (left panel), the alveolar epithelium (square) and fibroblast (oval) respond to the stretching of the alveolar wall by increasing surfactant production. In the kidney (right panel), the mesangium (oval) senses fluid pressure and regulates bloodflow in the glomeruli. In both cases, breakdown in cell–cell interactions causes these cells to become fibrotic (brown cell) due to upregulation of Wnt.
PTHrP and skin cell-molecular evolutionary homeostasis
PTHrP is essential for the development of skin through its paracrine interaction between melanocytes and keratinocytes [115], generating the stratum corneum as a dual water- and bacterial-barrier essential for preventing desiccation in terrestrial vertebrates [97]. It is noteworthy that the alveolar type II epithelial cells and the skin epithelium of the stratum corneum exhibit a functional homology at the cell/molecular level, packaging lipids together with host defense peptides, and secreting them in the form of lamellar bodies to generate lipid-based barriers against water loss (from the inside out), and host invasion (from the outside in) in both structures.
The evolutionary significance of the homology between lung and skin as barriers is further exemplified by the pathophysiology of asthma. Patients with asthma often have the skin disease atopic dermatitis. Both these phenotypes are common to humans and dogs, and have been mechanistically linked through a molecular defect in β-defensins, which mediate innate host defense in both skin and lung [116]. In dogs, β-defensins determine coat color, which serves a variety of adaptive advantages, ranging from protective coat coloration to associated reproductive strategies. The β-defensin CD103 has also been shown to cause atopic dermatitis in dogs, and possibly asthma, since it is also found in dog airway epithelial cells [117]. Therefore, hierarchically, host defense and reproduction take evolutionarily adaptive precedence over wheezing due to asthma.
The Goodpasture syndrome and barrier formation and function: exception that proves the rule?
Vertebrates transitioned from water to land approximately 300 million years ago, causing selection pressure for type IV collagen [118], which acts to physically maintain the integrity of the walls of the alveoli. Since the extracellular matrix forms during the process of cellular differentiation, it is highly likely that modification of the basement membrane occurred early in the evolutionary adaptation to land. Molecular evolutionary studies of Goodpasture syndrome [118] have shown that the 3α isoform of type IV collagen evolved during the phylogenetic transition from fish to amphibians due to selection pressure for specific amino acid substitutions that rendered the molecule more hydrophobic and negatively charged, providing a natural barrier against water loss. Goodpasture syndrome is an autoimmune disease caused by catastrophic failure of both the kidney and lung epithelial barriers, caused by pathogenic circulating autoantibodies targeted to a set of discontinuous epitope sequences within non-collagenous domain 1 (NC1) of the 3α chain of type IV collagen [3(IV)NC1], referred to as the “Goodpasture autoantigen”. Basement membrane extracted NC1 domain preparations from Caenorhabditis elegans, Drosophila melanogaster, and Danio rerio do not bind Goodpasture autoantibodies, while frog, chicken, mouse, and human 3(IV)NC1 domains bind autoantibodies. The α3 (IV) chain is not present in worms (C. elegans) or flies (D. melanogaster), and can first be detected in fish (D. rerio). Interestingly, native D. rerio 3(IV)NC1 does not bind Goodpasture autoantibodies. In contrast to the recombinant human 3(IV)NC1 domain, there is complete absence of autoantibody binding to recombinant D. rerio 3(IV)NC1. Three-dimensional molecular modeling of the human NC1 domain suggests that evolutionary alteration of electrostatic charge and polarity due to the emergence of critical serine, aspartic acid, and lysine amino acid residues, accompanied by the loss of asparagine and glutamine, contributed to the emergence of the two major Goodpasture epitopes on the human 3(IV)NC1 domain, as it evolved from fish over the ensuing 450 million years. The evolved 3(IV)NC1 domain forms a natural physicochemical barrier against the exudation of serum and proteins from the circulation into the alveoli and glomeruli, due to its hydrophobic and electrostatic properties, respectively, which likely provided the molecular selection pressure for the evolution of this protein, given the rising oncotic and physical pressures impinging on the evolving barriers of both the lung and kidney during the water-to-land transition. Taken together, the lung, kidney, and skin evolved critical physiologic barriers against desiccation in land-dwelling animals.
Internal and external selection, PTHrP, and the water-to-land transition
Another way to think about this co-option of cell-molecular mechanisms of evolution is as serial interactions between internal and external selection pressures. Such external environmental constraints to the transition from water-to-land as air breathing, gravitational orthostatic forces, and desiccation were all hypothetically adapted to via a common internal cell-molecular pathway for development and homeostasis– PTHrP and its cognate G protein-coupled receptor [35]. This model is also predictive, since PTHrP is a potent vasodilator [23], and an angiogenic factor (promotes capillary formation) [119], potentially explaining why glomeruli, as microvascular derivatives of the renal artery, may have evolved in the transition from fish to amphibians [114].
The significance of PTHrP in the vertebrate transition from water to land may be as follows: Such organisms must have been selected for being able to spontaneously over-express PTHrP signaling, initially for lung evolution from the swim bladder, specifically in physostomous fish like zebra fish, which possess a tracheal homolog, the pneumatic duct that connects the esophagus and swim bladder for gas filling and emptying. At the cell-molecular level, the smooth muscle that forms both the pneumatic duct and trachea are determined by FGF10 expression [120]. The PTHrP-mediated mechanisms in the kidney and skin must have followed suit, since they would have protected against desiccation during the terrestrial adaptation. Calcification of bone in response to increased gravitational force on land would have further facilitated adaptation to land life [121]; Wolff’s Law states that bone will adapt to the load under which it is placed [122]. PTHrP is a gravity-sensitive hormone [20] that is integral to bone development and homeostasis [123], determining bone calcium uptake and incorporation into cartilaginous structures, facilitating the adaptation of terrestrial organisms to environmental gravitational forces [51]. This scenario of an iterative process for the acquisition of traits that facilitated the water-to-land transition is consistent with data showing that vertebrates attempted the water-to-land transition several times [121]. It is reasonable to assume that the visceral organs had to also evolve in adaptation to land habitation. Based on parsimony, one can propose that these processes were all realized as a result of the PTHrP receptor gene duplication event that occurred during the water-to-land transition, beginning with the lung, by necessity [85], and that those organisms that could up-regulate their PTHrP/PTHrP receptor signaling through ligand-receptor-mediated paracrine mechanisms evolved as the forebears of contemporary land vertebrates. In contrast, individuals who were unable to accomplish this feat became extinct. This perspective is supported by our demonstration of the correlation between the cell/molecular genetic motifs common to ontogeny and phylogeny of the lung and major environmental epochs (Fig. 3). Note the apparently seamless alternations between internal and external selection mechanisms in association with major ecologic stresses; we postulate that there are no gaps between these genetic adaptations because the data are derived from contemporary land vertebrates; conversely, those members of the species who failed to adapt died off, and thus are not accounted for in this analysis.
In further support of this concept, it is noteworthy that many chronic lung diseases are typified by simplification- or ‘reverse evolution’. That mechanism is due to the loss of signaling between the epithelial and mesenchymal compartments of the alveoli, leading to increased diameter of the alveoli, seemingly reverting back to earlier ontogenetic-phylogenetic stages in lung evolution. At the cellular level, this is characterized by the atavistic expression of both the myofibroblast, and the Wingless/int (Wnt) pathway due to loss of signaling from neighboring epithelial cells. Moreover, experimentally providing the spatio-temporal signals that generated the mammalian alveolus- PTHrP, leptin, PPARγ- recapitulates the evolution of lung, providing solid evidence for this mechanism.
Tiktaalik
In 2006, Neil Shubin [92] announced the discovery of Tiktaalik, the fossilized remains of the organism that transitioned from water to land. But in the era of Quantum Mechanics and General Relativity, we have come to expect more than just a descriptive biologic “Big Bang.” The ability to live on land was highly physiologically demanding, but according to the Romer Hypothesis [124], it was necessitated by the increase in carbon dioxide in the primordial atmosphere, causing the Earth’s lakes, ponds and rivers to dry up, forcing our vertebrate ancestors to seek refuge on land, or face extinction. So from a physiologic perspective, how might a fish have evolved into a tetrapod? The biggest constraint was the ability to breathe air. It has long been thought, though controversial, that the swim bladder of fish evolved into the lungs of land vertebrates, since both are gas exchangers that are derived from the gut tube. The notion that evolution co-opted an organ for buoyancy into one that mediated oxygenation for metabolism is attractive, though there are certain anatomic constraints [125]. That controversy has been put to rest by a recent study showing that at the molecular level, the swim bladder expresses all the homologous genes of the developing lung [93], including PTHrP, which, as discussed above, is a gravity-sensing gene that is necessary for the formation of alveoli in mammals [46]. Thus, there is a functional genomic link between the water-to-land transition and PTHrP signaling, which underwent a gene duplication [95] sometime during the fish-amphibian transition, providing a mechanistic explanation for the evolution of the lung from the swim bladder.
As discussed above, equally important is the fact that the organs necessary for barrier function against desiccation are also PTHrP-dependent, both developmentally [126] and physiologically [127]. The skin [128], kidney [129], and gut [130] all express both PTHrP and its receptor in close proximity to one another, and the signaling between the mesoderm and epithelium of these organs is mediated by the PTHrP/PTHrP receptor [82], which determines the structural and functional development of these organs to form homeostatically-regulated physiologic barriers against water loss. Moreover, PTHrP is necessary for the calcification of cartilage [123]; so it would have facilitated the evolution of the boney tetrapod limbs of Tiktaalik to accommodate the increased gravitational force on its skeleton on land, exhibiting the plasticity similar to that of the lung, and skin. As an added note in support of this interrelationship, when the PTHrP gene is deleted in mice, they exhibit morphogenetic defects in lung, skin, and bone [131].
The angiogenic properties of PTHrP are another feature relevant to its utility in the water-to-land transition and organ adaptation. PTHrP promotes vascularization of bone [119] and skin [119], particularly when the vascular endothelium is cyclically distended, as in conditions of increased physiologic stress such as those involved in the water-to-land transition. PTHrP receptors exist in the lymphatic microcirculation as well [132], further aiding in the regulation of surfactant physiology since lymphatic drainage regulates the function of the surfactant complex in the alveolar hypophase. Additionally, PTHrP is a vasodilator, ultimately epistatically relieving tension on the remodeled microvasculature, while simultaneously providing increased perfusion for remodeling of the adjacent parenchyma in further adaptation to internal physiologic stress. Such a mechanism would be consistent with the progressive expansion of the surface area of the lung, and may help explain the evolution of the glomerulus, which is either small or absent from the kidneys of boney fish [114], but is omnipresent in amphibians, reptiles, mammals, and birds. Perhaps it was not merely fortuitous that there was a PTHrP receptor gene duplication “just in time” for Tiktaalik’s water-to-land migration. More likely, there were cumulative, episodic increases in shear stress on the microvessels of the organs that facilitated that transition– swim bladder, lung, adrenals, skin, kidney, skeleton (hence the multiple efforts to breach land based on skeletal fossil remains) - causing the generation of radical oxygen species (ROS) and lipid peroxides that affected those vascular beds. ROS cause DNA damage [133], giving rise to mutations such as cross-overs and gene duplications. ROS are also embryonic signal transducers [134] that may act to promote structural and functional remodeling through morphogenetic events. The endothelium is known to be highly heterogeneous, each endothelial cell acting like an adaptive, non-linear input/output device [135]. The input comes from the extracellular environment, consisting of biomechanical and biochemical forces. The output is the heterogeneity of the endothelial cell population, reflected by cell shape, calcium flux, protein expression, mRNA expression, migration, proliferation, apoptosis, vasomotor tone, hemostatic balance, release of inflammatory mediators, and leukocyte adhesion/transmigration [135].
Such dual mechanisms of development and mutation for evolutionary change would have provided robust pressures for both internal and external selection, depending on the nature and magnitude of the agent, suggesting a mechanistic way of thinking about “evolvability.” More importantly, organisms such as Tiktaalik exhibited the plasticity necessary for the remodeling of vital organs for adaptation to terrestrial life. Tiktaalik’s ancestors were thus preadapted for the “great leap forward” during vertebrate evolution, but because metazoans evolved from unicellular organisms [1, 2], Tiktaalik was preadapted for such a critical “sea change” in vertebrate evolution.
Cellular growth factors as the universal language of biology
This approach to a fundamental, a priori understanding of vertebrate physiology, not as a top-down descriptive process, but as a series of co-optations originating from the cell membrane of unicellular organisms, will lead to understanding of the FPPs based on their evolutionary origins [1]. The actualization of such FPPs would have numerous ramifications, including a predictive model for physiology and medicine [13], as well as a functional merging biology, chemistry, and physics into a common algorithm for the natural sciences. Such a perspective would allow us to deemphasize the human signature from our anthropocentric view of our physical environment, on the scale of the Copernican re-centering of the solar system on the Sun, which obscures our perception of our universe, and that of other universes.
What predictions follow from a cellular approach to evolution
Starting with the premise that ontogeny is the only biological process we know of that formally generates new structures and functions, we should exploit this process to understand evolution, since it does so throughout the phylogenetic history of the organism. By focusing on cell-cell interactions, particularly those mediated by soluble growth factors and their cognate receptors, one can deconvolute the evolution of the lung, functionally tracking it back to the swim bladder of physostomous fish [93]; unlike physoclistous fish, this category of boney fish has a tracheal homolog that connects the esophagus to the swim bladder. And Zebra Fish members have been shown to express all of the genes necessary for lung development.
Since the adaptation of fish to land was contingent on efficient atmospheric gas exchange, the lung can be seen as the cellular-molecular blueprint for the evolution of other physiologic adaptations to land life [13]. By systematically tracing the functional molecular homologies between the lung, adrenals, skin, kidney, gut, bone, and brain across developmental, phylogenetic, and pathophysiologic space and time, the FPPs can be determined [1, 2, 13]. Once such relationships are traced back to unicellular organisms, the underlying physiologic principles can be used to replay the evolutionary tape [8], and predict and prevent homeostatic failure as disease [1, 2]. Perhaps as E.O. Wilson has suggested [136], the reduction of biology to ones and zeros can offer the opportunity to merge biology, chemistry, and physics into one common user-friendly algorithm as a “periodic table of nature” [137].
The zygote as the level of selection for vertebrate evolution
Another prediction of this approach to evolution is the primacy of the unicellular state as the ideal mechanism of evolution. It provides the functional bauplan for the biota as the reference-point for sustaining life on Earth. In support of that notion, it is clearly possible that there were organisms that wandered away from this process, but I would submit that they are extinct. Witness bacteria, which continue to this day in the unicellular state; apparently pseudomulticellular states like quorum sensing and biofilm sufficed as modes of coping with eukaryotes.
Like the illumination of our physical place in the Universe by the shift to a Heliocentric perspective, ushering in the Enlightenment, our recognition that it is the unicellular state that is being selected for, and that all of the other aspects of the life cycle are in service to it, will enlighten us as to our biologic place in the Universe. That ‘frame-shift’ would inform us as to the relationship between Man and other biota as one seamless continuum, and permit us to make rational decisions about bioethical questions based on first principles of physiology rather than ‘guessing’ the right course of action. In short, the cellular perspective would herald a new Age of Enlightenment, and non too soon, given the exorbitant cost of healthcare, the pollution of our environment and Climate Change.
The multicellular state, that which Gould and Lewontin called ‘Spandrels’, is merely a biologic ‘probe’ for monitoring the environment between unicellular stages in order to register and facilitate adaptive changes. This consideration can be based on both a priori and empiric data. Regarding the former, emerging evidence for epigenetic inheritance demonstrates that the environment can cause heritable changes in the genome, but they only take effect phenotypically in successive generations. This would suggest that it is actually the germ cells of the offspring that are being selected for. The starvation model of metabolic syndrome may illustrate this experimentally. Maternal diet can cause obesity, hypertension and diabetes in the offspring. But they also mature sexually at an earlier stage due to the excess amount of body fat. Though seemingly incongruous, this may represent the primary strategy to accelerate the genetic transfer of information to the next generation (positive selection), effectively overarching the expected paucity of food. The concomitant obesity, hypertension and diabetes are unfortunate side effects of this otherwise adaptive process in the adults. Under these circumstances, it can be surmised that it is the germ cells that are being selected for; in other words, the adults are disposable, as Richard Dawkins has opined.
Hologenomic evolution theory provides yet another mechanism for selection emerging from the unicellular state. According to that theory, all complex organisms actually represent a vast collaborative of linked, co-dependent, cooperative and competitive localized environments and ecologies functioning as a unitary organism towards the external environment. These co-linked ecologies are comprised of both the innate cells of that organism, and all of the microbial life that is cohabitant with it. The singular function of these ecologies is to maintain the homeostatic preferences of their constituent cells. In this theory, evolutionary development is the further expression of cooperation, competition and connections between the cellular constituents in each of those linked ecologies in successive iterations as they successfully sustain themselves against a hostile external genetic environment. Ontogeny would then recapitulate phylogeny since the integrity of the linked environments that constitute a fully developed organism can only be maintained by reiterating those environmental ecologies in succession towards their full expression in the organism as a whole.
Another way to think about the notion of the unicellular state as the one being selected for is to focus on calcium signaling as the initiating event for all of biology. There is experimental evidence that increases in carbon dioxide during the Phanerozoic Era caused acidification of the oceans, causing leaching of calcium from the ocean floor. The rise in calcium levels can be causally linked to the evolution of the biota, and is intimately involved with nearly all biologic processes. For example, fertilization of the ovum by sperm induces a wave of calcium, which triggers embryogenesis. The same sorts of processes continue throughout the life cycle, until the organism dies. There seems to be a disproportionate investment in the zygote from a purely biologic perspective. However, given the prevalence of calcium signaling at every stage, on the one hand, and the participation of the gonadocytes in epigenetic inheritance on the other, the reality of the vectorial trajectory of the life cycle becomes apparent - it cannot be static, it must move either toward or away from change.
By using the cellular-molecular ontogenetic and phylogenetic approach described above for the water-land transition as a major impetus for evolution, a similar approach can be used moving both forward and backward from that critically important phase of vertebrate evolution In so doing, the gaps between unicellular and multicellular genotypes and phenotypes can realistically be filled in systematically. But it should be borne in mind that until experimentation is done, these linkages remain hypothetical. Importantly though, there are now model organisms and molecular tools to test these hypotheses, finally looking at evolution in the direction in which it occurred, from the earliest iteration forward. This approach will yield a priori knowledge about the First Principles of Physiology, and how they have evolved to generate form and function from their unicellular origins.
We are not just in this environment, we are of it
The realization that there are First Principles in Physiology, as predicted by the cellular-molecular approach to evolution is important because of its impact on how we think of ourselves as individual humans, as a species, and our relationship to other species. Once it is recognized and understood that we, as our own unique species, have evolved from unicellular organisms, and that this is the case for all of the other organisms on Earth, including plant life, the intense and intimate interrelationships between all of us must be embraced. This kind of thinking has previously been considered in the form of genes that are common to plants and animals alike, but not as part of a larger and even more elemental process of evolution from the physical firmament. This perspective is on par with the reorientation of Man to his surroundings once he acknowledged that the Sun, not the Earth was the center of the Solar System. That shift in thought gave rise to the Age of Enlightenment! Perhaps in the present age, such a frame-shift will provide insight to Dark Matter, String Theory and Multiverses.
In retrospect, it should have come as no surprise that we have misapprehended our own physiology. Many discoveries in biomedicine are serendipitous, medicine is post-dictive, and the Human Genome Project has not yet yielded any of its predicted breakthroughs. However, moving forward, knowing what we now do, we should countenance our own existence as part of the wider environment, that we are not merely in this world, but literally of this world, with an intimacy that we had never previously imagined.
This unicellular-centric vantage point is heretical, but like the shift from Geocentrism to Heliocentrism, our species would be vastly improved by recognizing this persistent, systematic error in self-perception. Man is not the pinnacle of biologic existence, and should be a better steward of the land and the planet, sharing resources with all his/her biological relatives. Perhaps through a fundamental, scientifically testable and demonstrable understanding of what we are and how we came to be so, more of us will behave more consistently with Nature’s needs instead of subordinating them to our own narcissistic whims. As we become deeply aware of our true place in the biologic realm, such as we are already witnessing through increasing recognition of an immense microbial array as fellow travelers with us as our microbiome, we may find a more ecumenical approach to life than we have been practicing for the last 5,000 years.
Bioethics based on evolutionary ontology and epistemology, not descriptive phenotypes and genes
By definition, a fundamental change in the way we perceive ourselves as a species would demand a commensurate change in our ethical behavior. Such thoughts are reminiscent of a comment in a recent biography of the British philosopher Derek Parfit in The New Yorker magazine, entitled “How to be Good”, in which he puzzles over the inherent paradox between empathy and Darwinian Survival of the Fittest. These two concepts would seem to be irreconcilable, yet that is only because the latter is based on a false premise. Darwin’s great success was in making the subject of evolution user-friendly by providing a narrative that was simple and direct. Pleasing as it may be, it is at best, entirely incomplete. Think of it like the transition from Newtonian Mechanics to Relativity Theory. As much is learned about the unicellular world with its surprising mechanisms and capacities, new pathways must be imagined. It is clear that we as humans are hologenomes, and all the other complex creatures are too. In fact, there are no exceptions. The reasons for this can only be understood properly through a journey from the ‘Big Bang’ of the cell forward, with all its faculties and strictures. By concentrating on cellular dynamics, an entirely coherent path is empowered. Tennyson’s line about ‘Nature, red in tooth and claw’ is only the tip of what the iceberg of evolution really constitutes. As pointed out above, we evolved from unicellular organisms through cooperation, co-dependence, collaboration and competition. These are all archetypical cellular capacities. Would we not then ourselves, as an example of cellular reiteration, have just those self-same and self-similar behaviors?
CONCLUSION
In summary, by looking at the process of evolution from its unicellular origins, the causal relationships between genotype and phenotype are revealed, as are many other aspects of biology and medicine that have remained anecdotal and counter-intuitive. That is because the prevailing descriptive, top-down portrayal of physiology under Darwinism is tautologic. In opposition to that, the cellular-molecular, bottom-up approach is conducive to prediction, which is the most powerful test of any scientific concept. Though there is not a great deal of experimental evidence for the intermediate steps between unicellular and multicellular organisms compared to what is known of ontogeny and phylogeny of metazoans, it is hoped that the perspectives expressed in this article will encourage more such fundamental physiologic experimentation in the future.
Footnotes
CONFLICT OF INTEREST STATEMENT
I have nothing to declare.
REFERENCES
- 1.Torday JS. Perspect. Biol. Med. 2013;56:455. doi: 10.1353/pbm.2013.0038. [DOI] [PubMed] [Google Scholar]
- 2.Torday JS, Rehan VK. Evolutionary Biology, Cell-Cell Communication and Complex Disease. Wiley; New Jersey: 2012. [Google Scholar]
- 3.Needleman D, Brugues J. Dev. Cell. 2014;29:135. doi: 10.1016/j.devcel.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Rehan VK, Liu J, Naeem E, Tian J, Sakurai R, Kwong K, Akbari O, Torday JS. BMC Med. 2012;10:129. doi: 10.1186/1741-7015-10-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cropley JE, Suter CM, Beckman KB, Martin DI. Proc. Natl. Acad. Sci. USA. 2006;103:17308. doi: 10.1073/pnas.0607090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olsson L, Levit GS, Hossfeld U. Naturwissenschaften. 2010;97:951. doi: 10.1007/s00114-010-0720-9. [DOI] [PubMed] [Google Scholar]
- 7.Smocovitis VB. Unifying Biology. Princeton University Press; Princeton, NJ: 1996. [Google Scholar]
- 8.Gould SJ. The Structure of Evolutionary Theory. The Belknap Press; Cambridge, MA: 2002. [Google Scholar]
- 9.Grobstein C. Natl. Cancer Inst. Monogr. 1967;26:279. [PubMed] [Google Scholar]
- 10.Stephenson RO, Rossant J, Tam PP. Cold Spring Harb. Perspect. Biol. 2012;1:4. doi: 10.1101/cshperspect.a008235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torday JS, Rehan VK. Am. J. Respir. Cell. Mol. Biol. 2004;31:8. doi: 10.1165/rcmb.2004-0019TR. [DOI] [PubMed] [Google Scholar]
- 12.Sorokin S. Dev. Biol. 1961;3:60. [Google Scholar]
- 13.Torday JS, Rehan VK. Physiol. Genomics. 2009;38:1. doi: 10.1152/physiolgenomics.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torday JS, Rehan VK. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;283:L130. doi: 10.1152/ajplung.00380.2001. [DOI] [PubMed] [Google Scholar]
- 15.Daniels CB, Orgeig S. News Physiol. Sci. 2003;18:151. doi: 10.1152/nips.01438.2003. [DOI] [PubMed] [Google Scholar]
- 16.Maina JN, West JB. Thin and strong! Physiol. Rev. 2005;85:811. doi: 10.1152/physrev.00022.2004. [DOI] [PubMed] [Google Scholar]
- 17.Liem KF. Am. Zool. 1988;28:739. [Google Scholar]
- 18.Maina JN. Anat. Rec. 2000;261:25. doi: 10.1002/(SICI)1097-0185(20000215)261:1<25::AID-AR6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 19.Maina JN. Adv. Anat. Embryol. Cell. Biol. 2002;163:1. doi: 10.1007/978-3-642-55917-4. [DOI] [PubMed] [Google Scholar]
- 20.Torday JS. Adv. Space Res. 2003;32:1569. doi: 10.1016/S0273-1177(03)90397-8. [DOI] [PubMed] [Google Scholar]
- 21.Torday JS, Rehan VK. Pediatr. Res. 2007;62:2. doi: 10.1203/PDR.0b013e31806772a1. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Esteban J, Tsai SW, Sang J, Qin J, Torday JS, Rubin LP. Am. J. Med. Sci. 1998;316:200. [PubMed] [Google Scholar]
- 23.Gao Y, Raj JU. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;289:L60. doi: 10.1152/ajplung.00411.2004. [DOI] [PubMed] [Google Scholar]
- 24.Holgate ST. Allergy Asthma Immunol. Res. 2013;5:343. doi: 10.4168/aair.2013.5.6.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies DE, Wicks J, Powell RM, Puddicombe SM, Holgate ST. J. Allergy Clin. Immunol. 2003;111:215. doi: 10.1067/mai.2003.128. [DOI] [PubMed] [Google Scholar]
- 26.Torday J. Semin. Perinatol. 1992;16:130. [PubMed] [Google Scholar]
- 27.Rehan VK, Fong J, Lee R, Sakurai R, Wang ZM, Dahl MJ, Lane RH, Albertine KH, Torday JS. Pediatr. Res. 2011;70:462. doi: 10.1203/PDR.0b013e31822f58a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berner RA, Vandenbrooks JM, Ward PD. Science. 2007;316:557. doi: 10.1126/science.1140273. [DOI] [PubMed] [Google Scholar]
- 29.Weibel ER, Taylor CR, Bolis L. Principles of animal design. Cambridge University Press; United Kingdom: 1998. [Google Scholar]
- 30.Duncker HR. Respir. Physiol. Neurobiol. 2004;144:111. doi: 10.1016/j.resp.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 31.Daniels CB, Orgeig S. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001;129:9. doi: 10.1016/s1095-6433(01)00303-8. [DOI] [PubMed] [Google Scholar]
- 32.Maina JN. J. Anat. 2002;201:281. doi: 10.1046/j.1469-7580.2002.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weibel ER, Taylor CR, Hoppeler H. Respir. Physiol. 1992;87:325. doi: 10.1016/0034-5687(92)90015-o. [DOI] [PubMed] [Google Scholar]
- 34.Torday JS. Am. J. Physiol. Cell. Physiol. 2013;305:C682. doi: 10.1152/ajpcell.00197.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubin LP, Kifor O, Hua J, Brown EM, Torday JS. Biochim. Biophys. Acta. 1994;1223:91. doi: 10.1016/0167-4889(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 36.Schultz CJ, Torres E, Londos C, Torday JS. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;283:L288. doi: 10.1152/ajplung.00204.2001. [DOI] [PubMed] [Google Scholar]
- 37.Torday JS, Sun H, Wang L, Torres E, Sunday ME, Rubin LP. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;282:L405. doi: 10.1152/ajplung.2002.282.3.L405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu K, Guo F, Zhang S, Liu C, Wang F, Zhou Z, Chen A. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2009;29:39. doi: 10.1007/s11596-009-0108-2. [DOI] [PubMed] [Google Scholar]
- 39.Chen HL, Demiralp B, Schneider A, Koh AJ, Silve C, Wang CY, McCauley LK. J. Biol. Chem. 2002;277:19374. doi: 10.1074/jbc.M108913200. [DOI] [PubMed] [Google Scholar]
- 40.Rehan VK, Torday JS. Drugs Today (Barc.) 2007;43:317. doi: 10.1358/dot.2007.43.5.1062665. [DOI] [PubMed] [Google Scholar]
- 41.Silva LF, Etchebarne BE, Nielsen MS, Liesman JS, Kiupel M, VandeHaar MJ. J. Dairy Sci. 2008;91:3034. doi: 10.3168/jds.2007-0761. [DOI] [PubMed] [Google Scholar]
- 42.Torday JS, Powell FL, Farmer CG, Orgeig S, Nielsen HC, and Hall, A. J. Respir. Physiol. Neurobiol. 2010;173:S37. doi: 10.1016/j.resp.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.West JB, Mathieu-Costello O. Annu. Rev. Physiol. 1999;61:543. doi: 10.1146/annurev.physiol.61.1.543. [DOI] [PubMed] [Google Scholar]
- 44.MacDonald BA, Sund M, Grant MA, Pfaff KL, Holthaus K, Zon LI, Kalluri R. Blood. 2006;107:1908. doi: 10.1182/blood-2005-05-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warburton D, Bellusci S, Del Moral PM, Kaartinen V, Lee M, Tefft D, Shi W. Respir. Res. 2003;4:5. doi: 10.1186/1465-9921-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rubin LP, Kovacs CS, De Paepe ME, Tsai SW, Torday JS, Kronenberg HM. Dev. Dyn. 2004;230:278. doi: 10.1002/dvdy.20058. [DOI] [PubMed] [Google Scholar]
- 47.Torday JS, Rehan VK, Hicks JW, Wang T, Maina J, Weibel ER, Hsia CC, Sommer RJ, Perry SF. Integr. Comp. Biol. 2007;47:601. doi: 10.1093/icb/icm069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.West JB, Watson RR, Fu Z. Eur. Respir. J. 2007;29:11. doi: 10.1183/09031936.00133306. [DOI] [PubMed] [Google Scholar]
- 49.Jüppner H. Curr. Opin. Nephrol. Hypertens. 1994;3:371. [PubMed] [Google Scholar]
- 50.Noda M, Takuwa Y, Katoh T, Kurokawa K. Curr. Opin. Nephrol. Hypertens. 1995;4:383. doi: 10.1097/00041552-199509000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Torday JS, Rehan VK. Cell Biochem. Biophys. 2003;37:235. doi: 10.1385/cbb:37:3:235. [DOI] [PubMed] [Google Scholar]
- 52.Prosser CG, Davis SR, Farr VC, Lacasse P. J. Dairy Sci. 1996;79:1184. doi: 10.3168/jds.S0022-0302(96)76472-X. [DOI] [PubMed] [Google Scholar]
- 53.Torday JS, Sanchez-Esteban J, Rubin LP. Am. J. Med. Sci. 1998;316:205. doi: 10.1097/00000441-199809000-00010. [DOI] [PubMed] [Google Scholar]
- 54.Orgeig S, Bernhard W, Biswas SC, Daniels CB, Hall SB, Hetz SK, Lang CJ, Maina JN, Panda AK, Perez-Gil J, Possmayer F, Veldhuizen RA, Yan W. Integr. Comp. Biol. 2007;47:610. doi: 10.1093/icb/icm079. [DOI] [PubMed] [Google Scholar]
- 55.Foot NJ, Orgeig S, Daniels CB. Respir. Physiol. Neurobiol. 2006;154:118. doi: 10.1016/j.resp.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 56.Rehan VK, Torday JS. Antioxid. Redox. Signal. 2014;21:1893. doi: 10.1089/ars.2013.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Torday JS, Rehan VK. Pediatr. Res. 2006;60:382. doi: 10.1203/01.pdr.0000238326.42590.03. [DOI] [PubMed] [Google Scholar]
- 58.Vaccaro C, Brody JS. Anat. Rec. 1978;192:467. doi: 10.1002/ar.1091920402. [DOI] [PubMed] [Google Scholar]
- 59.Brody JS, Vaccaro C. Fed. Proc. 1979;38:215. [PubMed] [Google Scholar]
- 60.Rehan VK, Sugano S, Wang Y, Santos J, Romero S, Dasgupta C, Keane MP, Stahlman MT, Torday JS. Exp. Lung Res. 2006;32:379. doi: 10.1080/01902140600880257. [DOI] [PubMed] [Google Scholar]
- 61.Torday J, Hua J, Slavin R. Biochim. Biophys. Acta. 1995;1254:198. doi: 10.1016/0005-2760(94)00184-z. [DOI] [PubMed] [Google Scholar]
- 62.Torday J, Rehan V. Exp. Lung Res. 2011;37:376. doi: 10.3109/01902148.2011.580903. [DOI] [PubMed] [Google Scholar]
- 63.Gewolb IH, Torday JS. Lab. Invest. 1995;73:59. [PubMed] [Google Scholar]
- 64.Torday JS, Torday DP, Gutnick J, Qin J, Rehan V. Pediatr. Res. 2001;49:843. doi: 10.1203/00006450-200106000-00021. [DOI] [PubMed] [Google Scholar]
- 65.Torday JS. Front. Pediatr. 2014;2:34. doi: 10.3389/fped.2014.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Torday JS, Rehan VK. Pediatr. Res. 2007;62:2. doi: 10.1203/PDR.0b013e31806772a1. [DOI] [PubMed] [Google Scholar]
- 67.Torday JS, Rehan VK. Med. Hypotheses. 2009;72:596. doi: 10.1016/j.mehy.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Torday JS, Torres E, Rehan VK. Pediatr. Pathol. Mol. Med. 2003;22:189. doi: 10.1080/pdp.22.3.189.207. [DOI] [PubMed] [Google Scholar]
- 69.Theodosiou NA, Tabin CJ. Dev. Biol. 2003;259:258. doi: 10.1016/s0012-1606(03)00185-4. [DOI] [PubMed] [Google Scholar]
- 70.Bruce MC, Honaker CE, Cross RJ. Am. J. Respir. Cell. Mol. Biol. 1999;20:228. doi: 10.1165/ajrcmb.20.2.3150. [DOI] [PubMed] [Google Scholar]
- 71.Awonusonu F, Srinivasan S, Strange J, Al-Jumaily W, Bruce MC. Am. J. Physiol. 1999;277:L848. doi: 10.1152/ajplung.1999.277.4.L848. [DOI] [PubMed] [Google Scholar]
- 72.Burri PH. Biol. Neonate. 2006;89:313. doi: 10.1159/000092868. [DOI] [PubMed] [Google Scholar]
- 73.Brody JS, Kaplan NB. Am. Rev. Respir. Dis. 1983;127:763. doi: 10.1164/arrd.1983.127.6.763. [DOI] [PubMed] [Google Scholar]
- 74.Maksvytis HJ, Niles RM, Simanovsky L, Minassian IA, Richardson LL, Hamosh M, Hamosh P, Brody JS. J. Cell. Physiol. 1984;118:113. doi: 10.1002/jcp.1041180203. [DOI] [PubMed] [Google Scholar]
- 75.Kaplan NB, Grant MM, Brody JS. Am. Rev. Respir. Dis. 1985;132:1307. doi: 10.1164/arrd.1985.132.6.1307. [DOI] [PubMed] [Google Scholar]
- 76.Phipps RP, Penney DP, Keng P, Quill H, Paxhia A, Derdak S, Felch ME. Am. J. Respir. Cell. Mol. Biol. 1989;1:65. doi: 10.1165/ajrcmb/1.1.65. [DOI] [PubMed] [Google Scholar]
- 77.Penney DP, Keng PC, Derdak S, Phipps RP. Anat. Rec. 1992;232:432. doi: 10.1002/ar.1092320312. [DOI] [PubMed] [Google Scholar]
- 78.Cerny L, Torday JS, Rehan VK. Lung. 2008;186:75. doi: 10.1007/s00408-007-9069-z. [DOI] [PubMed] [Google Scholar]
- 79.Rehan VK, Asotra K, Torday JS. Lung. 2009;187:281. doi: 10.1007/s00408-009-9158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gil J, McNiff JM. Am. J. Pathol. 1983;113:331. [PMC free article] [PubMed] [Google Scholar]
- 81.Königshoff M, Wilhelm A, Jahn A, Sedding D, Amarie OV, Eul B, Seeger W, Fink L, Günther A, Eickelberg O, Rose F. Am. J. Respir. Cell. Mol. Biol. 2007;37:640. doi: 10.1165/rcmb.2006-0379TR. [DOI] [PubMed] [Google Scholar]
- 82.Vilardaga JP, Romero G, Friedman PA, Gardella TJ. Cell. Mol. Life Sci. 2011;68:1. doi: 10.1007/s00018-010-0465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rehan VK. Indian J. Pediatr. 2006;73:1027. doi: 10.1007/BF02758312. [DOI] [PubMed] [Google Scholar]
- 84.Rehan VK, Torday JS. PPAR Res. 2012;2012:289867. doi: 10.1155/2012/289867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Torday JS, Rehan VK. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;292:L608. doi: 10.1152/ajplung.00379.2006. [DOI] [PubMed] [Google Scholar]
- 86.Chilosi M, Poletti V, Zamò A, Lestani M, Montagna L, Piccoli P, Pedron S, Bertaso M, Scarpa A, Murer B, Cancellieri A, Maestro R, Semenzato G, Doglioni C. Am. J. Pathol. 2003;162:1495. doi: 10.1016/s0002-9440(10)64282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.deLemos RA, Coalson JJ. Clin. Perinatol. 1992;19:521. [PubMed] [Google Scholar]
- 88.Hall JE. Guyton and Hall Textbook of Physiology. Saunders; Philadelphia PA: 2011. [Google Scholar]
- 89.Calkins K, Devaskar SU. Curr. Probl. Pediatr. Adolesc. Health Care. 2011;41:158. doi: 10.1016/j.cppeds.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rose CM, van den Driesche S, Meehan RR, Drake AJ. Biochem. Soc. Trans. 2013;41:809. doi: 10.1042/BST20120356. [DOI] [PubMed] [Google Scholar]
- 91.Laguna-Barraza R, Bermejo-Álvarez P, Ramos-Ibeas P, de Frutos C, López-Cardona AP, Calle A, Fernandez-Gonzalez R, Pericuesta E, Ramírez MA, Gutierrez-Adan A. Reprod. Fertil. Dev. 2012;25:38. doi: 10.1071/RD12262. [DOI] [PubMed] [Google Scholar]
- 92.Daeschler EB, Shubin NH, Jenkins FA., Jr Nature. 2006;440:757. doi: 10.1038/nature04639. [DOI] [PubMed] [Google Scholar]
- 93.Zheng W, Wang Z, Collins JE, Andrews RM, Stemple D, Gong Z. PLoS One. 2011;6:e24019. doi: 10.1371/journal.pone.0024019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pinheiro PL, Cardoso JC, Power DM, Canário AV. BMC Evol. Biol. 2012;12:110. doi: 10.1186/1471-2148-12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Torday JS, Sun H, Qin J. Am. J. Physiol. 1998;274:L106. doi: 10.1152/ajplung.1998.274.1.L106. [DOI] [PubMed] [Google Scholar]
- 96.Heinrich S, Hartl D, Griese M. Curr. Med. Chem. 2006;13:3239. doi: 10.2174/092986706778773112. [DOI] [PubMed] [Google Scholar]
- 97.Liu AY, Destoumieux D, Wong AV, Park CH, Valore EV, Liu L, Ganz T. J. Invest. Dermatol. 2002;118:275. doi: 10.1046/j.0022-202x.2001.01651.x. [DOI] [PubMed] [Google Scholar]
- 98.Bickel PE, Tansey JT, Welte MA. Biochim. Biophys. Acta. 2009;1791:419. doi: 10.1016/j.bbalip.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brasaemle DL, Barber T, Wolins NE, Serrero G, Blanchette-Mackie EJ, Londos C. J. Lipid Res. 1997;38:2249. [PubMed] [Google Scholar]
- 100.Gao J, Serrero G. J. Biol. Chem. 1999;274:16825. doi: 10.1074/jbc.274.24.16825. [DOI] [PubMed] [Google Scholar]
- 101.Sakurai R, Li Y, Torday JS, Rehan VK. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011;301:L721. doi: 10.1152/ajplung.00076.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Csete M, Walikonis J, Slawny N, Wei Y, Korsnes S, Doyle JC, Wold B. J. Cell. Physiol. 2001;189:189. doi: 10.1002/jcp.10016. [DOI] [PubMed] [Google Scholar]
- 103.Berner RA, VandenBrooks JM, Ward PD. Science. 2007;316:557. doi: 10.1126/science.1140273. [DOI] [PubMed] [Google Scholar]
- 104.Torday JS, Rehan VK. Mol. Biol. Evol. 2011;28:2973. doi: 10.1093/molbev/msr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Besnard V, Wert SE, Stahlman MT, Postle AD, Xu Y, Ikegami M, Whitsett JA. J. Biol. Chem. 2009;284:4018. doi: 10.1074/jbc.M805388200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Orgeig S, Daniels CB, Johnston SD, Sullivan LC. Reprod. Fertil. Dev. 2003;15:55. doi: 10.1071/rd02087. [DOI] [PubMed] [Google Scholar]
- 107.Schumacker PT, Samsel RW. Crit. Care Clin. 1989;5:255. [PubMed] [Google Scholar]
- 108.Garbrecht MR, Klein JM, Schmidt TJ, Snyder JM. Biol. Neonate. 2006;89:109. doi: 10.1159/000088653. [DOI] [PubMed] [Google Scholar]
- 109.Provost PR, Boucher E, Tremblay Y. J. Steroid Biochem. Mol. Biol. 2013;138:72. doi: 10.1016/j.jsbmb.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 110.Kvetnansky R, Lu X, Ziegler MG. Adv. Pharmacol. 2013;68:359. doi: 10.1016/B978-0-12-411512-5.00017-8. [DOI] [PubMed] [Google Scholar]
- 111.Endlich N, Nobiling R, Kriz W, Endlich K. Exp. Nephrol. 2001;9:436. doi: 10.1159/000052643. [DOI] [PubMed] [Google Scholar]
- 112.Bosch RJ, Rodríguez-Puyol D, Bover J, Rodríguez-Puyol M. Exp. Nephrol. 1999;7:212. doi: 10.1159/000020604. [DOI] [PubMed] [Google Scholar]
- 113.Magann EF, Sandlin AT, Ounpraseuth ST. J. Ultrasound Med. 2011;30:1573. doi: 10.7863/jum.2011.30.11.1573. [DOI] [PubMed] [Google Scholar]
- 114.Smith HW. From Fish to Philosopher. Ciba, Summit; NJ: 1959. [Google Scholar]
- 115.Errazahi A, Lieberherr M, Bouizar Z, Rizk-Rabin M. J. Steroid Biochem. Mol. Biol. 2004;89-90:381. doi: 10.1016/j.jsbmb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 116.Doss M, White MR, Tecle T, Hartshorn KL. J. Leukoc. Biol. 2010;87:79. doi: 10.1189/jlb.0609382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Erles K, Brownlie J. Vet. Immunol. Immunopathol. 2010;135:12. doi: 10.1016/j.vetimm.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.MacDonald BA, Sund M, Grant A, Pfaff KL, Holthaus K, Zon LI, Kalluri R. Blood. 2006;107:1908. doi: 10.1182/blood-2005-05-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Isowa S, Shimo T, Ibaragi S, Kurio N, Okui T, Matsubara K, Hassan NM, Kishimoto K, Sasaki A. Anticancer Res. 2010;30:2755. [PubMed] [Google Scholar]
- 120.Korzh S, Winata CL, Zheng W, Yang S, Yin A, Ingham P, Korzh V, Gong Z. Dev. Biol. 2011;359:262. doi: 10.1016/j.ydbio.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 121.Clack JA. Gaining Ground. Indiana University Press; Bloomington, IN: 2002. [Google Scholar]
- 122.Chen JH, Liu C, You L, Simmons CA. J. Biomech. 2010;43:108. doi: 10.1016/j.jbiomech.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 123.Sharan K, Siddiqui JA, Swarnkar G, Chattopadhyay N. Indian J. Med. Res. 2008;127:274. [PubMed] [Google Scholar]
- 124.Romer AS. Science. 1967;158:1629. doi: 10.1126/science.158.3809.1629. [DOI] [PubMed] [Google Scholar]
- 125.Perry SF, Sander M. Respir. Physiol. Neurobiol. 2004;144:125. doi: 10.1016/j.resp.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 126.Slominski A, Wortsman J. Minerva Endocrinol. 2003;28:135. [PubMed] [Google Scholar]
- 127.Karperien M, van Dijk TB, Hoeijmakers T, Cremers F, Abou-Samra AB, Boonstra J, de Laat SW, Defize LH. Mech. Dev. 1994;47:29. doi: 10.1016/0925-4773(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 128.Diamond AG, Gonterman RM, Anderson AL, Menon K, Offutt CD, Weaver CH, Philbrick WM, Foley J. J. Invest. Dermatol. 2006;126:2127. doi: 10.1038/sj.jid.5700338. [DOI] [PubMed] [Google Scholar]
- 129.Atchison DK, Harding P, Cecilia Ortiz-Capisano M, Peterson EL, Beierwaltes WH. Am. J. Physiol. Renal Physiol. 2012;303:F1157. doi: 10.1152/ajprenal.00269.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gentili C, Morelli S, de Boland AR. J. Cell Biochem. 2003;88:1157. doi: 10.1002/jcb.10472. [DOI] [PubMed] [Google Scholar]
- 131.Karaplis AC, Luz A, Glowacki J, Bronson RT, Tybulewicz VL, Kronenberg HM, Mulligan RC. Genes Dev. 1994;8:277. doi: 10.1101/gad.8.3.277. [DOI] [PubMed] [Google Scholar]
- 132.Mizuno R, Ono N, Ohhashi T. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H60. doi: 10.1152/ajpheart.2001.281.1.H60. [DOI] [PubMed] [Google Scholar]
- 133.Lü JM, Lin PH, Yao Q, Chen C. J. Cell Mol. Med. 2010;14:840. doi: 10.1111/j.1582-4934.2009.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dennery PA. 2007;81:155. doi: 10.1002/bdrc.20098. [DOI] [PubMed] [Google Scholar]
- 135.Aird WC. Cold Spring Harb. Perspect. Med. 2012;2:a006429. doi: 10.1101/cshperspect.a006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wilson EO. Knopf. New York: 1998. [Google Scholar]
- 137.Torday JS. The Scientist. 2004;18:32. [Google Scholar]