Abstract
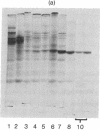
The terminal enzyme of the NADH-dependent stearyl coenzyme A desaturase system has been isolated from rat liver microsomes. This desaturase is a single polypeptide of 53,000 daltons containing 62% nonpolar amino-acid residues and one atom of non-heme iron. The purified protein forms high molecular weight aggregates that can be dispersed by detergent procedures. Desaturase activity requires NADH, stearyl coenzyme A, oxygen, lipid, and the three enzymes, cytochorme b5 reductase (EC 1.6.2.2), cytochrome b5, and desaturase. Cytochrome b5 is the direct electron donor to the desaturase, which appears to utilize the iron in the oxidation-reduction sequence during desaturation of stearyl coenzyme A.
Keywords: fatty acid desaturation, membrane-bound enzymes
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkin C. L., Thelander L., Reichard P., Lang G. Iron and free radical in ribonucleotide reductase. Exchange of iron and Mössbauer spectroscopy of the protein B2 subunit of the Escherichia coli enzyme. J Biol Chem. 1973 Nov 10;248(21):7464–7472. [PubMed] [Google Scholar]
- BEAVEN G. H., HOLIDAY E. R. Ultraviolet absorption spectra of proteins and amino acids. Adv Protein Chem. 1952;7:319–386. doi: 10.1016/s0065-3233(08)60022-4. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A., Vanderkooi G. The low polarity of many membrane proteins. Proc Natl Acad Sci U S A. 1972 Apr;69(4):930–932. doi: 10.1073/pnas.69.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. B., Kirkwood R., Kaufman S. Rat liver phenylalanine hydroxylase, an iron enzyme. J Biol Chem. 1972 Aug 25;247(16):5161–5167. [PubMed] [Google Scholar]
- GELLHORN A., BENJAMIN W. THE INTRACELLULAR LOCALIZATION OF AN ENZYMATIC DEFECT OF LIPID METABOLISM IN DIABETIC RATS. Biochim Biophys Acta. 1964 Apr 20;84:167–175. doi: 10.1016/0926-6542(64)90073-3. [DOI] [PubMed] [Google Scholar]
- Gurr M. I., Robinson M. P. Preliminary partial purification of hen liver microsomal stearoyl-CoA desaturase. Eur J Biochem. 1970 Aug;15(2):335–341. doi: 10.1111/j.1432-1033.1970.tb01012.x. [DOI] [PubMed] [Google Scholar]
- HOLLOWAY P. W., PELUFFO R., WAKIL S. J. ON THE BIOSYNTHESIS OF DIENOIC FATTY ACID BY ANIMAL TISSUES. Biochem Biophys Res Commun. 1963 Aug 1;12:300–304. doi: 10.1016/0006-291x(63)90300-0. [DOI] [PubMed] [Google Scholar]
- Holloway P. W., Katz J. T. A requirement for cytochrome b 5 in microsomal stearyl coenzyme A desaturation. Biochemistry. 1972 Sep 26;11(20):3689–3696. doi: 10.1021/bi00770a005. [DOI] [PubMed] [Google Scholar]
- Holloway P. W., Wakil S. J. Requirement for reduced diphosphopyridine nucleotide-cytochrome b5 reductase in stearly coenzyme A desaturation. J Biol Chem. 1970 Apr 10;245(7):1862–1865. [PubMed] [Google Scholar]
- Jones P. D., Holloway P. W., Peluffo R. O., Wakil S. J. A requirement for lipids by the microsomal stearyl coenzyme A desaturase. J Biol Chem. 1969 Feb 25;244(4):744–754. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Oshino N., Imai Y., Sato R. A function of cytochrome b5 in fatty acid desaturation by rat liver microsomes. J Biochem. 1971 Jan;69(1):155–167. doi: 10.1093/oxfordjournals.jbchem.a129444. [DOI] [PubMed] [Google Scholar]
- Oshino N., Imai Y., Sato R. Electron-transfer mechanism associated with fatty acid desaturation catalyzed by liver microsomes. Biochim Biophys Acta. 1966 Oct 17;128(1):13–27. doi: 10.1016/0926-6593(66)90137-8. [DOI] [PubMed] [Google Scholar]
- Oshino N., Sato R. Stimulation by phenols of the reoxidation microsomal bound cytochrome b5 and its implication to fatty acid desaturation. J Biochem. 1971 Jan;69(1):169–180. doi: 10.1093/oxfordjournals.jbchem.a129445. [DOI] [PubMed] [Google Scholar]
- Ozols J. Amino acid sequence of rabbit liver microsomal cytochrome b5. J Biol Chem. 1970 Oct 10;245(19):4863–4874. [PubMed] [Google Scholar]
- Pistorius E. K., Axelrod B. Iron, an essential component of lipoxygenase. J Biol Chem. 1974 May 25;249(10):3183–3186. [PubMed] [Google Scholar]
- Ruettinger R. T., Olson S. T., Boyer R. F., Coon M. J. Identification of the omega-hydroxylase of Pseudomonas oleovorans as a nonheme iron protein requiring phospholipid for catalytic activity. Biochem Biophys Res Commun. 1974 Apr 23;57(4):1011–1017. doi: 10.1016/0006-291x(74)90797-9. [DOI] [PubMed] [Google Scholar]
- Shimakata T., Mihara K., Sato R. Reconstitution of hepatic microsomal stearoyl-coenzyme A desaturase system from solubilized components. J Biochem. 1972 Nov;72(5):1163–1174. doi: 10.1093/oxfordjournals.jbchem.a130004. [DOI] [PubMed] [Google Scholar]
- Spatz L., Strittmatter P. A form of cytochrome b5 that contains an additional hydrophobic sequence of 40 amino acid residues. Proc Natl Acad Sci U S A. 1971 May;68(5):1042–1046. doi: 10.1073/pnas.68.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatz L., Strittmatter P. A form of reduced nicotinamide adenine dinucleotide-cytochrome b 5 reductase containing both the catalytic site and an additional hydrophobic membrane-binding segment. J Biol Chem. 1973 Feb 10;248(3):793–799. [PubMed] [Google Scholar]
- Steck T. L., Fairbanks G., Wallach D. F. Disposition of the major proteins in the isolated erythrocyte membrane. Proteolytic dissection. Biochemistry. 1971 Jun 22;10(13):2617–2624. doi: 10.1021/bi00789a031. [DOI] [PubMed] [Google Scholar]
- Strittmatter P., Rogers M. J., Spatz L. The binding of cytochrome b 5 to liver microsomes. J Biol Chem. 1972 Nov 25;247(22):7188–7194. [PubMed] [Google Scholar]
- Strittmatter P. The characterization and interconversions of two conformational states of cytochrome b5 reductase. J Biol Chem. 1971 Feb 25;246(4):1017–1024. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weiner A. M., Platt T., Weber K. Amino-terminal sequence analysis of proteins purified on a nanomole scale by gel electrophoresis. J Biol Chem. 1972 May 25;247(10):3242–3251. [PubMed] [Google Scholar]