Abstract
Mice rely on the sense of olfaction to detect food sources, recognize social and mating partners, and avoid predators. Many behaviors of mice including learning and memory, social interaction, fear, and anxiety are closely associated with their function of olfaction, and behavior tasks designed to evaluate those brain functions may use odors as cues. Accurate assessment of olfaction is not only essential for the study of olfactory system but also critical for proper interpretation of various mouse behaviors especially learning and memory, emotionality and affect, and sociality. Here we describe a series of behavior experiments that offer multidimensional and quantitative assessments for mouse’s olfactory function, including olfactory habituation, discrimination, odor preference, odor detection sensitivity, and olfactory memory, to both social and nonsocial odors.
Keywords: Olfaction, mouse, olfactory discrimination, olfactory learning and memory
Introduction
This unit outlines methods that are commonly used to assess olfactory functions in mice. Five basic protocols are described in this unit. They can be classified into two categories: the cotton tip-based olfactory tests and the sand-digging task-based olfactory tests. The cotton tip-based olfactory tests use cotton tips as the media to deliver odorants and the mouse’s spontaneous investigative activity is observed and quantified, whereas in the sand-digging based olfactory tests, food rewards are associated with odor cues and mice are trained for a task that requires the use of odor cues to retrieve food rewards.
Cotton tip-based olfactory habituation/dishabituation test, described in Protocol 1, can be used as the primary test for the evaluation of a mouse’s basic ability to detect and discriminate odors. To evaluate fine olfactory discrimination, structurally similar odorants can be presented during the habituation/dishabituation test. The same test can also be used to determine whether the mouse is able to detect the social odors in urine samples and discriminate male and female odors. Furthermore, urine collected and pooled from different groups of mice can be used to evaluate the ability for fine discrimination of social odors. Additionally, using the same odor-delivering medium, the cotton tips, a variety of other olfactory assays have been developed to evaluate the odorant detection sensitivity, odorant preference, and olfactory short-term memory in mice.
Despite the relatively simple setup and ease of these cotton tip-based olfactory assays, they are largely dependent upon an intrinsic curiosity and motivation to investigate novel odors. The level of curiosity and motivation may be confounding factors. In contrast, the sand digging-based olfactory discrimination test associates odor cues with a food reward buried in sand. Mice are food restricted prior to beginning these assays, which minimizes motivation variance and offers a more accurate assessment for odor discrimination. Again, both discrete and structurally similar odors can be used for this experiment.
Basic Protocol 1: Cotton tip-based olfactory habituation/dishabituation test
This test is used to examine the animal’s ability to detect odors and discriminate between different odors, including regular odors and social odors. It consists of sequential presentations of different odors by using cotton swabs (Sundberg et al., 1982; Trinh and Storm, 2003; Wang et al., 2006; Pan et al., 2012; Zou et al., 2012; Zou et al., 2013). Each odor (or vehicle) is presented in four consecutive trials, each for 1 min. The inter-trial interval is 2 min. Naïve mice are first habituated to the presence of a cotton swab soaked in plain mineral oil or water and then presented with three odorants sequentially, with four presentations for each odorant (Zou et al., 2012). All presentations are performed in the home cage. A high duration of sniffing upon the initial exposure of an odorant indicates that the animal detects the odorant. Sniffing declines in a step-wise fashion in subsequent trials because the odorant is no longer novel. A significant increase in the investigation time when a novel odorant is presented indicates the discrimination of the novel odorant from the familiar one.
Materials
Mice: 3–8 months old, housed in regular mouse cages with bedding, nestlets and food and water bottles placed over the wire tops
One stopwatch
One digital timer
Cotton-tipped wooden applicators (6 inch in length with one end wrapped with cotton, Allegiance, Cat. No. C15055-006)
A digital video recorder
Clean caps of 15 ml conical tubes
Odorants and vehicle (plain mineral oil or distilled water)
Protocol steps
Pre-test
Individually house the mice one week prior to the test and handle the mice for 2 min, twice a day for one week.
Change the cage one day before the test to reduce the level of background odor.
Assemble odorant-presenting applicators: drill a small hole on the cap of a 15 ml conical tube. Stick the bare end of a cotton-tipped applicator through the hole and slide the cap to the middle of the rod. Then over a clean cage with wire top, drop the cotton-tip end of the applicator between bars of the wire top toward one end of the cage and let the conical tube cap sit above the wire top. Slide the applicator down until the cotton-tip is 8 cm above the cage floor. Mark the position of cap on the applicator and use it as the reference for all other applicators (Fig. 1).
Prepare odorant solutions: dissolve odorants in either plain mineral oil or water, depending on the solubility. The concentration of the odorants should be optimized through pilot experiments using naïve mice with the same genetic background and at the same age. The concentration should be high enough for the naïve mice to smell but not overbearing such that the mice would prefer to stay away from the applicator rather than approaching the applicator.
Prepare the testing room: olfactory test should be done in a room separate from the housing room. The testing room should also be thoroughly ventilated before the experiment; this is especially important if that room has been used for olfactory tests recently.
Habituate mice to the testing room: on the day of testing, mice should be habituated in the testing room for at least one hour to familiarize them with the room odor and other room settings, for example, furniture and background noise.
Figure 1.
Cotton tip-based olfactory habituation/dishabituation test. Top view of the set up for cotton tip-based test.
During test
Remove the plastic top of the cage, lace a cotton-tipped applicator with mineral oil, assemble the applicator to the conical tube cap, and then put the applicator into the side of the cage remote from the nest. The cotton tip should be 3–5 cm away from the end wall, in the middle between the side walls, and 8 cm above the cage floor.
Use a stopwatch to record cumulative time spent sniffing the tip during a 1 min trial. Sniffing is scored when the mouse is oriented towards the cotton tip with its nose 1 cm or closer to the tip. Preferably, a video recorder is placed from the side of the cage and used to record the behavior, and the sniffing is scored off-line from the video, so the animal will not be disturbed by the noise from the stopwatch.
Remove the applicator at the end of trial and discard the applicator into an enclosed container. A new applicator should be used for each trial. The conical tube cap can be reused.
Repeat the mineral oil presentation for a total of four trials with a 2 min inter-trial interval.
Two minutes after the 4th trial of mineral oil presentation, introduce an odorant (1st odorant) to the mouse in the same manner as the mineral oil presentation and video record the behavior or use a stopwatch to record cumulative time spend sniffing the odorant. Repeat the odorant presentation for a total of four trials with a 2 min inter-trial interval.
Two minutes after the 4th trial of the 1st odorant presentation, introduce a novel odorant (the 2nd odorant) to the mouse in the same manner as the presentation of the mineral oil and the 1st odorant and repeat the presentation of the 2nd odorant for a total of four trials.
Present the 3rd odorant to the mouse for a total of four trials in the same manner as the presentation of the mineral oil and the 1st and 2nd odorants.
After test
Place the plastic top back and return the cage to the housing room.
Thoroughly ventilate the room using fans.
Alternative Protocol 1 for Basic Protocol 1: Cotton tip-based olfactory habituation/dishabituation test for fine odor discrimination
This test is used to examine whether the mouse is able to discriminate between structurally similar odorants (Moreno et al., 2009; Zou et al., 2012). Similar to Basic Protocol 1, this alternative protocol consists of sequential presentations of two odors by using cotton-tipped applicators. However, in this protocol, the two odors share similarity in their chemical structure, demanding a higher level of discrimination ability to distinguish between them. The first odorant is presented for four trials followed by one presentation for the second, and a significant increase in investigation time during the first presentation of the second odorant compared with the 4th presentation of the first odorant indicates the discrimination of the second odorant from the first odorant.
Materials
Same as those in Basic Protocol 1, except that pairs of structurally similar odorants at the same concentration should are used. For example, limonene (+) and limonene (−) represent a pair of enantiomers, while butanol and pentanol are alcohols with one carbon difference. These pairs of structurally similar odorants were used to assess fine odor discrimination.
Protocol steps
Follow “pre-test” steps 1 – 6 from Basic Protocol 1
Follow “during test” steps 1 – 4 from Basic Protocol 1
Two minutes after the 4th trial of mineral oil presentation, introduce an odorant (1st odorant) to the mouse in the same manner as the mineral oil presentation and video record the behavior or use a stopwatch to record cumulative time spend sniffing the odorant. Repeat the odorant presentation for a total of three trials with a 2 min inter-trial interval.
For the fourth trial, present a structurally similar odorant, at the same concentration and score the duration of sniffing.
Alternative Protocol 2 for Basic Protocol 1: Cotton tip-based olfactory habituation/dishabituation test for discrimination between male and female urinary odors
Male mice are capable of detecting and differentiating distinct pheromone-containing urine samples, such as those from male versus female mice, or from ovariectomized versus estrous female mice, when presented sequentially (Pankevich et al., 2004; Jakupovic et al., 2008; Zou et al., 2013). The alternative protocol 2 of basic protocol 1 is used to examine whether the male test mouse is able to discriminate between male and female odors carried in urine. Pooled and undiluted urine samples collected from groups of normal male and female mice are used for this test. The male test mouse is first presented with four presentations of male urine followed by four presentations of female urine. Similar to Basic Protocol 1, a high duration of sniffing upon the initial exposure of male urine indicates that the animal detected the odor information in the urine. Sniffing declined in a step-wise fashion in subsequent trials because the odor information is no longer novel. A higher level of investigation when a second urine sample, the female urine, is presented, compared with the 1st presentation of the male urine sample, indicates the discrimination of the female urine from the male urine.
Materials
10 urine samples from normal male mice between 3 – 8 months old
10 urine samples from normal female mice between 3 – 8 months old
Protocol steps
Collect urine samples individually from is collected from a group of 10 male and 10 female normal mice, between 3- to 8- months old. Pick up the mouse by holding the mouse’s skin on the back neck with one hand and immediately collect the urine with a 1.5 ml Eppendorf tube.
Undiluted urine samples are pooled within each gender with equal volume of urine from each individual, e.g. pool 20 µl of urine from each mouse for a total volume of 200 µl.
Use the pooled urine samples as male and female urine samples for this experiment. Store urine samples at −80°C till use. Avoid frequent freeze-thaw cycles.
Follow “pre-test” steps 1 – 6 from Basic Protocol 1.
Following “during test” steps 1 – 4 from Basic Protocol 1, Naïve male test mice are pre-trained with cotton-tipped applicators laced with water for four trials.
Present male urine sample for four trials for 1 minute per trial with a 2 minute inter-trial interval.
-
Two minutes after the 4th trial of male urine presentation, introduce female urine to the mouse in the same manner as the male urine and video record the behavior or use a stopwatch to record cumulative time spend sniffing the odorant. Repeat the female urine presentation for a total of four trials with a 2 min inter-trial interval.
Note: We store urine samples in −80C freezer and usually use the aliquots within a few weeks after collection. We have not tested the length of time samples can be stored, but they should be fairly stable under −80C as long as no frequent freeze-thaws.
Alternative Protocol 3 for Basic Protocol 1: Cotton tip-based olfactory habituation/dishabituation test for fine discrimination of urinary odors between different groups of mice
Mice are not only able to detect the differences of urinary odors of different genders but also the subtle differences in urinary odors of different individuals of the same gender (Zou et al., 2013). In this test, the male subject mouse is habituated with pooled urine collected from a group of ten male mice (male group A urine) and then is presented with pooled urine collected from a different group of ten male mice (male group B urine) to assess the ability for fine discrimination between the urinary odors from different group of mice. The male mouse can also be tested for the fine discrimination of urinary odors from different groups of female mice (female group A and B urine). Note that the subject mice are male when either male or female urine is tested. Similar to Alternative Protocol 2, an increase of sniffing upon the 1st exposure of group B urine compared with the 4th presentation of the group A urine indicates that the animal is able to discriminate the subtle differences in the urine from two groups of mice of the same gender.
Materials
10 urine samples from normal male mice between 3–8 months old (male group A)
10 urine samples from normal male mice between 3–8 months old (male group B)
10 urine samples from normal female mice between 3–8 months old (female group A)
10 urine samples from normal female mice between 3–8 months old (female group B)
Protocol steps
Collect urine samples as in Steps 1–3 from Alternative Protocol 2.
Following Steps 4–5 from Alternative Protocol 2, pre-train naïve mice with cotton-tipped applicators laced with water for four trials.
Present male group A urine sample for four trials for 1 minute per trial with a 2 minute inter-trial interval.
Two minutes after the 4th trial of male group A urine presentation, introduce male group B urine to the mouse in the same manner as the male group A urine and video record the behavior or use a stopwatch to record cumulative time spend sniffing the odorant.
The same protocol can be used for female urine fine discrimination using female group A and B urine.
Alternative Protocol 4 for Basic Protocol 1: Cotton tip-based olfactory habituation/dishabituation test for fine discrimination of urinary odors between ovariectomized and estrous female urine
This test is used to examine a male mouse’s ability to determine the reproductive status of a female mouse through the olfaction of pheromonal information in female urine, specifically, the ability to detect the pheromonal differences in the urine collected from ovariectomized females and estrous females (Pankevich et al., 2004; Zou et al., 2013). In this test, a subject male mouse is presented with 4 trials of ovariectomized urine followed by 1 trial of estrous urine. To avoid potential confounding volatile odors and/or pheromone components that are unrelated to the female reproductive stages, a cohort of ovariectomized female mice has been artificially cycled with estradiol and progesterone. The urine collected before hormone injection is designated as ovariectomized (OVX) urine, and urine collected after hormone injection is used as estrous urine. The differences in the two urine samples are likely due to different pheromones related to the reproductive stages of female mice since the mice have been maintained under identical conditions before and after hormone injection.
Materials
Five to ten of 3–7 month-old ovariectomized mice with their ovariectomy surgeries performed at 2 months old
Estradiol benzoate
Progesterone
Needles and syringes
Protocol steps
Collect ovariectomized urine sample as in Steps 1–3 from Alternative Protocol 2. Collect urine from ovariectomized mice individually, twice a day for 14 consecutive days, and pool with equal volume of urine from each individual. This pooled and undiluted urine sample is designated OVX urine.
Artificial cycling of the ovariectomized mice. Inject the ovariectomized mice intraperitoneally with 20 µg estradiol benzoate daily for seven consecutive days and 500 mg progesterone daily 24 h after each estradiol benzoate injection to achieve and maintain estrous status. Collect urine individually, daily between 4–7 h after the progesterone injection, and pool with equal volume of urine from each individual. This pooled urine is designated estrous urine.
Following Steps 4–5 from Alternative Protocol 2, pre-train Naïve mice with cotton-tipped applicators laced with water for four trials.
Present OVX urine sample for four trials for 1 minute per trial with a 2 minute inter-trial interval.
Two minutes after the 4th trial of OVX urine presentation, introduce estrous urine to the mouse in the same manner as the OVX urine and video record the behavior or use a stopwatch to record cumulative time spend sniffing the odorant.
Basic Protocol 2: Cotton-tip based olfactory preference test
Mice may be intrinsically more interested in some odorants than others. This test is used to compare the mouse’s level of interest toward two different odorants (Zou et al., 2012). Same as Basic Protocol 1, the odorants are presented using cotton-tipped applicators. However, in this test, different from Basic Protocol 1, the two odorants are presented for only one trial, side by side and simultaneously rather than sequentially. The preference is determined by comparing the cumulative sniffing duration between the two odorants.
Materials
Materials are similar as in Basic Protocol 1 except that two odorants (odorant 1 and 2) and two stopwatches are needed and plain mineral oil is not needed.
Protocol steps
Pre-test: Same as in Basic Protocol 1.
During test
Remove the plastic top of the cage, lace two cotton-tipped applicators with odorant 1 and 2, assemble the applicators to the conical tube caps, and then put the applicators side-by-side into the side of the cage remote from the nest. The two cotton tips should be apart by at least 10 cm, close to the left and right side walls, 3–5 cm away from the end wall, and 8 cm above the cage floor.
Use two stopwatches to record cumulative time spent sniffing each tip during a 3–5 min trial. At the end of the trial, remove the applicators and place the plastic cage top back.
The left and right orientation of the odorant 1 and 2 should be randomized between different subject mice.
The same cohort of mice may be tested for the preference for different pairs of odorants on different days.
After test: Same as in Basic Protocol 1.
Alternative Protocol 1 for Basic Protocol 2: Cotton-tip based olfactory preference test for preference between male and female urinary odors
Normal male wild type mice show strong preference to female urine over male urine when the two urine samples are presented simultaneously (Pankevich et al., 2004; Mandiyan et al., 2005). However, mice with deficient olfaction may not show such preference. As a variation of the olfactory preference test, a urine preference test has been developed to examine the pheromone preference of the male mice (Zou et al., 2013).
Materials
Same as those in Basic Protocol 2 except that the female and male urine samples are prepared as in Alternative Protocol 2 for Basic Protocol 2.
Protocol steps
Follow “pre-test” steps as in Basic Protocol 2
Follow “during test” steps 1 – 4 from Basic Protocol 2. Use 20 µl of undiluted male and female urine as odorant 1 and 2, respectively.
Follow “after test” step as in Basic Protocol 2.
Alternative Protocol 2 for Basic Protocol 2: Cotton-tip based olfactory preference test for preference between ovariectomized and estrous urinary odors
Normal male wild type mice are not only able to discriminate the subtle differences between ovariectomized urine and estrous urine but also prefer the estrous urine over the ovariectomized urine (Pankevich et al., 2004; Zou et al., 2013). This preference may not exist if the mouse’s olfaction is deficient due to the chemical or genetic ablation of main or accessory olfactory system (Pankevich et al., 2004; Zou et al., 2013). This alternative protocol is designed to assess the male mouse’s preference for estrous urinary odors.
Materials
Same as those in Basic Protocol 2 except that the OVX and estrous urine samples are prepared as in Alternative Protocol 3 for Basic Protocol 1.
Protocol steps
Follow “pre-test” steps from Basic Protocol 2
Follow “during test” steps 1 – 4 from Basic Protocol 2. Use 20 µl of OVX and estrous urine as odorant 1 and 2, respectively.
Follow “after test” step from Basic Protocol 2.
Basic Protocol 3: Cotton tip-based olfactory detection threshold test
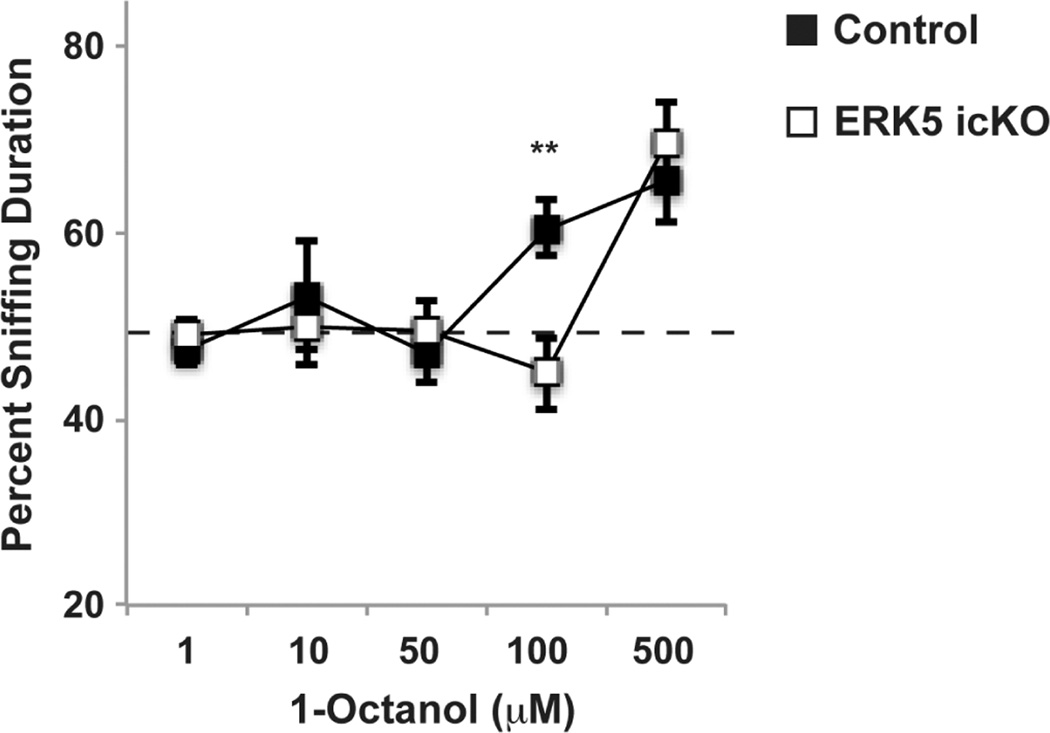
This test is used to determine how sensitive the mouse is to detect odors at low concentrations. This is performed on consecutive days as described with modifications (Breton-Provencher et al., 2009; Pan et al., 2012). Each day, an odorant solution and the vehicle are presented to the mouse simultaneously. Solutions of the same odorant but of ascending gradients are presented on consecutive days. If the mouse is unable to detect the odorant at the tested concentration, the mouse will spend equal amount of time sniffing the odorant and vehicle. However, once the concentration of the odorants is above the detection threshold, the mouse will spend more time sniffing the odorant than the vehicle (Fig. 2).
Figure 2.
Cotton tip-based olfactory threshold test. Mice were presented with two cotton swabs simultaneously: one laced with vehicle (mineral oil) and the other with increasing concentration of 1-octanol. The cumulative duration of sniffing on each cotton swab was quantified. There was a 24-h interval between two adjacent concentrations. For each concentration, the percentage of time spent sniffing the 1-octanol cotton swab in the total duration of sniffing on both cotton swabs was calculated. An above 50% sniffing duration of the octanol cotton tip indicateds detection of the odorant. Figure is adopted from our previous publication (Pan et al., 2012).
Materials
Mice: 3–8 months old, housed in regular mouse cages with bedding, nestlets and food and water bottles over the wire tops
Two stopwatches
One digital timer
Cotton-tipped wooden applicators (6 in. in length with one end wrapped with cotton, Allegiance, Cat. No. C15055-006)
A digital video recorder
Caps of clean 15 ml conical tubes
Protocol steps
-
Determine the range of odorant concentrations by a pilot study. The lowest concentration should be set at least one order of magnitude lower than the lowest concentration that a mouse can possibly detect, and the highest concentration should be set at least one order of magnitude higher than concentration that all mice are able to detect. The difference between adjacent concentrations should be at least 5 folds. Odorant solutions are made fresh daily before the test.
The lowest concentration of the odorant that a mouse can possibly detect is determined by trial. The ability to detect is not determined by how long the mouse sniff because there is a big variation among individual mice in terms of absolute sniffing time. It is determined by the ratio of time spent sniffing the odorant cotton-tip versus the vehicle cotton-tip.
On day 1, using two cotton-tipped applicators, present the odorant solution at the lowest concentration and vehicle to the mouse for 3 min and score the time spent on each applicator. Calculate the percentage of odorant sniffing with the following equation: the percentage of odorant sniffing = time spend on odorant / (time spent on odorant + time spent on vehicle) X 100.
From day 2 and on, repeat the session, one session per day, by presenting the same odorant but at a higher concentration each day. Calculate the percentage of odorant sniffing on each day.
With slight modifications, this protocol is suitable for measuring the detection sensitivity for TMT, a known fear-inducing predator odor for rodents (Fendt et al., 2005; Pan et al., 2012). Similarly, present TMT at increasing concentrations each day together with a vehicle control. And calculate the percentage of TMT sniffing at different concentrations. Since mice will avoid TMT when they detect it, a sniffing duration less than 50% indicates that the animal detected the fear odor.
Basic Protocol 4: Cotton tip-based olfactory short-term memory test
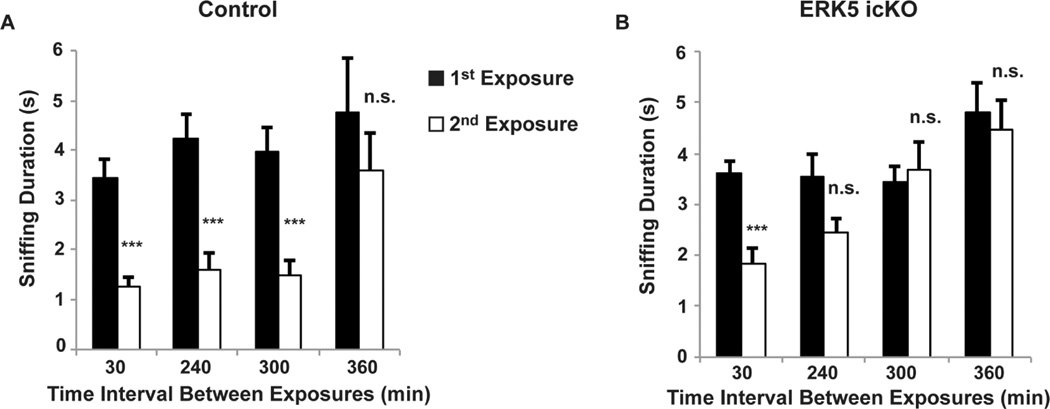
This test is used to examine the mouse’s memory retention to an odorant after a one-time exposure (Rochefort et al., 2002; Mechawar et al., 2004; Fendt et al., 2005; Pan et al., 2012). The mouse is presented with an odorant solution and the presentation is repeated after a period of time. A significant reduction of sniffing during the second presentation suggests the olfactory memory for the 1st presentation of the same odorant (Fig. 3).
Figure 3.
Cotton tip-based olfactory short-term memory test
Mice were presented with cotton swabs laced with the same odorants twice at the indicated time intervals. A different odor was used for each interval time point. The sniffing duration of the cotton swab was recorded for both exposure session. A decrease in investigation during the second exposure of the same odorant is suggestive of olfactory memory of the odorant. A. Data of control mice. B. Data of ERK5 icKO mice. Figure is adopted from our previous publication (Pan et al., 2012).
Materials
Same as in Basic Protocol 1.
Protocol steps
Habituate the animal in the behavior room for at least 1 h.
-
Remove the plastic top of the cage. Using a cotton-tipped applicator, present odorant 1 to the mouse for 5 min and score the duration of sniffing. Place the plastic top back.
The odorant is presented in the same manner as in Basic protocol 1 regarding the assemble of the applicator and the three-dimensional location in the mouse cage that the cotton-tip should be hung. However, the duration of the presentation and number of presentations are different from Basic Protocol 1.
Repeat the presentation of the same odorant 30 min later and score the duration of sniffing during the 2nd presentation. A reduction of the duration of sniffing during the 2nd presentation compared to that during the 1st presentation by pair-wised comparison is indicative of olfactory memory.
Twenty-four hours later, use a different odorant to test olfactory memory at a different time interval, for example, 1 h.
Olfactory memory retention at longer intervals, such as 2, 4, and 8 h, may be tested by using a different odorant for each interval, but only a single time interval should be tested on each day for each mouse to avoid cross interference of olfactory detection and memory.
Basic Protocol 5: Sand-digging task-based olfactory discrimination/learning test
Although the cotton-tip based olfactory habituation/dishabituation test, as described in Basic Protocol 1, offers a rapid assessment of olfactory discrimination in mice, this test relies on the mouse’s nature of curiosity and motivation to investigate novel odors. In such case, the individual variation of the level of curiosity/motivation may be a confounding factor.
Different from the cotton-tip based test, here, the sand digging-based olfactory discrimination test is based on the mouse’s drive for food when they are starved, and is not affected by the level of intrinsic curiosity (Mihalick et al., 2000; Wesson et al., 2008; Pan et al., 2012; Zou et al., 2012). In this test, the subject mouse is presented with two dishes - both are filled with sand and are scented with two different odorants (isoamyl acetate and citralva are used as examples in this protocol) but only one dish is baited with a food reward and the food reward is always associated with one odorant but not the other. The food-restricted mouse is trained to learn to use the odor cue to dig for the food reward (Fig. 4).
Figure 4.

Apparatus and set up for the assay of sand-digging task-based olfactory discrimination/learning test.
Since mice will be trained to learn to use the odor cues to detect food sources, this experiment also offers the assessment of olfactory associated learning (Pan et al., 2012).
Materials
A cohort of 3–7 month-old male mice, individually housed and pre-handled as in Basic Protocol 1
Lids of two 96-well culture dishes
Lids of two 35-mm culture dishes
Plexiglass sheets of various sizes:
55X50 mm, clear
20X32 cm, clear
25X20 cm taped to 25X18 cm, painted to opaque
The coat was very thin and it was completely dried and should not have any paint odors left.
Playground sand (Sarrete), autoclaved before the test
Bioserv mouse food pellets (Product No. F0071)
35-mm culture dishes
Odorant solutions: 1 mM isoamyl acetate and 1 mM citralva are used as examples in this protocol.
Glue: Super glue.
Protocol steps
Pre-test
Construction of the food-delivery apparatus: an apparatus is constructed and used to present food and sand (Fig. 4). This includes gluing the lids of two 96-well culture plates perpendicular to each other at the edges to form the platform base and handle, followed by gluing a 55 mm deep X 50 mm high Plexiglas divider between the base and handle which separates the platform base into right and left sides, and then mounting the lid of a 35 mm culture dish upside down on each side of the base platform. Next, place a clean 35 mm culture dish on top of the mounted lids on each side.
Food restriction for subject mice: before and during the training, subject mice to food restriction to maintain their body weights at 85–90% of free-feed level. First, subject mice for an overnight food deprivation for 18 h and then feed them with a half pellet of food (around 3 g) each day for around 5–7 days until they achieve their desired weight. Then the test may start but in the meanwhile continue the food restriction to maintain the body weight at 85–90% of free-feed level till the completion of the test.
Acclimate mice in the behavior room for at least one hour before each day’s training.
During test
Mice are first subjected for a 3-day pre-training followed by the 6–8-day olfactory discrimination acquisition.
Loading of the apparatus: Fill two dishes with sand to the top of the dish. Place a piece of food pallet in only one of the two dishes. Place the food pallet in the center of the dish. Depending on the stages of training, the food pallet may be placed on top of the sand, half buried, completely buried but right under the sand surface, or deeply buried down to the bottom of the dish.
The food delivery in each trial: Perform training in home cage. Remove the plastic top, wire top, and water bottle and cover the cage by a piece of 20 × 32 cm Plexiglas. To begin each trial, first remove the Plexiglas cover, and place an opaque barrier (the opaque plexiglass) inside of the cage to confine the mouse to one end of the cage (the end with nest). This barrier blocks the view of the mouse while lower the apparatus with food and sand into the other end of the cage, with the dishes facing toward the animal and the handle close to the end wall of the cage. Then quickly remove the barrier and reposition the Plexiglas cover. Score animal’s behavior by either the latency to retrieve the food or the correct or incorrect choice made, depending on the training stages as described below. Use a new dish for each mouse and each odorant. After the trial, remove the Plexiglas cover and apparatus and reposition the cage top. Clean the apparatus between animals by washing with water, wiping with 95% ethanol to remove any trace odorant, and spraying with 70% ethanol.
- Pre-train: The animals are pre-trained to learn to recognize the reward pellets as food and to retrieve the rewards from sand. This pre-training session consists of 3 consecutive days during which only one of the two dishes is filled with sand and baited with food reward. The left or right location of dish with food should be randomized among the trials. The criterion for graduating from the pre-training is that all mice should retrieve food, which is buried at the bottom of the dish, within 30 s time limit.
-
1)On day 1, place the food reward on top of the sand and train animals for four trials with 1 min inter-trial interval per block for two blocks with a 4 h inter-block interval, totaling eight trials. Record the latency to retrieve food in each trial by a stopwatch. If the food is not retrieved within 10 min, remove the apparatus and record the latency as 600 sec. By the end of eight trials on day 1, all animals should retrieve the food within 30 s, or an additional day of training is necessary.
-
2)On day 2, train animals for two trials (1 min inter-trial interval) with the reward partially buried in the sand followed by another two trials with the reward completely buried but just underneath the surface of the sand. Allow animals to explore for a maximum of 10 min to retrieve the food reward in each trial. Again, by the end of four trials on day 2, all animals should retrieve the food within 30 s, or an additional block of four trials is necessary.
-
3)On day 3, train animals for four trials per block for two blocks with the reward placed at the bottom of the dish and completely buried in sand. Record the latency to retrieve food in each trial as on day 1. By the end of training on day 3, all mice should retrieve the food within 30 s, or an additional day of training is necessary.
-
1)
- Olfactory discrimination/learning acquisition: The day after the completion of pre-training, subject animals to the olfactory discrimination acquisition session.
-
1)Load two dishes with sand to the top. Deeply bury a food pellet in the center and down to the bottom of one of the dishes.
-
2)Scent the two dishes with odorant solutions. As described previously (Zou et al., 2012), use 1 mM IAA as the positive odorant to scent the dish with the food reward, and use 1 mM citralva as the negative odorant to scent the dish without food reward. To scent the dish, add 100 µL odorant solution over the sand near the dish edge closest to the handle. The scented wet sand is distal from the animal in order to minimize the spill of scented sand into the cage during the digging and prevent the odor contamination in the cage.
-
3)Perform the olfactory discrimination acquisition training for four training trials per block, two blocks per day with an inter-block interval of 4 h, for 6–8 consecutive days.
-
4)Place the two dishes randomly on either the left or right side as long as each dish is placed on each side twice per block, but no more than three consecutive times in each day.
-
5)After the apparatus with the sand dishes is placed in the cage in each trial, if the animal’s first response is to dig the IAA-scented dish, allow the animal to dig until it retrieves the food and record the trial as “correct”. If the food reward is accidentally lost during the digging, drop one more piece of reward immediately into the dish for replacement. Allow the animal to eat the reward and then remove the apparatus from the cage. The trial is still recorded as “correct”. If the animal’s first response is to dig the citralva-scented dish, discontinue the digging immediately by removing the apparatus quickly from the cage and record the trial as “incorrect”.
-
6)Calculate the percentage of correct trials on each training day and graph a learning curve of the training using the data from consecutive days. Terminate the training until the average percentage of correct choices reaches above 90–95% or the learning curve reaches a plateau.
-
1)
Alternative Protocol 1 for Basic Protocol 5: Sand-digging task-based olfactory discrimination/learning test with structurally similar odorants
In order to identify subtle deficits of olfactory discrimination, the sand digging-based olfactory discrimination task could be alternatively performed by using a pair of structurally similar odorants (Zou et al., 2012).
Materials
Same as those in Basic Protocol 5 except that one pair of structurally similar odorants, such as limonene (+) and limonene (−), or butanol and pentanol, is used.
Protocol steps
Follow Steps 1–3 in “Pre-test” from Basic Protocol 5.
Follow Steps 1–3 in “During test” from Basic Protocol 5.
Follow Step 4 in “During test” from Basic Protocol 5, but use a pair of structurally similar odorants, such as limonene (+) and limonene (−), for olfactory discrimination acquisition training (Zou et al., 2012).
Alternative Protocol 2 for Basic Protocol 5: Sand-digging task-based olfactory discrimination/learning test with multiple negative odorants
To make the task more challenging, Basic Protocol 5 could be alternatively performed by using one positive odorant and multiple negative odorants. This will increase the power of identifying a mild deficit in olfactory discrimination.
Materials
Same as Basic Protocol 5 except that the preparation of odorant solutions includes one odorant solution as positive odorant and three odorant solutions as negative odorants. For example, use IAA at 1 mM as the positive odorants, while use citralva, octonol, benzaldehyde, all at 1 mM, as negative odorants.
Protocol steps
Follow Steps 1–3 in “Pre-test” from Basic Protocol 5.
Follow Steps 1–3 in “During test” from Basic Protocol 5.
Follow Step 4 in “During test” from Basic Protocol 5, but in each trial, include the presentation of the positive odorant and one of the negative odorants. Randomize the selection of the negative odorant in each trial so that each negative odorant should be presented for at least once but no more than twice in each block of 4 trials and no more than three times on each training day. The scoring of the correct and incorrect choice for each trial and the calculation of the percentage of correct choices is the same as that in the Basic Protocol 5.
Alternative Protocol 3 for Basic Protocol 5: Sand-digging task-based olfactory discrimination/learning test with multiple odorant pairs
As a more challenging version of the Alternative Protocol 1 for Basic Protocol 5, the odor discrimination/ learning acquisition could be alternatively performed by using multiple pairs of structurally similar odorants. This will increase the power of identifying a subtle deficit in olfactory discrimination.
Materials
Same as the Alternative Protocol 1 for Basic Protocol 5 except that three pairs of structurally similar odorants are used. For example:
Pair 1: Limonene (+) at 10mM is designated as the positive odorant and limonene (−) of the same concentration is designated as the negative odorants
Pair 2: Butanol and pentanol at 10mM are designated as the positive and negative odorant respectively.
Pair 3: Carvone (+) and (−) at 10mM are designated as the positive and negative odorant respectively.
Protocol steps
Follow Steps 1–3 in “Pre-test” from Basic Protocol 5.
Follow Steps 1–3 in “During test” from Basic Protocol 5.
Follow Step 4 in “During test” from Basic Protocol 5, but in each trial, include the presentation of one pair of positive odorant and negative odorant. Randomize the selection of the odorant pair and the right/left location of the positive/negative odorant in each trial, but the combination of the positive and negative odorants in each pair remains unchanged.
In order to increase the level of difficulty of the task and thus the power of identifying a mild deficit in olfactory discrimination, tag the food reward or no reward with different ratios of similar odorants. For example, use 60% limonene (+)/40% limonene (−) for food reward versus 40% limonene (+)/60% limonene (−) for no reward.
The scoring of the correct and incorrect choice for each trial and the calculation of the percentage of correct choices is the same as that in the Basic Protocol 5.
Commentary
Background Information
From birth, rodents rely heavily on their sense of smell for locating and identifying food, avoiding predators, establishing social hierarchies, finding mates, caring for their young and a host of other behaviors. Olfactory dysfunction is frequently associated with schizophrenia (Moberg and Turetsky, 2003; Turetsky et al., 2009), bipolar disorder (Hardy et al., 2012), depression (Negoias et al., 2010; Gopinath et al., 2011), posttraumatic stress disorder (Croy et al., 2010) and Parkinson’s and Alzheimer’s diseases (Rahayel et al., 2012). Assessment of olfaction is likely to be an essential component when mouse models of these diseases are investigated. Additionally, behavior paradigms designed for the assessment of other brain functions, such as learning and memory, may use odors as cues for the assays, and an intact olfaction for all the experimental mice is the pre-condition for the assay to be valid. For example, odor cues may be used as the control testing (cue testing) for the context fear conditioning testing, a commonly used behavior assay for hippocampus-dependent learning and memory (Curzon et al., 2009). Therefore a thorough and accurate assessment of olfaction is essential for the assessment of animal’s behaviors.
The first described olfactory behavioral test is buried food test, in which a piece of familiar palatable food is buried beneath the bedding of a clean cage and an overnight-fasted mouse is allowed to retrieve the food within limited time (Alberts and Galef, 1971; Del Punta et al., 2002). This assay has loss of favor because of the high false positive: mice with normal olfaction may fail to retrieve the food due to the novelty-induced anxiety. In contrast, the olfactory habituation/dishabituation test, first described in the early 1980s (Gregg and Thiessen, 1981), is an olfactory test with higher accuracy and has been used widely as the primary test for the evaluation of olfactory functions. The mouse general levels of curiosity, anxiety, and the adaptation can be first evaluated with the presentations of non-scented cotton-tips. Significant habituation and dishabituation is an indicator of normal olfaction. Both nonsocial and social odors can be used for this assay, and the structurally similar odorants or odorants mixtures of different ratios can be used to probe the subtle deficits of olfactory discrimination.
Using the same cotton-tip odor-delivering concept, the olfactory threshold assay, olfactory preference assay, and olfactory short-term memory assay were developed to provide multidimensional and quantitative assessment of mouse’s olfactory functions, in addition to the basic odor recognition and discrimination function.
Although it is a fundamental and widely used olfactory behavior test, the cotton tip-based olfactory habituation/dishabituation assay relies on the mouse’s intrinsic curiosity for novel odors, and variations in their general level of activity, curiosity, anxiety and adaptation can be confronting factors. In contrast, the sand-digging task-based olfactory discrimination/learning assay, first introduced in 2000 (Mihalick et al., 2000), uses the mouse’s drive for food as the basis for the experiment design and thus is independent of the mouse’s general activity and curiosity. It is a more complicated and time-consuming test, but it has a high power to detect the subtle deficits in olfactory discrimination and learning especially when pairs of structurally similar odors or multiple odor pairs are used (Pan et al., 2012; Zou et al., 2012).
Critical Parameters /Troubleshooting
Cotton-tip based olfactory habituation/discrimination assay
The purpose of this assay is to test the mouse’s ability to detect the odorant and discriminate between different odorants. The basic protocol of this assay is used as the screening test for olfactory deficits while the fine discrimination variant of this assay is to detect the subtle deficits in odor discrimination. The ability to smell is determined by the manifestation of the mouse’s interest to the first exposure to an odorant and the interest declines with repetitive presentations of the same odorant. The ability of odor discrimination is determined by the significant increase of the duration of sniffing every time a novel odorant is presented.
As in all the olfactory tests, sufficient familiarization is the key. This includes the familiarization of the mouse to both background odors and the odor-presentation media – the cotton swabs. It is also critical to keep the background odors to a low level. To this end, the mouse needs to be individually housed for at least one week prior to the test, and the bedding needs to be changed one day before the test to minimize the background odors without imposing significant stress to the mouse. The room needs to be fanned thoroughly before the mouse is moved in and the mouse needs to be acclimatized to the room odor for at least one hour before the test. The mouse needs to be familiarized to the cotton swabs before any odorant is presented to ensure that the sniffing of odor presentations is not a response to the novel object.
The concentration of the odorants should be optimized through pilot experiments using naive mice. The concentration should be high enough for the normal mouse to smell but not overbearing such that the normal mouse would prefer to stay away from the applicator rather than approaching the applicator. A simple way to estimate the concentration of odorants is to make serial dilutions of an odorant and let normal humans smell the odorants at ascending concentrations. The lowest concentration that humans are able to detect is likely to be an appropriate beginning concentration for this experiment.
When multiple odorants are tested sequentially, the order of the odor presentation needs to be carefully determined. Typically mice are more interested in pheromone-containing odorants while neutral to regular chemicals. When both chemicals and a social odor are tested in one assay, the social odor needs to be presented the last. When multiple chemical odorants need to be tested and mice are more interested in some odorants than others, the neutral odorants should be presented earlier while the preferred ones later. For example, ethyle vanillin should be presented after octanol and benzaldehyde because mice are more interested in it (Zou et al., 2012).
Cotton-tip based olfactory preference assay
This purpose of this assay is to determine whether the mouse prefers one odorant to another. This test is commonly used to eliminate the possibility that a deficit shown on another olfactory assay with two odorants involved is not due to the mouse’s preference to one odorant over the other. For example, in olfactory habituation/dishabituation assay, if mice failed to show an increase of sniffing when a novel odorant was presented, one explanation for this result is that the mice were able to recognize the novel odorant but they did not like the smell of it. To rule out this possibility, an olfactory preference test should be performed using the same odorants. An equal amount of time sniffing the two odorants indicates that the deficit of mice in habituation/dishabituation assay is not due to their dislike of the novel odorant.
The concentration of the two odorants should be the same if structurally similar odorants are compared, while if unrelated odorants are compared, the concentration for each odorant should be optimized with normal mice on pilot experiments. The left and right location of the two odorants needs to be balanced among the cohort. The cotton tips should be placed within 2–3 cm to the sidewall of the cage to ensure that the two odorants are well separated.
Cotton-tip based olfactory threshold assay
This assay is designed to assess the mouse’s sensitivity to detect odorants. The mouse is presented to the ascending concentrations of an odorant and the odorless vehicle. To start the test, a very low concentration of the odorant, that has been shown to be at least one order of magnitude below the detection threshold on pilot trial, should be used. Data are expressed as the percentage of the odorant sniffing in the total sniffing on both the odorant and vehicle cotton-tips. A value of about 50% indicates the chance sniffing - the mouse is unable to smell the odorant and thus spends equal amount of time sniffing both cotton-tips. The concentration of the odorant should be increased exponentially with typically 10 folds of increase from the previous concentration. Two adjacent concentrations should be spaced by at least 24 h. The detection threshold is determined by the concentration of the odorant at which the percentage of odorant sniffing rises up from the 50% chance level (typically at 70–85%) and it is increased significantly compared to that of the previous concentration (Fig. 2).
Same as the olfactory habituation/dishabituation assay, the olfactory threshold assay requires the optimal reduction of background odor levels and acclimation of the animals to the testing room to familiarize them with the background odors and reduce the stress to new environment.
The odorant selected for this assay should be a neutral or a slightly favorable one because an unfavorable odorant (eg. TMT, a predator odor) may cause reduced sniffing and avoidance, whereas a highly favorable odorant (eg. mouse urine of the opposite sex) may increase the mouse’s biting of the cotton tips and make the quantification difficult and unreliable.
Occasionally, experimenters may encounter a problem that the curve of the percentage of sniffing ramps up gradually along with the increase of the concentrations of the odorant but no threshold concentration could be determined. This problem could be caused by the individual variation of the detection threshold within the cohort. Some animals are able to smell the odorant at a lower concentration while others are still unable to detect, and thus the average percentage of sniffing among the cohort will vaguely rise above the 50% chance level. On the next day, when the same odorant was presented at a higher concentration, those who were able to detect the odorant on the previous day may still remember it and thus their interest to the odorant was reduced, while those who were unable to detect the odorant on the previous day might be able to smell it and their interest would increase. So, even when the concentration of the odorant has been raised to a level that apparently every animal is able to detect, the average percentage of sniffing at this concentration may not be significantly increased from the previous day.
Several strategies can be used to troubleshoot this problem. The concentration gradients of the odorant should be great enough to avoid ambiguous concentrations. The presentations of two adjacent concentrations should be spaced by a minimum of 24 hrs. If inconclusive data have been obtained, the test could be repeated with a novel odorant, and the interval between the presentations of adjacent concentrations could be increased to 48 h. Switching to a more favorable odorant might also be helpful, so even a mouse can remember the odorant from the presentation of the odorant on a previous day, it might still be interested in the same odorant at a higher concentration the next day. Nevertheless, this test should be assisted with other assays such as electro-olfactogram in order to evaluate an animal’s ability to detect odors.
Cotton-tip based olfactory short-term memory assay
This assay is to assess the mouse’s ability to memorize an odor. The mouse is presented to an odorant twice for 5 min each with an interval between the two presentations. A significant reduction of the sniffing time on the second presentation compared to the first one indicates that the mouse is able to recall the odorant after the time interval (Fig. 3).
Before the olfactory short-term memory test, all mice should be screened with the olfactory habituation/dishabituation test using discrete odorants to ensure that they retain the basic function of olfaction. Novel odorants should be used for the following memory test, and different odorants should be used when different time intervals are tested, with one odorant used for only one time-point. There should be at least a 24-h break between the tests of two time-points to avoid the interference of the memory. The concentration of the odorants used for olfactory memory test should exceed the detection thresholds of the odorants for all test mice.
One confronting factor for this assay is the mouse’s likeness to the odorant. If the mouse likes the odorant, the sniffing may not decline significantly on the second presentation even if the mouse can well recognized the odorant as a familiar one. Therefore, neutral odorants are preferred for this assay.
Sand-digging based olfactory discrimination/learning assay
Both cotton-tip based olfactory habituation/dishabituation assay and sand-digging based olfactory discrimination assay can be used to test the mouse’s ability to discriminate two discrete or similar odorants, however, only the sand-digging based assay is independent of the mouse’s motivation for exploration. The food-restricted mouse is trained to learn to use the odor cue to dig for the food reward, in which the ability to discriminate the positive and negative odorants is required.
Data are expressed as the percentage of correct choices over 8 training trials on a given day and the learning curve is obtained by plotting the data from each training day over the entire training session. The first data point of the curve, the percentage of correct choices on the first training day, should be at around 50%, which is the probability of making correct choice by chance only. The curve ramps up as the training goes on. Typically by the training day 5 to 8, the percentage of correct choices for normal mice could reach 90%. The training could be terminated when there is no progression for two days. Mice with deficient olfactory discrimination would show the learning curve staggering around 50% or significantly progressing slower than that of the normal mice. The sensitivity of the assay could be improved by using similar odorants and/or multiple odorants as described in the alternative protocols of the basic protocol.
While this assay is primarily used for evaluating the olfactory discrimination, it is also a test for the assessment of odor-associated learning. For this purpose, discrete odorant pairs should be used and mouse should first be tested for olfactory discrimination with olfactory habituation/dishabituation assay using the two odorants to ensure that all mice were able to discriminate the two odorants in the first place. Subsequently any retarded progress in sand-digging task-based olfactory assay could be safely explained by the deficiency in odor-associated learning.
In addition to food-restriction, the preparation of this assay includes the pre-training of the mouse for digging the food reward out from the sand with the graduating criteria that all the mice are able to retrieve the deeply-buried food within 30 s (Most mice are able to retrieve the food within 10 s). The first day of pre-train could be frustrating because mice do not recognize the food pellets and the food-delivering apparatus causes some anxiety to mice. In that case, a dish with sand and a few pieces of food pallets could be placed in the mouse cage overnight after the first pre-training day. Individual mice that are still not performing on the second pre-training day should be excluded from the test.
Anticipated Results
Cotton-tip based olfactory habituation/discrimination assay
Animal with normal olfaction should show step-wise reduction of sniffing on the 2–4th presentations of an odorant and a reinstatement of sniffing when a novel odor is presented. Significant habituation-dishabituation, rather than a high level of sniffing, is an indicator for odor recognition and discrimination between odorants (Zou et al., 2012).
Cotton-tip based olfactory preference assay
The animal is presented with two unfamiliar odorants simultaneously. The duration of sniffing toward each odorant is recorded. The significant discrepancy of sniffing between the two odorants indicates the preference for one odorant over the other. Normal mice typically do not prefer one odorant over the other if both odorants are neural odorants, such as IAA and citralva, or structurally similar odorants, such as limonene (+) and limonene (−), while male mice with normal olfaction should prefer female urine odors over male urine odors, and prefer urine odors from estrous mice over ovariectomized mice (Zou et al., 2012; Zou et al., 2013).
Cotton-tip based olfactory threshold assay
The first data point of the detection curve should be around 50%. The curve may wander around the 50% for a couple of more concentrations, depending on how low concentration the test started from and how big the gradient between concentrations. With the increase of the concentrations, the curve rises up steeply to a level of 70–85%, which signs that the concentration has exceeded the detection threshold (Fig. 2). However, the percentage of sniffing at the next concentration might plumb due to the partial memory of the odorant.
Cotton-tip based olfactory memory assay
Our experience using mice with C57Bl/6 and 129 mixed background showed that wild type mice were able to remember odors for up to 5–9 h. The memory of the odor diminished after 24 h (Fig. 3).
Sand-digging based olfactory discrimination/learning assay
Typically three days of pre-training is sufficient for mice to acquire the ability to retrieve food rewards from sand. On day 1 of the olfactory discrimination acquisition training, the percentage of correct choices for both normal mice and mice with olfaction deficits should be around the chance level of 50%. Normal mice may exhibit a significant progress on training day 3, typically no later than day 5, and finally reaches the end points on day 7 to 9 (the average percentage of correct choices reaches 90% and the learning curve plateaus). Mice with deficient olfactory discrimination or learning may present with lagged learning curve and reduced level of competency (decreased percentage of correct choices) at the end of training (Pan et al., 2012; Zou et al., 2012).
Time Considerations
All the olfactory tests described in this unit require the animals to be singly housed and handled for at least one week prior to the test. After this preparation, all the cotton tip-based olfactory tests can be finished within a few days, varying from 5 min for olfactory preference test to 3–5 days for olfactory detection threshold test. In contrast, the sand-digging task-based olfactory discrimination/learning tests are more time-consuming. It typically takes 1 week to perform food restriction and reach the goal body weight. The pre-training lasts for 3 days. The discrimination acquisition training lasts for 7–10 days for each odorant pair, and multiple pairs of odorants may be necessary before a conclusion can be made.
Acknowledgments
The authors would like to thank all members of the Xia and Storm Laboratories for their critical input, evolution and optimization of the protocols in this unit. This work was supported by the National Institutes of Health grant (R01 MH95840) to Z. X.
Literature Cited
- Alberts JR, Galef BG., Jr Acute anosmia in the rat: a behavioral test of a peripherally-induced olfactory deficit. Physiol Behav. 1971;6:619–621. doi: 10.1016/0031-9384(71)90218-6. [DOI] [PubMed] [Google Scholar]
- Breton-Provencher V, Lemasson M, Peralta MR, 3rd, Saghatelyan A. Interneurons produced in adulthood are required for the normal functioning of the olfactory bulb network and for the execution of selected olfactory behaviors. J Neurosci. 2009;29:15245–15257. doi: 10.1523/JNEUROSCI.3606-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy I, Schellong J, Joraschky P, Hummel T. PTSD, but not childhood maltreatment, modifies responses to unpleasant odors. Int J Psychophysiol. 2010;75:326–331. doi: 10.1016/j.ijpsycho.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Curzon P, Rustay NR, Browman KE. Cued and Contextual Fear Conditioning for Rodents. 2009 [PubMed] [Google Scholar]
- Del Punta K, Leinders-Zufall T, Rodriguez I, Jukam D, Wysocki CJ, Ogawa S, Zufall F, Mombaerts P. Deficient pheromone responses in mice lacking a cluster of vomeronasal receptor genes. Nature. 2002;419:70–74. doi: 10.1038/nature00955. [DOI] [PubMed] [Google Scholar]
- Fendt M, Endres T, Lowry CA, Apfelbach R, McGregor IS. TMT-induced autonomic and behavioral changes and the neural basis of its processing. Neurosci Biobehav Rev. 2005;29:1145–1156. doi: 10.1016/j.neubiorev.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Gopinath B, Anstey KJ, Sue CM, Kifley A, Mitchell P. Olfactory impairment in older adults is associated with depressive symptoms and poorer quality of life scores. Am J Geriatr Psychiatry. 2011;19:830–834. doi: 10.1097/JGP.0b013e318211c205. [DOI] [PubMed] [Google Scholar]
- Gregg B, Thiessen DD. A simple method of olfactory discrimination of urines for the Mongolian gerbil, Meriones unguiculatus. Physiol Behav. 1981;26:1133–1136. doi: 10.1016/0031-9384(81)90221-3. [DOI] [PubMed] [Google Scholar]
- Hardy C, Rosedale M, Messinger JW, Kleinhaus K, Aujero N, Silva H, Goetz RR, Goetz D, Harkavy-Friedman J, Malaspina D. Olfactory acuity is associated with mood and function in a pilot study of stable bipolar disorder patients. Bipolar Disord. 2012;14:109–117. doi: 10.1111/j.1399-5618.2012.00986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakupovic J, Kang N, Baum MJ. Effect of bilateral accessory olfactory bulb lesions on volatile urinary odor discrimination and investigation as well as mating behavior in male mice. Physiol Behav. 2008;93:467–473. doi: 10.1016/j.physbeh.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandiyan VS, Coats JK, Shah NM. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nat Neurosci. 2005;8:1660–1662. doi: 10.1038/nn1589. [DOI] [PubMed] [Google Scholar]
- Mechawar N, Saghatelyan A, Grailhe R, Scoriels L, Gheusi G, Gabellec MM, Lledo PM, Changeux JP. Nicotinic receptors regulate the survival of newborn neurons in the adult olfactory bulb. Proc Natl Acad Sci U S A. 2004;101:9822–9826. doi: 10.1073/pnas.0403361101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalick SM, Langlois JC, Krienke JD, Dube WV. An olfactory discrimination procedure for mice. J Exp Anal Behav. 2000;73:305–318. doi: 10.1901/jeab.2000.73-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg PJ, Turetsky BI. Scent of a disorder: olfactory functioning in schizophrenia. Curr Psychiatry Rep. 2003;5:311–319. doi: 10.1007/s11920-003-0061-x. [DOI] [PubMed] [Google Scholar]
- Moreno MM, Linster C, Escanilla O, Sacquet J, Didier A, Mandairon N. Olfactory perceptual learning requires adult neurogenesis. Proc Natl Acad Sci U S A. 2009;106:17980–17985. doi: 10.1073/pnas.0907063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negoias S, Croy I, Gerber J, Puschmann S, Petrowski K, Joraschky P, Hummel T. Reduced olfactory bulb volume and olfactory sensitivity in patients with acute major depression. Neuroscience. 2010;169:415–421. doi: 10.1016/j.neuroscience.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Pan YW, Kuo CT, Storm DR, Xia Z. Inducible and Targeted Deletion of the ERK5 MAP Kinase in Adult Neurogenic Regions Impairs Adult Neurogenesis in the Olfactory Bulb and Several Forms of Olfactory Behavior. PLoS One. 2012;7:e49622. doi: 10.1371/journal.pone.0049622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankevich DE, Baum MJ, Cherry JA. Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. J Neurosci. 2004;24:9451–9457. doi: 10.1523/JNEUROSCI.2376-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahayel S, Frasnelli J, Joubert S. The effect of Alzheimer’s disease and Parkinson’s disease on olfaction: a meta-analysis. Behav Brain Res. 2012;231:60–74. doi: 10.1016/j.bbr.2012.02.047. [DOI] [PubMed] [Google Scholar]
- Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22:2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg H, Doving K, Novikov S, Ursin H. A method for studying responses and habituation to odors in rats. Behav Neural Biol. 1982;34:113–119. doi: 10.1016/s0163-1047(82)91501-1. [DOI] [PubMed] [Google Scholar]
- Trinh K, Storm DR. Vomeronasal organ detects odorants in absence of signaling through main olfactory epithelium. Nat Neurosci. 2003;6:519–525. doi: 10.1038/nn1039. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Hahn CG, Borgmann-Winter K, Moberg PJ. Scents and nonsense: olfactory dysfunction in schizophrenia. Schizophr Bull. 2009;35:1117–1131. doi: 10.1093/schbul/sbp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Balet Sindreu C, Li V, Nudelman A, Chan GC, Storm DR. Pheromone detection in male mice depends on signaling through the type 3 adenylyl cyclase in the main olfactory epithelium. J Neurosci. 2006;26:7375–7379. doi: 10.1523/JNEUROSCI.1967-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DW, Donahou TN, Johnson MO, Wachowiak M. Sniffing behavior of mice during performance in odor-guided tasks. Chem Senses. 2008;33:581–596. doi: 10.1093/chemse/bjn029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Storm DR, Xia Z. Conditional Deletion of ERK5 MAP Kinase in the Nervous System Impairs Pheromone Information Processing and Pheromone-Evoked Behaviors. PLoS One. 2013;8:e76901. doi: 10.1371/journal.pone.0076901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Pan YW, Wang Z, Chang SY, Wang W, Wang X, Tournier C, Storm DR, Xia Z. Targeted Deletion of ERK5 MAP Kinase in the Developing Nervous System Impairs Development of GABAergic Interneurons in the Main Olfactory Bulb and Behavioral Discrimination between Structurally Similar Odorants. J Neurosci. 2012;32:4118–4132. doi: 10.1523/JNEUROSCI.6260-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]