Abstract
During a pregnancy complicated by diabetes, the human placenta undergoes a number of functional and structural pathologic changes, such as increased placental weight and increased incidence of placental lesions including villous maturational defects and fibrinoid necrosis. The pathologic findings reported have differed among studies, potentially reflecting differences in type of diabetes, study methodology, or glycemic control of study participants. Alternatively, these discrepancies may represent different biologic adaptations to distinct metabolic diseases. In order to clarify these distinctions, we conducted a comprehensive review of English language citations in Pubmed and Embase using the keywords “diabetes”, “placenta”, AND “pathology”. Abstracts were reviewed for relevance then full-text articles were reviewed in order to extract a comprehensive summary of current pathological findings associated with pregestational and gestational diabetes mellitus, as well as an understanding of the impact of glycemic control on placental pathology. Placental abnormalities most consistently associated with maternal diabetes are an increased incidence of villous immaturity, increased measures of angiogenesis, and increased placental weight. The literature suggests that, despite similarities in placental abnormalities, differences in placental pathology may reflect differences in pathophysiology among different types of diabetes. Consequently, standardization of terminology used to define placental lesions is warranted. Moreover, further research is needed to investigate the impact of pathophysiology, glycemic control and clinical factors, such as infant sex, weight and race, on placental structure and function.
Keywords: Placenta, pathology, histology, diabetes mellitus, type 1, type 2, gestational
1. INTRODUCTION
The human placenta is the critical organ responsible for the facilitation of nutrient uptake, waste elimination, and gas exchange between mother and fetus (1). The placenta is also a vital source of hormone production such as progesterone and human chorionic gonadotropin that maintain the pregnancy (1). Consequently, placental dysfunction can lead to a number of adverse fetal outcomes (2, 3). Moreover, because the placenta reflects the metabolic milieu of both mother and fetus, it serves as a valuable tool for studying the metabolic perturbations that may take place during pregnancy, such as diabetes mellitus.
The extent to which maternal glycemic control contributes to placental abnormalities remains unclear. Literature demonstrates that, when maternal glucose levels are well-controlled, the placentas from women affected by diabetes are normal as evaluated by routine light microscopy (4, 5). However, several studies have identified histopathologic placental abnormalities among women even with well-controlled pregestational (6-8) and gestational diabetes (9, 10). Moreover, placental abnormalities associated with maternal diabetes have been inconsistently reported in the literature, perhaps reflecting population differences in sample size (6, 11); glycemic control (7, 12); study methodology (13, 14); prenatal care quality (15, 16); or diabetes types (6, 17).
To our knowledge, there have been no systematic reviews evaluating the differences of placental histopathology between pregestational diabetes, defined as type 1 diabetes mellitus (T1DM) or type 2 diabetes mellitus (T2DM); and gestational diabetes (GDM), defined as diabetes diagnosed during pregnancy that is not clearly overt diabetes (18). Consequently, we have developed a comprehensive systematic review of the current literature in order to critically examine the gross and histopathologic findings associated with dysglycemia in pregnancy. The literature will be discussed with respect to diabetes type, pregestational or GDM, as well as by the control groups under investigation and the placental derangements demonstrated.
2. METHODS
2.1 Search strategy
Literature searches of MEDLINE (PubMed) and EMBASE databases were conducted through September 1, 2014 with the key terms “diabetes”, “placenta”, “pathology” and “histopathology”. Two investigators (JH and DD) independently reviewed titles, abstracts, and full-text articles. Additional articles were identified through searching the reference lists from included studies. Search results and included articles were verified by a third investigator (RB-L). Disagreements were resolved by consensus.
2.2 Eligibility criteria
Pre-specified inclusion criteria required that participants included pregnant women classified as having pregestational diabetes or GDM; the study compared findings in two or more comparison groups; and the outcome measure included gross or histopathologic placental abnormalities. Studies were excluded if they examined placental abnormalities in animals; were case-reports or review articles; were comprised of women with diabetes and other pregnancy complications such as preeclampsia or hypertension; did not have a valid comparison group; or did not specify diabetes type.
2.3 Data Extraction and Analysis
Data on population characteristics, diabetes class, and placental abnormalities were extracted. Two investigators performed the data extraction (JH and DD), which was then verified by a third investigator (RB-L). Because of the variability in GDM diagnostic criteria in use (18), data on the criteria used for defining GDM were also extracted. Because of the substantial heterogeneity in study methodology, placental abnormalities under investigation, and population characteristics, a quantitative meta-analysis of the data was not appropriate.
2.4 Exposure Definitions
For the purposes of this systematic review, we categorized study findings by diabetes type as reported by the study authors. Pregestational diabetes is defined as either T1DM or T2DM before pregnancy. T1DM is characterized by a severe deficiency in insulin production due to the autoimmune destruction of islet cells in the pancreas (18). Conversely, T2DM is a metabolic disorder characterized by insulin resistance and relative insulin deficiency (18). Similar to T1DM, it is also characterized by hyperglycemia; persistent hyperglycemia from both T1DM and T2DM has been associated with a number of well-described adverse clinical sequelae, such as retinopathy, nephropathy, peripheral neuropathy, and diabetic encephalopathy (19-21). These conditions reflect the damage hyperglycemia inflicts upon not only the nerves, but also the vasculature, which leads to impaired blood flow with subsequent end-organ damage. Consequently, the presence of maternal systemic vascular complications may also portend the impact to uterine vasculature affecting placental perfusion (22, 23).
GDM has been considered to be a transient insulin resistance potentially resulting from the influence of several pregnancy hormones, including progesterone, cortisol, placental lactogen, prolactin and growth hormone (24). After delivery, the insulin resistance improves, although GDM has been associated with an increased risk of subsequent T2DM. Because the metabolic derangements in GDM are more pronounced in the latter stages of pregnancy (25), GDM is generally responsible for fewer birth defects than pregestational diabetes (26). Nonetheless, GDM is associated with several fetal complications such as macrosomia and hypoglycemia; and maternal complications, including hypertension, preeclampsia, and an increased risk of Cesarean delivery (24).
Additionally, the White classification of diabetes in pregnancy, developed by Priscilla White in 1949 in order to predict perinatal outcomes (27), was used to aggregate populations in several studies included in this systematic review. These criteria, subdivided into different categories by the age of onset, duration of diabetes, and presence of vascular disease, are provided in Table 1. The original White classification did not include a category for GDM; however, Dr. White’s 1965 and 1978 revisions expanded the definition to include GDM (28, 29).
Table 1.
White Classification of Diabetes in Pregnancy1
| Class A | Abnormal glucose tolerance test at any age or of any duration treated only by diet therapy |
| A1 | Gestational diabetes; controlled by diet and exercise |
| A2 | Gestational diabetes; requires insulin |
| Class B | Onset at age 20 or older or with duration of less than 10 years |
| Class C | Onset at age 10-19 or duration of 10-19 years |
| Class D | Onset before age 10 or duration greater than 20 years |
| D1 | Onset before age 10 years |
| D2 | Duration over 20 years |
| D3 | Calcification of vessels of the leg (macrovascular disease) |
| D4 | Benign retinopathy (microvascular disease) |
| D5 | Hypertension (not preeclampsia) |
| Class R | Proliferative retinopathy or vitreous hemorrhage |
| Class F | Renal nephropathy with over 500 mg/d proteinuria |
| Class RF | Criteria for both classes R and F |
| Class G | Many pregnancy failures |
| Class H | Evidence of arteriosclerotic heart disease |
| Class T | Prior renal transplant |
2.5 Outcome Definitions
For the purpose of this systematic review, we categorized placental gross and histologic findings as reported by the study authors. Because placental gross and histologic findings varied among study papers, a true meta-analytical approach was not possible. However, we extracted data on whether or not definitions of placental variables were provided in the study and we listed these variables in the column, “Placental structure/abnormalities under investigation,” in Tables 2, 3, and 4.
Table 2.
Placental Histopathologic Abnormalities in Pregnancies Affected by Pregestational diabetes
| Authors, Year |
Ref | Study type | Population | Diabetes Type | Blinding of pathologist to diabetes diagnosis ? |
Glycemic control ? |
Placental structure/abnormalities under investigation |
Defined placental variables? | Results | |
|---|---|---|---|---|---|---|---|---|---|---|
| Diabetes | Control | |||||||||
| Fox, 1969 | (8) | Prospective | 48 | 234 | Pregestational diabetes |
Not specified | Not specified | Gross lesions (infarction, perivillous fibrin, calcification, retroplacental hematoma, intervillous thrombi); villous abnormalities (excess syncytial knots, villous fibrosis, excess villous fibrinoid necrosis, trophoblastic basement membrane thickening, excess Langhans cells); abnormalities of fetal stem arteries (fetal artery thrombosis, obliterative endarteritis, calcification) |
No | -No statistically significant difference in gross lesions. - Principal histologic abnormalities in placentas from diabetic women were an obliterative endarteritis of the fetal stem arteries, thickening of the trophoblastic basement membrane and villous fibrinoid necrosis. - Placentas in diabetic women had a higher frequency of villous fibrosis and excess syncytial knot formation. - No consistent pattern of disturbance in villous maturation. |
| Asmussen, 1982 |
(6) | Prospective | 9 | Not specified | White Class D, non-smokers |
Not specified | Well- controlled |
Morphological changes in terminal villi and fetal capillaries |
No | - Terminal villi of diabetic women showed changes in maturation, increased vascularization due to small vessels penetrating into the trophoblast, and glycogen accumulation within the stroma cells and pericytes. - Women with diabetes had 2-3 times as many capillaries per terminal villus compared to controls. |
| Bjork & Persson, 1982 |
(37) | Prospective | 17 | 20 | White Class B, C, D, F (all Insulin- dependent) |
Not specified | Not specified | Syncytial knots; vasculosyncytial membranes; hypovascular villi; intravillous hemorrhage; subsyncytial edema; the frequency of immature villi |
Yes | - Placentas from diabetic women had a higher frequency of hypovascular villi, subsyncytial edema, syncytial knots and immature villi |
| Teasdale, 1983 |
(11) | Prospective | 10 (5 with appropriate for gestational age infants; 5 with large for gestational age infants) |
5 | White Class B (not specified whether insulin- dependent) |
Not specified | Excellent | [Within the parenchyma, defined as the intervillous space, trophoblast layer (cytotrophoblast and syncytiotrophoblast) and the fetal capillaries of both peripheral and stem villi] Volume of intervillous space and fetal capillary bed; the number per unit area, surface densities and surface areas of the villi and their fetal capillaries; the number of syncytiovascular membranes per 100 peripheral villi |
Yes | - Placentas from diabetic women were significantly heavier. - Placenta for appropriate for gestational age infants morphologically similar to control group except for villous vascularization. - The placenta of large for gestational age infants had significantly greater accumulation of non- parenchymal tissue (sum of the decidual and chorionic plates, intercotyledonary septa, fetal vessels, connective tissue of the villi, fibrin deposits, and infarcts) and a moderate increase in parenchymal tissue compared to control group. |
| Bjork & Persson, 1984 |
(17) | Prospective | 13 | 13 | T1DM | Not specified | Not specified | Length and area of villi in three well-defined areas within a cotyledon (central, intermediate and lateral regions) |
Yes | - While normoglycemic controls showed consistent organization of the cotyledon with increasing villous length towards the periphery, villi were of even length through the cotyledon in women with T1DM. - Average surface area of the cotyledon due to increased branching of peripheral villi was significantly greater in women with T1DM. |
| Teasdale, 1985 |
(38) | Prospective | 10 | 5 | White Class C (not specified whether insulin- dependent) |
Not specified | Excellent | [Within the parenchyma, defined as the intervillous space, trophoblast layer (cytotrophoblast and syncytiotrophoblast) and the fetal capillaries of both peripheral and stem villi] Volume of intervillous space and fetal capillary bed; the number per unit area, surface densities and surface areas of the villi and their fetal capillaries; the number of syncytiovascular membranes per 100 peripheral villi |
Yes | - Placentas from diabetic women had significantly greater increase in parenchymal (intervillous space, the trophoblast layer, and fetal capillaries of both the peripheral and stem villi) and non-parenchymal tissue (sum of the decidual and chorionic plates, intercotyledonary septa, fetal vessels, connective tissue of the villi, fibrin deposits, and infarcts). |
| Boyd et al., 1986 |
(7) | Prospective | 14 | 22 | T1DM | Not specified | Moderate to good glycemic control |
Volume and surface areas of parenchymal tissue (consisting of villi, including fetal capillaries, and the maternal intervillous space) and non-parenchymal tissue (chorionic and decidual plates, fetal vessels of diameter >0.1 cm and intercotyledonary septa); villous surface area |
Yes | - Placentas from diabetic women had significantly increased volume of parenchymal tissue and decreased volume of non- parenchymal tissue. - Placentas from diabetic women had significantly increased villous surface area, with the mean value being 17.3 m2 compared to 11.4 m2 of the normoglycemic women. |
| Jirkovska, 1991 |
(30) | Prospective | 13 | 14 | T1DM | Not specified |
Good | Thickness of capillary basement membrane |
Yes | - Placentas of women with T1DM had increased number of capillaries in the terminal villi - Placenta of women with T1DM had significantly thinner capillary basement membrane |
| Mayhew et al., 1993 |
(39) | Prospective | 11 | 34 | White Class D with benign retinopathy (no proliferative retinopathy or other serious complications) |
Yes | Good | Volumes of the following tissue compartments: intervillous space (excluding fibrin deposits), peripheral villi, villous trophoblast, villous stroma, fetal capillaries and non-parenchymal tissue (comprising decidual and chorionic plates and intercotyledonary septa); total lengths and exchange surface areas of villi and fetal capillaries |
Yes | - Placentas in women with diabetes were 17% heavier. - Volume of fetal capillaries was 45% greater in women with diabetes and 30% larger in males. - Compared to Cesarean deliveries, vaginal deliveries had greater stromal diffusion distance (measured from the fetal aspect of trophoblast to the liminal aspect of capillary endothelium). |
| Mayhew, 2002 |
(31) | Prospective | 34 | 34 | T1DM | Not specified | Good | Volume, surface areas and lengths of peripheral villi and their capillaries |
Yes | - Placentas of women with T1DM had increased volume of fetal capillaries (19-45% greater) that is attributed to the increases in the combined length (12-47% greater) of capillaries and not to alteration of vessel cross- sectional area or perimeter. |
| Evers et al., 2003 |
(32) | Prospective | 71 | 38 | T1DM | Yes | Excellent or good in 82% of women |
Lymphohistiocytic villitis; ischemia; infarction; presence of nucleated fetal red blood cells; villous fibrinoid necrosis; degree of villous immaturity; chorangiosis; hydropic villi; fetal vessel thrombosis/avascular villi |
Yes | - Placentas of women with diabetes were significantly heavier. - Placentas of women with diabetes had increased incidence of nucleated fetal red blood cells, fibrinoid necrosis, villous immaturity, and chorangiosis. - Placenta of women with large for gestational age infants had a higher incidence of histological abnormalities compared to those of women with appropriate for gestational age infants. |
| Maly et al., 2005 |
(33) | Prospective | 10 | 13 | T1DM | Not specified | Good | Volume and surface area of placental villi and capillaries |
Yes | - Placentas of women with diabetes had more than 5-fold decrease in villous vascular volume |
| Jauniaux et al., 2006 |
(12) | Prospective | 12 | 10 | T1DM | Not specified | 5 patients with poor glycemic control |
Volume and surface area of placental villi and capillaries |
Yes | - Placentas of women with diabetes had a significant increase in fetal and placental weights, placental volume, volumes of the intervillous space and the trophoblast found in diabetic group compared to controls. |
| Nelson et al., 2009 |
(34) | Prospective | 88 | 39 | T1DM | Not specified | Not specified | Volume and surface areas of placental villi and capillaries |
Yes | - Placentas of women with diabetes were significantly heavier. - Placentas of women with diabetes had a significant increase in the intervillous space volume but villous, non- parenchymal, trophoblast and capillary volumes did not differ. |
| Jirkovska et al., 2012 |
(35) | Prospective | 17 | 14 | T1DM | Not specified | Not specified | Spatial arrangement of villous capillary bed and quantitative measures of capillary branching pattern |
Yes | - Placentas from mothers with T1DM had changed size and course of capillaries, altered structure of the villous stroma and enhanced sprouting angiogenesis in the terminal villi. |
| Higgins et al., 2012 |
(36) | Prospective | 74 (9 women with T2DM; 65 women with T1DM) |
77 | Pregestational diabetes |
Yes | Not specified | Delayed villous maturation |
Yes | - Placental diagnosis of delayed villous maturation was significantly increased in women with pregestational diabetes (28.4%) compared to controls (14.3%). |
Table 3.
Placental Histopathologic Abnormalities in Pregnancies Affected by Gestational Diabetes
| Authors, Year |
Ref | Study type | Population | Diabetes Diagnosis Criteria |
Blinding of pathologist to diabetes type? |
Glycemic control? | Placental structure/ abnormali ties under investigation |
Defined placental variables? |
Results | |
|---|---|---|---|---|---|---|---|---|---|---|
| GDM | Control | |||||||||
| Teasdale, 1981 |
(9) | Prospective | 5 | 5 | O' Sullivan and Mahan criteria |
Not specified | Good | Volume of intervillous space and fetal capillary bed; the number per unit area, surface densities and surface areas of the villi and their fetal capillaries; the number of syncytiovascular membranes per 100 peripheral villi |
Yes | - Significant increase of volumes and surface areas of parenchymal tissue components (intervillous space, trophoblast and fetal capillaries) in placentas of women with GDM. |
| Lao, 1997 | (40) | Retrospecti ve |
21 | 478 | WHO criteria |
Not specified | Not specified | Placental weight | Yes | - Placentas of women with GDM were significantly heavier. |
| Jirkovska, 2002 |
(41) | Prospective | 11 | 9 | Not specified | Not specified | Not specified | Vascular topography and branching |
Yes | - Basic arrangement of villous capillaries similar in both normal and diabetic placentas. - Proportion of simple forms of the capillary bed without redundant connections is significantly decreased and the mean number of redundant connections per villus increased in diabetic placentas compared to controls. |
| Taricco et al., 2003 |
(14) | Prospective | 132 | 143 | Carpenter -Coustan criteria |
No | Not specified | Placental weight | Yes | - Placentas of women with GDM had significantly higher placenta and lower fetus/placenta weight ratios. |
| Ashfaq, 2005 |
(42) | Prospective | 20 | 20 | Not specified |
Not specified | Not specified | Placental weight | Yes | - Placentas of women with GDM had significant increases in weight, central thickness and diameter of the placenta. |
| Daskalakis et al., 2007 |
(10) | Prospective | 40 | 40 | Carpenter -Coustan criteria |
Yes | Excellent to good | Lymphohistiocytic villitis, presence of nucleated red blood cells, ischemia, infarction, villous fibrinoid necrosis, villous immaturity, chorangiosis, hydropic villi, fetal vessel thrombosis, avascular villi |
Yes | - Placenta of women with GDM had increased incidence of fibrinoid necrosis and chorangiosis. - Placentas of women with GDM had increased incidence of villous immaturity and presence of nucleated fetal red blood cells. |
| Madazli et al., 2008 |
(43) | Prospective | 22 | 22 | 2001 ADA criteria |
Yes | Good | Villous immaturity, chorangiosis, lymphohistiocytic villi, villous fibrinoid necrosis, ischemia, infarction, the presence of nucleated red blood cells |
Yes | - Placentas of women with GDM had increased incidence of villous immaturity, chorangiosis and ischemia. - The incidence of fibrinoid necrosis, nucleated red blood cells counts, infarction and villitis increased in diabetic women but did not reach statistically significance. |
Table 4.
Placental Histopathologic Findings Comparisons
| Authors et al., Year |
Ref | Study type | Population | Diabetes Classification |
Blinding of pathologist to diabetes diagnosis? |
Glycemic control? |
Placental structure under investigation |
Defined placental variables? |
Results | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | F | R | Control | |||||||||
| Jacomo et al., 1976 |
(44) | Prospective | 5 | 11 | 13 | 9 | 2 | 2 | 20 | White’s Classification of Diabetes |
Not specified | Not specified | Placental capillaries and villi; Endothelial proliferation and edema, endothelial edema, muscular hypertrophy; stroma edema, stromal cellularity, size of the villi, and blood vessels of villous trunks |
Yes | - No statistically significant differences between occurrence of stromal edema, stromal cellularity, in the size of the villi between control and diabetic placenta, or between White classes. - Placentas from diabetic group had higher incidence of partial or total obstruction of the vascular lumen due to endothelial proliferation and edema compared to control group but no statistical difference found between White classes. - White classes A+B+C had higher percentage of total vascular lesions compared to controls but no difference found between White classes. |
| Stoz et al., 1987 |
(45) | Prospective | 22 | 22 | White’s Classification of Diabetes |
Not specified | Not specified | Volume and surface area of placental capillaries and villi |
Yes | - Significant increase in cross sectional surface area of terminal villi and villous circumference in diabetic placentas to normal placentas which were more prominent in women with White Classes A+B+C diabetes compared to White Class D diabetes. - Number of vessels in diabetic placentas is significantly decreased compared to control placentas and most often expressed in women with White Class B diabetes. |
|||||
| Mayhew et al., 1994 |
(46) | Prospective | 39 | 16 | 34 | White’s Classification of Diabetes |
Yes | Good | Volume and surface area of placental capillaries and villi |
Yes | - Diabetic placenta had a more voluminous fetal capillary bed of greater length, diameter and surface area compared to controls, but no significant differences detected between White classes. |
||||
| Mayhew, 1998 |
(47) | Prospective | 6 | 4 | 6 | 6 | White’s Classification of Diabetes |
Not specified | Good | Various morphometric variables (including the star volumes of villous ’domains’ and intervillous ’pores’ and trophoblast surface denudation) |
Yes | - No significant differences detected between control and diabetic placenta, or between White classes. |
|||
| Mayhew et al., 2000 |
(4) | Prospective | 7 | 4 | 6 | 8 | White’s Classification of Diabetes |
Not specified | Good | Volume and surface area of placental capillaries and villi |
Yes | - Volume densities of villi (terminal and peripheral), intervillous spaces and perivillous fibrin-type fibrinoid deposits did not differ between diabetic groups, but was statistically different in non-insulin-dependent placentas compared to controls |
|||
| Makhseed et al., 2002 |
(48) | Prospective | 40 | 36 | 7 | 65 | White’s Classification of Diabetes |
Not specified |
Not specified | Microscopic and macroscopic features of placenta, including immaturity, maternal floor infarction, intravascular thrombosis, villitus, infarction, funitis, chorioamnionitis, and calcification |
Yes | - Placental weight and mean placental surface area and perimeter significantly higher in diabetic groups White A and White B compared to control. - No statistical significant associations of microscopic abnormalities found between control and diabetic placenta, or between White classes. |
|||
| Authors et al., Year |
Ref | Study type | Population | Diabetes Comparison |
Blinding to pathologist |
Glycemic control? |
Placental structure under investigation |
Defined placental variables? | Results | ||||||
| GDM | T1DM | T2DM | Control | ||||||||||||
| Clarson et al., 1989 |
(49) | Prospective | 11 | 19 | 11 | Pregestational diabetes and GDM |
Not specified | Good | Placental weight | Yes | - No significant difference between placental weights between control and diabetic placenta, or between women with pregestational diabetes or GDM. |
||||
| Al-Okail et al., 1994 |
(50) | Prospective | 6 | 6 | 6 | Pregestational diabetes and GDM |
Not specified |
Poorly controlled GDM and well- controlled pregestational diabetes |
Histological changes in placental syncytiotrophoblast |
No | - Fibrin thrombi in syncytiotrophoblast, villous edema, hyperplasia, and thickening of basement membrane increased in women with poorly controlled GDM compared to pregestational diabetes and control. |
||||
| Younes et al., 1996 |
(51) | Prospective | 18 | 13 | 17 | Pregestational diabetes and GDM |
Not specified |
Variable | Histological changes in placental basement membrane; Volume and surface area of placental capillaries and villi |
No | - Placenta from diabetic women had a higher prevalence of villous immaturity, fibrinoid material, hypertrophy of the capillaries, and thickening of the basement membrane and the amniotic membrane compared to controls. - The amniotic cells were significantly tall columnar in shape in 90% of diabetic cases with distally located nuclei. - The thickening of the trophoblast basement membrane, capillary basement membrane and the increase in fibrinoid material was more prominent in cases of GDM compared to pregestational diabetes. |
||||
| Calderon et al., 2007 |
(52) | Prospective | 59 | 41 | 56 | Pregestational diabetes and GDM |
Yes | Not specified | Volume and surface area of placental capillaries and villi |
No | -In diabetic placentas, both the mean area and total area of villous vessels were smaller while the number of villous vessels was similar to that in control group. -The villous capillarization index was significantly lower in diabetic pregnancies. |
||||
| Dubova et al., 2011 |
(53) | Prospective | 11 | 12 | 6 | T1DM and GDM |
Not specified | Not specified | Area of intervillous space, areas and perimeters of the terminal and mature intermediate villi, their capillaries, and syncytiotrophoblast, and the number of capillaries |
Yes | -Similar changes in the studied parameters of placental villi in T1DM and GDM but deviation of the studied morphometric parameters of placental villi were most pronounced in T1DM. -67% of placentas with T1DM displayed villous immaturity compared to 46% placentas of women from GDM. - Area and perimeter of the villi were reduced by 17% and 12% in women with T1DM compared to 15% and 8% in women with GDM. |
||||
| Rudge et al., 2011 |
(55) | Prospective | 8 | 83 | 6 | Pregestational diabetes and GDM |
Yes | Variable | Histopathologic placental lesions (including lesions of circulatory pathology, lesions indicative of degeneration, lesions of proliferation, and lesions of inflammation) |
No | -Women with overt diabetes displayed all 22 examined histopathologic changes, 21 of which were already present in the preterm period. -Women with GDM had only nine histopathologic changes (cystoid degeneration, congestion, villous edema, intervillous fibrosis, calcification, focal hyaline degeneration, dysmaturity, focal amnionitis, endarteritis). -Additional histopathologic changes found only in women with pregestational diabetes included chorial edema, intima edema, interstitial hemorrhage, subchorial infarct, villous fibrosis, Hofbauer hyperplasia, chorioangiosis, syncytial nodes, villitis, phantom cells, two vessels, duplicate membrane, and cord hemorrhage. |
||||
| Higgins et al., 2011 |
(13) | Prospective | 10 | 8 | 10 | T1DM and T2DM |
Yes | 9 women in diabetic groups had poor glycemic control |
Volume, length and surface area of placental components |
Yes | -Terminal villous volume significantly increased in both diabetic groups compared to controls. -Capillary volume and length was significantly increased in T1DM pregnancies compared to controls and T2DM. |
||||
| Beauharnais et al., 2012 |
(15) | Retrospective | 53 | 45 | T1DM and T2DM |
Yes | Not specified but no difference in glycemic control between two groups |
Placental weight, placental immaturity, villous maturation, villitis of unclear etiology, histological evidence of placental infarction |
Yes | - Higher prevalence of placental infarcts and lower prevalence of placental immaturity in women with T2DM compared with T1DM. - No significant differences in placental weight, villous maturity, or villitis of unclear etiology. |
|||||
| Bentley- Lewis et al., 2014 |
(16) | Retrospective | White (GDM): 48 | Non-White (GDM): 78 |
White and non-white women with GDM |
Yes | Not specified | Gross parameters including placental weight, cord weight, cord length, and cord insertion site, villous immaturity, maternal inflammation, acute chroioamnionitis, measures of fetal stress, placental perfusion pathologies, decidual vasculopathy |
Yes | - White women had higher incidence of chorangiosis compared to non-white women. |
|||||
2.6 Methodological Quality Assessment
The study team assessed the methodological quality of the studies by comparing study design, inclusion criteria, and the blinding of investigators to pregnancy outcome or causation.
3. RESULTS
3.1 Study characteristics
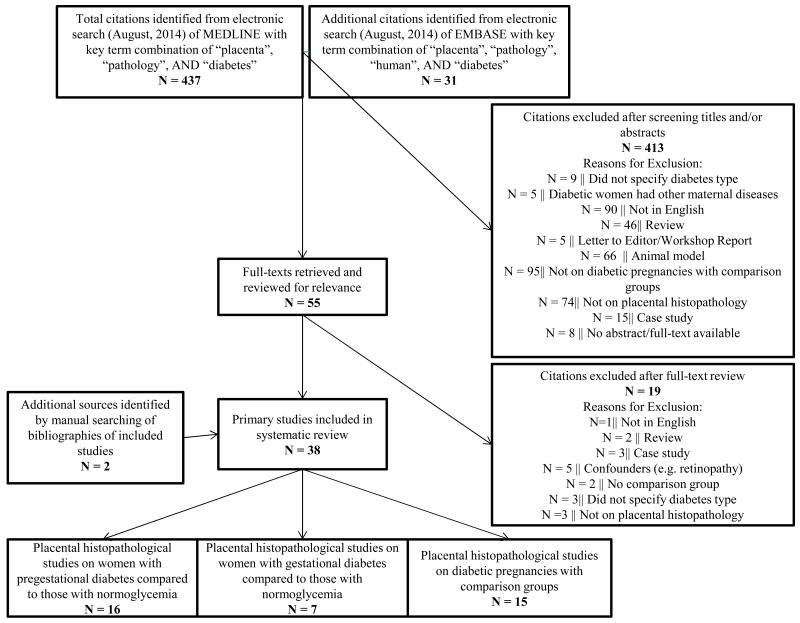
The selection algorithm for the 38 studies that met the inclusion and exclusion criteria for our systematic review is detailed in Figure 1. Study characteristics, diabetes type, placental abnormalities examined, and population characteristics for studies in pregnancies affected by pregestational diabetes are summarized in Table 2. Nine of the 16 studies included in this systematic review examined women with T1DM compared to normoglycemic controls (7, 12, 17, 30-35). Two other studies identified women as having pregestational diabetes (8, 36) while the remaining five studies defined the women by the White classification (6, 11, 27, 37-39). No studies were identified that specifically examined women with maternal T2DM compared to a normoglycemic population.
Fig. 1.
Systematic review selection algorithm.
The 38 studies that fulfilled the inclusion/exclusion criteria for this systematic review were selected from among 468 studies identified in the initial PubMed and Embase literature reviews. The selection algorithm is presented here.
Study characteristics, diagnostic criteria, placental abnormalities examined, and population characteristics for studies of pregnancies complicated by GDM are summarized in Table 3. Seven studies examined placental gross or histomorphometric features in women with GDM compared to those with normoglycemia (9, 10, 14, 40-43). Diagnostic criteria for GDM were inconsistent across studies: two studies used Carpenter-Coustan criteria (10, 14); one study used the 1964 O’Sullivan and Mahan criteria (9); one study used 2001 ADA criteria (43); one study used the WHO criteria (40); and two studies did not specify the diagnostic criteria used (41, 42).
Study characteristics, diabetes type, placental abnormalities examined, and population characteristics for studies examining more than one type of diabetes are summarized in Table 4. Six studies compared findings across different White classifications with a normoglycemic control group (4, 44-48); five studies compared findings between pregestational and GDM with a normoglycemic control group (49-52); one study compared findings between T1DM and GDM with a normoglycemic control group (53); two studies examined differences between T1DM and T2DM (13, 15); and one study examined placental histomorphometry in women with GDM using race/ethnicity as the basis for comparison (16). Common gross and histopathologic placental findings in pregestational diabetes and GDM compared to healthy controls are provided in Table 5.
Table 5. Common Gross and Histopathologic Placental Findings.
| Placental pathology | Progestational diabetes | Gestational diabetes | |
|---|---|---|---|
| Type 1 diabetes | Type 2 diabetes | ||
| Growth/weight | - Increased placental weight compared to healthy controls |
- Increased placental weight |
|
| Degree of villous maturity |
- Increased frequency of immature villi compared to healthy controls |
- Increased frequency of immature villi |
|
| Measures of angiogenesis |
- Increased number of capillaries in terminal villi compared to healthy controls - Increased mean number of redundant connections per terminal villi compared to healthy controls - Greater combined length of fetal capillaries compared to healthy controls |
- Increased mean number of redundant connections per terminal villi compared to healthy controls |
|
| Volume and surface areas of parenchymal tissue (contains the structure or compartments that are strictly concerned in metabolic exchanges between mother and fetus) |
- Increased volume of parenchymal tissue compared to healthy controls |
- Increased volume of parenchymal tissue compared to healthy controls |
|
| Maternal vascular (primary vascular and secondary vascular lesions) |
- Increased incidence of nucleated fetal red blood cells, fibrinoid necrosis, villous immaturity, and chorangiosis compared to healthy controls |
- Increased incidence of fibrinoid necrosis and chorangiosis |
|
3.2 Quality assessment
Quality of included studies varied considerably; only three out of 38 included studies were retrospective, case-controlled studies (15, 16, 40); twelve included studies specified whether or not pathologists were blinded to diabetes diagnosis or pregnancy outcomes and 22 out of 38 included studies provided information on the degree of glycemic control in their study population.
3.3 Placental abnormalities reported in studies of pregestational diabetes
Placental abnormalities reported in relation to pregestational diabetes varied considerably across studies, depending mostly on the placental anatomy under investigation. Increased villous immaturity (32, 36, 37) and increased volume and surface area of parenchyma tissue (7, 11, 38) were the placental abnormalities most consistently reported. Three studies measured the volume and surface areas of the parenchyma, described in 1966 by Aherne and Dunnill as the villi, including fetal vessels and the maternal intervillous space; and non-parenchymal tissue comprised of the chorionic and decidual plates, fetal vessels of diameter greater than 0.1 cm, and intercotyledonary septa (7, 11, 38, 54). Teasdale found increased non-parenchymal and parenchymal tissue in the placentas of women with well-controlled White class B and C diabetes (11, 38). In contrast, Boyd et al. found increased parenchymal volumes, on average 12% larger than placentas from normoglycemic controls, but a decrease in non-parenchymal tissue in women with well-controlled T1DM compared to normoglycemic controls (7). Three studies reported an association between the incidence of villous immaturity, defined as placentas with inadequate or absent terminal villi, and pregestational diabetes (32, 36, 37). The prevalence of placental immaturity in the normoglycemic obstetrical population has been reported to be 14% and the presence of pregestational diabetes nearly doubles the risk of delayed villous maturation (36).
3.4 Placental abnormalities reported in studies of GDM
Similar to studies of pregestational diabetes, placental histomorphometric abnormalities studied in relation to GDM varied considerably. Increased placental weight (14, 40, 42) was the placental abnormality most consistently studied and reported. Three studies reported heavier placentas in women with GDM compared to normoglycemic controls (14, 40, 42). Ashfaq et al. observed that placenta of women with GDM had 22% increase in weight, 33% increase in diameter, and 85% increase in central thickness compared to normal placentas (42). Taricco et al. also reported lower fetus to placenta weight ratios in women with GDM (14). Two studies examined the incidence of placental immaturity and histopathologic placental lesions (10, 43), reporting a significant increase in fibrinoid necrosis (10), chorangiosis (10, 43), and ischemia, defined by increased maturation band Tenney-Parker changes or microscopic/macroscopic infarcts (43), and villous immaturity (10, 43) in women with GDM compared to women with normoglycemia.
3.5 Placental abnormalities reported in studies of across diabetes type
Most studies using the White classification of diabetes did not observe significant histological differences in volume and surface area of terminal villi or growth and maturational status of villi among placentas across different White classifications (4, 44, 46-48). Stoz et al. found that the significant increase in the cross-sectional surface area of the terminal villi and villous circumference compared to those from normoglycemic placentas was more prominent in women with White classes A, B, or C compared to White class D (45). They also observed that while the number of vessels was significantly decreased across all White classifications compared to normoglycemic placentas, the number of vessels was most prominently decreased in women with White class B (45).
In studies of women with pregestational diabetes compared to those with GDM, Clarson et al. and Calderon et al. found no significant differences in placental weights (49) or in the mean and total area of villous vessels (52). Al-Okail et al. found increased fibrin thrombi in the syncytiotrophoblast, villous edema, hyperplasia, and thickening of the basement membrane in the placentas of women with poorly controlled GDM compared to those with pregestational diabetes (50). In contrast, Rudge et al. observed that women with GDM presented with nine types of histopathologic change (cystoid degeneration, congestion, villous edema, intervillous fibrosis, calcification, focal hyaline degeneration, dysmaturity, focal amnionitis, endarteritis) compared to 22 types of histopathologic change present in placentas of women with pregestational diabetes(55). Additional histopathologic changes found only in women with pregestational diabetes included chorial edema, intimal edema, interstitial hemorrhage, subchorial infarct, villous fibrosis, Hofbauer hyperplasia, chorioangiosis, syncytial knots, villitis, phantom cells, two vessels, duplicate membrane, and cord hemorrhage (55). Younes et al. found that the thickening of the trophoblast basement membrane, capillary basement membrane, and the increase in presence of fibrinoid material were more prominent in cases of GDM compared to pregestational diabetes (51).
3.7 Variations in Placental Variable Definitions/Descriptions
Placental variables under investigation were defined in 32 out of 38 papers. There was clear variability in definitions used for commonly studied placental abnormalities, especially found in descriptions of villous maturity, parenchymal tissue and measures of angiogenesis. While several papers reported a difference in villous maturation in women with diabetes compared to controls, definitions of villous maturity differed among studies. Fox (8) studied fetal stem arteries for the overall villous maturity, taking into account specifically the size of the villi, the number of syncytial knots, the degree of stromal condensation and the position and size of the fetal villous capillaries. By this definition, Fox (8) reported that 29.2% of pregestational diabetic placentas showed accelerated maturation, 27.1% delayed maturation, and 43.7% normal maturation. Bjork and Persson (37) observed a higher frequency of immature villi in pregestational diabetic placenta compared to controls. In this study, immature villi was also referred to as “syncytial sprouts”, defined as structures containing syncytial nuclei forming a bud that extends into the intervillous space with a narrow blood vessel at its base that clearly extended into this sprout (37). Evers et al. (32) also reported an increased incidence of villous immaturity that was defined as moderate where there was a decreased formation of terminal villi compared to healthy controls and a relatively increased presence of mature intermediate villi in relation to gestational age. Furthermore, in studies of women with GDM, Daskalakis et al. and Madazli et al. characterized villous immaturity as a decreased formation of terminal villi and increased presence of immature intermediate villi in relation to gestational age (10). Higgins et al., in a study comparing placentas of women with T1DM to T2DM, provided a more detailed description of immature villi where immature intermediate villi had diameter greater than 80 m containing arterioles and venules or capillaries whose main function is villous growth (13). Immature intermediate villi also contain reticular stroma (13).
Moreover, several studies compared the parenchymal tissue in diabetic women compared to healthy controls (7, 9, 11, 38). In women with White Class B, White Class C, and White Class A diabetes, Teasdale (9, 11, 38) found a significant increase in parenchymal tissue, defined as the intervillous space, the trophoblast layer, and fetal capillaries of both the peripheral and stem villi. Boyd et al. (7) also found an increased volume of parenchymal tissue; however, this definition did not include the trophoblast layer, and consisted only of the villi of the fetal capillaries and the maternal intervillous space.
Several articles included in this systematic review also investigated measures of angiogenesis (31, 35, 41). Two studies adopted the term “redundant capillary connections”, connections that can be removed without disconnecting from the villous capillary bed. Jirkovska et al. reported increased measures of angiogenesis, determined by an increase in the mean number of redundant connections per terminal villi, in women with GDM (35, 41) and in women with T1DM (35) compared to controls. While another study reported increased measures of fetoplacental angiogenesis, Mayhew et al. (31) determined this increase by comparing capillary volume, surface area and length. Mayhew et al. (31) characterized these changes in angiogenesis as exclusively longitudinal and not accompanied by any increase in vascular remodeling or changes in capillary caliber.
4. DISCUSSION
To our knowledge, this is the first comprehensive systematic review of placental histopathology in the setting of maternal diabetes. Strengths of this systematic review include the rigorous and detailed method of identification and review of the relevant literature. However, a notable limitation is that we were unable to use a meta-analytical approach to substantially compare data across studies spanning from 1969 to 2014 because of the varied placental abnormalities examined and study methodologies employed. This systematic review also detailed placental variables as reported by the authors of included studies. As there was also wide variability in the definitions of placental variables, we were further limited in our ability to conduct a true meta-analysis of the results.
To reduce the methodological variability across clinical studies of placental pathology, standardization of placental variable definitions is critical in directing future research. A recent review calls for a clinically oriented, unifying and simple placental pathology classification system; yet, more research is still needed to define international diagnostic criteria to describe placental morphology (56). Furthermore, future studies should clearly describe the methodology and definitions pathologists used to report placental abnormalities. Our systematic review uncovered that only 12 out of 38 included studies specified whether or not pathologists were blinded to diabetes diagnosis and pregnancy outcome. Moreover, 32 of 38 included studies defined the placental variables under investigation and there was evident variability in the definitions of villous immaturity, parenchyma, and measures of angiogenesis. Nonetheless, this critical examination of placental pathology across types of diabetes will enable us to demonstrate pathological patterns that may inform how we consider future clinical practice and research implications of pregestational and GDM.
The most common placental findings in the setting of pregestational diabetes and also reported in GDM in this systematic review are increased villous immaturity and increased measures of angiogenesis. Angiogenesis, defined as the process of new blood vessels arising from preexisting ones, is essential for normal fetal growth and placental development. Generally, there are two phases of angiogenesis: (1) branching angiogenesis with formation of tightly looped capillaries and (2) non-branching angiogenesis with formation of longer capillaries (57, 58). One study by Mayhew et al. reported increased non-branching angiogenesis, without a change in the cross-sectional caliber or shape of the capillaries, compared to controls in placentas affected with maternal T1DM despite adequate glycated hemoglobin levels (31). Conversely, Jirkovska et al. observed increased branching angiogenesis, hypovascular and hypervascular terminal villi, and enhanced capillary surface areas in terminal villi of placentas affected by maternal T1DM with good glycemic control (59). Four separate studies also reported an increase in the number of capillaries per villi in women with T1DM (30), White Class D (6), and GDM (9, 41). These findings suggest that placentas from pregnancies complicated by dysglycemia may display both increases in branching and non-branching angiogenesis. Additionally, increased incidence of placental villous immaturity was commonly seen in placentas affected by pregestational (8, 32, 37) and GDM (10, 43). This placental abnormality, which has been independently associated with an increased risk of perinatal mortality (60), may serve as a connection between maternal diabetes and an increased risk of fetal intrauterine death (61). Further study in these areas is warranted to elucidate etiologic mechanisms.
Although there were several similarities in placental histopathologic findings associated with T1DM, T2DM, and GDM, the different pathophysiology of these three diabetic conditions may differentially impact placentation, despite similar medical care and glycemic control. For example, hypertension (62) and inflammation (63), both more frequently associated with T2DM than T1DM (64, 65), are associated with distinct placental pathological abnormalities which may predominate in T2DM relative to T1DM placentas (26). In fact, one study reported that placentas of pregnancies complicated by T2DM had a significant increase in placental infarcts compared to T1DM (15), a vascular abnormality that has also been found in studies of hypertension in pregnancy (66). Moreover, this difference was found to be attenuated after controlling for the hypertension (15).
The time course of metabolic insult may also influence the degree of placental abnormality. For example, the metabolic derangements in GDM may be more pronounced in the latter stages of pregnancy (25), potentially implying fewer severe placental aberrations. Yet, placental abnormalities in women with GDM relative to those with pregestational diabetes have not been consistently studied or reported. One study observed a greater number of histopathologic lesions in women with pregestational diabetes compared to GDM and reported that most of these placental lesions were already present prior to 37 weeks gestational age, suggesting that distinct placental changes may vary based on the gestational period during which the diabetic insult occurred (55). However, other studies reported increased fibrinoid material (50, 51) and thickening of the basement membrane (50, 51) in women with GDM compared to those with pregestational diabetes.
Moreover, diabetes duration has been examined in the context of studies comparing women across different White classifications. However, there were no observed significant histological differences among placentas (4, 44, 46-48), which suggests that may not differentially impact placental pathology. Because the population sizes of these studies were small, ranging from 2 to 40 women within each diabetic subgroup, further research comparing placental abnormalities in women with pregestational and GDM across different White classifications is warranted.
Notably, although glycemic control has been considered integral to the pathophysiology of placental abnormalities, only 22 of 38 studies included in this review detailed the degree of glycemic control of their study populations, with 16 studies specifying moderate to good glycemic control. Poor glycemic control in the periconception period and first trimester of pregnancy has been linked to a number of maternal adverse outcomes, such as hypertension, preeclampsia, Cesarean section, and hypoglycemia, as well as numerous fetal adverse outcomes, including fetal and neonatal loss, fetal growth acceleration, macrosomia, stillbirth, and respiratory distress syndrome (67). Moreover, the correction of hyperglycemia might not prevent the development of placental abnormalities since several studies have shown that placental histopathologic changes persisted even in pregnancies with well-controlled diabetes (4, 6, 7, 9, 10, 30-33, 38, 39, 43, 46). Consequently, additional research on placental histopathology in maternal diabetes should include a consideration of the glycemic control of diabetic populations in order to maximally characterize the impact of dysglycemia on placental histopathology.
Furthermore, additional clinical factors must be considered when reporting on placental abnormalities. Some studies have shown that mode of delivery (39), sex of the infant (39), birth weight of infant (11, 32), and race (16) may be associated with distinct placental pathologies. Mode of delivery was observed to have significant main and interaction effects on the placental stromal diffusion distance. This was found to be 25% greater in vaginal deliveries (39). In addition, fetal sex has been implicated in placental pathologic changes. The fetal capillary volume was found to be 30% greater in males compared to female infants(39). Moreover, Teasdale et al. found that the placentas from diabetic pregnancies with appropriate-for-gestational age infants were morphologically similar to those from normoglycemic pregnancies except in the case of villous vascularization. However, the placenta of large-for-gestational age infants had significantly greater accumulation of non-parenchymal tissue, defined as the sum of the decidual and chorionic plates, intercotyledonary septa, fetal vessels, connective tissue of the villi, fibrin deposits, and infarcts; and a moderate increase in parenchymal tissue, described as the intervillous space, the trophoblast layer, and fetal capillaries of both the peripheral and stem villi, both of which contributed to a greater surface area of exchange between mother and fetus (11). Yet, Evers et al. found that the histological differences between diabetic and control placenta were most significant in pregnancies with appropriate-for-gestational age infants. Large-for-gestational age control placentas showed a high prevalence of histological abnormalities, including nucleated fetal red blood cells, fibrinoid necrosis, villous immaturity, and chorangiosis, which were almost comparable to the diabetic placentas (32).
A more novel factor for consideration is race/ethnicity. Only one study to date has examined the implications of race on placental pathology in women with maternal diabetes (16). Bentley-Lewis et al. observed that, among women with GDM, the prevalence of chorangiosis was greater among white compared to non-white women. However, because this study was unable to report on degree of insulin use or glycemic control of its study population and significant racial disparities in the rates of maternal and fetal adverse outcomes exist (16), further research on racial/ethnic differences in placental pathology is warranted.
Existing placental histomorphologic studies of maternal diabetes present varied and inconsistent findings regarding placental abnormalities. Some data suggest similarities, such as increased incidence of placental immaturity (8, 10, 32, 36, 37, 43) and increased angiogenesis (6, 9, 30, 31, 41, 59) compared to normoglycemic pregnancies, and differences in placental findings between women with pregestational diabetes to those with GDM, such as in the thickness of the synctiotrophoblast or basement membrane (50, 51) or severity of placental abnormalities (55). Although it is reasonable to consider that well-controlled diabetes will result in less placental pathology, concomitant maternal conditions are likely to contribute to the placental pathological changes seen in women with diabetes. Further investigations that compare across diabetic types are needed in larger, more racially/ethnically diverse populations.
Highlights.
-
-
Significant variability in study characteristics prevents quantitative meta-analysis
-
-
Pregestational diabetes promotes enhanced angiogenesis and villous immaturity
-
-
Gestational diabetes is often associated with increased placental weight
-
-
Glycemic control has variable impact on placental histopathologic changes
-
-
Clinical factors such as infant sex, weight, and race may inform pathologic findings
ACKNOWLEDGEMENTS
The authors would like to thank Rebecca Walmer, BA of the Diabetes Research Center at the Massachusetts General Hospital for her assistance in manuscript review. This work was funded in part by the NIH 1R03DK096152 and the Robert Wood Johnson Foundation Harold Amos Medical Faculty Development Program awarded to R.B.-L. and the Harvard Summer Clinical and Translational Research Program, Harvard Catalyst, Harvard Clinical and Translational Research Center awarded to D.D.
Abbreviations
- GDM
gestational diabetes mellitus
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The authors have no relevant disclosures.
AUTHOR CONTRIBUTIONS
All authors reviewed the included studies, drafted and critically revised the manuscript for important intellectual content, and approved final version of the manuscript. JH and DD conducted the initial literature review and contributed to study design. RB-L and DJR conceived the study. RB-L is responsible for the integrity of the work as a whole, and is the guarantor of this work. The authors confirm that they have no disclosures to report.
REFERENCES
- 1.Blackburn S. Maternal, Fetal & Neonatal Physiology. 4th ed. Saunders; Maryland Heights: 2013. Prenatal Period and Placental Physiology; pp. 79–85. [Google Scholar]
- 2.Roescher AM, Hitzert MM, Timmer A, Verhagen EA, Erwich JJ, Bos AF. Placental pathology is associated with illness severity in preterm infants in the first twenty-four hours after birth. Early Hum Dev. 2011;87(4):315–9. doi: 10.1016/j.earlhumdev.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 3.van Vliet EO, de Kieviet JF, van der Voorn JP, Been JV, Oosterlaan J, van Elburg RM. Placental pathology and long-term neurodevelopment of very preterm infants. Am J Obstet Gynecol. 2012;206(6):489.e1–7. doi: 10.1016/j.ajog.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Mayhew TM, Jairam IC. Stereological comparison of 3D spatial relationships involving villi and intervillous pores in human placentas from control and diabetic pregnancies. J Anat. 2000;197(Pt 2):263–74. doi: 10.1046/j.1469-7580.2000.19720263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mooney E, Doyle E, Gearhart P, Robboy S. Placenta - clinical scenarios. Robboy’s Pathology of the Female Reproductive Tract. 2nd ed Elsevier Limited; 2009. p. 871. [Google Scholar]
- 6.Asmussen I. Ultrastructure of the villi and fetal capillaries of the placentas delivered by non-smoking diabetic women (White group D) Acta Pathol Microbiol Immunol Scand A. 1982;90(2):95–101. doi: 10.1111/j.1699-0463.1982.tb00069_90a.x. [DOI] [PubMed] [Google Scholar]
- 7.Boyd PA, Scott A, Keeling JW. Quantitative structural studies on placentas from pregnancies complicated by diabetes mellitus. Br J Obstet Gynaecol. 1986;93(1):31–5. doi: 10.1111/j.1471-0528.1986.tb07809.x. [DOI] [PubMed] [Google Scholar]
- 8.Fox H. Pathology of the placenta in maternal diabetes mellitus. Obstet Gynecol. 1969;34(6):792–8. [PubMed] [Google Scholar]
- 9.Teasdale F. Histomorphometry of the placenta of the diabetic women: class A diabetes mellitus. Placenta. 1981;2(3):241–51. doi: 10.1016/s0143-4004(81)80007-0. [DOI] [PubMed] [Google Scholar]
- 10.Daskalakis G, Marinopoulos S, Krielesi V, Papapanagiotou A, Papantoniou N, Mesogitis S, et al. Placental pathology in women with gestational diabetes. Acta Obstet Gynecol Scand. 2008;87(4):403–7. doi: 10.1080/00016340801908783. [DOI] [PubMed] [Google Scholar]
- 11.Teasdale F. Histomorphometry of the human placenta in Class B diabetes mellitus. Placenta. 1983;4(1):1–12. doi: 10.1016/s0143-4004(83)80012-5. [DOI] [PubMed] [Google Scholar]
- 12.Jauniaux E, Burton GJ. Villous histomorphometry and placental bed biopsy investigation in Type I diabetic pregnancies. Placenta. 2006;27(4-5):468–74. doi: 10.1016/j.placenta.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Higgins M, Felle P, Mooney EE, Bannigan J, McAuliffe FM. Stereology of the placenta in type 1 and type 2 diabetes. Placenta. 2011;32(8):564–9. doi: 10.1016/j.placenta.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 14.Taricco E, Radaelli T, Nobile de Santis MS, Cetin I. Foetal and placental weights in relation to maternal characteristics in gestational diabetes. Placenta. 2003;24(4):343–7. doi: 10.1053/plac.2002.0913. [DOI] [PubMed] [Google Scholar]
- 15.Beauharnais CC, Roberts DJ, Wexler DJ. High rate of placental infarcts in type 2 compared with type 1 diabetes. J Clin Endocrinol Metab. 2012;97(7):E1160–4. doi: 10.1210/jc.2011-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bentley-Lewis R, Dawson DL, Wenger JB, Thadhani RI, Roberts DJ. Placental histomorphometry in gestational diabetes mellitus: the relationship between subsequent type 2 diabetes mellitus and race/ethnicity. Am J Clin Pathol. 2014;141(4):587–92. doi: 10.1309/AJCPX81AUNFPOTLL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Björk O, Persson B. Villous structure in different parts of the cotyledon in placentas of insulin-dependent diabetic women. A morphometric study. Acta Obstet Gynecol Scand. 1984;63(1):37–43. doi: 10.3109/00016348409156271. [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association Standards of Medical Care in Diabetes--2014. Diabetes Care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 19.Association AD. Standards of medical care for patients with diabetes mellitus. Diabetes Care. 2002;25:213–29. doi: 10.2337/diacare.25.1.213. [DOI] [PubMed] [Google Scholar]
- 20.Brands AM, Kessels RP, de Haan EH, Kappelle LJ, Biessels GJ. Cerebral dysfunction in type 1 diabetes: effects of insulin, vascular risk factors and blood-glucose levels. Eur J Pharmacol. 2004;490(1-3):159–68. doi: 10.1016/j.ejphar.2004.02.053. [DOI] [PubMed] [Google Scholar]
- 21.Fowler MJ. Microvascular and Macrovascular Complications of Diabetes. Clinical Diabetes. 2008;26:77–82. [Google Scholar]
- 22.Winer N, Hamidou M, El Kouri D, Philippe HJ. Maternal and obstetrical risk factors of placental vascular pathology (biologic and epidemiological data excluded) Ann Med Interne (Paris) 2003;154(5-6):316–24. [PubMed] [Google Scholar]
- 23.Salafia CM, Yampolsky M, Misra DP, Shlakhter O, Haas D, Eucker B, et al. Placental surface shape, function, and effects of maternal and fetal vascular pathology. Placenta. 2010;31(11):958–62. doi: 10.1016/j.placenta.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Setji TL, Brown AJ, Feinglos MN. Gestational Diabetes Mellitus. Clinical Diabetes. 2005;23:17–24. [Google Scholar]
- 25.Desoye G, Hauguel-de Mouzon S. The human placenta in gestational diabetes mellitus. The insulin and cytokine network. Diabetes Care. 2007;30(Suppl 2):S120–6. doi: 10.2337/dc07-s203. [DOI] [PubMed] [Google Scholar]
- 26.Correa A, Gilboa SM, Besser LM, Botto LD, Moore CA, Hobbs CA, et al. Diabetes mellitus and birth defects. Am J Obstet Gynecol. 2008;199(3):237.e1–9. doi: 10.1016/j.ajog.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White P. Pregnancy and Diabetes. In: Marbel AWP, Bradley RF, Krall LP, editors. Joslin’s Diabetes Mellitus. Lea and Febiger; Philadelphia: 1971. [Google Scholar]
- 28.WHITE P. PREGNANCY AND DIABETES, MEDICAL ASPECTS. Med Clin North Am. 1965;49:1015–24. doi: 10.1016/s0025-7125(16)33292-8. [DOI] [PubMed] [Google Scholar]
- 29.White P. Classification of obstetric diabetes. Am J Obstet Gynecol. 1978;130(2):228–30. doi: 10.1016/0002-9378(78)90373-3. [DOI] [PubMed] [Google Scholar]
- 30.Jirkovská M. Comparison of the thickness of the capillary basement membrane of the human placenta under normal conditions and in type 1 diabetes. Funct Dev Morphol. 1991;1(3):9–16. [PubMed] [Google Scholar]
- 31.Mayhew TM. Enhanced fetoplacental angiogenesis in pre-gestational diabetes mellitus: the extra growth is exclusively longitudinal and not accompanied by microvascular remodelling. Diabetologia. 2002;45(10):1434–9. doi: 10.1007/s00125-002-0927-1. [DOI] [PubMed] [Google Scholar]
- 32.Evers IM, Nikkels PG, Sikkema JM, Visser GH. Placental pathology in women with type 1 diabetes and in a control group with normal and large-for-gestational-age infants. Placenta. 2003;24(8-9):819–25. doi: 10.1016/s0143-4004(03)00128-0. [DOI] [PubMed] [Google Scholar]
- 33.Maly A, Goshen G, Sela J, Pinelis A, Stark M, Maly B. Histomorphometric study of placental villi vascular volume in toxemia and diabetes. Hum Pathol. 2005;36(10):1074–9. doi: 10.1016/j.humpath.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 34.Nelson SM, Coan PM, Burton GJ, Lindsay RS. Placental structure in type 1 diabetes: relation to fetal insulin, leptin, and IGF-I. Diabetes. 2009;58(11):2634–41. doi: 10.2337/db09-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jirkovská M, Kučera T, Kaláb J, Jadrníček M, Niedobová V, Janáček J, et al. The branching pattern of villous capillaries and structural changes of placental terminal villi in type 1 diabetes mellitus. Placenta. 2012;33(5):343–51. doi: 10.1016/j.placenta.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 36.Higgins MF, Russell NM, Mooney EE, McAuliffe FM. Clinical and ultrasound features of placental maturation in pre-gestational diabetic pregnancy. Early Hum Dev. 2012;88(10):817–21. doi: 10.1016/j.earlhumdev.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Björk O, Persson B. Placental changes in relation to the degree of metabolic control in diabetes mellitus. Placenta. 1982;3(4):367–78. doi: 10.1016/s0143-4004(82)80030-1. [DOI] [PubMed] [Google Scholar]
- 38.Teasdale F. Histomorphometry of the human placenta in Class C diabetes mellitus. Placenta. 1985;6(1):69–81. doi: 10.1016/s0143-4004(85)80034-5. [DOI] [PubMed] [Google Scholar]
- 39.Mayhew TM, Sørensen FB, Klebe JG, Jackson MR. The effects of mode of delivery and sex of newborn on placental morphology in control and diabetic pregnancies. J Anat. 1993;183(Pt 3):545–52. [PMC free article] [PubMed] [Google Scholar]
- 40.Lao TT, Lee CP, Wong WM. Placental weight to birthweight ratio is increased in mild gestational glucose intolerance. Placenta. 1997;18(2-3):227–30. doi: 10.1016/s0143-4004(97)90097-7. [DOI] [PubMed] [Google Scholar]
- 41.Jirkovská M, Kubínová L, Janácek J, Moravcová M, Krejcí V, Karen P. Topological properties and spatial organization of villous capillaries in normal and diabetic placentas. J Vasc Res. 2002;39(3):268–78. doi: 10.1159/000063692. [DOI] [PubMed] [Google Scholar]
- 42.Ashfaq M, Janjua MZ, Channa MA. Effect of gestational diabetes and maternal hypertension on gross morphology of placenta. J Ayub Med Coll Abbottabad. 2005;17(1):44–7. [PubMed] [Google Scholar]
- 43.Madazli R, Tuten A, Calay Z, Uzun H, Uludag S, Ocak V. The incidence of placental abnormalities, maternal and cord plasma malondialdehyde and vascular endothelial growth factor levels in women with gestational diabetes mellitus and nondiabetic controls. Gynecol Obstet Invest. 2008;65(4):227–32. doi: 10.1159/000113045. [DOI] [PubMed] [Google Scholar]
- 44.Jácomo KH, Benedetti WL, Sala MA, Alvarez H. Pathology of the trophoblast and fetal vessels of the placenta in maternal diabetes mellitus. Acta Diabetol Lat. 1976;13(5-6):216–35. doi: 10.1007/BF02581119. [DOI] [PubMed] [Google Scholar]
- 45.Stoz F, Schuhmann RA, Schmid A. Morphometric investigations of terminal villi of diabetic placentas in relation to the White classification of diabetes mellitus. J Perinat Med. 1987;15(2):193–8. doi: 10.1515/jpme.1987.15.2.193. [DOI] [PubMed] [Google Scholar]
- 46.Mayhew TM, Sørensen FB, Klebe JG, Jackson MR. Growth and maturation of villi in placentae from well-controlled diabetic women. Placenta. 1994;15(1):57–65. doi: 10.1016/s0143-4004(05)80236-x. [DOI] [PubMed] [Google Scholar]
- 47.Mayhew TM, Sisley I. Quantitative studies on the villi, trophoblast and intervillous pores of placentae from women with well-controlled diabetes mellitus. Placenta. 1998;19(5-6):371–7. doi: 10.1016/s0143-4004(98)90076-5. [DOI] [PubMed] [Google Scholar]
- 48.Makhseed M, Musini VM, Ahmed MA, Al-Harmi J. Placental pathology in relation to the White’s classification of diabetes mellitus. Arch Gynecol Obstet. 2002;266(3):136–40. doi: 10.1007/s004040100232. [DOI] [PubMed] [Google Scholar]
- 49.Clarson C, Tevaarwerk GJ, Harding PG, Chance GW, Haust MD. Placental weight in diabetic pregnancies. Placenta. 1989;10(3):275–81. doi: 10.1016/0143-4004(89)90028-3. [DOI] [PubMed] [Google Scholar]
- 50.al-Okail MS, al-Attas OS. Histological changes in placental syncytiotrophoblasts of poorly controlled gestational diabetic patients. Endocr J. 1994;41(4):355–60. doi: 10.1507/endocrj.41.355. [DOI] [PubMed] [Google Scholar]
- 51.Younes B, Baez-Giangreco A, al-Nuaim L, al-Hakeem A, Abu Talib Z. Basement membrane thickening in the placentae from diabetic women. Pathol Int. 1996;46(2):100–4. doi: 10.1111/j.1440-1827.1996.tb03585.x. [DOI] [PubMed] [Google Scholar]
- 52.Calderon IM, Damasceno DC, Amorin RL, Costa RA, Brasil MA, Rudge MV. Morphometric study of placental villi and vessels in women with mild hyperglycemia or gestational or overt diabetes. Diabetes Res Clin Pract. 2007;78(1):65–71. doi: 10.1016/j.diabres.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 53.Dubova EA, Pavlov KA, Yesayan RM, Nagovitsyna MN, Tkacheva ON, Shestakova MV, et al. Morphometric characteristics of placental villi in pregnant women with diabetes. Bull Exp Biol Med. 2011;151(5):650–4. doi: 10.1007/s10517-011-1406-9. [DOI] [PubMed] [Google Scholar]
- 54.Aherne W, Dunnill MS. Quantitative aspects of placental structure. J Pathol Bacteriol. 1966;91(1):123–39. doi: 10.1002/path.1700910117. [DOI] [PubMed] [Google Scholar]
- 55.Rudge MV, Lima CP, Damasceno DC, Sinzato YK, Napoli G, Rudge CV, et al. Histopathological placental lesions in mild gestational hyperglycemic and diabetic women. Diabetol Metab Syndr. 2011;3(1):19. doi: 10.1186/1758-5996-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turowski G, Berge LN, Helgadottir LB, Jacobsen EM, Roald B. A new, clinically oriented, unifying and simple placental classification system. Placenta. 2012;33(12):1026–35. doi: 10.1016/j.placenta.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Mayhew TM. Fetoplacental angiogenesis during gestation is biphasic, longitudinal and occurs by proliferation and remodelling of vascular endothelial cells. Placenta. 2002;23(10):742–50. doi: 10.1016/s0143-4004(02)90865-9. [DOI] [PubMed] [Google Scholar]
- 58.Kaufmann P, Mayhew TM, Charnock-Jones DS. Aspects of human fetoplacental vasculogenesis and angiogenesis. II. Changes during normal pregnancy. Placenta. 2004;25(2-3):114–26. doi: 10.1016/j.placenta.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 59.Jirkovská M, Janácek J, Kaláb J, Kubínová L. Three-dimensional arrangement of the capillary bed and its relationship to microrheology in the terminal villi of normal term placenta. Placenta. 2008;29(10):892–7. doi: 10.1016/j.placenta.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 60.Higgins M, McAuliffe FM, Mooney EE. Clinical associations with a placental diagnosis of delayed villous maturation: a retrospective study. Pediatr Dev Pathol. 2011;14(4):273–9. doi: 10.2350/10-07-0872-OA.1. [DOI] [PubMed] [Google Scholar]
- 61.Tennant PW, Glinianaia SV, Bilous RW, Rankin J, Bell R. Pre-existing diabetes, maternal glycated haemoglobin, and the risks of fetal and infant death: a population-based study. Diabetologia. 2014;57(2):285–94. doi: 10.1007/s00125-013-3108-5. [DOI] [PubMed] [Google Scholar]
- 62.Shams F, Rafique M, Samoo NA, Irfan R. Fibrinoid necrosis and hyalinization observed in normal, diabetic and hypertensive placentae. J Coll Physicians Surg Pak. 2012;22(12):769–72. [PubMed] [Google Scholar]
- 63.Jawerbaum A, González E. Diabetic pregnancies: the challenge of developing in a pro-inflammatory environment. Curr Med Chem. 2006;13(18):2127–38. doi: 10.2174/092986706777935302. [DOI] [PubMed] [Google Scholar]
- 64.Wong ND, Glovaci D, Wong K, Malik S, Franklin SS, Wygant G, et al. Global cardiovascular disease risk assessment in United States adults with diabetes. Diab Vasc Dis Res. 2012;9(2):146–52. doi: 10.1177/1479164112436403. [DOI] [PubMed] [Google Scholar]
- 65.Pedicino D, Liuzzo G, Trotta F, Giglio AF, Giubilato S, Martini F, et al. Adaptive immunity, inflammation, and cardiovascular complications in type 1 and type 2 diabetes mellitus. J Diabetes Res. 2013;2013:184258. doi: 10.1155/2013/184258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soma H, Yoshida K, Mukaida T, Tabuchi Y. Morphologic changes in the hypertensive placenta. Contrib Gynecol Obstet. 1982;9:58–75. [PubMed] [Google Scholar]
- 67.Negrato CA, Mattar R, Gomes MB. Adverse pregnancy outcomes in women with diabetes. Diabetol Metab Syndr. 2012;4(1):41. doi: 10.1186/1758-5996-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]