Abstract
Objective
To develop a better understanding on mechanisms of seizures and long-term epileptogenesis caused by neurocysticercosis.
Methods
A workshop was held bringing together experts in epilepsy and epileptogenesis and neurocysticercosis
Results
Human neurocysticercosis and parallel animal models offer a unique opportunity to understand basic mechanisms of seizures. Inflammatory responses to degenerating forms and in later stage calcified parasite granulomas are associated with seizures and epilepsy. Other mechanisms may also be involved in epileptogenesis as well.
Conclusions
Naturally occurring brain infections with neurocysticercosis offer a unique opportunity to develop treatments for one of the world’s most common causes of epilepsy and for the development of more general anti-epileptogenic treatments. Key advantages stem from the time course where an acute seizure heralds a start of the epileptogenic process and radiographic changes of calcification and perilesional edema provide biomarkers of a chronic epileptic state.
Search words: neurocysticercosis, epilepsy, seizure, Taenia solium, helminth
Neurocysticercosis (NCC) accounts for about 29% of all epilepsy in endemic regions and is the most common cause of adult acquired epilepsy worldwide [Nash 2011, Ndibumanzi 2011]. To develop a better understanding on mechanisms of seizures and long-term epileptogenesis caused by NCC, a workshop was held on October 23 and 24, 2013 in Lima, Peru to bring together experts in the areas of parasitology and epilepsy. A central conclusion reached was that human NCC and parallel animal models offer a unique opportunity to understand basic mechanisms of seizures and the transition to often life-long debilitating seizures. Inflammatory responses to degenerating forms and in later stage calcified parasite granulomas are associated with seizures and epilepsy. Other mechanisms may also be involved in epileptogenesis as well.
Life cycle of Taenia solium infection
Humans harbor the adult tapeworm, Taenia solium, which releases mature proglottids containing infectious ova or liberated ova in the feces. Free roaming pigs ingest ova that release larvae that enter the bloodstream and develop into cysts, mostly in the muscles and brain of the intermediate host. Following ingestion of cysts in raw or undercooked pork, humans become infected with the tapeworm 1. Tapeworm carriers can infect themselves or others around them who accidently ingest ova in contaminating feces. Almost all the clinical manifestations of cysticercosis are due to infection of the brain and spine although other tissues are also infected. Because of its complicated and exacting life cycle, which can be interrupted, and the development of high-grade immunity in swine to the infecting form, control of infection is possible. 2. Suitable control interventions include corralling pigs, avoiding ingestion of undercooked pork, or vaccination of pigs combined with treatment of human tapeworm carriers and/or treatment of pigs 2,3.
NCC is caused by infection of the central nervous system with the larval cyst of Taenia solium. The manifestations are variable, depending upon the location, number, and size of the cysts as well as the degree of accompanying inflammation. Infected people may be asymptomatic or may present with seizures due to parenchymal involvement, hydrocephalus and acute decompensation due to ventricular or basal subarachnoidal involvement or severe arachnoiditis, and focal neurological deficits as a result of cerebral infarcts due to subarachnoid located cysts, mass lesions with or without surrounding edema, or nerve entrapments.
Degenerating cysts invoke inflammation
Viable cysts in the brain parenchyma incite little inflammation and tend to be asymptomatic. When cysts begin to degenerate they provoke a variable inflammatory response. The onset likely reflects loss of parasite mechanisms suppressing the host response and parasite antigen-driven immune responses. This inflammation is associated with edema and abnormal enhancement in CT or MRI examination. Degenerating cysticerci tend to regress but recurrent seizures may herald an exacerbation of the surrounding inflammation. Calcification of degenerating cysts occurs late in the process. In studies of general populations in endemic regions calcified T. solium granulomas are the most frequent radiological finding in NCC. About 10–20% of randomly studied persons show brain calcifications on CT and only a small minority of these suffer from epilepsy. In those with seizures, however, calcified lesions can be foci of seizure activation. In a cohort of patients with only calcified lesions, a history of seizures and a positive serology, 36% had a seizure reoccurrence and half of these demonstrated perilesional edema and enhancement around one or more calcifications. 4 One of the major unresolved issues is the role of these calcifications in the development and maintenance of the chronic epileptic state. While some evidence suggests the perilesional edema is a transient inflammatory response to the calcified cyst either due to periodic antigen release, loss of immune suppression or a combination of both mechanisms, the calcifications themselves and the effects of ongoing focal seizures may also be playing roles 5. While seizures associated with perilesional edema around calcifications may due directly to inflammation, epilepsy associated with calcifications in the absence of overt MRI changes also occurs. How early in the degenerative process and the mechanisms involved is central to understand how epilepsy develops.
Antiparasitic treatment of NCC and seizures
Antiparasitic agents are commonly used in the treatment of viable NCC and these cysticidal agents may lead to a transient increase in the inflammatory response with clinical deterioration and seizures. For this reason, corticosteroids are commonly employed to abrogate clinical deterioration. Corticosteroid regimens have not been well standardized. In clinical practice, the choice of corticosteroid agent, dose, and duration varies considerably without clear evidence of superiority of one regimen over others. Even with the prophylactic use of steroids, there is a transient increase in seizure frequency especially during the first few weeks of antiparasitic therapy or at the time of corticosteroid tapering or withdrawal. This is one of the very few instances when seizures are predictably induced during a specific period of time. This time point may be an ideal target for studies to explore the mechanisms, epileptogenesis and brain damage as well as preventative measures.
Blood Brain Barrier dysfunction
One hypothesis for the genesis of seizures and epilepsy in NCC centers on blood-brain barrier (BBB) pathology and inflammation. This hypothesis suggests that host inflammation directed to degenerating cysts causes abnormal vascular permeability as well as neuronal dysfunction resulting in increased cortical excitability and acute seizures (Figure 1). How these acute effects lead to the development of a chronic, focal epileptic disorder, a common consequence of NCC, is not known. Possible mechanisms include early and/or continuing brain inflammation, reactive astrogliosis, cellular damage and increased BBB breakdown, which may further contribute to inflammation by creating a positive feedback loop. These together with changes in brain excitability might lead to the chronic epileptic state. Clinical, imaging and pathological evidence support these hypotheses
Figure 1.
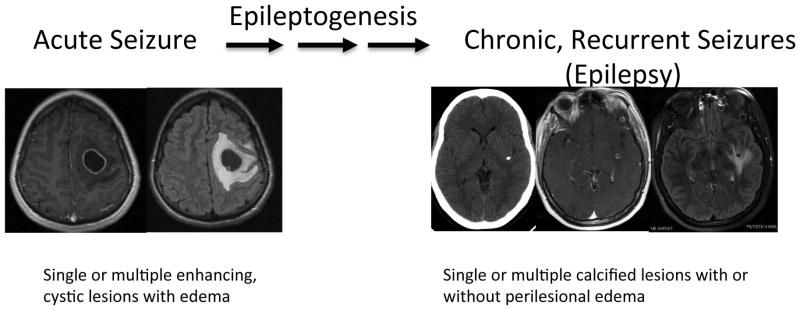
One proposed mechanism of development of chronic epilepsy from acute seizures due to a degenerating cyst (left panel) to a calcification (right panel, left image) associated with perilesional edema (right panel, right image) as one cause of epilepsy in these patients. Epilepsy can also occur without the presence of perilesional edema or without obvious association to observable abnormalities. Left panel shows the same lesion with enhancement (left) and edema (right) in a patient who presented with seizures and right-sided signs. The right panel has three images of another patient presenting with seizures. A CT demonstrating a single calcifications (left) with enhancement (middle) and perilesional edema (right) by MRI imaging (Images from T.E.N.).
NCC as a human model of epileptogenesis
There is a clear need for new models of ictogenesis and epileptogenesis. Although new antiepileptic drugs have been marketed in the last decade, they share mechanisms of action and with the possible exception of levetiracetam, offer no better efficacy than those available for 60 (phenytoin) to 100 (phenobarbital) years 10,11 offers many advantages over other human models of epileptogenesis ranging from status epilepticus to brain trauma, because of its predictable timing and clear focality.
NCC offers an excellent opportunity to study the natural history of the genesis of seizures and epilepsy prospectively in a human population, both epidemiologically and mechanistically. This is due to its unique epidemiology and timing of seizures associated with increased inflammation (despite reasonable attempts to suppress inflammation with corticosteroids and anti-seizure medications), a predictable occurrence of seizures in the treatment of parenchymal disease and subsequent development of epilepsy commonly localized to calcified lesions. It is likely that information gained from studies of NCC will be applicable to other conditions where inflammation plays a prominent causal role such as in brain trauma, infections and strokes, since they likely share common pathways that culminate in seizure activity.
The availability of animal models of NCC
In developing and testing therapeutics for epileptogenesis new animal models are also needed that closely parallel the human condition. For NCC, there are a number of animal model infections of T. solium and other cestodes that can be employed to focus human based studies. CNS models include intracranial inoculation of Mesocestoides corti, Taenia crassiceps, or granulomas associated with T. crassiceps. All are compromised by use of a different host and parasite than with human cysticercosis and the requirement for intracranial injection. T.solium models include naturally infected pigs, experimental oral infection of pigs, and T. solium oncospheres (invasive larvae) injected directly into the brain of rats or into the tissues of nude mice or uninfected pigs. All the models have significant and/or potential drawbacks. The naturally infected pig most closely resembles human infections. Brain involvement occurs commonly in heavily infected animals so that the natural inflammatory response to degenerating cysts and responses induced by anti-cysticidal treatment can be determined. Injection of T. solium cyst or oncospheres in the rat and mouse brain is easier to perform and analyze than pig studies since small animal models offer the possibility of larger numbers of animals, an extensive repertoire of reagents, and well-studied models for epilepsy. However, the T solium models are technically demanding, require a source of T. solium ova, and require 3–6 months for cyst maturation. Furthermore, the T. solium rat model employs neonatal rats whose immune system is still immature and lacks normal adult immune responses to the invading oncosphere.
Identification of antiepileptogenic drug targets for NCC
What characteristics make NCC a good model to study epilepsy treatments (and what are the obvious drug targets)? Recent studies have suggested that inflammation may be an important pathophysiologic process for both associated brain injury and epileptogenesis 12. There is also considerable evidence for a critical role of inflammation in ictogenesis in neurocysticercosis. The extent and intensity of perilesional inflammation and its effect on the surrounding brain tissue is easily discernible on imaging as well as histological studies 13,14. Other brain insults that commonly result in epilepsy such as trauma and stroke occur in the context of widespread, severe brain injury or systemic disease that complicates both the investigation and intervention. The lesions in NCC are less variable and more predictably similar to each other. They do not occur in the context of widespread, severe brain injury or systemic disease that complicates both the investigation and intervention. Cysts are frequently located at the junction of the cortical gray and subcortical white matter, are mostly limited in size to 1–2 cm in diameter, and consequently result in less brain damage. In a majority of instances patients also present with a single or limited number of lesions. The seizure focus, even in the presence of multiple lesions, is usually evident because of the presence of surrounding enhancement and/or edema. Seizures due to degenerating cysts are frequent and the most common presentation, facilitating selection for clinical trials. In these individuals, there usually is good localization of the epileptogenic zone. Serial imaging and EEG studies can be used as “biomarkers” of epileptogenesis, facilitating clinical trial design 15. The timing of the onset of the first seizure is an ideal zero point for therapeutic trials because whatever factors induce acute seizures are present at this point of time in all patients. The relative similarity in presentation and lesion characteristics would facilitate trials that might include anti-inflammatory, anti-synaptic plasticity and neuroprotective agents.
Important research questions to be considered include the following:
Question 1: What methods can best measure seizure activity, blood-brain barrier dysfunction, inflammation, gliosis and brain damage in vivo?
Comment: Modern neuroimaging techniques and EEG analysis methods in combination with biomarkers of inflammation or neurological pathology may be able to predict seizure development in patients at risk, and help determine the role of underlying processes such as inflammation and a compromised blood-brain barrier. For example studies have associated serum levels of matrix metalloproteases 2 and 9 symptomatic NCC patients with seizures 16. Polymorphisms in Toll-like receptor genes also correlate with seizures. Use of model cestode brain infections may be particularly useful.
Question 2: What factors determine whether inflammatory lesions provoke acute seizures?
Comment: Seizures can occur at any stage, but are uncommon until a cyst degenerates and shows accompanying inflammation. Even in the presence of degenerating cysts, only some persons suffer from overt seizures. Other lesions, even with varying degrees of inflammation, do not provoke observable seizures. The presence and frequency of subclinical seizures are unknown. Factors to consider include the extent, intensity and duration of the vascular/inflammatory and glial responses, location of the lesion and genetic disposition.
Question 3: Is there a genetic predisposition in people who develop seizures in NCC?
Comment: Only a portion of infected people develop seizures. Genetic susceptibilities for seizures may be consequence of enhanced ability to mount, control or resolve inflammatory response to the degenerating cyst 16 or to altered susceptibility to develop seizures/epilepsy 5, 17. A genetic propensity to become infected or have greater infection burden may also exist.
Question 4: Do anti-inflammatory measures prevent, decrease or control seizure activity?
Comment: If inflammation is important in the development of seizures and epilepsy, then prevention or control of inflammation would be expected to decrease their occurrence. Many potential drugs are available to test this hypothesis. Animal models may be helpful to determine the mechanisms involved, which should serve as a guide to decide what agents to test. On the other hand, an anti-inflammatory drug that is found to decrease seizure activity in humans would both be useful clinically and at the same time support the role of inflammation in the pathogenesis of epilepsy. Too much immunosuppression could also be detrimental. Inflammation is likely essential to control parasite growth, to degrade cysts, and to rid the brain of parasite products or, failing that, to effectively wall it off. One study suggested that blockade of substance P receptors could abrogate seizures in a T crassiceps granuloma model [18.
Questions 5: Does the amount and degree of brain damage correlate with development of seizure/epilepsy?
Comment: Whether the presence and level of brain damage associated with lesions that become seizure foci correlate with the risk of seizures is unproven but such a relationship is plausible. In some instances, people with high-grade inflammation involving specific lesions have subsequently developed gliosis and epilepsy associated with these lesions. The presence of gliosis correlates with the existence of refractory epilepsy [De Souza 2012]. Proving that inflammation leads to brain damage that in turn leads to seizures and epilepsy is, however, essential to develop therapeutic strategies to prevent and limit further seizures and perhaps epileptogenesis. Showing a direct role of inflammation in the development of epilepsy leads to an interesting but critical question of what level of inflammation, if any, should be tolerated in NCC or other analogous processes. The predictable nature of treatment induced seizures, makes it feasible to discover and test for systemic markers of inflammation and/or brain damage as biomarkers for risk of seizures.
Some groups have reported that mesial temporal sclerosis occurs more frequently than expected in NCC. If confirmed, this may happen because of prolonged or recurrent seizure activity, degeneration of cysts located in that region of the brain, genetic predisposition for its development, or other variables not yet considered 19,20.
Question 6: What is the role of calcified lesions in the genesis of epilepsy?
Comment: In endemic populations studies in rural regions epilepsy can affect 1–2% of the population and about 30% is due to NCC. In fact, calcifications are detected in 10–20% of randomly screened persons and in most patients with chronic epilepsy and cysticercosis. Viable lesions are much less frequent. Consequently, epilepsy will be associated with calcifications in most situations. The presence of intermittent perilesional edema events around calcifications implicate these lesions as foci of seizure activity and points to the pathophysiologic mechanism involved as a frequent cause of seizures and epilepsy in endemic populations. Others suggest that seizures themselves result in edema and inflammatory response. Prospective studies of humans with perilesional edema and clinical trials to prevent inflammation are important to identify treatments and preventions. The clinical observation that abrupt cessation of corticosteroids, which are frequently used to treat edema of the brain from multiple causes, results in rebound as well a new edema around previously uninvolved calcifications [Mejia 2013] suggests that active immune suppression mechanisms are involved. Loss of suppression could lead to presence of unrestrained inflammation, edema, and seizure reoccurrence.
Recurrent seizures are also found in about half of the patients with only calcifications who do not show perilesional edema 4. While this may suggest that the pathophysiology of epilepsy in this group is different from those with perilesional edema, more sensitive imaging studies recently developed, may be able to distinguish if lesions with and without perilesional edema cause seizures by unique mechanisms or if the inflammation is too mild to be detected with existing methods.
Question 7: How well do anti-seizure medications prevent seizures in NCC and do some anti-seizure medications work better in NCC than others?
Comment: No differences in efficacy of any particular anti-seizure medication are known. Cysticidal treatment commonly provokes seizure activity that is roughly maximal during and shortly after cysticidal treatment ceases. The ability of known or other potential anti-seizure drugs to suppress seizure activity could be compared or tested during this time period to evaluate effectiveness.
Question 8: What co-morbidities are associated with epilepsy associated with NCC?
Comment: It is well established that significant co-morbidities such as depression or altered cognition exist in people with epilepsy. NCC usually presents in late adolescence or in adulthood. Analyses before and after the development of epilepsy in individuals with NCC can be employed to define co-morbidities, which develop or are inherent in the individual.
Summary and specific recommendations
The purpose of the workshop as reported here are to provide rational arguments to the neurology and epilepsy community for the idea that naturally-occurring brain infections with NCC offer a unique opportunity not only to develop treatments for one of the world’s most common causes of epilepsy, but also for the development of more general antiepileptogenic treatments. Key advantages stem from the time course where an acute seizure heralds a start of the epileptogenic process and radiographic changes of calcification and perilesional edema provide biomarkers of a chronic epileptic state. In addition, strong epidemiological programs both in South America and India already study fairly uniform patient populations and there are evolving parallel animal models available for both clinical and preclinical drug development. We propose the following next steps:
Foster the development and use of pertinent animal models, as delineated above that could shed information on pathophysiological mechanisms and focus the nature of clinical human trials.
Enable synergy between neuroscientists interested in epilepsy, applied neuroscience, neuroinflammation with physicians/scientists knowledgeable in neurocysticercosis
Develop, support or enhance study centers and networks of interested investigators to regions or areas where sufficient patients exist and clinical studies can be performed. Apply state of the art imaging/studies and neuroscience to neurocysticercosis.
Perform prospective studies in patients with parenchymal NCC at the onset of treatment, when seizures are most likely to occur, employing quantitative measures of blood-brain barrier disruption, brain damage, inflammation, anatomical changes (e.g. vasogenic and cytotoxic edema), and seizure activity over the course of disease. The measures may include MRI/MRS, PET inflammatory markers, and quantitative EEG.
In defined populations, study known candidate genes associated with seizures that may predict genetic propensity for seizures in NCC.
Acknowledgments
Funding: Funded in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, the Office of Rare Diseases Research, National Center for Advancing Translational Sciences, and Fogarty International Center Training Grant D43 TW00114, all from the National Institutes of Health.
Footnotes
There are no financial conflicts and no commercial sponsors.
All authors participated in a conference held in Lima, Peru from October 23-4, 2013 that led to the content of the manuscript
T.E. Nash was primarily responsible for writing and editing the manuscript.
S. Mahanty (smahanty@niaid.nih.gov) contributed to the writing and editing of the manuscript
J.A. Loeb (aloeb@uic.edu) contributed to the writing and editing of the manuscript
W.H. Theodore (theodorw@ninds.nih.gov)contributed to the writing and editing of the manuscript
A. Friedman (alonf@exchange.bgu.ac.il)contributed to the writing and editing of the manuscript
J.W. Sander (ley_sander@yahoo.co.uk)contributed to the writing and editing of the manuscript
G. Singh (gagandeep_si@yahoo.co.uk)contributed to the writing and editing of the manuscript
E. Cavalheiro (esper.cavalheiro@unifesp.br)contributed to the content of the manuscript
O.H. Del Brutto (oscardelbrutto@hotmail.com)contributed to the writing and editing of the manuscript
O. Takayanagui (omtakay@fmrp.usp.br) contributed to the content of the manuscript
A. Fleury (afleury@biomedicas.unam.mx) contributed to the writing and editing of the manuscript
M. Verastegui (mveraste@jhsph.edu) contributed to the content of the manuscript
PM. Pruex (pierre-marie.preux@unilim.fr) contributed to the content of the manuscript
E.J. Pretell (ejpretell@hotmail.com) contributed to the content of the manuscript
S. Montano (silvia.montano@med.navy.mil) contributed to the content of the manuscript
A.C. White (acwhite@utmb.edu) contributed to the writing and editing of the manuscript
A. Gonzales (agonzale@jhsph.edu) contributed to the writing and editing of the manuscript
R. Gilman (gilmanr@yahoo.com) contributed to the writing and editing of the manuscript
H.H. Garcia (hgarcia@jhsph.edu) was primarily responsible for writing and editing the manuscript
References
- 1.Del Brutto O, Garcia H. Neurocysticercosis. Handbook of Neurology. 2013;114:313–325. doi: 10.1016/B978-0-444-53490-3.00025-X. [DOI] [PubMed] [Google Scholar]
- 2.Schantz PM, Cruz M, Sarti E, Pawlowski Z. Potential eradicability of taeniasis and cysticercosis. Bull Pan Am Health Organ. 1993;27:397–403. [PubMed] [Google Scholar]
- 3.Gilman RH, Gonzalez AE, Llanos-Zavalaga F, Tsang VCW, Garcia HH Cysticercosis Working Grp P. Prevention and control of Taenia solium taeniasis/cysticercosis in Peru. Pathogens and Global Health. 2012;106:312–318. doi: 10.1179/2047773212Y.0000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nash TE, Pretell EJ, Lescano AG, et al. Perilesional brain oedema and seizure activity in patients with calcified neurocysticercosis: a prospective cohort and nested case-control study. Lancet Neurology. 2008;7:1099–1105. doi: 10.1016/S1474-4422(08)70243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta RK, Awasthi R, Rathore RK, et al. Understanding epileptogenesis in calcified neurocysticercosis with perfusion MRI. Neurology. 2012;78:618–625. doi: 10.1212/WNL.0b013e318248deae. [DOI] [PubMed] [Google Scholar]
- 6.Nash TE, Garcia HH. Diagnosis and treatment of neurocysticercosis. Nature Reviews Neurology. 2011;7:584–594. doi: 10.1038/nrneurol.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chawla S, Husain N, Kumar S, Pal L, Tripathi M, Gupta RK. Correlative MR imaging and histopathology in porcine neurocysticercosis. J Magn Reson Imaging. 2004;20:208–215. doi: 10.1002/jmri.20105. [DOI] [PubMed] [Google Scholar]
- 8.Henneberg R. Die tierischen Parasiten des Zentralnerven-systems. In: Lewandowsky M, editor. Handbuch der Neurologie. Berlin: Verlag von Julius Springer; 1912. pp. 642–683. [Google Scholar]
- 9.Sikasunge CS, Johansen MV, Phiri IK, Willingham AL, 3rd, Leifsson PS. The immune response in Taenia solium neurocysticercosis in pigs is associated with astrogliosis, axonal degeneration and altered blood-brain barrier permeability. Vet Parasitol. 2009;160:242–250. doi: 10.1016/j.vetpar.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Loscher W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure: the journal of the British Epilepsy Association. 2011;20:359–368. doi: 10.1016/j.seizure.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 11.French JA, White HS, Klitgaard H, et al. Development of new treatment approaches for epilepsy: unmet needs and opportunities. Epilepsia. 2013;54 (Suppl 4):3–12. doi: 10.1111/epi.12294. [DOI] [PubMed] [Google Scholar]
- 12.Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nature reviews Neurology. 2011;7:31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ooi WW, Wijemanne S, Thomas CB, Quezado M, Brown CR, Nash TE. Short Report: A Calcified Taenia solium Granuloma Associated with Recurrent Perilesional Edema Causing Refractory Seizures: Histopathological Features. American Journal of Tropical Medicine and Hygiene. 2011;85:460–463. doi: 10.4269/ajtmh.2011.11-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nash TE, Bartelt LA, Korpe PS, Lopes B, Houpt ER. Case Report: Calcified Neurocysticercus, Perilesional Edema, and Histologic Inflammation. American Journal of Tropical Medicine and Hygiene. 2014;90:318–321. doi: 10.4269/ajtmh.13-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engel J, Jr, Pitkanen A, Loeb JA, et al. Epilepsy biomarkers. Epilepsia. 2013;54 (Suppl 4):61–69. doi: 10.1111/epi.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verma A, Prasad KN, Gupta RK, et al. Toll-Like Receptor 4 Polymorphism and Its Association with Symptomatic Neurocysticercosis. Journal of Infectious Diseases. 2010;202:1219–1225. doi: 10.1086/656395. [DOI] [PubMed] [Google Scholar]
- 17.Gupta RK, Awasthi R, Garg RK, et al. T1-weighted dynamic contrast-enhanced MR evaluation of different stages of neurocysticercosis and its relationship with serum MMP-9 expression. AJNR American journal of neuroradiology. 2013;34:997–1003. doi: 10.3174/ajnr.A3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson P, Garza A, Weinstock J, et al. Substance P causes seizures in neurocysticercosis. PLoS pathogens. 2012;8:e1002489. doi: 10.1371/journal.ppat.1002489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rathore C, Thomas B, Kesavadas C, Abraham M, Radhakrishnan K. Calcified neurocysticercosis lesions and antiepileptic drug-resistant epilepsy: a surgically remediable syndrome? Epilepsia. 2013;54:1815–1822. doi: 10.1111/epi.12349. [DOI] [PubMed] [Google Scholar]
- 20.Bianchin MM, Velasco TR, Wichert-Ana L, Takayanagui OM, Leite JP, Sakamoto AC. How frequent is the association of neurocysticercosis and mesial temporal lobe epilepsy with hippocampal sclerosis? Epilepsia. 2010;51:2359–2360. doi: 10.1111/j.1528-1167.2010.02735.x. [DOI] [PubMed] [Google Scholar]


