Abstract
Purpose
To examine local definitive therapy for non-metastatic breast cancer using the Centers for Disease Control and Prevention’s National Program of Cancer Registries Patterns of Care Breast and Prostate Cancer (POCBP) study.
Patients and Methods
POCBP medical record data were re-abstracted in seven state/ regional registry systems (GA, NC, KY, LA, WI, MN and CA) to verify data quality and assess treatment patterns in the population. National Comprehensive Cancer Network clinical practice treatment guidelines were aligned with American Joint Committee on Cancer stage at diagnosis to appraise care.
Results
6,505 of 9142 patients with registry confirmed breast cancer were coded as primary disease with 0-IIIA stage tumors and were included for study. Approximately 90% received guideline concordant loco-regional treatment; however this outcome varied by age group as 92.9% of women < 65 years and 85.2% ≥ 65 years received standard care (p <0.0001). Characteristics which best discriminated receipt of guideline concordant care in receiver operating curve (ROC) analyses (C-value) were receipt of BCS versus mastectomy (C = 0.70), patient age (C=0.62), greater tumor stage (C= 0.60), public insurance (C= 0.58) and presence of at least mild comorbidity (C = 0.55). RT following BCS was the most omitted treatment component causing non-concordance in the study population. In multivariable regression, effects of treatment facility, DCIS, race, and comorbidity on non-concordant care differed by age group.
Conclusion
Patterns of underuse of standard therapies for breast cancer vary by age group and BCS use, where omission of RT is at risk.
Keywords: breast cancer, non-metastatic, BCS, disparity, cancer registry
Introduction
Breast cancer is one of the most commonly diagnosed and prevalent cancers among women1 accounting for over 206,000 newly diagnosed cases and nearly 40,000 deaths in the U.S. in 20102. Treatment guidelines for non-metastatic breast cancers (e.g. the National Comprehensive Cancer Network (NCCN)) provide evidence-based recommendations from clinical trials about local definitive therapy with primary surgery, node dissection and use of radiation therapy based on stage of disease. Receipt of standard of care has been observed to vary across studies for non-clinical factors such as age, race and geographic location3–10, suggesting a need for organizations, facilities and insurers to systematically study care patterns. The present study on non-metastatic breast cancer care was conducted from data collected in the PoC breast and prostate cancers study (PoC-BP) to report on receipt of guideline-concordant local definitive therapy (surgical treatment and radiation therapy) pooled from 7 state or regional cancer registries (Georgia, North Carolina, Kentucky, Louisiana, Wisconsin, Minnesota, and Los Angeles and San Bernardino counties in California). A unique focus of this study was to identify patient characteristics related to receipt of evidence-based local definitive management of non-metastatic breast cancer in a large all-inclusive population.
Methods and materials
Data Sources
The study population included all POC-BP cases diagnosed with breast cancer in 2004 in the participating state and regional registries11. Standard registry data were re-abstracted following CDC National Program of Cancer Registries guidelines for training data abstractors, who reviewed data completeness, performed re-abstractions using Abstract Plus software, and obtained new or expanded data from healthcare record sources, including: comorbidities (diagnostic categories), body height and weight, health insurance status, cancer treatment facility site per procedure (surgery, chemotherapy, and radiation therapy). Missing or uncertain data entries were followed up by study site-specific methods that included on-site chart abstraction by trained staff, physician surveys mailed to the clinic or practice, and phone calls. The study was approved or exempted by institutional review boards at participating institutions from the seven states and the CDC.
Study Cases
Selected for study were all microscopically confirmed first primary breast cancers, stage 0 (ductal carcinoma in situ, (DCIS)) through IIIA breast cancer (International Classification of Diseases for Oncology, third edition, site codes C50.0–C50.9) among women aged 20 years and older. Excluded were cases diagnosed at Veteran’s Health Administration hospitals, cases identified through autopsy or death certificate, and women with prior cancers.
Definition of Guideline-Concordant Local Treatment
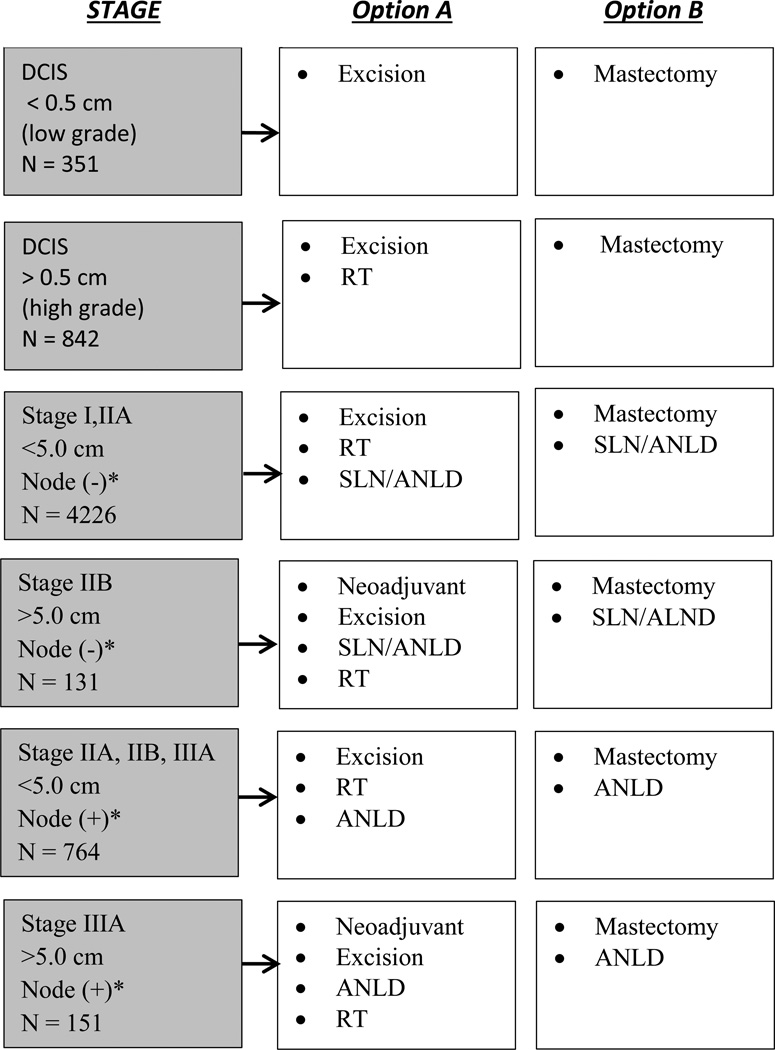
Standard of care for local treatment for stages 0 through IIIa breast cancer was determined from 2003 NCCN Clinical Practice Guidelines in Oncology. Components for surgery and radiation therapy (which is a required component following s breast conserving surgery) by staging group defined by tumor size nodal status (see Figure 1). Patients who received recommended treatments or procedures recorded in either re-abstracted cancer registry data, supplemental data by chart review or verification with treating physicians were considered guideline concordant. Standard cancer registry data were applied to Figure 1 treatment pathways. For axillary node dissection (ALND) and sentinel lymph node testing (SLN), a recognized data limitation reported for scope of regional lymph node surgery12 required that we accepted evidence of either SLN or ALND as lymph node dissection. We did not include SLN as a requirement in the quality of care assessment.
Figure 1.
*Clinical Node Status
Explanatory Variables
Patient age was modeled as five-level age groups and as < 65 versus 65+ years to examine potential less aggressive care for older women13 and for relevance to population research with SEER-Medicare data. Patient health insurance status at diagnosis was categorized as: private or private supplements, Medicare (with or without public co-insurance plans of TRICARE, military insurance, Veterans Affairs, or Indian Health Service coverage), Medicaid (including Medicare co-insurance and other government programs), none, and unknown. Area based measures were constructed from 2000 U.S. Census data linked to the census tract of the patient’s residence and included percent of the population with an income below the federal poverty level (FPL), with less than a high school education for adults aged 25 or older, and living in urban areas. Poverty was categorized as less than 20% of census tract population living below FPL versus ≥ 20%; educational attainment was classified as < 25% versus with a high-school education versus > 25%. Patient’s location of residence was classified as urban/rural from U.S. census data on population density on block groups and summarized within census tracts as ‘rural’ (100%), urban (100%) or rural/urban mix (< 100% urban). Commission on Cancer (CoC) status was categorized on the basis of the facility in which the woman received her breast cancer surgery. Comorbidity was collected by using the Adult Comorbidity Evaluation-27 (The ACE-27) developed by Piccirillo et al14,15 and was chosen for its range of coexisting conditions and disease severity relevant to cancer therapy choice and outcome.
Statistical Analysis
Bivariate and multivariate logistic regression models were fit to the data by age subgroup (<65, >=65) and weighted using post stratification weights reflecting the population sizes from which the registry samples were drawn to approximate a representative sample from each state. Multivariable logistic regression models include terms for node status, tumor size and stage to estimate main effects of each clinical tumor feature on the outcome of local therapy. SAS® software system Version 9.3 procedure SURVEYLOGISTIC (SAS Institute, Inc., Cary, North Carolina) was used to fit the models and derive 95% confidence intervals. To identify the most important predictors of guideline concordant care, the predictive capability of each independent variable and treatment component was assessed using the C-value in the bivariate data, representing the area under the sensitivity/specificity curve (ROC) of the logistic model, where a perfect model would have a C-value fit of 1.0.
Missing data
Missing data were imputed from clinical staging information where possible (i.e., evidence of positive lymph nodes), otherwise probabilistic imputation was performed with regression calibration procedures16 using the Markov Chain Monte Carlo method for initial imputation of variables.
Results
Of 9142 cases of primary breast cancer in POCBP study population, 6,505 women met the criteria of a diagnosis of stage 0 through IIIA primary breast cancer and were selected for study. In Table 1, approximately 27% of study cases were less than age 50; 38% ages 50–64 years, and 36 % ages 65 years and over. Most (72%) were white, with private health insurance (55%); treated within a CoC approved facility (51%); and classified as having at least one mild, moderate or severe more comorbidity (56%). Approximately 15% were black and 5% Hispanic; and 17% lived in a high poverty census tract.
Table 1.
Characteristics of Women Diagnosed with Loco-regional Breast Cancer
| Characteristics | Count | Weighted % | |
|---|---|---|---|
| All patients | 6505 | 100 | |
| Age at diagnosis (yrs) | |||
| <40 | 404 | 5.54 | |
| 40–49 | 1450 | 21.00 | |
| 50–64 | 2425 | 37.45 | |
| 65–69 | 670 | 10.60 | |
| 70+ | 1556 | 25.41 | |
| Race/ethnicity | |||
| White/non-Hispanic | 3507 | 72.29 | |
| Black/non-Hispanic | 1878 | 14.77 | |
| Hispanic | 453 | 4.91 | |
| Other race/ethnicity | 390 | 3.45 | |
| Unknown | 277 | 4.59 | |
| Health Insurance | |||
| Private | 3440 | 55.41 | |
| Medicare w/ supplement | 1347 | 22.72 | |
| Medicaid/Medicare only/Dual | 1269 | 15.18 | |
| None/Uninsured/Unknown | 449 | 6.69 | |
| Census-tract Povertyb | |||
| < 20% | 4878 | 82.79 | |
| 20% + | 1614 | 16.99 | |
| Unknown | 13 | 0.23 | |
| State of Residence | |||
| NC | 784 | 19.57 | |
| GA | 1722 | 20.15 | |
| LA | 1215 | 9.06 | |
| KY | 419 | 10.06 | |
| WI | 550 | 8.31 | |
| MN | 718 | 11.96 | |
| CA | 1097 | 20.89 | |
| Surgical facility CoC status | |||
| Yes | 3375 | 50.71 | |
| No | 2295 | 35.53 | |
| Other | 835 | 13.76 | |
| Clinical Tumor stage | |||
| DCIS < 0.5 | 351 | 5.61 | |
| DCIS > 0.5 | 842 | 12.96 | |
| Stage I (N0, < 5cm) | 3148 | 50.40 | |
| Stage IIA (N0, <5cm) | 1118 | 17.21 | |
| Stage IIA (N1, < 5cm) | 652 | 8.85 | |
| Stage IIB (N0, > 5cm) | 131 | 1.64 | |
| Stage IIIA (N2, < 5cm) | 112 | 1.46 | |
| Stage IIIA (N1, > 5cm) | 119 | 1.52 | |
| Stage IIIA (N2, > 5cm) | 32 | 0.35 | |
| Lymph node status | |||
| Negative | 3631 | 57.35 | |
| Positive | 1775 | 25.85 | |
| Undetermined | 1099 | 16.80 | |
| Comorbidity | |||
| No | 2725 | 42.58 | |
| Mild | 2757 | 42.63 | |
| Moderate/Severe | 905 | 13.13 | |
| Unknown | 118 | 1.66 | |
| Surgical approach | |||
| Mastectomy only | 2070 | 31.83 | |
| BCS only | 723 | 11.17 | |
| Mastectomy + RT | 573 | 7.78 | |
| BCS + RT | 2998 | 47.18 | |
| None | 80 | 1.06 | |
| Undetermined | 61 | 0.99 | |
| Neoadjuvant Chemotherapy | |||
| With T3 | 89 | 1.19 | |
| With Other(T1,T2) | 190 | 2.85 | |
| Other/Not performed | 6226 | 95.96 |
High poverty was defined as 20% or more of persons with income below the federal-defined poverty level.
Guideline concordant care
Bivariate results
Among study cases, 88% received guideline concordant loco-regional therapy, thus approximately 12% (N = 813) had some form of non-concordant cancer care (Table 2). Patient characteristics linked to lack of receipt of concordant loco-regional care included older age (p < 0.01 for years of age), Medicare or Medicaid versus other forms of insurance (p < 0.01), living in a low poverty census tract vs. higher poverty tract (p =0.04), living in a urban vs rural residence (p = 0.02), and having a moderate/severe level of comorbidity (p < 0.01). Lack of receipt of concordant care was highest for treatment guidelines involving stage 0 disease (i.e., ductal carcinoma in-situ) greater than 0.5 cm, stage IIB tumors, BCS (p < 0.01), and for node dissection (P < 0.01). ROC analysis showed that for the bivariate patterns described above, the best discriminators of concordant versus non-concordant care by order of importance were surgical approach (C = 0.70); tumors requiring lymph node dissection (C= 0.68) ; patient age (C=0.62); tumor stage (C= 0.60); insurance status (C= 0.58); and comorbidity level (C = 0.55).
Table 2.
Treatment Concordance by Patient Characteristics
| Characteristics | Treatment Discordant |
Weighted % | ||
|---|---|---|---|---|
| ALL PATIENTS | 813 | 12.4 | ||
| Age at diagnosis (yrs) | P <0.01 | |||
| <40 | 35 | 7.96 | ||
| 40–49 | 120 | 7.56 | ||
| 50–64 | 243 | 10.45 | ||
| 65–69 | 83 | 11.34 | ||
| 70+ | 332 | 20.50 | ||
| Race/ethnicity | P =0.11a | |||
| White | 417 | 12.00 | ||
| Black | 264 | 14.18 | ||
| Hispanic | 48 | 13.04 | ||
| Other race/ethnicity | 61 | 15.86 | ||
| Unknown | 23 | 7.82 | ||
| Insurance | P <0.01 | |||
| Private | 316 | 11.52 | ||
| Medicare w/ supplement | 220 | 9.65 | ||
| Medicaid/Medicare only/Dual | 214 | 17.88 | ||
| None/Uninsured/Unknown | 63 | 15.51 | ||
| Census-tract Povertyb | P=0.04a | |||
| < 20% (ref) | 575 | 11.93 | ||
| 20% + | 235 | 14.30 | ||
| Unknown | 3 | 20.48 | ||
| Urban Rural Residence | P =0.02a | |||
| Urban | 428 | 13.75 | ||
| Urban Rural Mix | 269 | 10.73 | ||
| Rural | 113 | 10.96 | ||
| Unknown | 3 | 21.92 | ||
| Census-tract Educationc | P =0.20a | |||
| High | 450 | 11.91 | ||
| Low | 360 | 13.27 | ||
| Unknown | 3 | 20.48 | ||
| State of Residence | P <0.01 | |||
| NC | 95 | 10.28 | ||
| GA | 194 | 10.27 | ||
| LA | 143 | 11.23 | ||
| KY | 52 | 11.86 | ||
| WI | 76 | 15.62 | ||
| MN | 66 | 8.59 | ||
| CA | 187 | 17.88 | ||
| Surgical facility CoC status | P = 0.08a | |||
| Yes | 362 | 11.15 | ||
| No | 316 | 13.12 | ||
| Unknown | 135 | 14.80 | ||
| Clinical Tumor stage | P <0.01 | |||
| DCIS < 0.5 | 5 | 2.51 | ||
| DCIS > 0.5 | 162 | 19.50 | ||
| Stage I (N0, < 5cm) | 369 | 11.16 | ||
| Stage IIA (N0, <5cm) | 162 | 14.54 | ||
| Stage IIA (N1, < 5cm) | 54 | 8.82 | ||
| Stage IIB (N0, > 5cm) | 33 | 28.45 | ||
| Stage IIIA (N2, < 5cm) | 7 | 5.57 | ||
| Stage IIIA (N1, > 5cm) | 16 | 10.95 | ||
| Stage IIIA (N2, > 5cm) | 5 | 18.58 | ||
| Lymph node status | P =0.26a | |||
| Negative | 269 | 7.63 | ||
| Positive | 149 | 8.79 | ||
| Undetermined | 395 | 33.94 | ||
| Comorbidity | P <0.01a | |||
| No | 284 | 10.18 | ||
| Mild | 326 | 11.86 | ||
| Moderate/Severe | 167 | 18.48 | ||
| Unknown | 36 | 32.34 | ||
| Surgery | P <0.01 | |||
| Mastectomy | 52 | 2.11 | ||
| BCS | 681 | 17.85 | ||
| Lymph node dissection | No | P< 0.01 | 417 | 35.43 |
| Yes | 395 | 7.62 | ||
Ignores ‘Unknown’ or ‘Undetermined’ category
High poverty was defined as 20% or more of persons with income below the federal-defined poverty level.
Low education was defined as 25% or more of adults (aged 25 years and older) with less than a high school education.
Borderline ER or PR was grouped with the ER+ and/or PR+ group.
Age stratified models
Discordant treatment was found among 9.3% of cases ≤ 65 years of age vs. 17.8% among > 65 years (p <0.0001) (data not shown). Multivariable regression analyses stratified by age group (Table 3) revealed that for women aged < 65 years (n=4,279), the odds of receipt of discordant care were significantly higher with increased age, but lower with rural residence (OR = 0.51, p=0.005), treatment in a CoC approved facility (OR = 0.68, p= 0.009), and tumor stage other than DCIS (e.g., versus stage I: OR = 0.55 p <0 .002). Among women > 65 years (N=2226) the odds of having discordant care were highest with increased age (OR = 1.11, p < 0.001), for black women (OR = 1.41, p= 0.049), and with moderate/severe comorbidity (OR= 1.63, p = 0.030). Assessment of variance inflation factors as an indicator of multicollinearity among interrelated staging variables found that all values were below 3.6, were values of 5 or greater are often used to indicate appreciable multicollinearity17.
Table 3.
Multivariate Logistic Model Predicting Discordant Care (N=6,515 Total)
| Age<65 (N = 4,279) | Age 65+ (N = 2,226) | |||||
|---|---|---|---|---|---|---|
| Predictors | OR | 95% CI | P-value | OR | 95% CI | P-value |
| Age(per year) | 1.02 | 1.00–1.04 | 0.017 | 1.11 | 1.09–1.14 | <.001 |
| Race | ||||||
| White (ref) | ||||||
| Black | 1.19 | 0.90–1.58 | 0.224 | 1.41 | 1.00–1.98 | 0.049 |
| Hispanic | 1.15 | 0.72–1.84 | 0.568 | 1.68 | 0.88–3.18 | 0.114 |
| Other | 1.16 | 0.74–1.83 | 0.526 | 1.57 | 0.78–3.13 | 0.205 |
| Health Insurance | ||||||
| Public (ref) | ||||||
| Private | 0.87 | 0.62–1.23 | 0.438 | 1.13 | 0.72–1.79 | 0.591 |
| Uninsured/NA | 0.90 | 0.54–1.50 | 0.687 | 0.96 | 0.52–1.80 | 0.907 |
| Census tract education | ||||||
| Low (ref) | ||||||
| High | 0.93 | 0.65–1.32 | 0.669 | 0.95 | 0.65–1.39 | 0.781 |
| Rural/Urban Residence | ||||||
| Urban (ref) | ||||||
| Urban RuralMix | 0.84 | 0.61–1.15 | 0.268 | 0.83 | 0.58–1.17 | 0.274 |
| Rural | 0.51 | 0.32–0.81 | 0.005 | 1.01 | 0.67–1.53 | 0.963 |
| Census tract poverty | ||||||
| < 20% (ref) | ||||||
| 20%+ | 0.87 | 0.62–1.24 | 0.447 | 0.99 | 0.64–1.53 | 0.958 |
| Comorbidity index score | ||||||
| None (ref) | ||||||
| Mild | 0.77 | 0.56–1.05 | 0.097 | 1.05 | 0.71–1.55 | 0.817 |
| Moderate/Severe | 1.14 | 0.74–1.75 | 0.552 | 1.63 | 1.05–2.53 | 0.030 |
| CoC Surgery facility status | ||||||
| No (ref) | ||||||
| Yes | 0.68 | 0.50–0.91 | 0.009 | 1.04 | 0.76–1.43 | 0.795 |
| Tumor size (cm) | ||||||
| < 0.5 (ref) | ||||||
| 0.5–1 | 1.12 | 0.60–2.11 | 0.718 | 0.70 | 0.37–1.32 | 0.272 |
| > 1 – 2 | 1.07 | 0.60–1.90 | 0.833 | 0.62 | 0.34–1.12 | 0.116 |
| > 2 – 5 | 1.34 | 0.72–2.51 | 0.359 | 0.74 | 0.37–1.46 | 0.384 |
| > 5 | 2.03 | 0.86–4.79 | 0.106 | 1.23 | 0.46–3.30 | 0.684 |
| Lymph node status | ||||||
| Negative (ref) | ||||||
| Positive | 1.11 | 0.73–1.69 | 0.613 | 1.03 | 0.67–1.58 | 0.891 |
| Stage | ||||||
| DCIS (ref) | ||||||
| Stage I | 0.55 | 0.38–0.80 | 0.002 | 0.80 | 0.51–1.26 | 0.341 |
| Stage II | 0.66 | 0.40–1.09 | 0.104 | 0.66 | 0.36–1.18 | 0.162 |
| Stage IIIA | 0.42 | 0.19–0.95 | 0.038 | 0.35 | 0.09–1.27 | 0.110 |
Surgical Approach
Overall, 60.9% (n=2138) of women aged < 65 years of age and 67.2% of women aged ≥ 65 years (n= 1,284) with tumor size < 5 cm received BCS as primary therapy. In Table 4, the odds of BCS versus mastectomy among women < 65 years of age were higher for increasing year of age (OR = 1.01, p=0.031), black race/ethnicity (OR = 1.24, p=0.043), private health insurance (OR = 1.37, p=0.023), and receipt of breast surgery in a CoC approved facility (OR = 1.26, p=0.028). Tumor characteristics associated with receipt of mastectomy included positive node status (OR = 0.70. p=0.002), residence in an urban/rural mixed county vs. others (OR=0.79, p=0.028), greater than stage I tumors vs. stage 0 (OR = 0.49 and 0.21, p=< 0.002 for stage II and IIIa respectively). Among women ≥ 65 years of age, receipt of BCS was higher with residence in a census tract where > 25% of adults had completed high school (OR = 1.54, p=0.013). Treatment with mastectomy was more likely for tumors larger than 1.0 cm (OR = 0.52 for 1–2 cm, p =0.012, and 0.36 for 2–5 cm, p=0.001), patients who resided in rural counties (OR = 0.68, p= 0.048), patients having positive clinical node status (OR = 0.53, p = 0.002), and AJCC derived IIIA (OR = 0.14, p= 0.002). CoC facility, poverty and race were not associated with receipt of BCS among older women. Among N= 5309 women who would be recommend to receive lymph node dissection in Figure 1, a total of 4,940 (93%) actually received some form of lymph node dissection (data not shown).
Table 4.
Use of BCS versus mastectomy in patients with non-metastatic disease < 5 cm.
| Age<65 (N=3,511) | Age 65+ (n=1,910) | |||||
|---|---|---|---|---|---|---|
| Predictors | OR | 95% CI | P-value | OR | 95% CI | P-value |
| Age (per year) | 1.01 | 1.00 –1.03 | 0.031 | 0.99 | 0.97–1.01 | 0.297 |
| Race | ||||||
| White (ref) | ||||||
| Black | 1.24 | 1.01–1.53 | 0.043 | 1.14 | 0.85–1.53 | 0.397 |
| Hispanic | 1.21 | 0.83–1.76 | 0.336 | 1.34 | 0.72–2.47 | 0.356 |
| Other | 0.83 | 060–1.14 | 0.253 | 0.66 | 0.38–1.16 | 0.147 |
| Health Insurance | ||||||
| Public (ref) | ||||||
| Private | 1.37 | 1.04–1.80 | 0.023 | 1.19 | 0.81–1.75 | 0.373 |
| Uninsured/NA | 1.43 | 0.96–2.12 | 0.076 | 1.76 | 0.95–3.26 | 0.071 |
| Census tract education | ||||||
| Low (ref) | ||||||
| High | 1.20 | 0.93–1.54 | 0.154 | 1.54 | 1.09–2.16 | 0.013 |
| Rural/Urban Residence | ||||||
| Urban (ref) | ||||||
| Urban Rural Mix | 0.79 | 0.64–0.98 | 0.028 | 0.75 | 0.55–1.01 | 0.055 |
| Rural | 0.72 | 0.53–0.96 | 0.028 | 0.68 | 0.46–1.00 | 0.048 |
| Census tract poverty | ||||||
| 20% + (ref) | ||||||
| < 20% | 0.99 | 0.74–1.32 | 0.953 | 0.79 | 0.55–1.13 | 0.189 |
| Comorbidity index score | ||||||
| None (ref) | ||||||
| Mild | 0.99 | 0.80–1.22 | 0.921 | 0.86 | 0.63–1.18 | 0.343 |
| Moderate/Severe | 1.36 | 0.99–1.88 | 0.060 | 1.11 | 0.74–1.66 | 0.631 |
| CoC Surgery facility status | ||||||
| No (ref) | ||||||
| Yes | 1.26 | 1.03–1.54 | 0.028 | 1.30 | 0.96–1.75 | 0.095 |
| Tumor size (cm)1 | ||||||
| < 0.5 (ref) | ||||||
| 0.5–1 | 1.15 | 0.79–1.66 | 0.467 | 0.92 | 0.53–1.59 | 0.833 |
| 1 – 1.9 | 0.92 | 0.66–1.29 | 0.643 | 0.52 | 0.31–0.87 | 0.012 |
| 2 – 4.9 | 0.80 | 0.55–1.18 | 0.260 | 0.36 | 0.20–0.65 | 0.001 |
| Lymph node status | ||||||
| Negative (ref) | ||||||
| Positive | 0.70 | 0.56–0.88 | 0.002 | 0.53 | 0.38–0.74 | 0.002 |
| Stage | ||||||
| DCIS (ref) | ||||||
| Stage I | 0.90 | 0.67–1.22 | 0.508 | 0.71 | 0.45–1.11 | 0.132 |
| Stage II | 0.49 | 0.34–0.71 | 0.002 | 0.59 | 0.34–1.02 | 0.057 |
| Stage IIIA | 0.21 | 0.11–0.42 | <0.001 | 0.14 | 0.04–0.47 | 0.002 |
Tumor size sample restricted to < 5 cm per algorithm in Figure 1.
Post-surgery RT
Among 3,721 women with breast tumors treated with BCS, 80% had receipt of adjuvant RT recorded, including 85.0% of women aged < 65 years of age (N=2,065) versus 72.3% (N= 933) of women 65 years and older (p < .0001). In multivariable regression (Table 5), predictors of RT following BCS among women < 65 years of age were surgical treatment received at a CoC approved facility (OR= 1.46, p= 0.037), having a tumor size of .5 to 1 cm (OR= 2.22, p= 0.008) or stage of disease other than DCIS: cases with stage I breast cancer were almost 3 times more likely to receive RT following BCS than those with DCIS (OR= 2.94, p < 0.001). Among women aged 65 years and older, the odds of RT following BCS were lower with increased year of age (OR= 0.88, p < 0.001), Hispanic ethnicity (OR= 0.40, p= 0.012), and having moderate/severe comorbidity (OR= 0.52, p=0.012).
Table 5.
Receipt of Breast Conservation Surgery with RT versus No RT
| Age<65 (N=2,430) | Age 65+ (N=1,291) | |||||
|---|---|---|---|---|---|---|
| Predictors | OR | 95% CI | P-value | OR | 95% CI | P-value |
| Age (per year) | 0.98 | 0.96–1.00 | 0.053 | 0.88 | 0.85–0.90 | <.001 |
| Race | ||||||
| White (ref) | ||||||
| Black | 0.91 | 0.66–1.26 | 0.566 | 0.71 | 0.47–1.07 | 0.117 |
| Hispanic | 0.95 | 0.56–1.62 | 0.855 | 0.40 | 0.21–0.82 | 0.012 |
| Other | 0.82 | 0.49–1.37 | 0.447 | 0.50 | 0.22–1.19 | 0.101 |
| Health Insurance | ||||||
| Public (ref) | ||||||
| Private | 1.22 | 0.81–1.83 | 0.350 | 0.75 | 0.44–1.27 | 0.283 |
| Uninsured/NA | 0.99 | 0.54–1.80 | 0.962 | 1.05 | 0.55–2.00 | 0.891 |
| Census tract education | ||||||
| Low (ref) | ||||||
| High | 1.20 | 0.80–1.82 | 0.376 | 1.26 | 0.76–2.08 | 0.370 |
| Rural/Urban Residence | ||||||
| Urban (ref) | ||||||
| Urban Rural Mix | 0.98 | 0.69–1.38 | 0.896 | 1.22 | 0.82–1.83 | 0.334 |
| Rural | 1.51 | 0.91–2.51 | 0.107 | 1.14 | 0.68–1.91 | 0.613 |
| Census tract poverty | ||||||
| 20% + (ref) | ||||||
| <20% | 1.13 | 0.75–1.70 | 0.566 | 1.04 | 0.60–1.80 | 0.885 |
| Comorbidity index score | ||||||
| None (ref) | ||||||
| Mild | 1.38 | 0.97–1.96 | 0.072 | 0.97 | 0.62–1.52 | 0.894 |
| Moderate/Severe | 0.86 | 0.54–1.36 | 0.519 | 0.52 | 0.31–0.87 | 0.012 |
| CoC Surgery facility status | ||||||
| No (ref) | ||||||
| Yes | 1.46 | 1.03–2.07 | 0.037 | 1.08 | 0.75–1.56 | 0.673 |
| Tumor size (cm) | ||||||
| < 0.5 (ref) | ||||||
| 0.5–1 | 2.22 | 1.25–3.94 | 0.008 | 1.65 | 0.91–3.01 | 0.103 |
| > 1 – 2 | 1.89 | 1.07–3.34 | 0.034 | 1.60 | 0.92–2.75 | 0.094 |
| > 2 – 5 | 1.22 | 0.68–2.21 | 0.512 | 1.07 | 0.54–2.11 | 0.857 |
| > 5 | 0.47 | 0.11–1.89 | 0.285 | 0.90 | 0.13–6.25 | 0.914 |
| Lymph node status | ||||||
| Negative (ref) | ||||||
| Positive | 0.67 | 0.43–1.04 | 0.077 | 0.70 | 0.40–1.21 | 0.204 |
| Stage | ||||||
| DCIS (ref) | ||||||
| Stage I | 2.94 | 2.03–4.26 | <0.001 | 2.16 | 1.39–3.35 | 0.001 |
| Stage II | 1.96 | 1.15–3.31 | 0.013 | 2.37 | 1.19–4.75 | 0.015 |
| Stage IIIA | 6.03 | 1.56–23.3 | 0.009 | 2.90 | 0.22–39.0 | 0.422 |
Discussion
The identification of characteristics of patients not receiving guideline recommended therapy based on national guidelines is critically important so that these disparities can be strategically addressed and all patients receive appropriate therapy. A strength of this study is its large multi-state sample size, including all adult age groups, and chart re-abstraction to increase improve data collection from smaller hospitals or freestanding centers and to improve data such as commorbidities across all reporting faciulities. Approximately 12% of women in our study were found to have non-concordant loco-regional treatment for breast cancer. From the ROC analysis, the two most common deviations from guidelines were omission of RT when the surgical approach was BCS, and lymph node dissection. In some surgical facilities, BCS is a failure-risk for omission of RT, such as facilities that lack comprehensive services or integrated referral systems for cancer services. BCS Characteristics linked to non-concordant care differed by age group, as did rates of non-concordant care. Older women (age > 65 years) were significantly more likely to have non-concordant care than younger women (17.8% vs. 9.3%) as were women who lived in more affluent census tracts. For older women, non-concordant care was associated mostly with comorbidity status and race/ethnicity; whereas for younger women the correlates were non-rural residence, being treated at a non-CoC approved surgery facility and having a small tumor at diagnosis (i.e., DCIS). The finding of more guideline compliant care among younger women in CoC facilities is interesting. In exploratory analyses we found that compared to CoC facilities, women treated in non-COC facilities were more likely to be rural, live in poverty or low-educational attainment census tracts and have a high level of comobidities. Further, Non-CoC facilities were more likely to perform mastectomies and deliver BCS not followed with RT than CoC facilities in our study. In reports on adjuvant treatment, Bickel et al19 found similar barriers for racial disparities and Wu et al10 suggested that patients treated at non-CoC facilities may face barriers to multidisciplinary oncology consultations. These patterns point to a need for greater care coordination and patient support in rural regions where patients often face multiple barriers to access or treatment adherence.
Overall, approximately 60% of adult women with early stage breast cancer received breast conserving surgery. BCS has been viewed as a preferred treatment for most women with early stage breast cancer for decades based on evidence from prospective randomized trials20 but there increasing use of mastectomy21,22 in recent years perhaps due to perceived or actuarial risk for recurrence and patient preference23, distance or local access to RT services which may be challenging in rural locations24, surgeon preference or skill, and treatment recommendations for women with BRCA positivity. Like others21,25,26 we found that receipt of BCS varied considerably by non-clinical factors such as region (rurality), insurance status, and treatment facility type. With recent evidence of a possible survival benefit among women aged 50 years and older receiving BCS and radiotherapy versus mastectomy alone regardless of age or HR status27, a better understanding of how to effectively promote appropriate use of BCS may be needed. Our finding that black women were more likely to receive BCS than white women is consistent with previous reports9 but is difficult to interpret without patient reports as it may depend upon clinical status (e.g. tumor stage or size), cultural and treatment access (e.g., near versus far distances), hospital size or accreditation (e.g., larger or CoC accredited facilities), urban/rural status, and health insurance status28–31. Some studies have demonstrated no differences in surgical treatment between black and white women32–34. The finding of more concordant care with increased area affluence in the bivariate results was not present in multivariate models after accounting for age, comorbidity and tumor status.
That approximately 81% of patients in this study treated with BCS also received RT is discouraging as apparently little progress had been made over receipt of RT from earlier time periods reported in the literature by the time of study36. A study of the SEER registry data between 1983 and 1995 showed that the proportion of women receiving recommended primary therapy fell from 88% in 1983–89 to 78% by the end of 199535. This pattern may reflect emerging treatment guidelines in 2004 that endorsed the use of endocrine therapy with BCS instead of RT in patients older than 70 years with low grade, node negative, ER positive tumors < 2 cm in size,18 and gave recognition to poorer survival among older patients with breast cancer due to concomitant causes that may justify less aggressive treatment to preserve quality of life. We found that women > 65 years with moderate or severe comorbidity had only half the odds of RT than those with no comorbidity. However, women of any age who were treated at a CoC approved site for their initial breast cancer surgery were more likely to receive RT than those treated at non-approved sites. This could reflect better access to care through insurance plans37 or to comprehensive care within the surgical facility location and coordination of treatment with lab tests and pathology reports. Other studies have found associations of non-clinical factors and care access, such as delays in treatment for breast cancer among black women.38–41
Some limitations of registry data studies include potential omission of records when not available for review or missing sought. In our models assessing guideline concordance, 71% of the cases had complete data, 23% had 1 variable with missing values, 5% had 2 variables and <1% had 3 or more variables with missing values. Instead of discarding cases with missing data, multiple imputation procedures were applied but are subject to the assumption of a ‘missing at random‘ data mechanism. Further, the study data do not allow for the influence of patient choice in treatment decisions as not all DCIS patients or women with early stage <2cm ER+ cancers >70 years require radiation therapy. Another limitation is that results of this study are for the 2004 diagnosis year and thus do not reflect current use of guideline care due to recent guideline changes or trends in care. Finally, because of data quality issues in registry data we were unable to examine sentinel lymph node assessments as a separate component of care, thus we have likely underestimated care concordance.
In conclusion, we found both common and distinct patterns of underuse of standard therapies and important locoregional treatment variation for non-metastatic breast cancer for older versus younger women. Increased age and DCIS was associated with more discordant loco-regional treatment among all women. Older women with higher comorbidity levels or black race/ethnicity were the most likely to have had discordant breast cancer treatment. For younger women, living in rural locations or having surgery in a COC center reduced the odds for discordant cancer treatment. Improving access to recommended care guidelines is important so that all patients receive appropriate therapy.
Acknowledgement
The Breast and Prostate Cancer Data Quality and Patterns of Care Study was supported by the Centers for Disease Control and Prevention through cooperative agreements with the California Cancer Registry (Public Health Institute) (1-U01-DP000260), Emory University (1-U01-DP000258), Louisiana State University Health Sciences Center (1-U01-DP000253), Minnesota Cancer Surveillance System (Minnesota Department of Health) (1-U01-DP000259), Medical College of Wisconsin (1-U01-DP000261), University of Kentucky (1-U01-DP000251), and Wake Forest University (1-U01-DP000264). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Contributor Information
Roger T. Anderson, University of Virginia School of Medicine, Charlottesville VA
Cyllene Morris, California Cancer Reporting and Epidemiologic Surveillance Program, Institute for Population Health Improvement, UC Davis Health System
Gretchen Kimmick, Internal Medicine, Medical Oncology, Multidisciplinary Breast Program Duke University Medical Center
Amy Trentham-Dietz, University of Wisconsin Carbone Cancer Center, Madison, WI.
Fabian Camacho, Penn State College of Medicine, Hershey PA
Xiao-Cheng Wu, Epidemiology Program, School of Public Health, LSU Health Sciences Center
Susan A. Sabatino, Division of Cancer Prevention and Control, Centers for Disease Control and Prevention, Atlanta, GA.
Steven T. Fleming, Department of Epidemiology, University of Kentucky College of Public Health
Joseph Lipscomb, Rollins School of Public Health and Winship Cancer Institute, Emory University, Atlanta GA.
Literature Cited
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2010 Incidence and Mortality Web-based Report. Atlanta (GA): Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; 2013. Available at: http://www.cdc.gov/uscs. [Google Scholar]
- 3.Roetzheim RG, Gonzalez EC, Ferrante JM, et al. Effects of health insurance and race on breast carcinoma treatments and outcomes. Cancer. 2000;89:2202–2213. doi: 10.1002/1097-0142(20001201)89:11<2202::aid-cncr8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 4.Hawley S, Hofer TP, Janz NK, Fagerlin A, Schwartz K, Liu L, Deapen D, Morrow M, Katz SJ. Correlates of Between-Surgeon Variation in Breast Cancer Treatments. Medical Care. 2006 Jul;44(7):609–616. doi: 10.1097/01.mlr.0000215893.01968.f1. [DOI] [PubMed] [Google Scholar]
- 5.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 6.Mandelblatt JS, Kerner JF, Hadley J, et al. Variations in breast carcinoma treatment in older medicare beneficiaries: Is it black or white? Cancer. 2002;95:1401–1414. doi: 10.1002/cncr.10825. [DOI] [PubMed] [Google Scholar]
- 7.Sariego J. Regional variation in breast cancer treatment throughout the United States. Am J Surg. 2008;196:572–574. doi: 10.1016/j.amjsurg.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 8.Kimmick G, Camacho F, Foley K, Levine E, Balkrishnan R, Anderson R. Racial Differences in Patterns of Care Among Medicaid-Enrolled Breast Cancer Patients. Journal of Oncology Practice. 2006 doi: 10.1200/jop.2006.2.5.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson RT, Kimmick GG, Camacho F, Whitmire JT, Dickinson C, Levin EA, Tori FM, Balkrishnan R. Health System Correlates of Receipt of Radiation Therapy After Breast-Conserving Surgery Among Low-Income Medicaid-Enrolled Women. The American Journal of Managed Care. 2008 Oct;14(10):644–652. [PubMed] [Google Scholar]
- 10.Wu XC, Lund MJ, Kimmick GG, Richardson LC, Sabatino SA, Chen VW, Fleming ST, Morris CR, Huang B, Trentham-Dietz A, Lipscomb J. Influence of race, insurance, socioeconomic status, and hospital type on receipt of guideline-concordant adjuvant systemic therapy for loco-regional breast cancers. Journal of Clinical Oncology. 2012;30(2):142–150. doi: 10.1200/JCO.2011.36.8399. [DOI] [PubMed] [Google Scholar]
- 11.German RR, Wike JM, Bauer KR, Fleming ST, Trentham-Dietz A, Namiak M, Almon L, Knight K, Perkins C for the Patterns of Study Group. Quality of Cancer Registry Data: Findings from CDC-NPCR’s Breast and Prostate Cancer Data Quality and Patterns of Care Study. Journal of Registry Management. 2011;38(2):75–86. 2011. [PubMed] [Google Scholar]
- 12.Commission on Cancer. [3/9/2012]; http://www.facs.org/cancer/ncdb/scope-regional-lymph-node-surgery.pdf. [Google Scholar]
- 13.Muss HB, Busby-Whitehead J. Older women with breast cancer: Slow progress, great opportunity, now is the time. J Clin Oncol. 2011;29:4608–4610. doi: 10.1200/JCO.2011.38.6888. [DOI] [PubMed] [Google Scholar]
- 14.Piccirillo JF, Costas I, Claybour P, et al. The measurement of comorbidity by cancer registries. J Reg Mngt. 2003;30:8–14. [Google Scholar]
- 15.Piccirillo JF, Tierney RM, Costas I, et al. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2006;291:2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 16.Yucel RM, Yulei He, Zaslavsky AM. Using calibration to improve rounding in multiple imputation. The American Statistician. 2008;62(2):125–129. [Google Scholar]
- 17.Rogerson PA. Statistical methods for geography. London: Sage; 2001. [Google Scholar]
- 18.Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351:971–977. doi: 10.1056/NEJMoa040587. [DOI] [PubMed] [Google Scholar]
- 19.Bickell NA, Wang JJ, Oluwole S, Schrag D, Godfrey H, Hiotis K, Mendez J, Guth AA. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006 Mar 20;24(9):1357–1362. doi: 10.1200/JCO.2005.04.5799. [DOI] [PubMed] [Google Scholar]
- 20.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, Jeong JH, Wolmark N. Twenty-Year Follow-Up of a Randomized Trial Comparing Total Mastectomy, Lumpectomy, and Lumpectomy Plus Irradiation for the Treatment of Invasive Breast Cancer. New England Journal of Medicine. 2002;347(16):1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 21.Gomez SL, Lichtensztajn D, Kurian AW, Telli ML, Chang ET, Keegan THM, et al. Increasing mastectomy rates for early-stage breast cancer? population-based trends from California. J Clin Oncol. 2010;28:e155–e157. doi: 10.1200/JCO.2009.26.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katipamula R, Degnim AC, Hoskin T, et al. Trends in mastectomy rates at the Mayo Clinic Rochester: Effect of surgical year and preoperative magnetic resonance imaging. J Clin Oncol. 2009;27:4082–4088. doi: 10.1200/JCO.2008.19.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molenaar S, Oort F, Sprangers M, et al. Predictors of patients' choices for breast-conserving therapy or mastectomy: a prospective study. Br J Cancer. 2004;90:2123–2130. doi: 10.1038/sj.bjc.6601835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Celaya MO, Rees JR, Gibson JJ, Riddle BL, Greenberg ER. Travel distance and season of diagnosis affect treatment choices for women with early stage breast cancer in a predominantly rural population. Cancer causes Control. 2006;17:851–856. doi: 10.1007/s10552-006-0025-7. [DOI] [PubMed] [Google Scholar]
- 25.Morris CR, Cohen R, Schlag R, et al. Increasing trends in the use of breast-conserving surgery in California. Am J Public Health. 2000;90:281–284. doi: 10.2105/ajph.90.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goel MS, Burns RB, Phillips RS, et al. Trends in breast conserving surgery among Asian Americans and Pacific Islanders, 1992–2000. J Gen Intern Med. 2005;20:604–611. doi: 10.1111/j.1525-1497.2005.0090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang ES, Lichtensztajn DY, Gomez SL, Fowble B, Clarke CA. Survival after lumpectomy and mastectomy for early stage invasive breast cancer: the effect of age and hormone receptor status. Cancer. 2013;119:1402–1411. doi: 10.1002/cncr.27795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michalski TA, Nattinger AB. The influence of black race and socioeconomic status on the use of breast-conserving surgery for Medicare beneficiaries. Cancer. 1997;79:314–319. [PubMed] [Google Scholar]
- 29.Satariano ER, Swanson GM, Moll PP. Nonclinical factors associated with surgery received for treatment of early-stage breast-cancer. Am J Public Health. 1992;82:195–198. doi: 10.2105/ajph.82.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yood MU, Johnson CC, Blount A, et al. Race and differences in breast cancer survival in a managed care population. J Natl Cancer Inst. 1999;91:1487–1491. doi: 10.1093/jnci/91.17.1487. [DOI] [PubMed] [Google Scholar]
- 31.Yao N, Matthews SA, Marianne Hillemeier M, Anderson RT. Radiation Therapy Resources and Guideline-Concordant Radiotherapy for Early-Stage Breast Cancer Patients in an Underserved Region. Health Services Research. 2013;48(4):1433–1449. doi: 10.1111/1475-6773.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Velanovich V, Szymanski W. Quality of life of breast cancer patients with lymphedema. Am J Surg. 1999;177:184–187. doi: 10.1016/s0002-9610(99)00008-2. [DOI] [PubMed] [Google Scholar]
- 33.Desch CE, Penberthy LT, Hillner BE, McDonald MK, Smith TJ, Pozez AL, et al. A sociodemographic and economic comparison of breast reconstruction, mastectomy, and conservative surgery. Surgery. 1999;125:441–447. [PubMed] [Google Scholar]
- 34.Lund MJ, Butler EN, Bumpers HL, et al. High prevalence of triple-negative tumors in an urban cancer center. Cancer. 2008;113:608–615. doi: 10.1002/cncr.23569. [DOI] [PubMed] [Google Scholar]
- 35.Nattinger AB, Hoffmann RG, Kneusel RT, et al. Relation between appropriateness of primary therapy for early-stage breast carcinoma and increased use of breast-conserving surgery. Lancet. 2000;356:1148–1153. doi: 10.1016/S0140-6736(00)02757-4. [DOI] [PubMed] [Google Scholar]
- 36.Du Xianglin L, Gor BJ. Racial disparities and trends in radiation therapy after breast-conserving surgery for early-stage breast cancer in women, 1992 to 2002. Ethn Dis. 2007;17:122–128. [PMC free article] [PubMed] [Google Scholar]
- 37.Breen N, Wesley MN, Merrill RM, et al. The relationship of socio-economic status and access to minimum expected therapy among female breast cancer patients in the National Cancer Institute Black-White Cancer Survival Study. Ethnicity Disease. 1999;9:111–125. [PubMed] [Google Scholar]
- 38.Caplan LS, May DS, Richardson LC. Time to diagnosis and treatment of breast cancer: Results from the National Breast and Cervical Cancer Early Detection Program, 1991–1995. Am J Public Health. 2000;90:130–134. doi: 10.2105/ajph.90.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dennis CR, Gardner B, Lim B. Analysis of survival and recurrence vs. patient and doctor delay in treatment of breast cancer. Cancer. 1975;35:714–720. doi: 10.1002/1097-0142(197503)35:3<714::aid-cncr2820350326>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 40.Gwyn K, Bondy ML, Cohen DS, et al. Racial differences in diagnosis, treatment, and clinical delays in a population-based study of patients with newly diagnosed breast carcinoma. Cancer. 2004;100:1595–1604. doi: 10.1002/cncr.20169. [DOI] [PubMed] [Google Scholar]
- 41.Ramirez AJ, Westcombe AM, Burgess CC, et al. Factors predicting delayed presentation of symptomatic breast cancer: A systematic review. Lancet. 1999;353:1127–1131. doi: 10.1016/s0140-6736(99)02142-x. [DOI] [PubMed] [Google Scholar]