Abstract
Background
Tooth extraction is performed for a wide variety of reasons as we know. Several techniques aiming at enhancing the regeneration process in the extraction socket have been adopted such as filling the socket with autogenous bone grafts or bone substitutes. We know platelets play a central role in hemostasis and healing processes but relative contradictory effect of platelet in bone regenerating capacity have been published in different in vitro and in vivo studies.
Method
To explore this we used platelet-rich plasma (PRP) (autogenous) alone in empty extraction socket of bilateral impacted mandibular third molars. For that we selected five patients having bilateral impacted teeth. Out of two sockets one was used as intervention by filling with PRP and the other was allowed to heal without PRP. All patients were followed for clinical and radiological evaluation by using digital OPG view after 1 week, 1, 2 and 4 months period.
Result and conclusion
PRP enhanced the osteogenic response in initial bone healing at 1 month duration but there was no added benefit in late bone healing at 4 months period compared in both intervention and control groups. However PRP significantly improved the soft tissue healing in PRP treated sites compared to control group.
Keywords: Platelet-rich plasma (PRP), Early and late bone healing, Extraction socket
Introduction
Tooth extraction is performed for a wide variety of reasons, such as tooth decay that may destroy enough tooth structure to prevent restoration, or periodontal disease, which may severely affect the tooth-supporting tissues. Extraction of impacted or problematic wisdom teeth is routinely performed, as is extraction of some permanent teeth to make space for orthodontic treatment. The presence of fractured teeth or roots can be a further indication for extraction. In this case, the most conventional and predictable protocol dictates that implants are placed in the extraction sites after completion of the healing process, but parallel to ingrowth of bone into the socket a consistent resorption of the alveolar ridge occurs physiologically during the healing and remodelling phase of the extraction socket. In order to reduce alveolar bone dimensional changes, several techniques aiming at enhancing the regeneration process in the extraction socket have been adopted, such as filling the socket with autogenous bone grafts or bone substitutes and, guided bone regeneration (GBR) [1] with resorbable or non-resorbable barriers, and the use of various bone promoting molecules such as enamel matrix derivative, recombinant growth and differentiation factors, and autologous platelets concentrates.
Platelets play a central role in hemostasis and healing processes. Upon their activation, platelet α-granules release over 30 cytokines, including 7 fundamental protein growth factors including platelet-derived growth factor (PDGF) which include the 3 isomers (PDGFαα, PDGFββ and PDGFαβ), 2 of the numerous transforming growth factors (TGF-β1 and TGF-β2), vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), and epidermal growth factor (EGF) as well as active substances such as serotonin, catecholamines, von Willebrand factor, proaccelerin, osteonectin, and antimicrobial proteins [2–9]. By concentrating platelets, platelet-rich plasma (PRP) with higher levels of growth factors might be reached, which could stimulate the healing processes. PRP is an autologous concentration of human platelets in a small volume of plasma.
Platelet-rich plasma development via centrifugation has been greatly simplified so that it can be used in the office setting as well as the operating room. However, the centrifugation process must be sterile and precisely suited to platelet separation from red blood cells and their sequestration in high concentration without lysing the platelets or damaging them so that they no longer can actively secrete their growth factors.
Some authors have reported significant increase in bone formation after application of PRP; others did not observe any improvement. Despite controversies in the literature regarding the added benefit of this procedure, recent investigations have confirmed osteoinductive properties of PRP in vitro and vivo studies in animals and few in human beings. First attempt was made by Marx and Garg in 1998 [8] to standardize platelet concentration in PRP and showed improved healing in their experimental study of mandibular marginal bony defect healing with autogenous graft with the use of PRP. Similarly Freymiller and Aghaloo [10] have pointed out that PRP acts as a biologic adhesive that holds the bone particles together, thereby making manipulation of the graft material much easier. PRP was first introduced to the oral surgery community by Whitman et al. in their 1997 [11] article entitled “Platelet Gel: An Autologous Alternative to fibrin Glue with application in Oral and Maxillofacial Surgery”. Over the past decade autologous platelets have been shown to promote tissue repair as a source of healing factors in several clinical situations in maxillofacial surgery, oral implantology, orthopedics, ulcer treatment, sports medicine and ophthalmology among others.
In the present study, our aim is to determine the influence of PRP on healing of third molar impacted tooth extraction sockets. For this we used PRP alone without autogenous or synthetic graft. To our knowledge, there is very little study to report PRP application without grafts in surgical defects.
Materials and Methods
The study is designed as a prospective, single blinded clinical trial. The graft materials used in this study is PRP (platelet rich plasma) (autogenous). This was prepared in Oral Pathology Lab, Govt Dental College, Thiruvananthapuram under standard aseptic conditions. Study was conducted in Department of Oral & Maxillofacial Surgery, Government Dental College, Thiruvananthapuram. The study was conducted over a period of 1 year (2011–2012). Five patients having bilateral impacted mandibular third molar teeth and requiring surgical extraction of impacted lower third molar teeth on bilateral side who reported to the Out Patient clinic of Govt. Dental College, Thiruvananthapuram and who voluntarily consented to participate were studied.
Aims and objective
To determine the efficacy of PRP on bone healing in extraction socket of impacted teeth.
To evaluate the safety of the graft substitute PRP in the treatment of bone defects after extraction of impacted teeth.
To evaluate the soft tissue healing with use of PRP.
Inclusion criteria
Bilateral impacted mandibular third molars irrespective of angulation.
No previous surgical treatment (like operculectomy, odontoplasty, chemical cauterization).
A state of good general body and oral health.
Regular attendance at control visits.
Exclusion criteria
Patients with systemic disease like impaired calcium metabolism, blood dyscrasias and uncontrolled systemic diseases.
Patients with extensive surgical defects.
Patients having single impacted tooth.
Patients with periapical infection or lesion with respect to mandibular third molars.
Presence of opposing traumatic occlusion or impinging upper third molars.
Patients with unacceptable oral hygiene.
Study outcome variables
Age.
Gender.
Total platelet count in whole blood and PRP.
Clinical and radiological examination pre-operatively.
Clinical and radiological examination post operatively.
Materials
Preparation of PRP gel
Armamentarium
Centrifugation machine.
Test tubes and test tube holder rack.
IV canula, needles.
Syringes 10 ml
C.P.D.A. (citrate phosphate dextrose adenine) anticoagulant.
10 % CaCl2
Tourniquet
Sterile gloves
Informed consent regarding the benefits and protocol of the study was taken from all patients (Figs. 1, 2, 3).
Fig. 1.

Centrifugation machine
Fig. 2.
Armamentarium for PRP preparation
Fig. 3.

Armamantarium for tooth extraction
Study Methodology
Five patients satisfying inclusion and exclusion criteria were used for this study. Before starting the trial, the nature of the study (including benefits, risks and alternative treatments available) was explained to the patients. Informed consent was obtained from those patients who expressed their willingness to participate in the study.
Preoperatively following radiographs were taken for all patients
Intra oral periapical radiographs (IOPARs) with relation to impacted lower third molars.
-
Panoramic radiographs.
Pre-operatively all patients were evaluated for blood routine examination, blood sugar level, platelet count, HBsAg and HIV test.
All patients underwent bilateral surgical removal of impacted third molars and PRP that was prepared prior to start of the procedure was activated to form PRP gel which was placed into one of the extraction sockets randomly selected by the author.
All patients were recalled after 1, 4, 8 and 16 weeks postoperatively for follow-up study by clinical and radiological examination.
The data obtained were stored for statistical analysis.
Preparation of PRP Gel
1st Step: Collection of blood
Under aseptic techniques, 12 ml of blood was withdrawn intravenously from anticubital region of patients, forearm in syringe containing anticoagulant CPDA (citrate phosphate dextrose adenine). 10 ml syringe containing 1 ml CPDA anticoagulant was used for collection of 6 ml blood. Thus two syringes were used for each patient. The syringes were thoroughly shaken to ensure mixture of anticoagulant with the drawn blood (Fig. 4).
Fig. 4.
Withdrawing of blood
2nd Step: Preparation of PRP
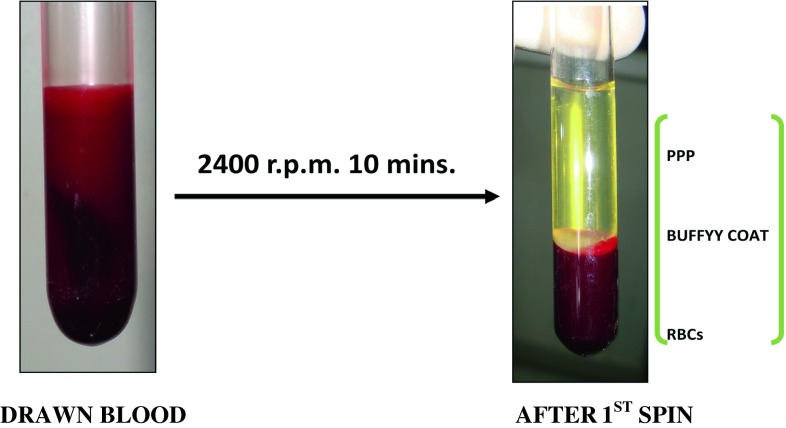
The collected blood was centrifuged at 2,400 rpm for 10 min. The supernatant formed was platelet poor plasma (PPP) and Buffy coat and bottom part consisted of RBCs (Fig. 5).
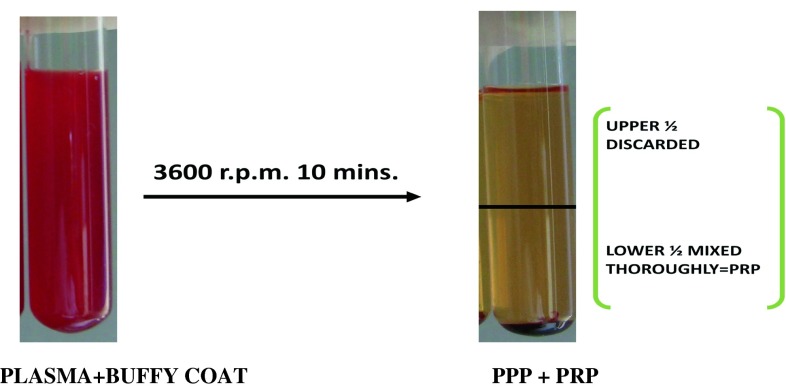
PPP and Buffy coat along with upper 1 mm RBC layer was collected in a fresh test tube and again centrifuged at 3,600 rpm for 10 min. The upper half was discarded and lower half mixed thoroughly to yield PRP (Fig. 6).
Fig. 5.
First spin centrifugation
Fig. 6.
Paltelet poor plasma and platelet rich plasma after second spin centrifugation
3rd Step: PRP GEL preparation
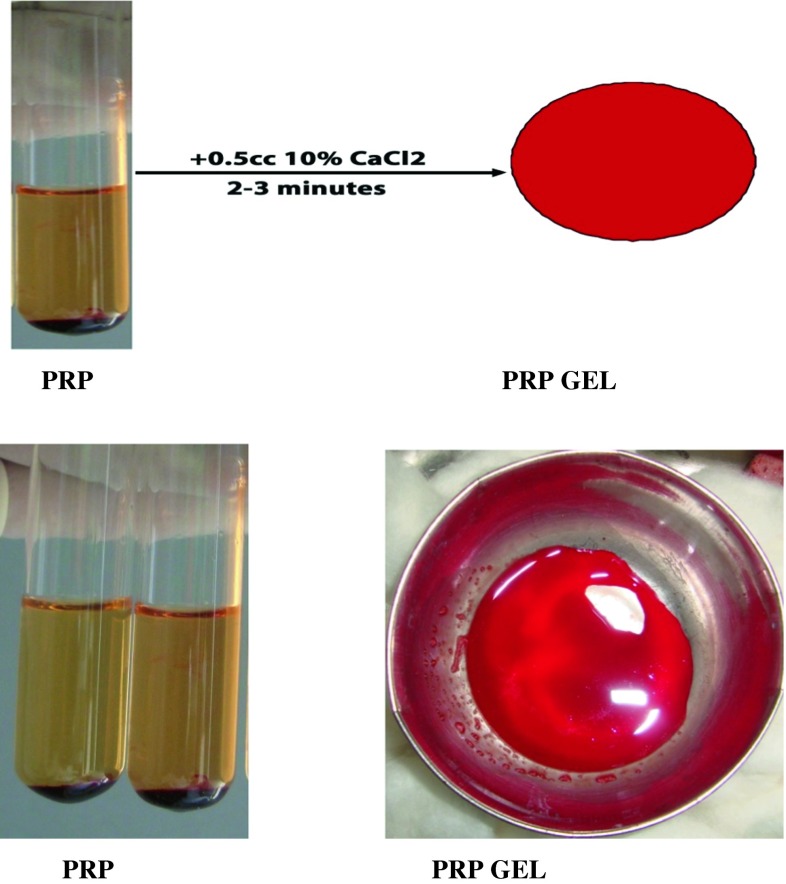
PRP gel was prepared by adding 10 % CaCl2 just before insertion into the surgical defect (Fig. 7).
Fig. 7.
Platelet rich plasma gel
Surgical Procedure
The patient’s face was prepared with betadine solution and was draped in aseptic condition (Fig. 8).
Anaesthesia Inferior alveolar nerve block (lingual nerve block) and long buccal nerve block were administered using 2 % lignocain hydrochloride with 1:80,000 adrenalines.
Incision and mucoperiosteal flap reflection Standard Ward’s incision was followed in all the cases. Full thickness mucoperiosteal flap was raised to expose sufficient bone on lateral and distal aspect of the impacted molar.
Removal of surrounding bone Removal of bone surrounding tooth was carried out with surgical bur. Constant irrigation was done with normal saline while using bur to prevent thermal necrosis.
Extraction Surgical removal of tooth done and if needed sectioning of tooth was carried out with surgical bur in selected cases.
Wound toilet The surrounding bone was smoothened. The wound was gently irrigated with sterile normal saline solution and checked for any small detached fragments of bone or tooth pieces.
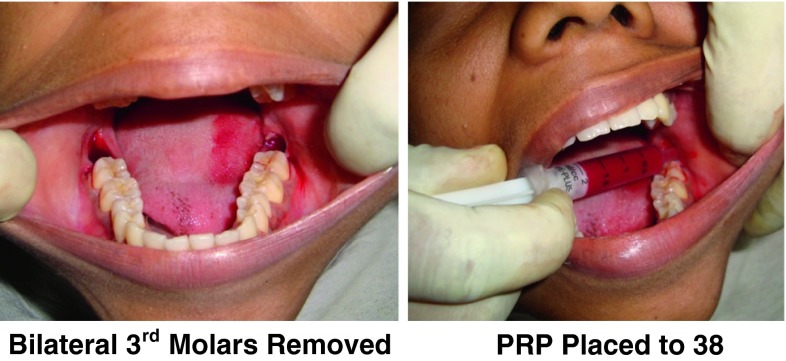
Surgical removal of bilateral impacted mandibular third molar Surgical removal of impacted mandibular 3 rd molar was done on the opposite side in the similar way in the same sitting and one of the extraction sockets was randomly chosen by the operator for PRP placement into the extraction socket (Fig. 8).
PRP placement The pre processed PRP was taken into the sterile stainless steel bowl and 0.5 ml of CaCl2 was mixed to obtain the PRP gel, which was placed into the selected extraction socket and primary closure was done.
Wound closure The irregular margins of the wound were trimmed and wound was closed with 3–0 black braided silk interrupted suture. Pressure pack was given.
Fig. 8.
Bilateral 3rd molars removed, PRP placed to 38
Post Operative Instructions
Regular post extraction instructions were given.
Medication prescription:
Cap. Amoxicillin 500 mg TID × 5 days
Tab. Pyroxicam 500 mg OD × 3 days
Each patient was followed for regular intervals at 1 week and 4, 8 and 16 weeks for evaluation on the basis of clinical and radiologic findings for healing of soft tissue and hard tissue respectively (Fig. 9).
Fig. 9.
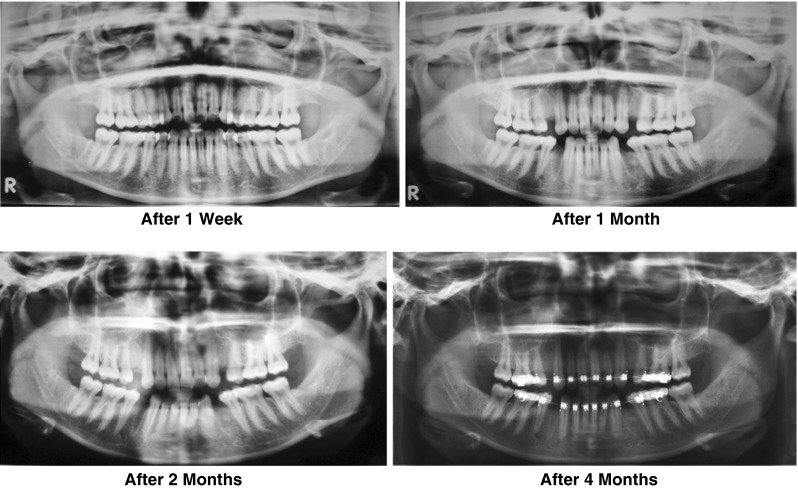
Panoromic views: PRP applied to left 3rd molar socket
1 week post operative:
Intraoral wound was examined for any wound dehiscence, infection, swelling, etc.
Following evaluation criteria were used to assess soft tissue healing:
One week later patients entered the degree of pain and swelling on the record, day by day, from zero to three, answering a questionnaire based on a personal evaluation comparing both surgical sides.
Patient did not know on which side PRP was inserted.
On the same day an author not involved in the study gave an evaluation from 0 to 3 on the type of wound healing observed after 1 week. The author assessed a score to the soft tissue healing from grade 0 to 3. Zero corresponded to post extraction alveolitis, one for initial healing, two for secondary closure and three for primary closure of the flap.
Intra oral sutures were removed.
Any fresh complaints were noted.
Digital OPG was taken which acts as baseline x-ray to evaluate changes in extraction socket healing in subsequent x-ray.
Digital OPG were taken post operatively at 4, 8 and 16 weeks to assess the radiographic changes at the extracted sites (Tables 1, 2, 3, 4, 5).
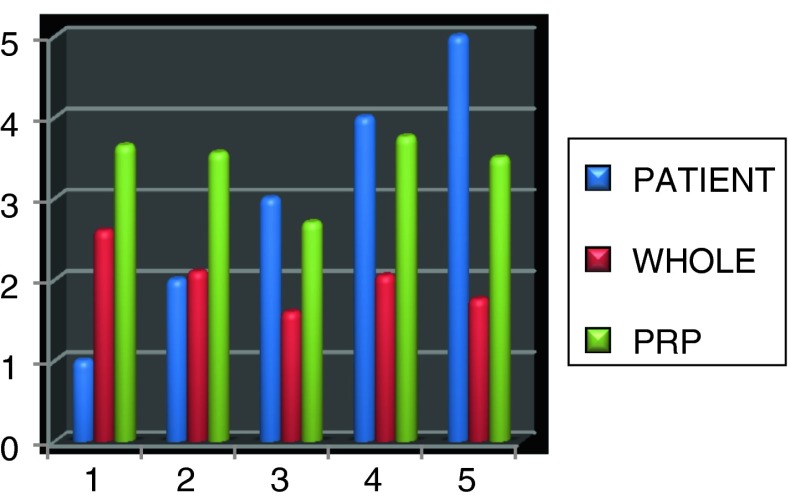
Table 1.
Total platelet count in whole blood and PRP
| Patient | Whole blood | PRP |
|---|---|---|
| 1 | 260,000 | 365,000 |
| 2 | 210,000 | 356,000 |
| 3 | 160,000 | 270,000 |
| 4 | 205,000 | 376,000 |
| 5 | 176,000 | 350,000 |
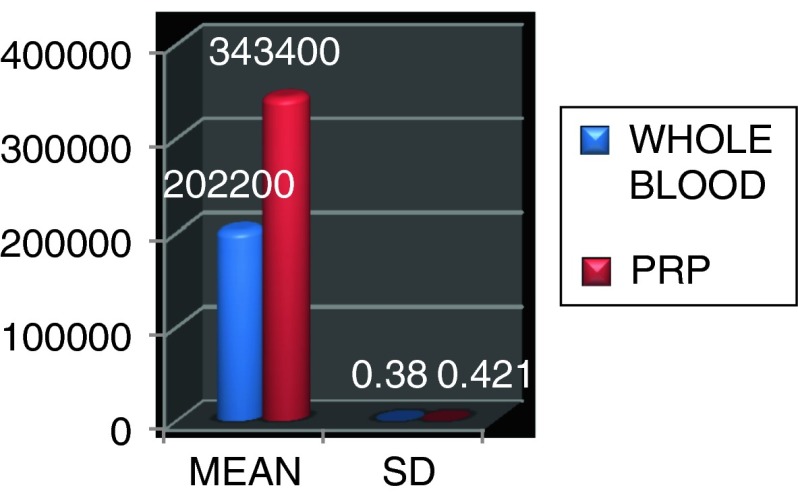
For whole blood: Mean 202,200 ± 0.38, For PRP: Mean 343,400 ± 0.421
Table 2.
Visual analog scale score for swelling (score 0–3)
| Day | Intervention group | Control group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 3 | 0 | 0 |
| 4 | 0 | 1 | 1 | 1 | 1 | 2 | 2 | 3 | 2 | 1 |
| 5 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 1 |
| 6 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 3 | 1 | 1 |
| 7 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 1 | 0 |
(0- no swelling, 1- mild swelling, 2- moderate swelling, 3- swelling)
Soft tissue parameters for extraction socket healing
Table 3.
Visual analog scale score for pain (score 0–3)
| DAY | Intervention group | Control group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 1 | 1 | 1 | 0 | 1 | 2 | 1 | 3 | 2 | 2 |
| 4 | 1 | 1 | 1 | 0 | 1 | 3 | 1 | 3 | 2 | 2 |
| 5 | 1 | 0 | 0 | 0 | 0 | 2 | 1 | 3 | 1 | 3 |
| 6 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 3 | 1 | 2 |
| 7 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 3 | 1 | 1 |
0 No pain, 1 mild pain, 2 moderate pain, 3 sever pain
Table 4.
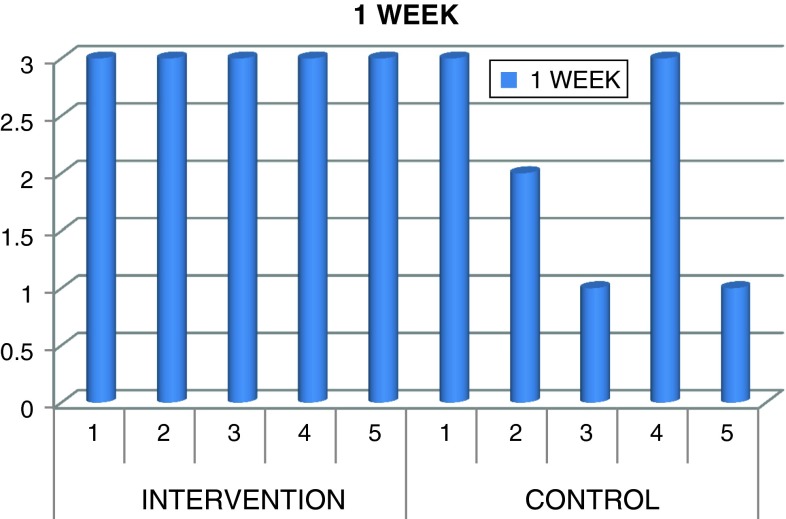
Wound healing score given by non author
| Time | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 Week | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 1 | 3 | 1 |
0 Post extraction alveolitis, 1 initial healing, 2 secondary closure, 3 primary closure
Table 5.
Radiographic evaluation of extraction socket healing
| Time | Intervention group | Control group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
| 1 Week | ↑ | ↓ | ↑ | ↑ | ≡ | ↓ | ↑ | ↓ | ↓ | ≡ |
| 4 Week | ↑ | ↓ | ↑ | ↑ | ↓ | ↓ | ↑ | ↓ | ↓ | ↑ |
| 8 Week | ↑ | ↓ | ≡ | ≡ | ↓ | ↓ | ↑ | ≡ | ≡ | ↑ |
| 16 Week | ↑ | ↓ | ≡ | ≡ | ↓ | ↓ | ↑ | ≡ | ≡ | ↑ |
Increased density ↑
Decreased density ↓
No changes ≡
Statistical Analysis
Statistical analysis using Statistical Package for Social Sciences version 10 was used for computing, tabulating and analyzing the data.
Outcome Variables

Observations and Results
Five patients (2 females and 3 males) were studied for effectiveness of PRP in third molar extraction sockets healing which includes two. The mean age of the sample population was 22.88 ± 5.263 years (range 19–32 years). There was no statistically significant difference in the age, gender and type of impaction between intervention and control groups.
All patients were evaluated for total platelet count in whole blood and in PRP. Platelet count on average in PRP ranged from 202,200 ± 0.38 platelets/ml3 (in whole blood) to 343,400 ± 0.421 platelets/ml3. Whole blood baseline measurements ranged from 160,000 to 260,000 platelets/ml3. Concentration procedures resulted in an average 1.5 folds increase in platelet concentration in PRP, the maximum platelet count of PRP was 376,000 and minimum was 270,000 platelets/ml (Figs. 10, 11; Table 1).
Fig. 10.
Total platelet count in whole blood and PRP
Fig. 11.
Total platelet count with mean and standard deviation
All tooth extraction sites healed without postoperative complications such as pain or infection or trismus except in one case which showed infection and pus discharge and associated swelling after 10 days of surgical procedure which was managed by medication Edema was within acceptable range in both groups. Surgical extraction sockets wound healed satisfactorily were graded as 3 in all cases of intervention group. Two cases were graded as 3, one case as 2 and remaining two cases as 1 in control group according to healing of extraction sockets (Fig. 12; Table 4). Complete healing was observed in all cases in both intervention and control groups after 4 weeks period. No patient was lost during follow up. There was significant difference in swelling and pain on 6th and 7th day with increased swelling and pain on 6th day and increased pain on 7th day in control group compared to intervention group which showed better soft tissue healing with minimal pain and swelling which was not significant (Tables 2, 3, 6). In wound healing there was significant difference with P value of 0.038 (Table 6) in intervention group compared to control group which showed better healing than control group (Table 6).
Fig. 12.
Wound healing at 1 week visit
Table 6.
Chi square test for soft tissue analysis
| Variable | Chi square value | P value |
|---|---|---|
| S6 | 10.00 | 0.007 |
| P6 | 10.00 | 0.019 |
| P7 | 10.00 | 0.007 |
| WH | 4.286 | 0.038 |
(S6-swelling on 6th day, P6-pain on 6th day, P7-pain on 7th day, WH-wound healing) view within article
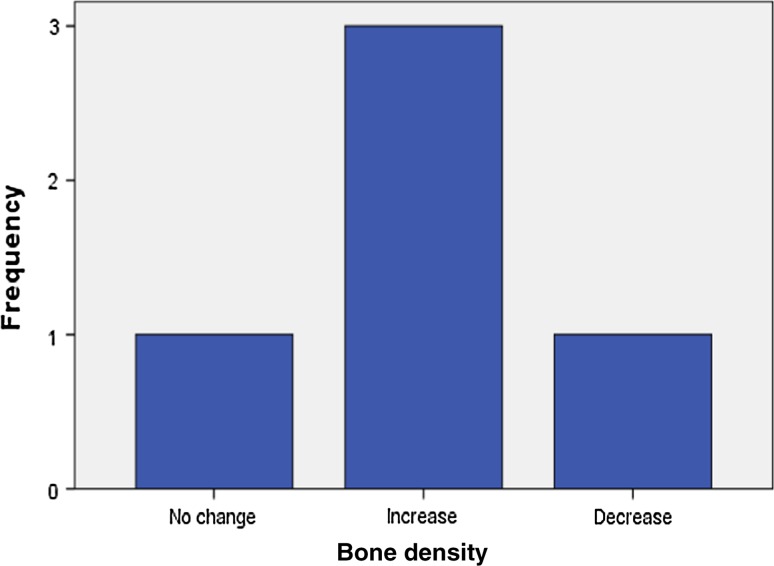
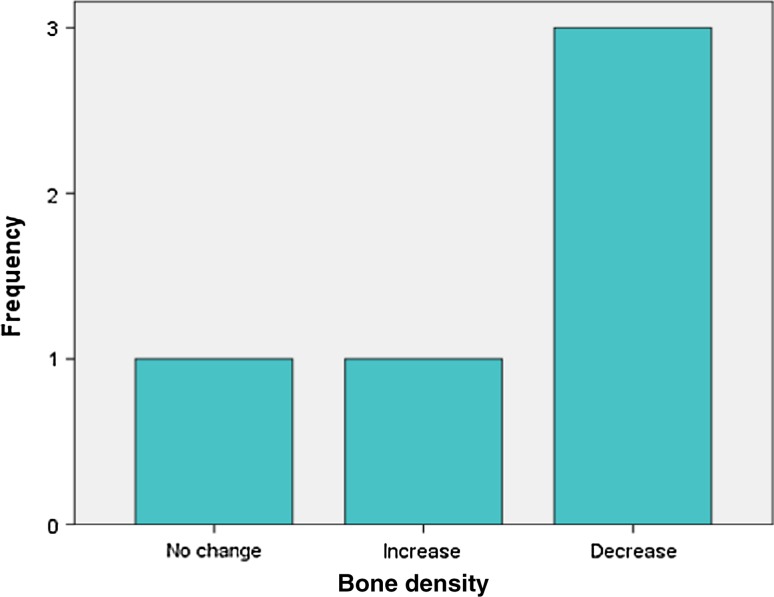
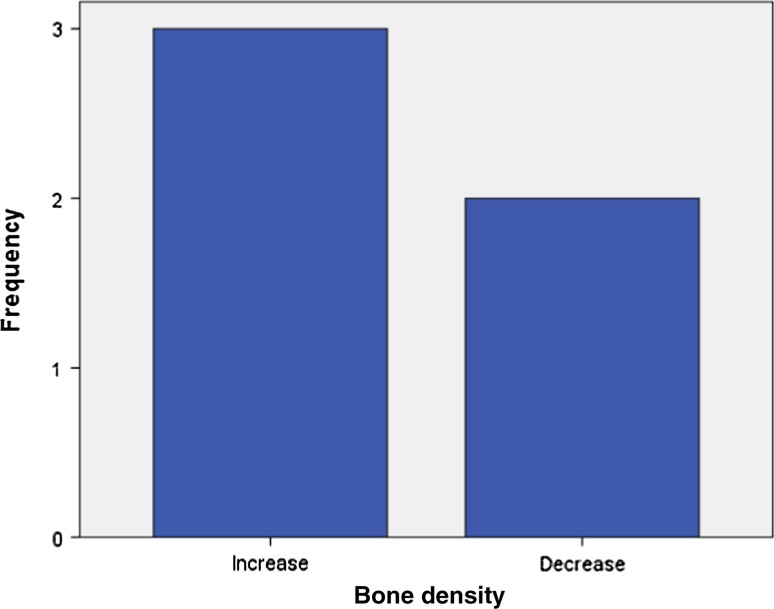
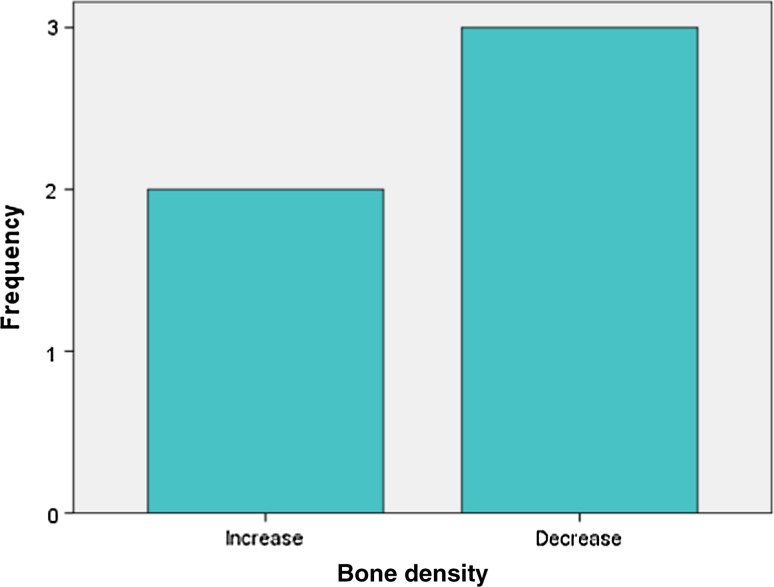
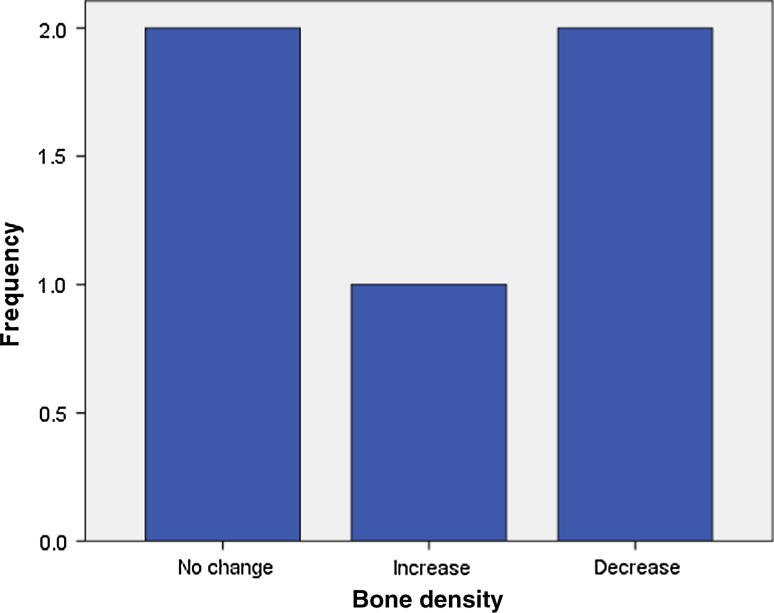
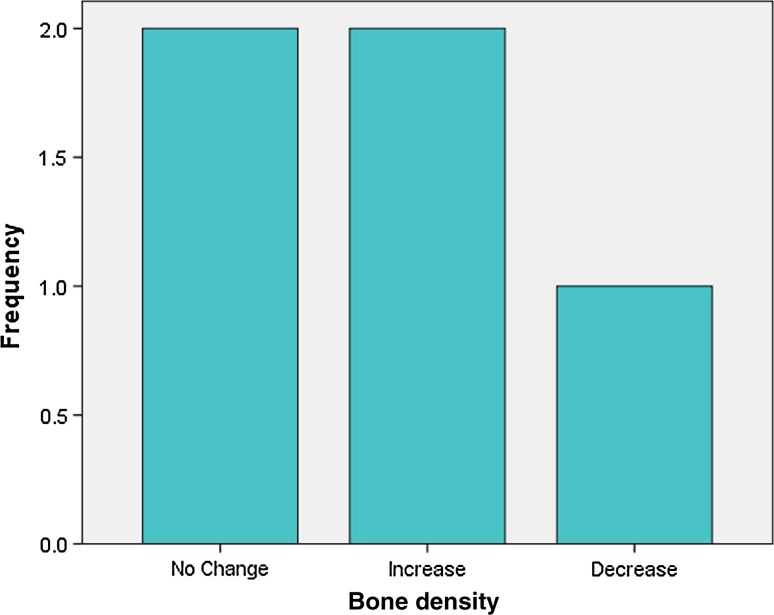
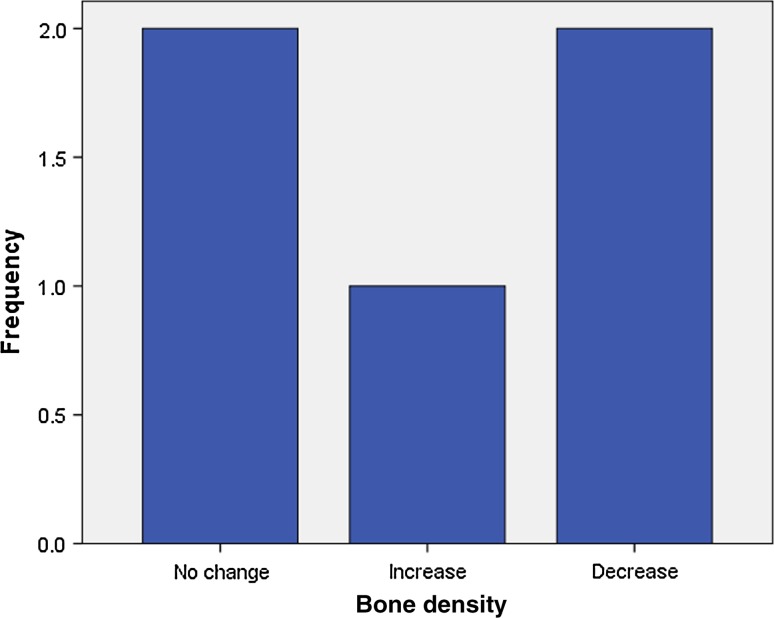
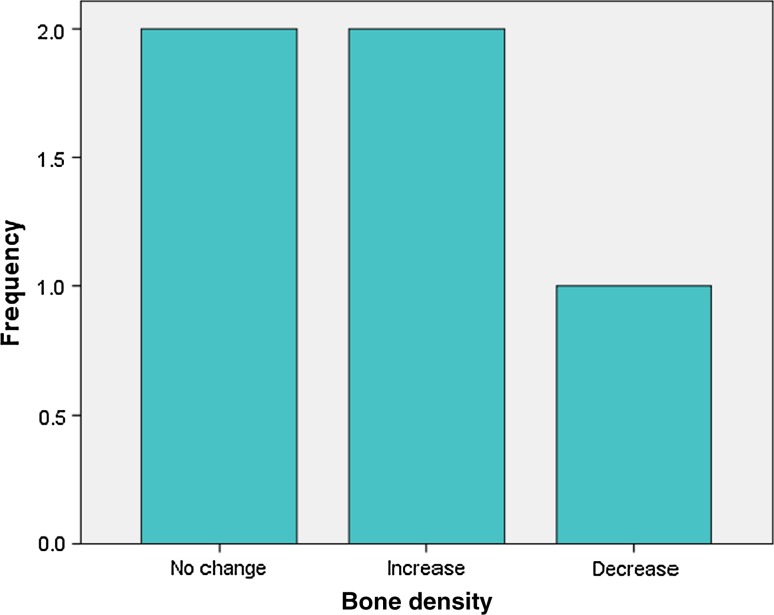
Analysis of radiographic bone density after 1, 4, 8 and 16 weeks showed significant differences between PRP and non-PRP groups by examining digital OPG view. Out of 5 cases studied only one case in intervention group showed significantly increased radiographic bone density at surgical site treated with PRP compared to non-PRP site at all four visits of follow-up. Two cases showed no beneficial effect of PRP compared to non-PRP site, instead non-PRP sites showed increased radiographic density at 4, 8 and 16 weeks follow-up visits. In one case there was no significant change in density after 1 week in both intervention and control groups. In two cases there were no significant changes in density after 8 and 16 weeks in both intervention and control groups. In three cases at 1 and 4 week visits, with P value 0.368 and 0.527 (Table 7) respectively, showed increased radiographic density in PRP treated sites than non-PRP sites.
Table 7.
Chi Square test for association of PRP status and bone density
| Variable | Chi Square value | P value |
|---|---|---|
| BD1 | 2 | 0.368 |
| BD4 | 0.40 | 0.527 |
| BD8 | 0.667 | 0.717 |
| BD16 | 0.667 | 0.717 |
(BD1- bone density after 1 week, BD4- bone density after 4 weeks, BD8-bone density after 8 weeks, BD16-bone density after 16 weeks)
All cell contain n < 5, without continuity correction
Statistical Analysis
Data were analyzed using computer software, statistical package for social sciences (SPSS) version 10. Data are expressed in frequency and percentage. To elucidate the association and comparison between different parameters, Chi Square(X2) test was used as non-parametric test. For all statistical evaluations a two-tailed probability value < 0.05 was considered significant (Figs. 13, 14, 15, 16, 17, 18, 19, 20).
Fig. 13.
Bone density after 1 week in intervention group
Fig. 14.
Bone density after 1 week in control group
Fig. 15.
Bone density after 4 weeks in intervention group
Fig. 16.
Bone density after 4 weeks in control group
Fig. 17.
Bone density after 8 weeks in intervention group
Fig. 18.
Bone density after 8 weeks in control group
Fig. 19.
Bone density after 16 weeks in intervention group
Fig. 20.
Bone density after 16 weeks in control group
Discussion
Reconstruction of bone defects represents a challenging problem for the surgical community. Many defects in facial skeleton may significantly impair proper prosthetic and functional rehabilitation of the stomatognathic system. Recently, much has been learned about the mechanisms involved in the regeneration and repair of some tissues. Thus, investigation of new methods of bone reconstruction of the maxillomandibular complex is always valid [12]. In 1998, Marx and Garg [8] proposed the local application of platelet-rich plasma (PRP) to enhance the maturation of bone grafts. Autologous PRP is an inexpensive and widely available modality to increase hard and soft tissue healing potentials. The clinician’s ability to use the known properties of TGF-β and PDGF contained in the PRP preparation has dramatically changed the surgical community’s understanding of bone healing and physiology and improved the expected outcomes in minor and major oral and maxillofacial surgery.
One of the main materials investigated and used to accelerate the growth of new bone tissue is autologous platelet-rich plasma (PRP). There are 3 type of platelets product invented, platelet rich plasma (PRP), platelet rich in growth factor (PRGF) and paltelet rich in fibrin (PRF) [13]. Its therapeutic strategy is based on acceleration of healing by concentrations of growth factors, which are universal initiators of nearly all healing events [3, 5, 12]. The growth factors present in PRP are well-known, including transforming growth factor-β (TGF-β1 and TGF-β2), vascular endothelial growth factor, 3 isomers of platelet-derived growth factor (PDGF-αα, PDGF-ββ, and PDGF-αβ), and endothelial growth factor. These growth factors are considered to have the ability to accelerate chemotaxis, mitogenesis, angiogenesis, and synthesis of collagen matrix and favor tissue repair when applied on bone wounds [12]. Instead of this it has been noted that in addition to growth factors released from platelets and their demonstrated positive effect on wound healing, thrombospondin TSP is also abundant in the α-granules of platelets. TSP family consists of five members TSP-1 through TSP-5. Chia-Wen Hsu et al. [4] noted that TSP-1 and weakly TSP-2 which inhibits adhesion, proliferation and tube formation of endothelial cells in culture, ultimately lead to inhibitory function on wound healing. It may be considered as a causative factor responsible for no beneficial effect in our study.
PRP is a promising biotechnology in tissue healing and has been the subject of increasing investigations for its ability to stimulate and accelerate bone formation. However, further studies should be performed to investigate the possible clinical application of PRP because this graft has been marketed. Thus, the present study investigated, radiographically, the performance of autologous PRP after its application instead of blood clot in dental sockets of impacted mandibular third molars.
The present results clearly show that the application of autologous PRP in surgical bone wounds after tooth extraction allows variable amount of bone healing, as indicated by data with statistical differences. In some cases it shows accelerated bone healing, in some cases no comparable bone healing and in some cases no changes in intervention and control group. Some results are compatible with earlier reports on PRP by Ronaldo Célio-Mariano et al. [12] who observed earlier bone formation with the use of PRP. PRP alone was used as a graft material in few studies conducted to investigate the effect on bone formation. They got variable results as in our study, among these variable results noted in soft tissue and hard tissue healing in third molar extraction socket. Some studies had more beneficial effect on soft tissue healing than bone healing, while in others no added benefit in bone healing was noticed as in some cases of our study. Allograft material or other source of bone may represent the osteoconductive support to the growth factors released by PRP gel, without that support the growth factors alone released during the platelet degranulation may lose part of their bone regeneration potentialities [13]. It is not quite clear why the PRP treated sites exhibited decreased bone formation when compared with non-PRP treated sites in some of our cases. The explanation may be related to the concentration of platelets in PRP. Variations in platelet concentration are known to influence bone healing [8]. Study conducted by Bahadir Gürbüzer et al. [14] showed that healing of extraction sockets treated with PRP alone had failed to show improved healing which was evaluated by bone scintigraphy method. Similar result was noted in some of our cases evaluated with digital OPG (Fig 9).
In fact, there are controversies in the literature regarding the potential benefits of this procedure. Some investigators have reported significant improvements in tissue healing and bone formation using PRP [1, 4, 7, 8, 11, 12, 15–31] whereas others did not observe improvements [2, 5, 13, 14, 32–36]. Some have given no conclusion regarding effect of PRP application in maxillofacial region than periodontal defects [37]. Such discrepancies are probably related to the lack of suitable standardization and definition of the different PRP preparations; the protocols and biological techniques used in the elaboration and administration of PRP differ widely. Variations in some key properties of PRP, including platelet concentration, type of clot activator, leukocyte content, and the time that the fibrin scaffold is put into place around the tissue after clotting has started, can influence the different biological effects markedly [12]. Schmitz and Hollinger questioned the actual isoforms and the biological action of the various growth factors concentrated in PRP and stated their opinion that at this time, basic research does not strongly endorse the ability of PRP to promote healing [11].
Concerning the achievement of PRP, the present study used sodium citrate as an anticoagulant and calcium chloride as a coagulant. There are studies that used different materials for activation of platelets before insertion of PRP in surgical defect. Some have used 10 % CaCl2 alone [2, 12, 14, 15, 18, 25, 29] as we have used. Anitua was the first who began the trend of using only CaCl2. One study had used calcium gluconate [13] with satisfactory result. Some had used 10 % CaCl2 and bovine thrombin [3, 10, 14, 20, 21, 31, 32, 34, 38] at the same sitting some have used thrombin alone [35, 37]. Landesberg et al. [2] opposed the use of bovine thrombin, considering the risk of developing antibodies that cross react with factors V, XI and thrombin and induce coagulopathies. Considering these material there was no significant relation between use of different coagulants and outcome. The most important is activation of platelets to follow coagulation cascade to facilitate early release of factors. Calcium is used to activate the coagulation cascade and thus the release of growth factors and the formation of 3D fibrin scaffold. Therefore potentially toxic substances like bovine thrombin are avoided. In addition calcium provides a reduced burst effect in comparison with thrombin, facilitating a more progressive growth factor release.
In addition, use of adequate centrifugation forces is necessary because greater forces can lead to the release of growth factors at the time of PRP preparation [12]. Centrifugation speed is a relevant variable for platelet count, it was not standardized among the authors and large variation can be noted in the protocols. In some studies there is no information about of centrifugation parameters and the reports of centrifugation parameters in rpm (rotation per minute) mode is another important aspect that makes the comparison of the results difficult [2]. In our study we have used 3,600 rpm for 10 min for separation of platelets that could facilitate bone healing. A similar study showed that by the use of 3,000 rpm for 10 min the PRP prepared was able to intensify proliferation of stem cells and osteoblasts in cultures and enhance matrix secretion by the osteogenic cells [2].
How much platelet count is sufficient to get positive result? There is still controversy regarding ideal platelet count [2]. In our study mean platelet count in PRP was 34,000 cells/ml3 which was very low considering the value by Whitman et al. who stated that the platelet count in PRP should oscillate between 500,000 and 1,000,000 cell/μL. In the initial work of Marx and Garg [8] mean PRP platelet count achieved was 7, 85,000 cell/μL. He was the first who attempted to standardize a platelet concentration for PRP. He also stated that best biological response occurs when a 4 to 5 fold increase over the baseline platelet number is achieved, but in our study the mean increase was only 1.5 fold which may be considered for decreased biological response in response to bone healing. Graziani et al. [4] showed that increased PRP concentration reduced the proliferation of osteoblasts and that optimal proliferation occurred at platelet concentration 2.5 times higher than normal baseline. Study suggested that the positive effect PRP on bone regeneration might have a limited concentration range. At lower concentration PRP effect was suboptimal, while higher concentration had a paradoxically inhibitory effect [3]. Gernot Weibrich et al. [39] conclude that there is significant but irrelevant influences of sex on platelet concentration, but no influence of age was detected. Similarly in our study there was no significant difference in platelet concentration considering the sex and mean age of patient.
Because the life span of a platelet in a wound and direct influence of its growth factors is < 5 days (range 5–8 days) a pronounced effect of PRP would be expected to occur especially in the early stage of bone healing [14, 32]. Study conducted by David Gerard et al. reported that healing of mandibular defect treated with autologous bone graft with PRP in dogs results in early (1 and 2 months) beneficial effects, but 3 and 6 months time period did not have any added beneficial effect [10]. Morphology of platelets changed as bench setting time increased as reported by Rutkowski et al. [9]. It shows that from 0 to 2 h platelets appear normal without or little aggregation but after 2 h form more number of small clusters. These effects were more dramatically evident by 6 h, indicating a change in their morphology as function of bench set time, these are accompanied by changes in growth factors content of the PRP. Prepared PRP should be inserted in surgical defect within at least 2 h to get maximum benefit of PRP. 95 % of growth factors in PRP are released in first hour after clot initiation. The PRP must therefore be developed in an anticoagulated state and be used within 10 min of clot initiation to get maximum advantages [8].
Periodontal complications at the distal root surface adjacent to the second molar that follow an impacted mandibular third molar in deep extraction have been judged to be related to the age of a patient and the angulation and severity of the inclusion. The possibility to concentrate growth factors in a surgical wound seems to allow activation of cellular events, providing a final gain in quality and time of healing. The use of PRP has been shown to be a useful tool in aiding periodontal and bone regeneration in vivo because of the high content of growth factors [16]. Results showed that PRGF contains a pool of growth factors that significantly stimulated the proliferation of tenocytes and induced the paracrine secretion of both VEGF and HGF. The latter is particularly important as these agents play an active role in angiogenesis and as antifibrotic molecule respectively. Another important consideration of this treatment is that cells return to their normal fate of proliferation once the PDGF are withdrawn, which is an obligatory biosafety requirement if the cells are to be transplanted into humans.
The use of autologous growth factors from platelet gel seems to be one of the most promising methods for the treatment of bone, cartilage and soft tissue defects in the future. Undoubtedly all clinicians have high hope that PRP will eventually prove to be of great benefit in bone graft healing. The easy preparation protocols, the biosafety and versatility of the platelet-based preparation and their reduced cost have also stimulated the research and interest by the scientific community. The conflicting results in today’s literature make in overwhelmingly evident that more research is needed before evidence-based surgeon can feel confident in recommending this procedure to their patients. It is anyway a quite invasive method which cannot be applied daily in private practice for office basis patient.
Summary and Conclusion
Platelets play a central role in hemostasis and healing process in all wound healing. The different growth factors present in α- granules of platelets plays an important role in differentiation and proliferation of native cells in wound site such as fibroblast, endothelial cell and osteoblast and other cells; which are prerequisite for wound healing. Considering this important role of platelets many research works are going on to elaborate the qualitative and quantitative effect of platelets in different wound healing such as in maxillofacial surgery, oral implantology, orthopedics, ulcer treatment, sports medicine and ophthalmology among others. PRP which can be easily prepared and used for office out basis patient due to availability of different machineries which are cost effective. But collection of blood for the PRP preparation is quite an invasive procedure so it may restrict its use on regular basis for all patients. Along with the beneficial effect we should also consider inhibitory effect of PRP, like TSP-I factor which is inhibitory in nature.
The application of PRP alone in impacted third molar extraction sockets showed increased bone density in initial period of healing, but there was no beneficial effect at late wound healing period. This may correlate with life span of platelets and early release of most growth factors at the wound site, also questionable role of platelets in osteogenesis through differentiation and proliferation of osteoblasts. However PRP significantly improves the soft tissue healing compared to non-PRP group. This may be due to more and intense differentiation and proliferation of fibroblasts and endothelial cells. No patient had any systemic infection after the use of PRP as PRP is an autogenous preparation, hence chances of transmission of diseases or immunogenic reaction are very rare. CaCl2 used for activation of platelets in the study should be popularized considering the immunogenic and some detrimental effect on platelets itself by the use of bovine thrombin used in earlier literature. CaCl2 is equally effective in activation of platelets as bovine thrombin. Considering all these, PRP significantly increases the healing quality of wound which further reduces the period of healing with less complications like infection, delayed healing, dehiscence of wound, etc.; which ultimately enhances the quality of life of patient.
The limitations of the study are less number of sample sizes, use of digital OPG view for evaluation of bone density on the basis of visual perception. This may contribute bias in study as subjective variation may occur. Further longitudinal studies with larger sample sizes and controls are needed to determine whether the addition of PRP and its various growth factors would offer earlier bone formation and increase the predictability of regenerative procedure. Also further investigation and clarification are needed to popularize the PRP as a graft material in general.
Study concludes that, PRP enhanced the initial bone healing at one month duration but there is no added benefit in late bone healing at four months period, in mandibular third molar extraction sockets compared in both intervention and control groups. However PRP significantly improved the soft tissue healing in PRP treated sites compared to the control group.
Acknowledgments
I would like to thank the following people for their contribution to this study. I express my sincere gratitude to Dr. C.R. Sobhana, MDS, MDS, MOSRCS (EDINBURGH, UK) Professor and Head, Department of Oral and Maxillofacial Surgery for guiding me to carry out this study as a my post graduate guide and also thanks to other teaching staff in the department. Dr. K C Usha, Professor and Head, Dept of Transfusion Medicine, Govt Medical College, Thiruvananthapuram for her immense help and guidance in this study. Dr. V T Beena, Prof and Head, Dept of Oral Pathology and also Lab assistant staff for their time-to-time help in preparation of PRP and analyzing platelet value for my study. Dr Shrinivas for his help with the statistical analyses. Above all, my sincere and thankful prayers to the Almighty who guides me always to the right path in my life.
References
- 1.Simon BI, Zatcoff AL, Kong JJW and O’Connell SM (2009) Clinical and histological comparison of extraction socket healing following the use of autologous platelet-rich fibrin matrix (PRFM) to ridge preservation procedures employing demineralized freeze dried bone allograft material and membrane. Open Dent J. 3: 92–99 1874-2106 [DOI] [PMC free article] [PubMed]
- 2.Andrade MGS, de Freitas Brandao CJ, Sa CN, de Bittencourt TCBSC, Sadigursky M. Evaluation of factors that can modify platelet-rich plasma properties. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(1):e5–e12. doi: 10.1016/j.tripleo.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 3.Plachokovaa AS, van den Doldera J, van den Beuckena JJJP, Jansen JA. Research paper bone regenerative properties of rat, goat and human platelet-rich plasma. Int J Oral Maxillofac Surg. 2009;38(8):861–869. doi: 10.1016/j.ijom.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Hsu C-W, Yuan K, Tseng C–C. The negative effect of platelet-rich plasma on the growth of human cells is associated with secreted thrombospondin-1. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2009;107(2):185–192. doi: 10.1016/j.tripleo.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Ogino Y, Ayukawa Y, Kukita T, Koyano K. The contribution of platelet-derived growth factor, transforming growth factor-β1, and insulin-like growth factor-I in platelet-rich plasma to the proliferation of osteoblast-like cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2006;101(6):724–729. doi: 10.1016/j.tripleo.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 6.Kanno T, Takahashi T, Tsujisawa T, Ariyoshi W, Nishihara T. Scientific articles Platelet-rich plasma enhances human osteoblast-like cell proliferation and differentiation. J Oral Maxillof Surg. 2005;63(3):362–369. doi: 10.1016/j.joms.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Anituaa E, Sanchezb M, Orivea G, Andiaa I. The potential impact of the preparation rich in growth factors (PRGF) in different medical fields. Biomaterials. 2007;28(31):4551–4560. doi: 10.1016/j.biomaterials.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 8.Marx RE, Garg AK (2004) ‘‘Platelet-rich Plasma: evidence to Support Its Use’’ dental and craniofacial application. J Oral Maxillofac Surg 62:489–496 [DOI] [PubMed]
- 9.Rutkowski JL, Thomas JM, Bering CL, Speicher JL, Radio NM, Smith DM, Johnson DA (2008) An analysis of a rapid, simple, and inexpensive technique used to obtain platelet-rich plasma for use in clinical practice. J Oral Implantol 34(1):25–33 [DOI] [PubMed]
- 10.Mozzati M, Martinasso G, Pol R, Polastri C, Cristiano A, Muzio G, Canuto R (2010) The impact of plasma rich in growth factors on clinical and biological factors involved in healing processes after third molar extraction. J Biomed Mater Res Part A 95(3):741–746 [DOI] [PubMed]
- 11.Freymiller EG, Aghaloo TL. Platelet-rich plasma: ready or not? J Oral Maxillofac Surg. 2004;62:484–488. doi: 10.1016/j.joms.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 12.Celio-Mariano R, de Melo WM, Carneiro-Avelino C. Comparative radiographic evaluation of alveolar bone healing associated with autologous platelet rich plasma after impacted mandibular third molar surgery. J Oral Maxil Surg. 2012;70(1):19–24. doi: 10.1016/j.joms.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 13.Mozzati M, Scoletta M, Gallarato I (2007) Clinical application of autologous platelet rich plasma (P.R.P.) in the extraction of third impacted mandibular molar. Revista Romana De Stomatologie LIII, NR. 2
- 14.Gurbuzer B, Pikdoken L, Urhan M, Süer BT, Narin Y. Scintigraphic evaluation of early osteoblastic activity in extraction sockets treated with platelet-rich plasma. J Oral Maxillofac Surg. 2008;66(12):2454–2460. doi: 10.1016/j.joms.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Rutkowski JL, Fennell JW, Kern JC, Madison DE, Johnson DA (2007) Inhibition of alveolar osteitis in mandibular tooth extraction sites using platelet-rich plasma. J Oral Implantol 33(3):116–121 [DOI] [PubMed]
- 16.Aloy-Prósper A, García-Mira B, Larrazabal-Morón C, Peñarrocha-Diago M (2010) Distal probing depth and attachment level of lower second molars following surgical extraction of lower third molars: a literature review. Med Oral Patol Oral Cir Bucal 15(5):e755–e759 [DOI] [PubMed]
- 17.Choukroun J, Diss A, Simonpieri A, Girard M-O, Schoeffler C, Dohan SL, Dohan AJJ, Mouhyi J, Dohan DM. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:E56–E60. doi: 10.1016/j.tripleo.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Nazaroglou I, Stavrianos C, Kafas P, Matoulas E, Upile T, Barlas I, Jerjes W (2009) Radiographic evaluation of bone regeneration after the application of plasma rich in growth factors in a lower third molar socket: a case report. Cases J 2(1):9134 [DOI] [PMC free article] [PubMed]
- 19.Ogino Y, Ayukawa Y, Tsukiyama Y, Koyano K. The effect of platelet-rich plasma on the response of cellular rat bone marrow cells in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2005;100(3):302–307. doi: 10.1016/j.tripleo.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Roussy Y, Bertrand Duchesne M-P, Gagnon G. Activation of human platelet-rich plasmas: effect on growth factors release, cell division and in vivo bone formation. Clin Oral Impl Res. 2007;18:639–648. doi: 10.1111/j.1600-0501.2007.01385.x. [DOI] [PubMed] [Google Scholar]
- 21.Frechette J-P, Martineau I, Gagnon G. Platelet-rich plasmas: growth factor content and roles in wound healing. J Dent Res. 2005;84(5):434–439. doi: 10.1177/154405910508400507. [DOI] [PubMed] [Google Scholar]
- 22.Shayesteh YS, Shafiee Ardestani MA, Khorsand A, Motahhary P, Dehghan M. Evaluation of platelet-rich plasma in combination with deproteinized bovine bone mineral in the rabbit cranium. Perio. 2006;3(4):295–303. [Google Scholar]
- 23.Torres J, Tamimi F, Martinez P–P, Alkhraisat MH, Linares R, Hernandez G, Torres-Macho J, Lo′pez-Cabarcos E. Effect of platelet-rich plasma on sinus lifting: a randomized-controlled clinical trial. J Clin Periodontol. 2009;36:677–687. doi: 10.1111/j.1600-051X.2009.01437.x. [DOI] [PubMed] [Google Scholar]
- 24.Pessoa RS, Oliveira SR, Menezes HHM, de Magalhaes D (2009) Effects of platelet-rich plasma on healing of alveolar socket: Split-mouth histological and histometric evaluation in Cebus apella monkeys. Indian J Dent Res 20(4):442 [DOI] [PubMed]
- 25.Rutkowski JL, Johnson DA, Radio NM, Fennell JW (2010) Platelet rich plasma to facilitate wound healing following tooth extraction. J Oral Implantol 36(1):11–23 [DOI] [PubMed]
- 26.Schuckert K-H, Jopp S, Osadnik M. The use of platelet rich plasma, bone morphogenetic protein-2 and different scaffolds in oral and maxillofacial surgery—literature review in comparison with own clinical experience. J Oral Maxillofac Res. 2011;2(1):e2-1. doi: 10.5037/jomr.2011.2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garg A (2011) The platelet: uses in dentistry. Dent Implantol Update 22(8):57–62 [PubMed]
- 28.Nevins ML, Camelo M, Schupbach P, Kim DM, Camelo JMB, Nevins M. Human histologic evaluation of mineralized collagen bone substitute and recombinant platelet-derived growth factor-BB to create bone for implant placement in extraction socket defects at 4 and 6 months: a case series. Int J Periodontics Restorative Dent. 2009;29:129–139. [PubMed] [Google Scholar]
- 29.McAllister BS, Haghighat K, Prasad HS, Rohrer MD. Histologic evaluation of recombinant human platelet-derived growth factor-BB after use in extraction socket defects: a case series. Int J Periodontics Restorative Dent. 2010;30:365–373. [PubMed] [Google Scholar]
- 30.Simon BI, Gupta P, Tajbakhsh S. Quantitative evaluation of extraction socket healing following the use of autologous platelet-rich fibrin matrix in humans. Int J Periodontics Restorative Dent. 2011;31:285–295. [PubMed] [Google Scholar]
- 31.Ogundipe OK, Ugboko VI, Owotade FJ. Can autologous platelet-rich plasma gel enhance healing after surgical extraction of mandibular third molars? J Oral Maxillof Surg. 2011;69(9):2305–2310. doi: 10.1016/j.joms.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 32.Mooren RECM, Merkx MAW, Bronkhorst EM, Jansen JA, Stoelinga PJW. The effect of platelet-rich plasma on early and late bone healing: an experimental study in goats. Int J Oral Maxillof Surg. 2007;36(7):626–631. doi: 10.1016/j.ijom.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 33.Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489–496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Fennis JPM, Stoelinga PJW, Jansen JA. Reconstruction of the mandible with an autogenous irradiated cortical scaffold, autogenous corticocancellous bone-graft and autogenous platelet-rich-plasma: an animal experiment. Int J Oral Maxillofac Surg. 2005;34:158–166. doi: 10.1016/j.ijom.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Alissa R, Esposito M, Horner K, Oliver R. The influence of platelet-rich plasma on the healing of extraction sockets: an explorative randomised clinical trial. Eur J Oral Implantol. 2010;3(2):121–134. [PubMed] [Google Scholar]
- 36.Roldán JC, Jepsen S, Miller J, Freitag S, Rueger DC, Açil Y, Terheyden H (2004) Bone formation in the presence of platelet-rich plasma versus bone morphogenetic protein-7. Bone 34(1):80–90 [DOI] [PubMed]
- 37.Toscano N, Holtzdaw D (2008) Surgical considerations in the use of platelet-rich plasma. Comp Cont Educ Dent 29(3):182 [PubMed]
- 38.Sánchez AR, Sheridan PJ, Kupp LI. Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants. 2003;18:93–103. [PubMed] [Google Scholar]
- 39.Weibrich G, Kleis WKG, Kunz-Kostomanolakis M, Loos AH, Wagner W. Correlation of platelet concentration in platelet-rich plasma to the extraction method, age, sex, and platelet count of the donor. Int J Oral Maxillofac Implants. 2001;16:693–699. [PubMed] [Google Scholar]