Abstract
Background and Aim
As far as research regarding any disease is concerned, each and every aspect poses a challenge. One such entity that poses a challenge in our arena is oral submucous fibrosis (OSF) as no effective treatment is available for this progressively disabling condition with high malignant potential. Hence the present study was undertaken with the aim to determine the use of pentoxifylline (PTX) on the clinical and histopathologic course of OSF.
Method
Thirty clinically confirmed OSF patients were categorized randomly into group I and group II. In group I, drug PTX was administered as an adjunct along with other conventional therapies. Group II patients were advised conventional therapies only. Pre- and post-treatment biopsies were obtained for the following parameters:
Micro-vascular density (MVD),
Area percentage of blood vessels,
Severity of fibrosis, and
Inflammatory components.
Results
On comparing MVD in groups I and II there was no significant difference in pre- and post-treatment.
On comparing the average area percentage occupied by blood vessels, significant difference was seen in pre- and post-treatment biopsies in group I.
On assessment of mouth opening and tongue protrusion, there was no significant improvement in either of the groups individually or in comparison. But when burning sensation of mucosa was assessed, pre- and post-treatment, both groups showed quite significant improvement individually.
Conclusion
Use of pentoxifylline seemed to be questionable, and taking into consideration the long administration time, its use is not recommended for the treatment of OSF patients.
Keywords: Blood vessels, Micro-vascular density, Oral submucous fibrosis, Pentoxifylline
Introduction
Over the years, man backed by his scientific temper and skills has been able to derive treatment and cures for various diseases, but certain diseases continue to pose a serious challenge to medical science and defies all rational. One such condition is oral submucous fibrosis (OSF). After years of painstaking research and efforts of undeterred clinicians and researchers, satisfactory modes of treatment for OSF are still awaited. It is therefore a cause of concern as it is a crippling disease of mouth with high malignant potential [1]. This disease is all the more important since it is one of the most common oral conditions seen in Indian subcontinent [2]. In 2002, the statistics from Indian subcontinent alone was about 5 million people (0.5 % of the population of India) [3]. In an epidemiological study on oral cancer and precancerous lesions in rural Indian population conducted over a 17-year period, 7.6 % malignant transformation rate in OSF is reported [4].
Oral submucous fibrosis has its first reference since the time of Sushrutha (1952) as ‘vidari’ [2]. It is a chronic, insidious, disabling and irreversible disease. Although the etiology is not known, the current concept suggests etiology to be multifactorial with the role of areca nut chewing, hereditary, nutritional deficiencies and immunological factors being few of them. The disease leads to fibroelastic transformation of the lamina propria and epithelial atrophy of the oral mucosa [5]. It has been demonstrated that gender, age, location and distribution of this disease constitute a regional variation, which is attributable to differences in the areca nut chewing between the genders and in geographic areas [6]. Although vesicle formation is an early sign, patients’ usual complaint is burning sensation and inability to tolerate hot and spicy food [7]. In advanced cases restricted tongue and jaw movement can also be seen [8].
The characterization of its pathogenesis is still met with difficulties. The degree of vascularity of the diseased mucosa in OSF has always been a matter of dispute and epithelial atrophy in OSF is based on the assumption of an ischemic epithelium resultant to poorly vascularised stroma due to fibrosis. Good case-control studies on the integrity and patency of microvasculature is still lacking [9].
Since etiology and pathogenesis of this premalignant condition is not well understood, management of this condition has still not been satisfactory for the simple reason that the fibrotic changes which have taken place with severe epithelial atrophy cannot be reversed. Current treatment modalities available are mostly symptomatic including injection of hyaluronidase, hydrocortisone, triamcinolone, placental extract, plus vitamin and iron supplements being most popular. Surgical treatment is the method of choice in patients with marked limitation of opening and in cases where biopsy has revealed dysplastic or neoplastic changes. Although various surgical modalities were proposed for the management but in long run patient shows recurrence of restricted mouth opening. Hence drug therapy forms an inseparable part of OSF management which apart from increasing mouth opening relieves early stage patients from severe fibrotic changes. Hence the present clinical study was undertaken to evaluate the efficacy of pentoxifylline (PTX), a methylxanthine derivative, in patients with OSF.
Aim and Objectives
The present study was undertaken with the aim to determine the effect of PTX on the clinical and histopathologic course of OSF.
The objectives were:
To determine the effect of PTX on the clinical and histopathologic course of OSF, and
To assess the acceptability of PTX as an adjunct in conservative management of OSF patients.
Materials and Methods
The research protocol for this study was reviewed and approved by the Institutional Review Board at KLE VK Institute of Dental Sciences (KLE VKIDS), Belgaum, India.
Source of Data
Patients of either sex, above 18 years of age, reporting to the outpatient Department of Oral Medicine and Radiology at KLE VKIDS, Belgaum, were included in the study after obtaining a written informed consent from the patient.
Method of Collection of Data
The inclusion criteria included (a) 30 clinically confirmed cases of OSF (b) habitual chewers of areca-quid or pan masala and (c) more than 18 years of age. The following exclusion criteria were considered (a) patients with history of any systemic disease like diabetes, hypertension, etc. (b) Pregnant or lactating mothers (c) patients who have previously exhibited intolerance to PTX or other xanthines such as caffeine, theophylline, etc. (d) patients below 18 years of age, and (e) patients with any developmental defects or TMJ disorders.
Thirty patients fulfilling the criteria set for patient selection were randomly selected and data was collected regarding socio-demographic factors, any oral habits and oral hygiene practices. The severity of the condition was clinically graded using Khanna and Andrade [10] classification and the patients were categorized into two groups in the following manner:
Group I: Clinically diagnosed OSF patients in whom PTX was administered (interventional group; n = 15).
Group II: Age and sex matched patients in whom PTX was not administered (control group; n = 15).
Method
In the present study 30 patients were enrolled, and were categorized randomly into group I (n = 15) and group II (n = 15). All the cases underwent detailed history as per the case history proforma and thorough oral examination. In group I, drug PTX was administered as an adjunct along with other conventional therapies that included intralesional corticosteroid, hyaluronidase and placentrix injections, along with local heat therapy and mouth stretching exercises. The drug PTX was administered as an inductive regime for the initial 15 days at a reduced dosage of 2 tablets daily. At the end of the period, all the patients in group I underwent routine blood and systemic examinations to record untoward effects, if any. Thereafter the dose was hiked to 3 tablets daily. The control group was advised conventional therapies only. Biopsies were obtained from buccal mucosa and histopathological examinations were done by three different oral pathologists and were assessed for the following parameters:
Micro-vascular density (MVD),
Area percentage of blood vessels,
Severity of fibrosis, and
Inflammatory components.
For every biopsy taken, five high power fields were selected and Leica Q win software was used to outline the vessels and measure their total number and also the area percentage covered by blood vessels for every given field. An average of all five fields was taken for both the parameters, i.e., MVD and area percentage. Also follow-up was done for every 15 days for each subject and clinical OSF status was checked using following criteria [11]
Subjective
Relief from intolerance to spicy food and ‘burning mouth’,
Improvement in salivation, rigidity of the mucosa and de-papillation of dorsum of tongue, and
Improvement from tinnitus.
The patients were questioned about the degree of burning sensation they observed upon ingestion of spicy food, tobacco, hot beverages, etc., and following scores were assigned.
0 No burning sensation
1 Mild burning sensation
2 Moderate burning sensation
3 Severe burning sensation
Objective
Improvement in mouth opening, and
Improvement in tongue protrusion base-line reference to vermillion lip.
After an average of about 4 months, biopsies were repeated from the area as close as possible to the previous site of biopsy in all patients and histopathological examinations were carried out in the similar method as described before.
Clinical course of the disease and the effectiveness of the treatment were assessed after comparing the pre- and post-clinical and histopathologic findings based on established pathologic parameters (Figs. 1–4).
Fig. 1.
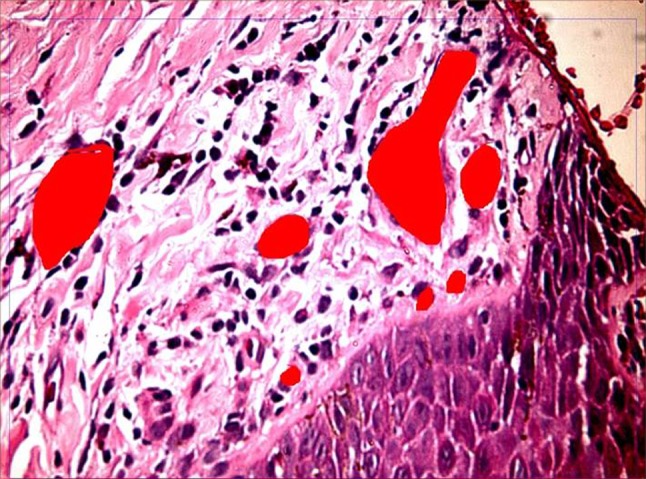
Pre-therapy biopsy for studying disease progression and morphometry in control group
Fig. 4.
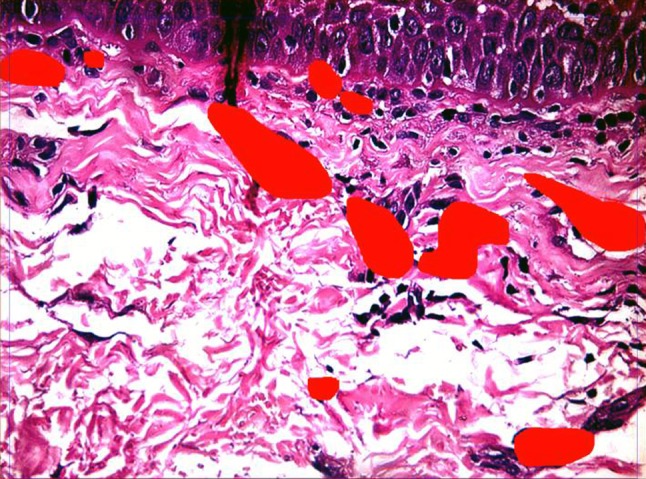
Post-therapy biopsy for studying disease progression and morphometry in interventional group
Fig. 2.
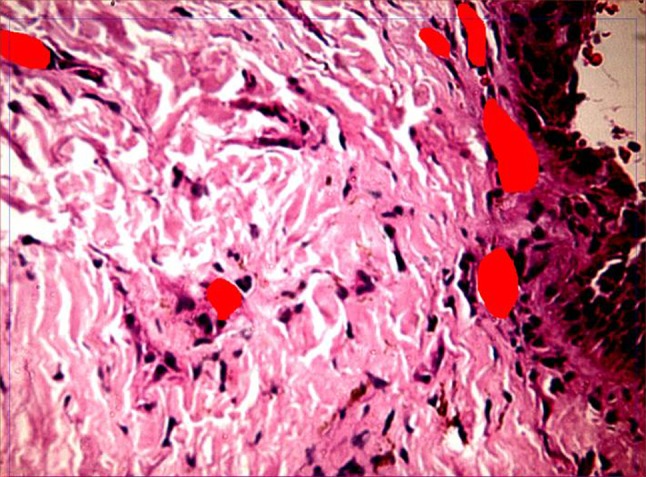
Post-therapy biopsy for studying disease progression and morphometry in control group
Fig. 3.
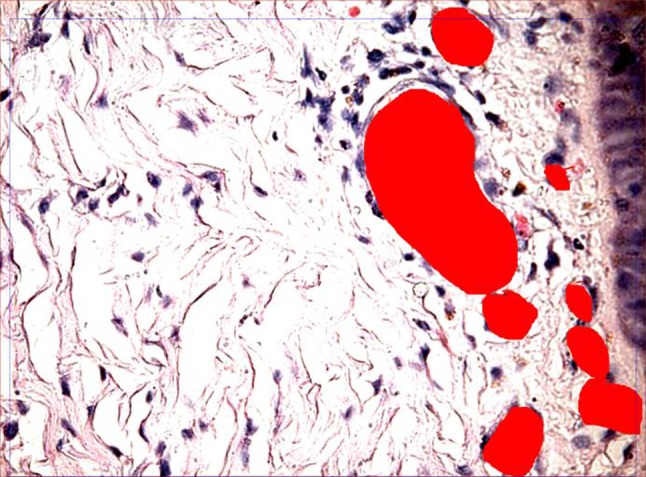
Pre-therapy biopsy for studying disease progression and morphometry in interventional group
Statistical Analysis
The data was then analyzed by means of the Statistical Package for Social Sciences (SPSS PC Version 15.0). Unpaired and paired t-tests were applied for evaluating the pre-, post-treatment and difference between the two groups.
Results
The results of our study showed that the MVD in either of the groups (interventional or control) showed no significant difference in pre- and post-treatment (Tables 1, 2). But on comparing the average area percentage occupied by blood vessels, significant difference was seen in pre- and post-treatment biopsies of patients in interventional group (Tables 3, 4). On comparison of mouth opening (Tables 5, 6) and tongue protrusion (Tables 7, 8), there was no significant improvement in either of the groups individually or in comparison. But when burning sensation of mucosa (Tables 9, 10) was assessed, pre- and post-treatment, both groups showed quite significant improvement individually. But when improvement between two groups was compared, interventional group gave no better results than control group.
Table 1.
Comparison of interventional and control groups at pre-, post-treatment and difference with respect to average microvascular density by unpaired t test
| Variables | Groups | n | Mean | SD | t-value | p value |
|---|---|---|---|---|---|---|
| Pre-treatment | Interventional | 15 | 6.8267 | 1.8030 | −0.9501 | 0.3502 |
| Control | 15 | 7.4000 | 1.4871 | |||
| Post- treatment | Interventional | 15 | 6.8933 | 1.9536 | −0.9076 | 0.3718 |
| Control | 15 | 7.4533 | 1.3763 | |||
| Change | Interventional | 15 | −0.0667 | 2.1296 | −0.0159 | 0.9874 |
| Control | 15 | −0.0533 | 2.4442 |
Table 2.
Comparison of pre- and post-treatment with respect to average microvascular density scores in interventional and control groups by paired t test
| Groups | Treatment | Mean | SD | Mean Diff. | SD Diff. | % of change | Paired t | p value |
|---|---|---|---|---|---|---|---|---|
| Interventional | Pre treatment | 6.83 | 1.80 | −0.07 | 2.13 | −0.98 | −0.1212 | 0.9052 |
| Post treatment | 6.89 | 1.95 | ||||||
| Control | Pre treatment | 7.40 | 1.49 | −0.05 | 2.44 | −0.72 | −0.0845 | 0.9338 |
| Post treatment | 7.45 | 1.38 |
Table 3.
Comparison of interventional and control groups at pre-, post-treatment and difference with respect to average area %age by unpaired t test
| Variables | Groups | n | Mean | SD | t-value | p value |
|---|---|---|---|---|---|---|
| Pre-treatment | Interventional | 15 | 8.3280 | 3.0427 | 2.3225 | 0.0277* |
| Control | 15 | 6.3800 | 1.1376 | |||
| Post- treatment | Interventional | 15 | 6.7251 | 1.7524 | 0.7470 | 0.4613 |
| Control | 15 | 6.2713 | 1.5693 | |||
| Change | Interventional | 15 | 1.6029 | 2.3901 | 1.9565 | 0.0604 |
| Control | 15 | 0.1087 | 1.7427 |
* p < 0.05
Table 4.
Comparison of pre- and post-treatment with respect to average area %age scores in interventional and control groups by paired t test
| Groups | Treatment | Mean | SD | Mean Diff. | SD Diff. | % of change | Paired t | p value |
|---|---|---|---|---|---|---|---|---|
| Interventional | Pre treatment | 8.33 | 3.04 | 1.60 | 2.39 | 19.25 | 2.5975 | 0.0211* |
| Post treatment | 6.73 | 1.75 | ||||||
| Control | Pre treatment | 6.38 | 1.14 | 0.11 | 1.74 | 1.70 | 0.2415 | 0.8127 |
| Post treatment | 6.27 | 1.57 |
* p < 0.05
Table 5.
Comparison of interventional and control groups at pre-, post-treatment and difference with respect to mouth opening by unpaired t test
| Variables | Groups | n | Mean | SD | t-value | p value |
|---|---|---|---|---|---|---|
| Pre-treatment | Interventional | 15 | 19.9333 | 3.1275 | −2.4539 | 0.0206* |
| Control | 15 | 22.9333 | 3.5550 | |||
| Post- treatment | Interventional | 15 | 21.0000 | 4.1231 | −2.1493 | 0.0404* |
| Control | 15 | 23.8000 | 2.9081 | |||
| Change | Interventional | 15 | 0.9333 | 2.7894 | 0.1485 | 0.8830 |
| Control | 15 | 0.8000 | 2.0771 |
* p < 0.05
Table 6.
Comparison of pre- and post-treatment mouth opening scores in interventional and control groups by paired t test
| Groups | Treatment | Mean | SD | Mean Diff. | SD Diff. | % of change | Paired t | p value |
|---|---|---|---|---|---|---|---|---|
| Interventional | Pre treatment | 19.93 | 3.13 | −1.07 | 3.13 | −5.35 | −1.3209 | 0.2077 |
| Post treatment | 21.00 | 4.12 | ||||||
| Control | Pre treatment | 22.93 | 3.56 | −0.87 | 2.17 | −3.78 | −1.5491 | 0.1437 |
| Post treatment | 23.80 | 2.91 |
Table 7.
Comparison of interventional and control groups at pre-, post-treatment and difference with respect to tongue protrusion by unpaired t test
| Variables | Groups | n | Mean | SD | t-value | p value |
|---|---|---|---|---|---|---|
| Pre-treatment | Interventional | 15 | 32.6667 | 4.2706 | −0.4875 | 0.6297 |
| Control | 15 | 33.3333 | 3.1320 | |||
| Post- treatment | Interventional | 15 | 32.5333 | 4.4379 | −1.0965 | 0.2822 |
| Control | 15 | 34.0667 | 3.1045 | |||
| Change | Interventional | 15 | 0.5333 | 1.1872 | −0.1254 | 0.9011 |
| Control | 15 | 0.6000 | 1.6818 |
Table 8.
Comparison of pre- and post-treatment tongue protrusion scores in interventional and control groups by paired t test
| Groups | Treatment | Mean | SD | Mean Diff. | SD Diff. | % of change | Paired t | p value |
|---|---|---|---|---|---|---|---|---|
| Interventional | Pre treatment | 32.67 | 4.27 | 0.13 | 2.13 | 0.41 | 0.2420 | 0.8123 |
| Post treatment | 32.53 | 4.44 | ||||||
| Control | Pre treatment | 33.33 | 3.13 | −0.73 | 1.79 | −2.20 | −1.5854 | 0.1352 |
| Post treatment | 34.07 | 3.10 |
Table 9.
Comparison of interventional and control groups at pre- post-treatment and difference with respect to burning sensation by Mann–Whitney u test
| Variables | Groups | n | Mean | SD | Median | Sum of ranks | T-value | Z-value | p value |
|---|---|---|---|---|---|---|---|---|---|
| Pre-treatment | Interventional | 15 | 0.93 | 0.88 | 1.00 | 227.50 | −0.2074 | 0.8357 | |
| Control | 15 | 1.00 | 0.85 | 1.00 | 237.50 | 107.50 | |||
| Post- treatment | Interventional | 15 | 0.40 | 0.51 | 0.00 | 240.00 | −0.3111 | 0.7557 | |
| Control | 15 | 0.33 | 0.49 | 0.00 | 225.00 | 105.00 | |||
| Change | Interventional | 15 | 0.53 | 0.83 | 0.00 | 222.50 | −0.4148 | 0.6783 | |
| Control | 15 | 0.67 | 0.72 | 1.00 | 242.50 | 102.50 |
Table 10.
Comparison of pre and post treatment burning sensation scores in interventional and control groups by Wilcoxon matched pairs test
| Groups | Treatment | Mean | Std.Dv. | Mean Diff. | SD Diff. | % of change | Z-value | p value |
|---|---|---|---|---|---|---|---|---|
| Interventional | Pre treatment | 0.93 | 0.88 | |||||
| Post treatment | 0.40 | 0.51 | 0.53 | 0.83 | 57.14 | 2.0304 | 0.0423* | |
| Control | Pre treatment | 1.00 | 0.85 | |||||
| Post treatment | 0.33 | 0.49 | 0.67 | 0.72 | 66.67 | 2.5205 | 0.0117* |
* p < 0.05
Discussion
The backbone of science is various queries. The endless series of questions and critical evaluation of answers to every question has lead us to the current era of extensive modern dental sciences. As far as research regarding any disease is concerned, each and every aspect, that is, epidemiology, clinical manifestation, habit pattern, ecological factors, etiology, pathophysiology and treatment pose a challenge. In the current era of evidence based scientific disciple, the queries are extensive dealing from individual to molecular level. One such entity that poses a challenge in our arena is OSF. Although many factors have been elicited and worked upon, no concrete etiology/pathophysiology has been elicited and thus no effective treatment is available for this progressively disabling condition with high malignant potential [12]. Since the treatment available for this disease is limited, hence the present study was undertaken to determine the use of PTX on the clinical and histopathologic course of OSF.
Pentoxifylline (PTX) is a methylxanthine derivative and a non-specific type IV phosphodiesterase inhibitor. It is used clinically to treat patients with peripheral vascular diseases [13]. Its pharmacological mechanisms are not completely understood but has been shown to reduce production of collagen, expression of interleukin-6 and transforming growth factor-beta 1 (TGFβ1) in rat hepatic stellate [14]. In a cultured HK-2 tubular epithelial cell line, PTX directly upregulated the expression of vascular endothelial growth factor mRNA by stabilization of its mRNA and directly prolonging its half life [15]. This is a substantial finding when considering the atrophic and ischemic condition of oral mucosa in patients of OSF. Epithelial atrophy in OSF is based on the assumption of an ischemic epithelium resultant to poorly vascularised stroma. The atrophic epithelium is envisaged to pre-dispose to malignant transformation when brought in contact with oral carcinogens [9]. In addition, PXT acts by increasing red cell deformability, leukocyte chemotaxis, anti-thrombin and anti-plasmin activities, and also decreases red cell and platelet aggregation, granulocyte adhesion, fibrinogen levels, and whole blood viscosity [16]. Pentoxifylline has also been shown to increase the production of prostaglandins (specifically E2 and I2) by vascular epithelium, important in maintaining cellular integrity and homeostasis after acute injury [17–19]. The degree of vascularity of the diseased mucosa in OSF has always been a matter of dispute while good case–control studies on the integrity, patency and density of microvasculature are still lacking. Hence the present study was undertaken at providing an evidence for clinical usage of PTX as an adjunct in treatment of OSF and to investigate histologically whether PTX has any effect of microvasculature for reversing the condition.
Fedorowicz et al. [20] reviewed the trial and found the following lacunae:
Since the patients also received local heat therapy and underwent forceful mouth stretching exercises it could not be deciphered whether the improvement was due to the drug or to associated heat therapy and stretching exercises.
In addition the authors did not mention any assessment of improvement in the range of jaw movement.
Changes in severity of burning sensation were reported, but these parameters were poorly defined not based on any recognized and validated pain scale and the reports did not provide any reliable information on how the assessments were made or how the scores were calculated.
They assess ‘relief from difficulty of speech’ and whilst it included data for both intervention groups the report contained no information on how these speech evaluations were carried out.
Randomization of patients, allocation concealment and blinding of patients/investigators as well as intention to treat were unclear.
Also few of the previous studies on treatment of OSF with PTX have shown improvement in the condition based on clinical assessment, but scales defining the improvement or histological evidence supporting the clinical findings are not mentioned [21].
The present study corroborated these findings and strict criteria were applied for blinding the observers as well as the patients. Our study compared the effect of the drug clinically as well as histologically laying emphasis on the MVD, area percentage occupied by blood vessels, reversal in fibrosis, and other important clinical parameters like mouth opening, tongue protrusion and burning sensation.
In our study of 30 OSF patients 29 (96.66 %) were males and 1 (3.33 %) was female. Similar male predominance has been reported by Ranganathan and Mishra [21], Chaturvedi and Marathe [22], Shah and Sharma [23] and Pindborg et al. [24]. In contrast female predominance has been reported by Canniff et al. [25] and Bhosle et al. [26]. Most of the patients in our study were in the second and third decades of life. This is in agreement with Phatak [27], and Gupta et al. [28]. The higher prevalence of OSF patients in younger age group is explained by popularity of refined areca nut products, which are readily available commercially, among teenagers. All the patients in our study had at least one areca nut based chewing habit. Similar observations were made by Shear et al. [29], and Van Wyk et al. [30]. Areca nut chewing is identified as the most important etiological factor.
The results of our study showed that the MVD in either of the groups (interventional or control) showed no significant difference in pre- and post-treatment. This exhibited the limited role of PTX having any effect on vasculature of tissue in OSF. But on comparing the average area percentage occupied by blood vessels, significant difference was seen in pre- and post-treatment biopsies of patients in interventional group. According to the observations of Rajendran et al. [8], diameter of the blood vessels increased with the progression of disease, while in this study increase in the area of blood vessels was noticed with decrease in progression of disease. This phenomenon was not observed in the control group, which is worth highlighting, as this might be contributed to the vasodilating property of PTX. Although vasodilating effect was seen in interventional group, reversal of hyalinization and/or fibrosis, and decrease in inflammatory infiltrate in submucosal layer did not exhibited much change in either of the groups. Thus the effect of PTX as an adjunct to intra-lesional drugs showed no added advantage over other local therapies.
On clinical assessment of mouth opening and tongue protrusion, there was no significant improvement in either of groups individually or in comparison. But when burning sensation of mucosa was assessed, pre- and post-treatment, both groups showed quite significant improvement individually. But when improvement between two groups was compared, interventional group gave no better results than control group.
Several studies have reported central nervous system (dizziness, headache, tremor, anxiety, and confusion) and gastro-intestinal (dyspepsia, nausea and/or vomiting, bloating, flatus, and bleeding) side effects caused by PTX that are dose related and are therefore minimized by dose reduction [31]. In an initial placebo-controlled clinical trial, the overall incidence of adverse effects was higher in patients who received PTX in capsule form than in those who received a commercially available sustained release tablet (SRT) [16] as the latter formulation slows drug delivery and minimizes gastric intolerance. No adverse side effects which warranted cessation of therapy was observed in this clinical trial that was attributed mainly to adherence to the threshold dosage and SRT form of medication. It is postulated that the improvement in the signs and symptoms even in the control group could possibly be due to the cessation of habits as part of counseling of the patients during follow-up visits.
One point which needs to be emphasized and has not been considered in other studies is the duration of the drug for which it is being administered to the patients and the following patient compliance. It is clear that patients often fail to take the medication in the way in which it was prescribed. Socrates in 400 BC cautioned physicians to be aware that patients will lie about taking the medications prescribed [32]. Many studies have demonstrated that patient compliance decreases with increasing number of pills per day, over the period of time. When prescription is for once daily administration, patient compliance is approximately 80 %, when it is necessary to take the pill twice daily, it decreases to 69 % and drops even further to 35 % for four times daily. Therefore if there is a choice, the clinician should prescribe drugs that can be given fewer times to increase compliance [33]. In accordance to this observation, it was noticed in our study also that reliance on patient regarding self administration of the drug was questionable, especially as the duration increased from weeks to months and so on.
Preliminary results by Mehrotra et al. [1] showed a statistically significant improvement in the group of OSF patients receiving PTX. However, the study also mentions that before its use can be recommended, a multi-institutional double-blind prospective study for assessment of effects of PTX treatment should be carried out.
Summary and Conclusion
Although primary or adjunctive therapy with PTX has been suggested for a multitude of disorders that include cases of pathological fibrosis, there are few controlled clinical trials to confirm its efficacy. Treatment for OSF the use of PTX remains a challenge. It is said that once the disease has developed, there is neither regression nor any effective treatment. Consequently, improved oral opening and relief of symptoms form the main objectives of treatment. An attempt and expedition towards better treatment options of this enigmatic disease has brought PTX in the long list of drugs used for its treatment. The present study attempted to evaluate its efficacy in greater depth including its study on microscopic level. Even though PTX does show vasodilatation at histological level, clinical improvement is at par with other drugs and local therapies used. Hence its use seems to be questionable, and taking into consideration its long administration time extending over several months its use is not recommended for the treatment of OSF patients. However further evaluation in this direction with larger sample size is necessary to give greater insight into the management of this condition and to decrease the malignant transformation rate.
Contributor Information
Namdeo Prabhu, Email: drpranam@gmail.com.
Sanjay S. Rao, Email: snajsamuel@yahoo.com
S. M. Kotrashetti, Email: kotra27@yahoo.co.in
Shridhar D. Baliga, Email: baliga1974@rediffmail.com
Seema R. Hallikerimath, Email: drseemah@indiatimes.com
Punnya V. Angadi, Email: punnya_angadi@rediffmail.com
Rakhi Issrani, Phone: +918565842350, Email: dr.rakhi.issrani00@gmail.com.
References
- 1.Mehrotra R, Singh HP, Gupta SC, Singh M, Jain S. Pentoxifylline therapy in the management of oral submucous fibrosis. Asian Pac J Cancer Prev. 2011;12:971–974. [PubMed] [Google Scholar]
- 2.Mehta AK, Panwar SS, Verma RK, Pal AK. Buccal fat pad reconstruction in oral submucosal fibrosis. Med J Armed Forces India. 2003;59:340–341. doi: 10.1016/S0377-1237(03)80151-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrawal D, Gupta S, Agarwal D, Gupta OP, Agarwal M. Role of GSTM1 and GSTT1 polymorphism: susceptibility to oral submucous fibrosis in the north Indian population. Oncology. 2010;79:3–4. doi: 10.1159/000318533. [DOI] [PubMed] [Google Scholar]
- 4.Nair U, Bartsch H, Nair J. Alert for an epidemic of oral cancer due to use of the betel quid substitutes gutkha and pan masala: a review of agents and causative mechanisms. Mutagenesis. 2004;19(4):251–262. doi: 10.1093/mutage/geh036. [DOI] [PubMed] [Google Scholar]
- 5.Reddy V, Wanjari PV, Banda NR, Reddy P. Oral submucous fibrosis: correlation of clinical grading to various habit factors. Int J Dent Clin. 2011;3(1):21–24. doi: 10.4103/2231-0754.115770. [DOI] [Google Scholar]
- 6.Gupta PG, Ray CS. Epidemiology of betel quid usage. Ann Acad Med Singapore. 2004;33:315–365. [PubMed] [Google Scholar]
- 7.Nataraj S, Guruprasad Y, Shetty JN. A comparative clinical evaluation of buccal fat pad and collagen in surgical management of oral submucous fibrosis. Arch Dent Sci. 2011;2(4):17–24. [Google Scholar]
- 8.Rajendran R, Rani V, Shaikh S. Pentoxifylline therapy. A new adjunct in the treatment of oral submucous fibrosis. Indian. J Dent Res. 2006;17(4):190–198. doi: 10.4103/0970-9290.29865. [DOI] [PubMed] [Google Scholar]
- 9.Mahajan AD, Tatu RJ, Shenoy NA, Sharma VS. Surgical management of oral submucous fibrosis in an edentulous patient: a procedural challenge. Natl J Maxillofac Surg. 2010;1(2):161–163. doi: 10.4103/0975-5950.79221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khanna JN, Andrade NN. Oral submucous fibrosis—a new concept in surgical management. Int J Oral Maxillofac Surg. 1995;24:433–439. doi: 10.1016/S0901-5027(05)80473-4. [DOI] [PubMed] [Google Scholar]
- 11.Maher R, Aga P, Johnson NW, Sankaranarayanan R, Warnakulasuriya S. Evaluation of multiple micronutrient supplementation in the management of oral submucous fibrosis in Karachi, Pakistan. Nutr Cancer. 1997;27(1):41–47. doi: 10.1080/01635589709514499. [DOI] [PubMed] [Google Scholar]
- 12.Taneja L, Nagpal A, Vohra P, Anja V. Oral submucous fibrosis: an oral physician approach. J Innovative Dentistry. 2011;1(3):78–81. [Google Scholar]
- 13.Magnusson M, Gunnarsson M, Berntorp E, Bjorkman S, Hoglund P. Effects of pentoxifylline and its metabolites on platelet aggregation in whole blood from healthy humans. Eur J Pharmacol. 2008;581:290–295. doi: 10.1016/j.ejphar.2007.11.054. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez E, Correa A, Bucio L, Souza V, Kershenobich D, Gutierrez-Ruiz MC. Pentoxifylline diminished acetaldehyde induced collagen production in hepatic stellate cells by decreasing interleukin-6 expression. Pharmacol Res. 2002;46:435–443. doi: 10.1016/S1043661802002025. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Q, Zheng F, Hou F. Inhibition of tubulointerstitial fibrosis by pentoxifylline is associated with improvement of vascular endothelial growth factor expression. Acta Pharmacol Sin. 2009;30(1):98–106. doi: 10.1038/aps.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward A, Clissold SP. Pentoxifylline: a review of its pharmacodynamic and pharmacokinetic properties and its therapeutic efficacy. Drug Eval. 1987;34:50–97. doi: 10.2165/00003495-198734010-00003. [DOI] [PubMed] [Google Scholar]
- 17.Streiter RM, Remick DG, Ward PA, et al. Cellular and molecular regulation of tumor necrosis factor alpha production by pentoxifylline. Biochem Biophys Res Comm. 1988;155:1230–1236. doi: 10.1016/S0006-291X(88)81271-3. [DOI] [PubMed] [Google Scholar]
- 18.Fahr A, Langer R, Ziegoleit S. Influence of pentoxifylline administered in vivo on the synthesis of prostacyclin in human varicose veins. Biomed Biochem Acta. 1988;14:29–34. [PubMed] [Google Scholar]
- 19.Matzky R, Darins H, Sehroar K. The release of prostacyclin by pentoxifylline from human vascular tissue. Arzneimittelforschung. 1982;32:1315–1318. [PubMed] [Google Scholar]
- 20.Fedorowicz Z, Chan Shih-Yen E et al (2008) Interventions for the management of oral submucous fibrosis. Cochrane Database Syst Rev 4: CD007156 [DOI] [PubMed]
- 21.Ranganathan K, Mishra G. An overview of classification schemes for OSMF. J Oral Maxillofac Pathol. 2006;10:55–58. [Google Scholar]
- 22.Chaturvedi VN, Marathe NG. Serum globulins and immunoglobulin in oral submucous fibrosis. Indian Practitioner. 1988;41:399–403. [Google Scholar]
- 23.Shah N, Sharma PP. Role of chewing and smoking habits in the aetiology of oral submucous fibrosis (OSF): a case control study. J Oral Pathol Med. 1998;27:475–479. doi: 10.1111/j.1600-0714.1998.tb01915.x. [DOI] [PubMed] [Google Scholar]
- 24.Pindborg JJ, Mehta FS, Gupta PC, Daftary DK. Prevalence of oral submucous fibrosis among 50,915 Indian villagers. Br J Cancer. 1968;22(4):646–654. doi: 10.1038/bjc.1968.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canniff JP, Harvey W, Harris M. Oral submucous fibrosis: its pathogenesis and management. Br Dent J. 1986;160(12):429–434. doi: 10.1038/sj.bdj.4805876. [DOI] [PubMed] [Google Scholar]
- 26.Bhonsle RB, Murti PR, Pindborg JJ, Daftary DK, Mehta FS. Focal vascular dilatations and petechiae in oral submucous fibrosis. Scand J Dent Res. 1981;89:270–274. doi: 10.1111/j.1600-0722.1981.tb01682.x. [DOI] [PubMed] [Google Scholar]
- 27.Phatak AG. Serum proteins and immunoglobulins in oral submucous fibrosis. Indian J Otolaryngol. 1978;30(1):1–4. [Google Scholar]
- 28.Gupta DS, et al. Estimation of major immunoglobulin profile in oral submucous fibrosis by radial immune-diffusion. Int J Oral surg. 1985;14:533–537. doi: 10.1016/S0300-9785(85)80060-0. [DOI] [PubMed] [Google Scholar]
- 29.Shear M, Lemmar J, Dockrat I. Oral submucous fibrosis in South African Indians—An epidemiological study. S Afr J Med Sci. 1967;32:41–46. [PubMed] [Google Scholar]
- 30.Van Wyk CW, et al. Collagen in submucous fibrosis: an electron-microscope study. J Oral Pathol Med. 1990;19:182–187. doi: 10.1111/j.1600-0714.1990.tb00821.x. [DOI] [PubMed] [Google Scholar]
- 31.Samlaska CP, Winfield EA. Pentoxyfylline. J Am Acad Derm. 1994;30(4):603–621. doi: 10.1016/S0190-9622(94)70069-9. [DOI] [PubMed] [Google Scholar]
- 32.Sclar DA, Tartaglione TA, Fine MJ. Overview of issues related to medical compliance with implications for the outpatient management. Infect Agents Dis. 1994;3:266–269. [PubMed] [Google Scholar]
- 33.Raz R, Elchanan G, Colodner R, et al. Antibiotics twice daily vs four times daily in treatment of streptococcal pharynigitis. Clinical Infect Dis Pract. 1995;4:50–54. doi: 10.1097/00019048-199501000-00015. [DOI] [Google Scholar]


