Abstract
Introduction
Decreasing the time needed for osseointegration has always been a big challenge for modern implantodontics. The main factor which helps to decrease the time needed for osseointegration is the newly developed surfaces being used, as well as their microstructures, in relation to their osseoinductive properties. The aim of this work is to clinically evaluate the osseointegration of the implants when using The anodized surfaces in humans, following a 60 days-period of osseointegration.
Methodology
Forty-Five implants were placed in different kinds of bones, according to the technique recommended by the manufacturer. Those implants were opened after 60 days of osseointegration. The success of evaluation was made through assessing the counter torque resistance of 25 Ncm. The implants which could withstand the applied torque were considered osseointegrated.
Results
Of the forty-five implants made in different kinds of bones, only one failed to present osseointegration, resulting in a success rate of 97.7 %.
Conclusions
With this methodology it was possible to conclude that anodized surface implants present primary osseointegration after 60 days of healing, after which they can function normally.
Keywords: Dental implants, Osseointegration, Surfaces
Introduction
The conventional protocol proposed by Bränemark for treatment utilizing dental implants requires implant procedures to be performed in two phases, maintaining an interval between procedures of 3 months for mandibular treatment and 6 months for maxillary treatment to ensure osseointegration [1–3].
Implant surfaces allow for the acceleration of osseointegration. The morphology, topography, surface roughness, surface energy, and chemical composition and potential have a significant influence on the reaction of the bone tissue during osseointegration [2–7].
A surface roughness of up to 0.5 μm is necessary for fibroblast adhesion, while a roughness ranging from 0.5 to 1.5 μm allows for osteoblast adhesion [8, 9]. As technology has developed, these special surfaces have diminished the time needed for osseointegration while maintaining an acceptable success rate. To ensure the migration of osteogenic cells to the implant surface, fibrin retention must occur [10]. To ensure fibrin retention, several texturization techniques may be utilized, such as etching, etching followed by acid texturing, acid texturing associated with fluorine deposition and anodization [7, 11]. Further, anodization is another important factor for faster osseointegration because it incorporates Ca and P ions on the implant surface [3–5, 12–19].
Vulcano Surface Actives® implants, produced by Connection, utilize an anodization treatment. This treatment, which produces a roughness of 1.26 μm [20], allows for the incorporation of Ca and P [21, 22]. This surface treatment increases the wettability capacity by increasing the contact surface area by 10 % relative to surfaces treated with acid [23] (fig.1).
Fig. 1.

Vulcano Actives surface (magnification 5.000×)
The main anodized surface in the market is TiUnite (Nobel Biocare), which was clinically shown to last 10 years with 97.96 % survival [24]. In a recent study, the structure of the Vulcan Actives’ surface was compared to that of TiUnite via electron microscopy. The study concluded that while the roughness of the two was similar, the treatment area obtained by the Vulcan Actives surface was significantly greater [20]. The authors noted that although these values suggest good clinical performance, such performance was not found by studies evaluating Vulcan Actives’ surface. This work is presented to address this issue and examine the clinical performance of Vulcan Actives’ surface, a topic not yet addressed by the present literature [20].
This work seeks to clinically assess the level of osseointegration of Vulcano Actives® implants 60 days after their placement in patients.
Methodology
Selection of patients and number of implants
The sample was selected from those patients attending a clinic for a specialization course on implantology at UNOESC, Joaçaba campus, who required implant rehabilitation of up to a maximum of three implants in each hemi-arch, provided that they did not have any systemic problems that could contraindicate implant rehabilitation. Further, the patients must not have been treated through the immediate load technique or have been in need of bone grafting. All patients were required to accept the terms of the research agreement. Forty-five Connection ARs and Morse ARs Vulcano Actives® surfaces were placed over the course of the study.
Pre-surgical preparation
Patients were evaluated through imaging (X-ray and tomography), and plaster models were made. A final diagnosis was then made to determine the number and position of implants to be placed. The patients’ systemic condition was evaluated by blood tests, including complete blood count and fasting glucose.
Two grams of amoxicillin was administered orally 1 h before the surgical procedure, and the patients gargled with chlorhexidine digluconate 0.12 % twice a day, beginning 1 day before the surgery. Patients who were allergic to penicillin were medicated with clindamycin 600 mg 1 h before surgery. Post-surgery, 750 mg of paracetamol was administered every 6 h for 24 h to control pain. Patients continued to gargle with chlorhexidine digluconate 0.12 % twice a day for 7 days after the procedure, as prescribed.
Surgical technique
The implants were selected on a case-by-case basis, as determined by recommendations according to the length, thickness, and type of connection. The insertion technique was performed as recommended by the manufacturer. The implant placement data, including positioning, bone quality, and insertion torque, were noted on the patient’s record.
Assessment
After 60 days of osseointegration, reopenings were performed. During this procedure, a torque test was performed so as to assess the osseointegration, with the help of a ratchet extender for the placement of implants, which was assembled in a prosthetic ratchet made by the Conexão Sistema de Próteses®. The implants were submitted to a torque of 25 Ncm.
The implants which did not withstand the counter torque test were removed, and prosthetic procedures were performed in a conventional way for the remaining implants.
Results
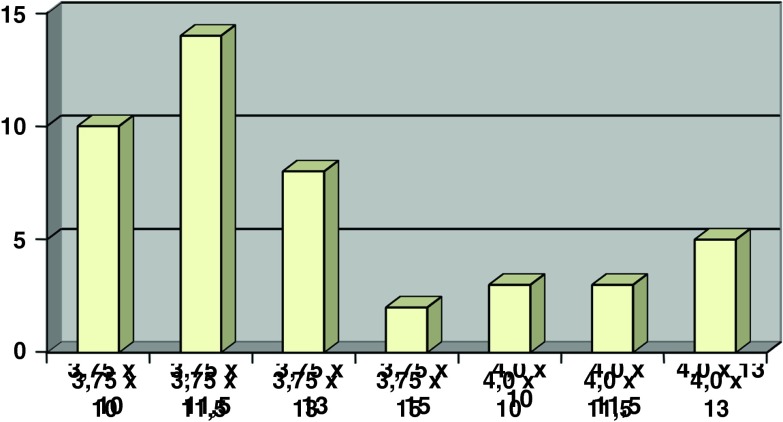
From September 2008 to December 2008, 45 implants were placed in 25 patients. The distribution of the implant locations in the maxilla and the mandible are given in Figs. 2, 3 and 4 respectively.
Fig. 2.
Distribution of implants according to their location in the maxilla
Fig. 3.
Distribution of implants according to their location in the mandible
Fig. 4.
Distribution of implants according to size and thickness
Of the 45 implant placements, only one placement failed to achieve osseointegration, thus yielding a 97.7 % success rate. Table 1 presents data referring to the 45 implant placements’ location, insertion torque, reopening time, and result.
Table 1.
Distribution of implants according to location, size of implant, type of prosthetic connection, bone quality, initial stability, and result
| Location | Size of implant | Connection | Bone quality | Reopening period (days) | Initial stability | Result | |
|---|---|---|---|---|---|---|---|
| 1 | 46 | 3.75 × 11.5 | HI | II | 60 | 70 Ncm | Success |
| 2 | 35 | 3.75 × 13 | HI | I | 58 | 60 Ncm | Success |
| 3 | 35 | 3.75 × 13 | HI | I | 58 | 70 Ncm | Success |
| 4 | 36 | 4.0 × 10 | CM | II | 62 | 50 Ncm | Success |
| 5 | 46 | 4.0 × 10 | HI | I | 60 | 60 Ncm | Success |
| 6 | 36 | 3.75 × 11.5 | HI | I | 60 | 60 Ncm | Success |
| 7 | 45 | 3.75 × 10 | HI | I | 60 | 50 Ncm | Success |
| 8 | 35 | 3.75 × 11.5 | HI | I | 60 | 55 Ncm | Success |
| 9 | 24 | 3.75 × 13 | CM | III | 60 | 50 Ncm | Success |
| 10 | 36 | 4.0 × 11.5 | HI | II | 59 | 40 Ncm | Success |
| 11 | 36 | 4.0 × 13 | HI | II | 60 | 60 Ncm | Success |
| 12 | 14 | 3.75 × 11.5 | HI | III | 60 | 50 Ncm | Success |
| 13 | 15 | 3.75 × 10 | HI | III | 60 | 35 Ncm | Success |
| 14 | 16 | 3.5 × 10 | HI | III | 60 | 60 Ncm | Success |
| 15 | 34 | 3.75 × 11.5 | HI | II | 59 | 45 Ncm | Success |
| 16 | 46 | 3.75 × 13 | HI | I | 60 | 50 Ncm | Failure |
| 17 | 36 | 3.75 × 10 | CM | II | 60 | 40 Ncm | Success |
| 18 | 34 | 3.75 × 10 | HI | II | 60 | 60 Ncm | Success |
| 19 | 36 | 4.0 × 13 | HI | II | 60 | 30 Ncm | Success |
| 20 | 44 | 3.75 × 10 | HI | II | 60 | 70 Ncm | Success |
| 21 | 46 | 4.0 × 11.5 | HI | II | 60 | 30 Ncm | Success |
| 22 | 36 | 4.0 × 11.5 | HI | II | 61 | 50 Ncm | Success |
| 23 | 34 | 3.75 × 15 | HI | II | 60 | 55 Ncm | Success |
| 24 | 36 | 4.0 × 13 | HI | II | 60 | 40 Ncm | Success |
| 25 | 36 | 3.75 × 11.5 | CM | II | 58 | 60 Ncm | Success |
| 26 | 47 | 3.75 × 11.5 | CM | II | 58 | 70 Ncm | Success |
| 27 | 24 | 3.75 × 13 | HI | III | 61 | 30 Ncm | Success |
| 28 | 15 | 3.75 × 11.5 | HI | IV | 61 | 25 Ncm | Success |
| 29 | 16 | 4.0 × 10 | HI | IV | 61 | 50 Ncm | Success |
| 30 | 22 | 3.75 × 11.5 | HI | III | 61 | 50 Ncm | Success |
| 31 | 16 | 4.0 × 13 | HI | II | 61 | 70 Ncm | Success |
| 32 | 45 | 4.0 × 13 | HI | II | 61 | 60 Ncm | Success |
| 33 | 47 | 3.75 × 13 | HI | II | 61 | 70 Ncm | Success |
| 34 | 36 | 3.75 × 11.5 | HI | II | 60 | 80 Ncm | Success |
| 35 | 36 | 3.75 × 11.5 | HI | III | 61 | 40 Ncm | Success |
| 36 | 11 | 3.75 × 11.5 | CM | III | 60 | 40 Ncm | Success |
| 37 | 21 | 3.75 × 10 | CM | III | 60 | 30 Ncm | Success |
| 38 | 23 | 3.75 × 11.5 | CM | III | 60 | 40 Ncm | Success |
| 39 | 36 | 3.75 × 10 | CM | II | 60 | 50 Ncm | Success |
| 40 | 46 | 3.75 × 10 | CM | II | 60 | 40 Ncm | Success |
| 41 | 14 | 3.75 × 13 | CM | III | 60 | 50 Ncm | Success |
| 42 | 16 | 3.75 × 10 | CM | IV | 60 | 30 Ncm | Success |
| 43 | 35 | 3.75 × 13 | HI | III | 60 | 40 Ncm | Success |
| 44 | 37 | 3.75 × 15 | HI | II | 60 | 70 Ncm | Success |
| 45 | 46 | 3.75 × 11.5 | HI | II | 60 | 50 Ncm | Success |
Bone quality and primary stability, which are two of the main factors that influence the success of osseointegration, are given in Tables 2 and 3, respectively.
Table 2.
Distribution of implants according to bone quality and their respective success rate
| Bone quality | Number of implants | Failed implants | % of success |
|---|---|---|---|
| Type I | 07 | 1 | 85.7 |
| Type II | 23 | 0 | 100 |
| Type III | 12 | 0 | 100 |
| Type IV | 3 | 0 | 100 |
Table 3.
Distribution of implants according to primary stability of implants and their respective success rates
| Primary stability (N/cm2) | Number of implants | Failed implants | % of success |
|---|---|---|---|
| 20 a 35 | 07 | 0 | 100 |
| 40 a 55 | 22 | 1 | 95.4 |
| 60 a 80 | 16 | 0 | 100 |
Discussion
When osseointegrated implant treatments were first introduced, the initial goal was to discover a metal that would bind well to bone. The material originally considered to be most promising was commercially pure titanium [3, 5, 24]. Today, other materials, such as tantalum, niobium, and titanium alloys, are known to have the capacity to achieve osseointegration [25]. Further, ceramics are known to permit greater bone bonding with the implant than titanium [26, 27].
Several surface treatments have been described in the literature. Initially, with the aim of increasing the bone/implant contact area, hydroxyapatite and titanium etchings were used [24]. Acid conditioning was also employed to create roughness in the implants and to increase the bone/implant contact area [28].
The difference in texturization is directly responsible for the cell behavior on the implant surface [8]. It influences not only the quality and the quantity of bone formation but also the speed of both bone formation and implant binding [29]. The main factors that allow for faster osseointegration are the nano-topography of the surfaces and the chemical modification resulting from the incorporation of calcium and phosphate ions [3–5, 12–19].
Albrektsson and Wennenberg [4, 5] stated that moderate roughness presented little or statistically insignificant advantages and that the anticipated performance should originate from the bioactive surfaces. Superficial changes with bisphosphonates and collagen seem to precociously reinforce peri-implant bone formation [12, 18, 19], and they improve cicatrization in the first 5 weeks [30]. To diminish osseointegration time, thus altering the biological behavior of implant, it is necessary to maintain the implant in an isotonic surface to eliminate the titanium oxide layer [31–33]. Maintaining both the implant and those surfaces bio-activated by bisphosphonates in an isotonic solution in animals presented significant differences in neither the quantity of bone formation nor the percentage of bone/implant contact [34].
This study used anodized surfaces, which showed 97.96 % success. This treatment produces a roughness of 1.26 μm, yielding a surface with nanometric features [20]; moreover, its shape diminishes the surface energy and increases the wettability capacity, improving the contact between the bone and the implant by 10 % compared to the surfaces obtained by double acid treatment [22]. This topography is associated with the incorporation of calcium and phosphate ions; in addition to improving the bone/implant contact, it brings about faster results and diminishes osseointegration time. Thus, we can characterize this surface as being bioactive and having medium roughness [22, 34, 35].
When compared to implants treated with etching and acid conditioning placed in rabbits’ tibias, the anodized surfaces showed a smaller contact angle between the bone and the implant, and they required a greater removal torque after 12 weeks of osseointegration [34]. In humans, 2 months following implant placement, the surface presented greater bone/implant contact than machined surface implants [35].
There are many works in the relevant literature on animals that address the aspect of speed of osseointegration on bioactive surfaces [14, 18, 31, 36–38], but there are few works on humans defining the necessary amount of time needed before placing a load on such implants [3]. Therefore, in this study, we chose to verify the secondary stability of implants produced by the company Connection, using a Vulcano surface (anodization) in humans. A torque of 25 N/cm was considered for this technique. In conventional implants, the insertion torque for the prosthetic component is 20 N/cm. Because it was 25 N/cm, it was possible to perform the prosthetic procedures following the manufacturer’s instructions. Despite only making assessments during the reopening procedure, no fixation was lost during the prosthetic stabilization and placement procedures.
Primary osseointegration was obtained regardless of the bone quality, insertion torque, and implant location. The only implant that did not present osseointegration had been placed in the posterior part of the mandible, in type I bone. We believe that the failure to establish osseointegration was due to problems that occurred during the implant placement, contamination, or prosthetic denaturation through the milling process, which, considered in the context of this work, does not impact the positive results obtained.
We believe that this methodology allows for the safe use of tested implants, regardless of the type of bone or implant placement location, resulting in a 60-days osseointegration period and confirming the expectation reported by the authors in the assessment of surface electronic microscopy [20].
Conclusions
The proposed methodology led us to conclude that the primary osseointegration success rate, tested using a counter torque of 25 N/cm on the Vulcano surface implants, was 97.7 % after 60 days of cicatrization.
References
- 1.Albrekson T, Branemark P-I, Hansson HÁ, Lindstöm J. Osseointegrated titanium implants. Requirements for ensuring long-lasting direct bone-to-implant anchorage in man. Acta Orthop Scan. 1981;52:155–170. doi: 10.3109/17453678108991776. [DOI] [PubMed] [Google Scholar]
- 2.Elias CN, Meirelles L. Improving osseointegration of dental implants. Expert Rev Med Devices. 2010;7:241–256. doi: 10.1586/erd.09.74. [DOI] [PubMed] [Google Scholar]
- 3.Albrektsson T, Sennerby L, Wennerberg A. State of the art of oral implants. Periodontology. 2000;2008(47):15–26. doi: 10.1111/j.1600-0757.2007.00247.x. [DOI] [PubMed] [Google Scholar]
- 4.Albrektsson T, Wennenberg A. Oral implant surfaces: Part 1—review focusing on clinical knowledge of different surface. Int J Prosthodont. 2004;17:536–543. [PubMed] [Google Scholar]
- 5.Albrektsson T, Wennenberg A. Oral implant surfaces: Part 2—review focusing on clinical knowledge of different surface. Int J Prosthodont. 2004;17:544–564. [PubMed] [Google Scholar]
- 6.Brunetto DM. The effects of implant surface topography on the behavior of cell. Int J Oral Maxillofac Implants. 1988;3:231–246. [PubMed] [Google Scholar]
- 7.Esposito M, Coulthard P, Thomsen P, Worthington HV. The role of implant surface modifications shape and material on the success of osseointegrated dental implants. A Cochrane systematic review. Eur J Prosthodont Restor Dent. 2005;13:15–31. [PubMed] [Google Scholar]
- 8.Joos U, Wiesmann HP, Szuwart T, Meyer U. Mineralization at the interface of implants. Int J Oral Maxillofac Surg. 2006;35:783–790. doi: 10.1016/j.ijom.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Inonue T, et al. Effect of the surface geometry of smooth and porous coated titanium alloy on the orientation of fibroblasts in vitro. J Biomed Mater Res. 1987;21:107–126. doi: 10.1002/jbm.820210114. [DOI] [PubMed] [Google Scholar]
- 10.Davies JE. Mechanisms of endosseus integration. Int J Prosthodont. 1998;11:391–401. [PubMed] [Google Scholar]
- 11.Testori T, Del Fabbro M, Szmukler-Moncler S, Francetti L, Weinstein RL. Immediate occlusal loading of osseotite implants in the completely edentulous mandible. Int J Oral Maxillofac Implants. 1997;12:319–324. [PubMed] [Google Scholar]
- 12.Ferguson SJ, Langhoff JD, Voelter K, Rechenberg B, Scharnweber D, Bierbaum S, Schnabelrauch M, Kautz AR, Frauchiger VM, Mueller TL, Lenthe H, Schlottig F. Biomechanical comparison of different surface modifications for dental implants. J Oral Maxillofac Implants. 2008;23:1037–1046. [PubMed] [Google Scholar]
- 13.Collaert B, de Bruyn H. Immediate functional loading of TiOblast dental implants in full-arch edentulous maxillae: a 3-year prospective study. Clin Oral Implant Res. 2008;19:1254–1260. doi: 10.1111/j.1600-0501.2008.01586.x. [DOI] [PubMed] [Google Scholar]
- 14.Marin C, Granato R, Suzuki M, Gil JN, Piatelli A, Coelho PG. Removal torque and histomorphometric evaluation of bioceramic grit-blasted/acid-etched and dual acid-etched implant surfaces: an experimental study in dogs. J Periodontol. 2008;79:1942–1949. doi: 10.1902/jop.2008.080106. [DOI] [PubMed] [Google Scholar]
- 15.Orsini G, Piatelli M, Scarano A, Petrone G, Kenealy J, Piatelli A, Caputi S. Randomized, controlled histologic and histomorphometric evaluation of implants with nanometer-scale calcium phosphate added to the dual acid-etched surface in the human posterior maxilla. J Periodontol. 2007;78:209–218. doi: 10.1902/jop.2007.060297. [DOI] [PubMed] [Google Scholar]
- 16.Barrere F, Layrolle P, van Blitterswijk CA, de Groot K (1999) Biomimetic calcium phosphate coatings on Ti6AI4V: a crystal grown study of octacalcium phosphate and inhibition by Mg2+ and HCO3−. Bone 25:107S–111S [DOI] [PubMed]
- 17.Barrere F, van der Valk CM, Dalmeijer RA, van Blitterswijk CA, de Groot K, Layrolle P. In vitro and in vivo degradation of biomimetic octacalcium phosphate and carbonate apatite coatings on titanium implants. J Biomed Mater Res, Part A. 2003;64:378–387. doi: 10.1002/jbm.a.10291. [DOI] [PubMed] [Google Scholar]
- 18.Guehennec LL, Goyenvalle E, Lopez-Heredia MA, Weiss P, Amouriq Y, Layrolle P. Histomorphometric analysis of the osseointegration of four different implant surfaces in the femoral epiphyses of rabbits. Clin Oral Implant Res. 2008;19:1103–1110. doi: 10.1111/j.1600-0501.2008.01547.x. [DOI] [PubMed] [Google Scholar]
- 19.Schliephake H, Scharnweber D, Roesseler S, Dard M, Sewing A, Aref A. Biomimetic calcium phosphate composite coating of dental implants. Int J Oral Maxillofac Implants. 2006;21:738–746. [PubMed] [Google Scholar]
- 20.Rosa MG, Albrekson T, Francischone CE, Schwartz Filho HO, Wennerberg A. Micrometric characterization of the implant surfaces from the five largest companies in Brazil, the second largest worldwide implant market. Int J Oral Maxillofac Implants. 2013;28:358–365. doi: 10.11607/jomi.2791. [DOI] [PubMed] [Google Scholar]
- 21.McAlamery ME, Oshiro MA, McAlarney CV. Effects of titanium dioxide passive film crystal structure, thickness and crystallinity on C3 adsorption. Int J Oral Maxillofac Implants. 1996;11:73–80. [PubMed] [Google Scholar]
- 22.Bathamarco RV, Solorzano G, Elias CN, Prioli R. Anatomic force microscopy analysis of the different surface treatments of Ti dental implant surfaces. Appl Surf Sci. 2004;233:29–34. doi: 10.1016/j.apsusc.2004.04.007. [DOI] [Google Scholar]
- 23.Degidi M, Nardi D, Piatelli A. 10-year follow-up of immediately loaded implants with TiUnite porous anodizes surface. Clin Implant Dent Relat Res. 2012;14:828–838. doi: 10.1111/j.1708-8208.2012.00446.x. [DOI] [PubMed] [Google Scholar]
- 24.Karabuda C, et al. Histologic and histomorphometric comparison of immediately placed hydroxyapatite-coated and titanium plasma-sprayed implants: a pilot study in dogs. Int J Oral Maxillofac Implants. 1999;14:510–515. [PubMed] [Google Scholar]
- 25.Johansson CB, Hansson Há, Albrekson T. Qualitative interfacial study between bone and tantalum, niobium or commercially pure titanium. Biomaterials. 1990;11:277–280. doi: 10.1016/0142-9612(90)90010-N. [DOI] [PubMed] [Google Scholar]
- 26.Oliva J, Oliva X, Oliva JD. One-year follow-up of first consecutive 100 zirconia dental implants in humans: a comparison of 2 different rough surfaces. Int J Oral Maxillofac Implants. 2007;22:430–437. [PubMed] [Google Scholar]
- 27.Akagawa Y, Ichikawa Y, Nikal H, Tsuru H. Interface histology of unload and early loaded partially stabilized zirconia endosseous implant in initial bone healing. J Prosthet Dent. 1993;69:599–604. doi: 10.1016/0022-3913(93)90289-Z. [DOI] [PubMed] [Google Scholar]
- 28.Testori T, Wiseman L, Woolfe S, Porter S. A prospective multicenter clinical study of the osseotite implant: four-year interim report. Int J Oral Maxillofac Implants. 2001;16:193–200. [PubMed] [Google Scholar]
- 29.Brunski JB. Biomechanical factors affecting the bone-dental implant interface. Clin Mater. 1992;10(153):201. doi: 10.1016/0267-6605(92)90049-y. [DOI] [PubMed] [Google Scholar]
- 30.Stmad J, Urban K, Povysil C, Stmad Z. Secondary stability assessment of titanium implants with an alkali-etched surface: a resonance frequency analysis study in beagle dogs. J Oral Maxillofac Implants. 2008;23:502–512. [PubMed] [Google Scholar]
- 31.Schwarz F, Herten M, Sager M, Wieland M, Dard M, Becker J. Bone regeneration in dehiscence-type defects at chemically modified (SLActive) and conventional SLA titanium implants: a pilot study in dogs. J Clin Periodontol. 2007;34:78–86. doi: 10.1111/j.1600-051X.2006.01008.x. [DOI] [PubMed] [Google Scholar]
- 32.Oates T, Valderrama P, Bischof M, Nedir R, Jones A, Simpson J, Toutenburg H, Cochran DL. Enhanced implant stability with a chemically modified SLA surface: a randomized pilot study. J Oral Maxillofac Implants. 2007;22:755–760. [PubMed] [Google Scholar]
- 33.Vicente JC, Recio O, Martin-Villa L, Junquera LM, Lopez-Arranz JS. Histomorphometric evaluation of guided bone regeneration around implants with SLA surface: an experimental study in beagle dogs. Int J Oral Maxillofac Surg. 2006;35:1047–1053. doi: 10.1016/j.ijom.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Elias CN, Oshida Y, Cavalcanti JH, Muller CA (2008) Relationship between surface properties (roughness, wettability and morphology) of titanium and dental implant removal torque. J Mech Behavior Biomed Mat 1:234–242 [DOI] [PubMed]
- 35.Shibli JA, Grassi S, Figueiredo LC, Feres M, Marcantonio Jr. E, Lezzi G, Piatelli A (2007) Influence of implant surface topography on early osseointegration: a histological study in human jaws. J Biomed Mater Res Part B: Appl Biomater 80:377–385 [DOI] [PubMed]
- 36.Schliephake H, Aref A, Scharnweber D, Bierbaum S, Sewing A. Effect of modifications of dual acid-etched implant surfaces on peri-implant bone formation. Part I: organic coatings. Clin Oral Implants Res. 2009;20:31–37. doi: 10.1111/j.1600-0501.2008.01603.x. [DOI] [PubMed] [Google Scholar]
- 37.Schliephake H, Aref A, Scharnweber D, Bierbaum S, Sewing A. Effect of modifications of dual acid-etched implant surfaces on peri-implant bone formation. Part II: calcium phosphate coating. Clin Oral Implants Res. 2009;20:38–44. doi: 10.1111/j.1600-0501.2008.01616.x. [DOI] [PubMed] [Google Scholar]
- 38.Lai HC, Zhuang LF, Zhang ZY, Wieland M, Liu X. Bone apposition around two different sandblasted, large-grit and acid-etched implant surfaces at sites with coronal circumference defects: an experimental study in dogs. Clin Oral Implants Res. 2009;20:247–253. doi: 10.1111/j.1600-0501.2008.01651.x. [DOI] [PubMed] [Google Scholar]