Abstract
Background
Treatment for head and neck cancer (HNC) may cause substantial local and systemic symptomatic morbidities, but many patients have high symptom levels before treatment begins. Knowledge of disease-related (treatment-naive) symptom status would aid evaluation of the symptomatic benefit or burden of HNC therapies.
Methods
We retrospectively examined symptoms, quality of life, and health status reported by HNC patients who were naïve to any treatment. We explored symptoms by anatomical site and correlated disease factors with symptom severity and interference. We used a clustering algorithm to identify a subset of highly symptomatic patients and examined the effects of disease site, tumor stage, and demographic variables on membership in this high-symptom group.
Results
We identified 748 treatment-naïve patients with mucosal (n=434), nonmucosal (n=272), or skull-base (n=42) tumors who had rated symptoms using the MD Anderson Symptom Inventory. Most were white non-Hispanic (82%) and male (68%) with a median age of 59 years. Approximately one third had high pretreatment symptom burden. Pain, fatigue, distress, and disturbed sleep were the most-severe symptoms, regardless of tumor stage or site. Symptom burden was higher in patients with more-advanced disease. Predictors of high symptom burden included having a mucosal tumor and being female.
Conclusions
The high prevalence of moderate-to-severe symptoms in this study demonstrates the importance of assessing patient-reported symptoms routinely before treatment is initiated and emphasizes the need for symptom management in parallel with disease treatment. Baseline characterization of symptom status should be incorporated into clinical trials that may affect symptom burden.
Keywords: head and neck cancer, patient-reported outcomes, symptom assessment, symptom burden, symptom severity, symptom interference, treatment-naïve, cancer site, cancer stage, MD Anderson Symptom Inventory
INTRODUCTION
Cancer and its treatment contribute significantly to patient burden. Many cancer patients often report distressing symptoms such as fatigue, poor appetite, and disrupted sleep. Cleeland et al1 recently identified a set of commonly reported cancer-related symptoms (constipation, diarrhea, distress, disturbed sleep, dry mouth, fatigue, lack of appetite, nausea, pain, numbness/tingling, sadness, and shortness of breath) derived from a national multicenter study detailing the percentages of patients with moderate-to-severe symptoms. Patients in that study rated symptoms using the MD Anderson Symptom Inventory (MDASI).2 An independent report from symptom-assessment experts from the Center for Medical Technology Policy3 also identified a set of common symptoms that overlapped considerably with the items of the MDASI, suggesting that these symptoms might form a “core” set of cancer symptoms.
Identifying important core symptoms is an essential first step toward providing better supportive care for patients with cancer and for developing effective patient-reported outcome measures of symptom endpoints in clinical trials of cancer therapies.1 Although there has been research describing the pretreatment symptoms of patients with head and neck cancer (HNC),4–8 the symptomatic status of treatment-naïve patients (ie, those who have not yet received treatment for their cancer) was not a focus of these studies, such that treatment-naïve patients were either a subset of a heterogeneous sample or not differentiated at all. For example, In one study4 that found that pretreatment health-related quality of life (QOL) is a significant predictor of mortality, it was difficult to distinguish treatment-naïve patients within the sample. In another study of HNC treatment-specific symptom clusters, treatment-naïve patients were not included.5 Other studies6–8 were focused on cachexia and weight loss and reported only nutrition-specific symptoms.
We recently reported the symptomatic status of HNC patients prior to radiation or chemoradiation therapy.9 The relatively high symptom levels reported by these patients underline the need for a larger symptoms study in treatment-naïve patients with HNC. A limitation of this report, however, was a confounding effect from the various treatment modalities. To avoid this confounding in the current study, we included HNC patients regardless of subsequent treatment, which also allowed us to examine symptoms in a larger range of disease sites and stages.9 By doing so, we aimed to help clinicians identify disease-related symptoms that need to be addressed from the time treatment is initiated.
At The University of Texas MD Anderson Cancer Center in Houston, Texas, the MDASI is used in the Head & Neck Care Center to assess each patient’s symptoms at their initial visit. We took advantage of this to examine the severity of these core symptoms in a study of treatment-naïve patients with HNC. The symptoms reported by patients who have yet to begin treatment are likely to be a result of the disease process, not confounded by the toxic effects of treatment. The study’s objectives were to (1) assess symptom severity and interference in treatment-naïve HNC patients, (2) explore symptoms in various HNC subgroups by anatomic site, and (3) report disease factors that correlate with symptom severity and interference in treatment-naïve patients.
METHODS
Patients
This single-institution, questionnaire-based study was conducted after approval by the MD Anderson Institutional Review Board. The study was a retrospective analysis of symptom data from treatment-naïve patients with malignant tumors at their initial presentation at the MD Anderson Head & Neck Care Center. The data were derived from routine intake measurement of patient-reported symptoms using the MDASI. We defined a treatment-naïve patient as one who had received no prior cancer therapy.
Measures
Demographic variables (eg, age, sex, race) and clinical characteristics (eg, cancer stage, disease site) were abstracted from the MD Anderson electronic medical record.
Symptom Measure: The MD Anderson Symptom Inventory
The MDASI is a brief and easily understood multisymptom assessment measure developed for use in the general cancer population.2 Patients rate the intensity of 13 common cancer-related symptoms (pain, fatigue, nausea, disturbed sleep, distress, shortness of breath, difficulty remembering, lack of appetite, drowsiness, dry mouth, sadness, vomiting, and numbness/tingling) and six items related to how much symptoms have interfered with general activity, mood, work, relations with others, walking, and enjoyment of life.2, 10 Symptoms are rated on 0–10 numeric scales ranging from “not present” to “as bad as you can imagine,” and interference is rated on 0–10 numeric scales ranging from “did not interfere” to “interfered completely.” The MDASI has been shown to be valid and reliable.2
Quality of Life and Functional Well Being
Patients were asked to rate their overall QOL on a 0–10 scale, with lower scores corresponding to poorer QOL. Social support, emotional well-being, and physical well-being were also rated on a 0–10 scale, with lower scores indicating poorer function. These 3 scales measuring different domains of overall QOL have a long history of use in National Cancer Institute-sponsored cancer-control studies.11 Sloan et al12 have shown that simple 1-item questions are valid and appropriate to use in cancer studies.
Statistical Analysis
Symptoms, Interference, and Quality of Life
For this study, the MDASI items “nausea” and “vomiting” were combined into 1 item in the electronic medical record. Hence, 12 items were analyzed. We defined a moderate-to-severe symptom as one rated ≥5 on the MDASI’s 0–10 scale. This cutpoint was based on results from various studies showing that “pain at its worst” is related to greater interference with function when rated ≥5 by cancer patients1, 13, 14 and community samples.15 Although the cutpoint used to delineate “moderate-to-severe” varies somewhat across studies and even among symptoms, ranging from 4–6, we reasoned that a cutpoint of 5 would provide analytical consistency across all symptoms. We chose a cutpoint of 7 to delineate a severe symptom,13, 16 on the basis of work by Serlin et al13 demonstrating that a cutpoint of 7 separates moderate from severe pain, and by Mendoza et al16, 17 demonstrating that a cutpoint of 7 optimally differentiates between moderate and severe fatigue. A rating of ≥7 to define severe pain and fatigue has also been used in routine clinical practice18 and to describe symptom prevalence in a large multicenter cooperative study.19 Pain rated ≥7 is considered a clinically significant problem that requires immediate clinical intervention.18, 19 To aid clinical interpretability, we used these cutpoints to report the proportions of patients with moderate-to-severe symptoms (rated ≥5 on the 0–10 scale) and severe symptoms (rated ≥7), categorized by disease site and stage.
Descriptive statistics (means, standard deviations, 95% confidence intervals (CI)) were used to summarize patient demographic and clinical characteristics.
Data on tumor stage was categorized according to TNM staging. T1 and T2 were combined into 1 group (early stage, in terms of size, extent, or depth of penetration) and T3 and T4 into another (advanced stage). Staging categories were collapsed in this way because some categories had only a few patients. Similarly, patients with no clinical evidence of lymph node metastasis (N0) formed a group, while all other nodal involvements (N1, N2, N3) formed a second group. For M staging, almost all patients had no distant metastases (M0), and therefore separate groups were not formed. Bonferroni adjustments were made for multiple comparisons within each symptom.
All P values are 2-tailed and were considered significant if <.05. Analyses were performed using IBM SPSS Statistics 21 (IBM Corporation, Armonk, NY, USA).
Cluster Analysis
We performed 2-step cluster analysis using all symptom severity and interference items to investigate whether a group of patients with high overall symptom burden could be identified. We determined the optimal number of clusters based on Kaufman and Rousseeuw’s interpretation of cluster structures.20 The silhouette measure of cohesion and separation measures the distance of a patient’s symptom response from its cluster center relative to other clusters. This measure ranges from –1 (very poor) to 1 (good).
Predictors of High Symptom Burden
We performed backward logistic regression with the variables age, sex, disease site, and tumor stage to determine significant predictors of membership in the high-symptom group. We also included interaction terms for sex-by-TNM-staging as well as sex-by-disease-site. Residual diagnostics were performed and changes in log-likelihood ratio were noted in evaluating model fit.
RESULTS
We identified 748 treatment-naïve patients with malignant HNC tumors who had completed the MDASI. See Table 1 for demographic and disease characteristics. Most patients were white non-Hispanic (n=613, 82%) and male (n=509, 68%); the median age was 59 years. Patients were categorized into 3 groups based on tumor site: those with mucosal tumors (n=434, 58%), including 122 lip and oral cavity and 312 pharynx/larynx tumors; those with nonmucosal tumors (n=272, 36%), including 24 eye, 36 salivary gland, 120 skin/subcutaneous, and 60 thyroid/endocrine tumors, along with 32 tumors of unknown origin; and those with skull-base tumors (n=42, 6%).
Table 1.
Patient Demographic and Disease Characteristics (N=748)
| Variable | n | % |
|---|---|---|
| Median age, years (SD) | 59 (14.6) | |
| Sex | ||
| Women | 239 | 32 |
| Men | 509 | 68 |
| Race | ||
| White non-Hispanic | 613 | 82 |
| Black non-Hispanic | 41 | 6 |
| Others | 94 | 12 |
| Disease stage | ||
| T stagea | ||
| T1, T2 | 325 | 60 |
| T3, T4 | 212 | 40 |
| N stageb | ||
| N0 | 264 | 46 |
| N1, N2, N3 | 316 | 54 |
| Disease site | ||
| Mucosal | 434 | 58 |
| Lip and oral cavity | 122 | |
| Pharynx/larynx | 312 | |
| Nonmucosal | 272 | 36 |
| Eye | 24 | |
| Salivary gland | 36 | |
| Skin/subcutaneous | 120 | |
| Thyroid/endocrine | 60 | |
| Unknown primary | 32 | |
| Skull base | 42 | 6 |
T stage was missing for 160 patients, was T0 (no evidence of a primary tumor) for 26 patients, and was TX (primary tumor cannot be assessed) for 25 patients.
N stage was missing for 160 patients and was NX (regional lymph nodes could not be assessed) for 8 patients.
Symptoms, Interference, and Quality of Life
Table 2 presents symptoms in order of decreasing mean severity. The 7 most-severe MDASI symptoms were disturbed sleep, distress, fatigue, pain, sadness, drowsiness, and dry mouth. More than 30% of the patients reported moderate-to-severe disturbed sleep, distress, and fatigue (rated ≥5 on the 0–10 scale). All symptoms with the exception of nausea/vomiting were reported to be moderate-to-severe by at least 10% of the patients. Severe disturbed sleep and pain (rated ≥7) were reported by 20% and 21% of patients, respectively. Distress, fatigue, sadness, and drowsiness were reported to be severe by at least 10% of the patients.
Table 2.
Descriptive Statistics for Symptoms, Interference, and Quality of Life (N=748)
| Percentages of Patients Reporting: | ||||
|---|---|---|---|---|
| Symptom | Mean Severitya |
Standard Deviation |
Moderate-to- Severe Symptomsa |
Severe Symptoms |
| Disturbed sleep | 3.11 | 3.30 | 35 | 20 |
| Distress | 2.99 | 3.11 | 32 | 17 |
| Fatigue | 2.86 | 2.98 | 31 | 16 |
| Pain | 2.79 | 3.33 | 28 | 21 |
| Sadness | 2.64 | 3.12 | 27 | 17 |
| Drowsiness | 2.09 | 2.79 | 22 | 11 |
| Dry mouth | 1.72 | 2.73 | 18 | 9 |
| Difficulty remembering | 1.59 | 2.37 | 14 | 6 |
| Shortness of breath | 1.55 | 2.67 | 14 | 8 |
| Lack of appetite | 1.41 | 2.49 | 16 | 8 |
| Numbness or tingling | 1.21 | 2.42 | 12 | 7 |
| Nausea/vomiting | 0.63 | 1.70 | 6 | 3 |
| Symptom interference | ||||
| Mood | 2.87 | 3.10 | ||
| Enjoyment of life | 2.85 | 3.32 | ||
| Work | 2.65 | 3.28 | ||
| General activity | 2.46 | 3.16 | ||
| Relations with others | 2.06 | 3.04 | ||
| Walking | 1.61 | 2.87 | ||
| Quality of lifeb | ||||
| Physical well-being | 6.54 | 3.13 | ||
| Emotional well-being | 6.69 | 3.08 | ||
| Overall quality of life | 6.87 | 3.14 | ||
| Social support | 7.83 | 3.19 | ||
The 7 most severe symptoms were also the 7 most prevalent.
Lower scores indicate poorer quality of life.
On the symptom interference scale, mood and enjoyment of life were the functional domains most affected by symptoms, whereas walking was the least affected. Interference ratings were less severe for work, general activity, and relations with others than they were for mood or enjoyment of life.
Physical well-being was reported to be the poorest QOL domain. This was followed by emotional well-being and overall QOL. Social support was reported to be the best QOL domain.
Proportions of Moderate-To-Severe Symptoms, by Disease Site
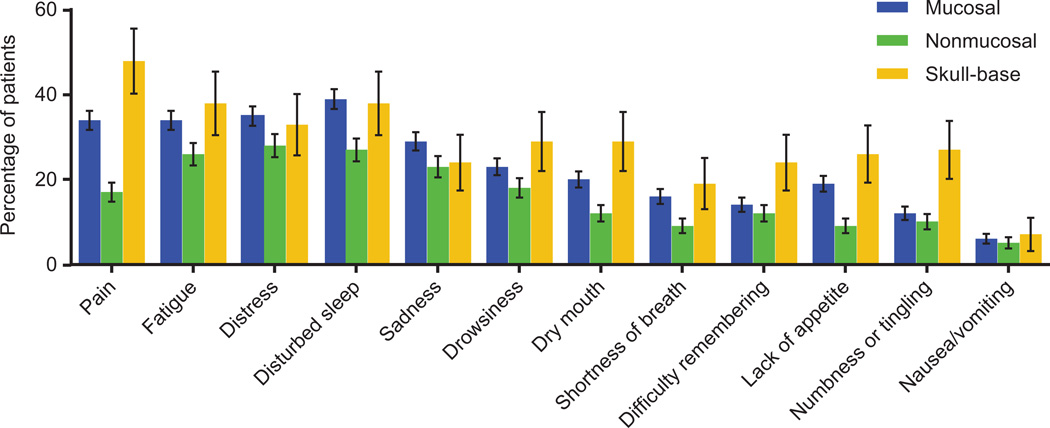
The proportions of patients reporting moderate-to-severe symptoms by the 3 major disease sites are presented in Fig. 1. Pain level differed significantly by disease site: a lower percentage of patients with nonmucosal tumors (17%) reported moderate-to-severe pain, whereas 34% and 48% of patients with mucosal or skull-base tumors, respectively, reported moderate-to-severe pain. In addition to pain, a significantly lower proportion of patients with nonmucosal tumors had moderate-to-severe dry mouth (12% vs 20% and 29%, respectively) and lack of appetite (9% vs 19% and 26%, respectively). In contrast, moderate-to-severe numbness or tingling was reported by a significantly greater number of patients with skull-base tumors (27%) than by patients with mucosal or nonmucosal tumors (12% and 27%, respectively).
Figure 1.
Percentages of patients reporting moderate-to-severe symptoms, by disease site. Of the 748 patients included, 434 (58%) had mucosal tumors, 272 (36%) had nonmucosal tumors, and 42 (6%) had skull-base tumors.
The proportions of patients with moderate-to-severe disturbed sleep were significantly lower for those with nonmucosal disease than for those with mucosal disease (27% vs 39%, respectively). However, no significant difference was found when we compared patients with skull-base tumors and patients with either of the other tumor sites, likely due to the small sample of patients with skull-base tumors. We observed a similar trend for shortness of breath (9% nonmucosal vs 16% mucosal).
No significant differences among disease sites were observed in the percentages of patients with moderate-to-severe levels of fatigue, distress, sadness, drowsiness, difficulty remembering, or nausea/vomiting.
Differences in Symptoms, Interference, and Quality of Life by Tumor Staging
N staging
As shown in Table 3, 46% of patients (n=264) had N0 stage tumors and 54% (n=316) had N1, N2, or N3 stage tumors. The percentages of patients reporting moderate-to-severe pain and fatigue differed significantly by N stage: 25% and 27% of patients without lymph node involvement (N0) reported moderate-to-severe pain and fatigue, respectively, compared with 36% and 36% of patients with lymph node metastases (N1–N3). No other symptoms were significantly different by N stage. Interference ratings were significantly different between the N0 and N1–N3 groups for general activity (2.1 vs 3.2, respectively; P <.004), work (2.3 vs 3.3; P <.018), and enjoyment of life (2.6 vs 3.6; P <.013).
Table 3.
Percentages of Patients Reporting Moderate-to-Severe Symptoms, by Tumor Staging
| Symptom | Percentages of Patients Reporting Moderate-to-severe Symptoms | |||
|---|---|---|---|---|
| N Stage | T Stage | |||
| N0 | N1, N2, N3 | T1, T2 | T3, T4 | |
| n=264 (46%) | n=316 (54%) | n=325 (60%) | n=212 (40%) | |
| Pain | 25 | 36a | 20 | 49a |
| Fatigue | 27 | 36a | 25 | 44a |
| Distress | 30 | 36 | 24 | 46a |
| Disturbed sleep | 32 | 39 | 26 | 52a |
| Sadness | 25 | 30 | 20 | 38a |
| Drowsiness | 21 | 23 | 18 | 31a |
| Dry mouth | 14 | 21 | 12 | 27a |
| Shortness of breath | 14 | 16 | 10 | 24a |
| Difficulty remembering | 14 | 13 | 13 | 16 |
| Lack of appetite | 14 | 20 | 11 | 28a |
| Numbness or tingling | 11 | 14 | 8 | 19a |
| Nausea or vomiting | 4 | 7 | 3 | 11a |
Significantly different between stages at P<.05.
Of the QOL measures, mean ratings of overall QOL (6.3 vs 7.1; P <.038) and physical well-being (5.9 vs 6.7; P<.038) were significantly worse for the patients with lymph node metastases than for patients with no nodal involvement, respectively.
T staging
Most patients (n=325, 60%) had stage T1 or T2 tumors; 39% (n=212) had stage T3 or T4 tumors. See Table 3. For all symptoms except difficulty remembering, significantly more patients in the T3–T4 group than in the T1–T2 group reported moderate-to-severe levels. The T3–T4 group also reported significantly worse interference from symptoms. Mean ratings for interference with general activity (3.7 vs 1.8), mood (3.9 vs 2.4), work (3.8 vs 2.0), relations with others (3.1 vs 1.5), walking (2.4 vs 1.2), and enjoyment of life (4.1 vs 2.3) reported by the T3–T4 group and the T1–T2 group, respectively, differed significantly (all P<.001).
In addition, mean ratings for all QOL measures were significantly different by T-stage group. Compared with the T1–T2 group, the T3–T4 group had significantly worse ratings for overall QOL (7.3 vs 6.0, respectively; P<.001), social support (8.0 vs 7.3; P<.015), emotional well-being (7.0 vs 6.1; P<.001) and physical well-being (7.0 vs 5.7; P<.001).
M staging
Because 97% (n=565) of patients had stage M0 HNC and only 3% (n=23) had either M1 staging (distant metastases) or MX staging (indeterminate metastases), group comparisons based on M staging were not performed.
Cluster analysis
Using 2-step cluster analysis, we determined the optimal number of patient groups clustered by symptom severity to be 2, on the basis of the silhouette measure of cohesion and separation. According to criteria set forth by Kaufman and Rousseeuw,20 the cluster quality is fair. In addition, the standardized effect size between the 2 patient clusters for symptom severity was 1.6, suggesting that a large effect size separated the patient groups.
The 2 patient clusters were based on proportions of patients reporting moderate-to-severe symptoms (rated ≥5 on the MDASI’s 0–10 scale) vs mild symptoms (rated <5). Approximately 39% of patients (n=248) clustered into the high overall symptom burden group, whereas 61% (n=393) clustered into the low-symptom group.
Predictors of high symptom burden
Logistic regression analysis showed that T staging, N staging, sex, and disease site were significant predictors of membership in the high overall symptom burden group. Patients with T3–T4 staging were 2.2 times (95% CI=1.3–3.7; P<.005) more likely to be in the high-symptom group. Patients with N1–N3 staging were 1.9 times (95% CI=1.1–3.7; P<.023) more likely to be in the high-symptom group. Patients in the high-symptom group were 3.1 times more likely to be female (95% CI=1.8–5.4; P<.001) and twice as likely to have mucosal cancer (95% CI=1.1–3.6; P<.029).
Our final model had a Nagelkerke21 R2 of 0.167 and correct classification rate of approximately 70%.
DISCUSSION
Our results indicate that more than one third of treatment-naïve patients with HNC are burdened by moderate-to-severe symptoms caused by their disease and are in need of symptom management at the time of treatment initiation. It is reasonable to expect that symptoms will worsen as patients begin cancer treatment.
Measurement of patient-reported symptoms in routine clinical care settings has not been widely adopted in head and neck oncology, although the need for regular symptom assessment in patients with cancer is a strong recurrent theme.1, 22, 23 Several accounts in the literature address the pretreatment status of patients with HNC. Schmidt et al7 used the Head and Neck Symptom Checklist to report the prevalence of pain and other symptoms for treatment-naïve patients. Siddiqui et al24 reported poorer QOL, poorer function, and more-severe symptoms from pretreatment to posttreatment in HNC patients with no distant metastasis, and Shepherd and Fisher25 reported symptom change from diagnosis to 3 months posttreatment in patients with oral or oropharyngeal cancer. However, a specific definition of “treatment-naïve” or “pretreatment” was lacking in these reports, and it was unclear if the concept of “pretreatment” in these clinical trials reflected no previous treatment or was simply a reference point for the start of the trial. The patients included in these studies might have had recurrent disease or might have been treated previously.
We stratified patients by tumor type (mucosal, nonmucosal, or skull-base) because we hypothesized that patients with tumors at different sites would have different symptom profiles. For example, patients with mucosal or skull-base tumors would likely have more pain because of the extensive sensory innervation in the region and the deeply invasive nature of these tumors. As expected, our investigation revealed higher percentages of patients with moderate-to-severe symptoms, particularly pain and disturbed sleep, when tumors were located in the mucosa of the upper aerodigestive tract or the skull-base region, versus being located in the nonmucosal region. Although there were slight differences in symptom severity by disease site, clustering of symptoms did not differ by disease site. For example, patients in all 3 groups perceived distress and sadness as closely related.
Our findings regarding the effects of cancer stage on symptom burden were expected and aligned with results from previous studies of patients with HNC. For example, symptom burden scores worsened for patients with stage III or stage IV cancer versus early-stage cancer.26, 27 Greater weight loss was seen in patients with advanced-stage cancer versus early-stage cancer.28 A meta-analysis review of 52 studies on the prevalence of pain concluded that 64% of patients with metastatic or advanced stage disease experienced pain and that the highest prevalence was found in the patients with HNC(70%); furthermore, the pain was moderate-to-severe for more than one third of patients despite longstanding pain-management recommendations from the World Health Organization.29 Nonetheless, a limitation of our study is that it assays symptoms across cancer types and treatments using a symptom screening tool. More in-depth symptom and functional assessment might further characterize pretreatment symptomatic and functional deficits.
Understanding the symptom burden being experienced by treatment-naïve patients with HNC is vital, for several reasons. Management of symptoms is essential for improving or retaining overall QOL. Also, symptom assessment is useful when done at first contact, especially for patients who have not had any prior treatment, as the symptoms reported by these patients are very likely caused by their cancer. The US Food and Drug Administration is particularly interested in symptomatic changes produced by the treatments it evaluates, but change can be characterized only if we can tease out disease-related symptoms from those caused by treatment. This information would facilitate decision making about the symptomatic benefit of a given treatment.
These data highlight the relatively high percentage of patients who are experiencing moderate-to-severe symptoms at first clinical contact. These symptoms should be addressed in parallel with treatment initiation, and well-accepted guidelines (such as those developed by the National Comprehensive Cancer Network) for assessment and treatment of several of these symptoms (pain, emotional distress, fatigue, sleep disturbance) are available.
A limitation to this retrospective investigation is that nausea and vomiting, although separate MDASI symptoms, were combined in the electronic health record at MD Anderson. Modifications to the electronic record (to separate nausea/vomiting and include additional HNC-related MDASI symptoms) are underway.
CONCLUSION
This study examined the early disease-related symptoms in patients with HNC. Approximately one third of treatment-naïve HNC patients presented with moderate-to-severe symptoms (particularly, pain, fatigue, distress, and disturbed sleep), underscoring the importance of routine assessment of patient-reported symptoms throughout the course of clinical care. Identifying symptoms before any treatment commences is critical for understanding the symptom burden induced by cancer therapy.
Acknowledgements
The authors acknowledge the editorial assistance of Jeanie F. Woodruff, BS, ELS.
Funding: Supported by grants from the National Cancer Institute, including R01 CA026582 to Charles Cleeland and MD Anderson Cancer Center Support Grant P30 CA016672. The sponsor had no role in the study design, the collection, analysis and interpretation of data, the writing of the report, or the decision to submit the article for publication.
Footnotes
Conflicts of Interest: None.
REFERENCES
- 1.Cleeland CS, Sloan JA, Cella D, et al. Recommendations for including multiple symptoms as endpoints in cancer clinical trials: a report from the ASCPRO (Assessing the Symptoms of Cancer Using Patient-Reported Outcomes) Multisymptom Task Force. Cancer. 2013;119:411–420. doi: 10.1002/cncr.27744. [DOI] [PubMed] [Google Scholar]
- 2.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the MD Anderson Symptom Inventory. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 3.Basch E, Abernethy AP, Mullins CD, et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J.Clin.Oncol. 2012;30:4249–4255. doi: 10.1200/JCO.2012.42.5967. [DOI] [PubMed] [Google Scholar]
- 4.Osthus AA, Aarstad AK, Olofsson J, Aarstad HJ. Prediction of survival by pretreatment health-related quality-of-life scores in a prospective cohort of patients with head and neck squamous cell carcinoma. JAMA Otolaryngol.Head Neck Surg. 2013;139:14–20. doi: 10.1001/jamaoto.2013.1056. [DOI] [PubMed] [Google Scholar]
- 5.Xiao C, Hanlon A, Zhang Q, et al. Symptom clusters in patients with head and neck cancer receiving concurrent chemoradiotherapy. Oral Oncol. 2013;49:360–366. doi: 10.1016/j.oraloncology.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kubrak C, Olson K, Baracos VE. The head and neck symptom checklist(c): an instrument to evaluate nutrition impact symptoms effect on energy intake and weight loss. Support.Care Cancer. 2013;21:3127–3136. doi: 10.1007/s00520-013-1870-z. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt KN, Olson K, Kubrak C, Parliament M, Ghosh S. Validation of the Head and Neck Patient Symptom Checklist as a nutrition impact symptom assessment tool for head and neck cancer patients. Support.Care Cancer. 2013;21:27–34. doi: 10.1007/s00520-012-1483-y. [DOI] [PubMed] [Google Scholar]
- 8.Ganzer H, Touger-Decker R, Parrott JS, Murphy BA, Epstein JB, Huhmann MB. Symptom burden in head and neck cancer: impact upon oral energy and protein intake. Support.Care Cancer. 2013;21:495–503. doi: 10.1007/s00520-012-1542-4. [DOI] [PubMed] [Google Scholar]
- 9.Gunn GB, Mendoza TR, Fuller CD, et al. High symptom burden prior to radiation therapy for head and neck cancer: A patient-reported outcomes study. Head Neck. 2012 doi: 10.1002/hed.23181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cleeland C. The MD Anderson Symptom Inventory User Guide. Available from URL: http://www.mdanderson.org/education-and-research/departments-programs-and-labs/departments-and-divisions/symptom-research/symptom-assessment-tools/MDASI_userguide.pdf.
- 11.Buchanan DR, White JD, O'Mara AM, Kelaghan JW, Smith WB, Minasian LM. Research-design issues in cancer-symptom-management trials using complementary and alternative medicine: lessons from the National Cancer Institute Community Clinical Oncology Program experience. J.Clin.Oncol. 2005;23:6682–6689. doi: 10.1200/JCO.2005.10.728. [DOI] [PubMed] [Google Scholar]
- 12.Sloan JA, Loprinzi CL, Kuross SA, et al. Randomized comparison of four tools measuring overall quality of life in patients with advanced cancer. J.Clin.Oncol. 1998;16:3662–3673. doi: 10.1200/JCO.1998.16.11.3662. [DOI] [PubMed] [Google Scholar]
- 13.Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61:277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 14.Anderson KO. Role of cutpoints: why grade pain intensity? Pain. 2005;113:5–6. doi: 10.1016/j.pain.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 15.Palos GR, Mendoza TR, Mobley GM, Cantor SB, Cleeland CS. Asking the community about cutpoints used to describe mild, moderate, and severe pain. J.Pain. 2006;7:49–56. doi: 10.1016/j.jpain.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Mendoza TR, Laudico AV, Wang XS, et al. Assessment of fatigue in cancer patients and community dwellers: validation study of the Filipino version of the brief fatigue inventory. Oncology. 2010;79:112–117. doi: 10.1159/000320607. [DOI] [PubMed] [Google Scholar]
- 17.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 18.Hubbard JM, Grothey AF, McWilliams RR, Buckner JC, Sloan JA. Physician Perspective on Incorporation of Oncology Patient Quality-of-Life, Fatigue, and Pain Assessment Into Clinical Practice. J.Oncol.Pract. 2014;10:248–253. doi: 10.1200/JOP.2013.001276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cleeland CS, Body JJ, Stopeck A, et al. Pain outcomes in patients with advanced breast cancer and bone metastases: results from a randomized, double-blind study of denosumab and zoledronic acid. Cancer. 2013;119:832–838. doi: 10.1002/cncr.27789. [DOI] [PubMed] [Google Scholar]
- 20.Kaufman L, Rousseeuw PJ. Finding Groups in Data: An Introduction to Cluster Analysis. Hoboken, New Jersey: Wiley; 2009. [Google Scholar]
- 21.Nagelkerke NJD. Maximum likelihood estimation of functional relationships. Springer-Verlag; 1992. [Google Scholar]
- 22.Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106:337–345. doi: 10.1016/j.pain.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Patrick DL, Ferketich SL, Frame PS, et al. National Institutes of Health State-of-the-Science Conference Statement: Symptom Management in Cancer: Pain, Depression, and Fatigue, July 15–17, 2002. J.Natl.Cancer.Inst. 2003;95:1110–1117. doi: 10.1093/jnci/djg014. [DOI] [PubMed] [Google Scholar]
- 24.Siddiqui F, Pajak TF, Watkins-Bruner D, et al. Pretreatment quality of life predicts for locoregional control in head and neck cancer patients: a radiation therapy oncology group analysis. Int.J.Radiat.Oncol.Biol.Phys. 2008;70:353–360. doi: 10.1016/j.ijrobp.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 25.Shepherd KL, Fisher SE. Prospective evaluation of quality of life in patients with oral and oropharyngeal cancer: from diagnosis to three months post-treatment. Oral Oncol. 2004;40:751–757. doi: 10.1016/j.oraloncology.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Aarstad HJ, Aarstad AK, Heimdal JH, Olofsson J. Mood, anxiety and sense of humor in head and neck cancer patients in relation to disease stage, prognosis and quality of life. Acta Otolaryngol. 2005;125:557–565. doi: 10.1080/00016480510027547. [DOI] [PubMed] [Google Scholar]
- 27.Aarstad HJ, Aarstad AK, Lybak S, Monge O, Haugen DF, Olofsson J. The amount of treatment versus quality of life in patients formerly treated for head and neck squamous cell carcinomas. Eur.Arch.Otorhinolaryngol. 2006;263:9–15. doi: 10.1007/s00405-005-0961-y. [DOI] [PubMed] [Google Scholar]
- 28.Hanna E, Alexiou M, Morgan J, et al. Intensive chemoradiotherapy as a primary treatment for organ preservation in patients with advanced cancer of the head and neck: efficacy, toxic effects, and limitations. Arch.Otolaryngol.Head Neck Surg. 2004;130:861–867. doi: 10.1001/archotol.130.7.861. [DOI] [PubMed] [Google Scholar]
- 29.Van den Beuken-van Everdingen M, De Rijke J, Kessels A, Schouten H, Van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann.Oncol. 2007;18:1437–1449. doi: 10.1093/annonc/mdm056. [DOI] [PubMed] [Google Scholar]