Abstract
Lesions of the lateral hypothalamus (LH) cause hypophagia. However, activation of glutamatergic neurons in LH inhibits feeding. These results suggest a potential importance for other LH neurons in stimulating feeding. Our current study in mice showed that disruption of GABA release from adult LH GABAergic neurons reduced feeding. LH GABAergic neurons project extensively to the paraventricular hypothalamic nucleus (PVH), and optogenetic stimulation of GABAergic LH → PVH fibers induced monosynaptic IPSCs in PVH neurons, and potently increased feeding, which depended on GABA release. In addition, disruption of GABA-A receptors in the PVH reduced feeding. Thus, we have identified a new feeding pathway in which GABAergic projections from the LH to the PVH promote feeding.
Keywords: feeding, GABA, LH, optogenetics, PVH
Introduction
Recent studies have identified important, neuropeptide-expressing hypothalamic neurons that regulate feeding (Luquet et al., 2005; Leinninger et al., 2011; Atasoy et al., 2012; Krashes et al., 2014). However, a large number of neurons that do not express known peptides may also have critical roles in feeding (Xu and Tong, 2011). Lesions of the lateral hypothalamus (LH) lead to hypophagia (Bernardis and Bellinger, 1996), suggesting that the net effects of LH neurons are to promote eating. However, it has been recently shown that selective activation of glutamatergic neurons in the LH inhibits feeding (Jennings et al., 2013). These contrasting results suggest a potential importance for other types of LH neurons in promoting feeding.
Increasing evidence supports an importance for GABA as a neurotransmitter, in mediating feeding behavior. However, the vast majority of previous studies regarding GABA action focus on GABAergic AgRP neurons (Wu et al., 2009; Atasoy et al., 2012; Krashes et al., 2013). At the same time, the functions of numerous non-AgRP GABAergic neurons elsewhere in the hypothalamus have not been elucidated. Interestingly, our previous study showed a selective role for GABA release from a subset of Arc GABAergic neurons, which express Cre driven by the rat insulin promoter (Rip-Cre) (Song et al., 2010), in energy expenditure regulation, but not in feeding (Kong et al., 2012), suggesting the importance of nonpeptidergic GABAergic hypothalamic neurons in regulating metabolism. However, the potential role of GABAergic neurons in other hypothalamic sites, including LH, remains largely unknown.
Previous pharmacological studies suggest a role for GABA in the paraventricular nucleus of hypothalamus (PVH) in feeding regulation (Pu et al., 1999; Stanley et al., 2011). However, it is unknown whether the GABA action is mediated directly or indirectly by PVH neurons given the potential widespread diffusion associated with the GABA administration. Further electrophysiological studies suggest that GABA may mediate the effects of NPY on PVH neurons (Melnick et al., 2007), and optogenetic studies demonstrate that GABA release from AgRP neurons onto PVH neurons directly mediates feeding behavior (Atasoy et al., 2012). However, the physiological relevance of GABA release to PVH neurons is not clear. Of note, GABA release from AgRP neurons is surprisingly not required for feeding behavior (Krashes et al., 2013), suggesting that other redundant AgRP neural pathways exist for promoting food intake.
We and others have shown that Cre expression driven by the pancreas duodenum homeobox 1 promoter (Pdx1) exhibits abundant expression in the LH region caudal to the PVH, dorsomedial hypothalamus (DMH), arcuate nucleus, and a few other brain sites (Honig et al., 2010; Song et al., 2010). By taking advantage of this Cre expression, our current study identified a potent role of LH GABAergic neurons in promoting feeding through projections to the PVH, in which GABA release is required. Our findings suggest that the physiological function of this projection is to promote nocturnal feeding.
Materials and Methods
Animal care.
Mice were housed at 21°C–22°C with a 12 h light/12 h dark cycle with food and water provided ad libitum. Animal care and procedures were approved by the University of Texas Health Science Center at Houston Institutional Animal Care and Use Committee. Rip-Cre and Pdx1-Cre mice were described previously (Song et al., 2010). Mice with Cre expression driven by the single-minded 1 gene promoter (Sim1-Cre) were described previously and these mice show Cre expression mainly in the PVH, but also in the other brains sites, including the amygdala and nucleus of lateral olfactory tract (Balthasar et al., 2005). Mice with the conditional allele of vesicular GABA transporter (Vgat, also named Slc32a1) and mice with the conditional allele of GABA-A receptor γ2 subunit were reported previously (Wulff et al., 2007; Tong et al., 2008). Breeding pairs (male Pdx1-Cre:Vgatflox/flox mice and female Vgatflox/flox mice; and male Sim1-Cre:γ2flox/flox mice and female γ2flox/flox mice) were maintained to generate the study subjects. Male and female Vgatflox/+ mice were interbred to generate wild-type and Vgatflox/flox mice for AAV-Cre injection study described below. In addition, Sim1-Cre mice were bred to Ai9 reporter mice (Madisen et al., 2010) to generate Sim1-Cre:Ai9 mice for electrophysiological recording.
Studies with stereotaxic injections.
At the start of surgical procedures, mice were anesthetized with ketamine and xylazine and placed on a stereotaxic frame (David Kopf Instruments). AAV-Cre-GFP vectors were purchased from the viral core facility of the University of Pennsylvania and were stereotaxically injected into bilateral LH (50 nl) with the following coordinates: bregma −1.6 mm; midline ±1.0 mm; dorsal surface −5.1 mm, of wild-type or Vgatflox/flox mice using a 0.5 μl Hamilton syringe controlled by a nano-injector (Stoelting). The injection speed was 0.5 nl/min, and the syringe was withdrawn 15 min after the final injection. Mice were used for experiments after a 2 week period recovery. We aimed to delete Vgat in the LH region with Pdx1-Cre expression. Because Pdx1-Cre is only expressed in the LH region located caudal to the PVH (Song et al., 2010), we targeted AAV-Cre-GFP to this specific LH region. To avoid the concern that Vgat deletion in nearby non-LH regions affects feeding, we excluded all mice with obvious AAV-Cre-GFP expression in non-LH regions as defined by Paxinos and Franklin (2004). We scored those mice with AAV-Cre-GFP expression limited to bilateral LH posterior to the PVH but across at least 1 mm in the rostral–caudal dimension of LH as hit.
In situ hybridization.
Then RNAscope Multiple Fluorescent Assay, a novel in situ hybridization technique with a unique “double Z” probe design, which greatly increases signal-to-noise ratio and can visualize RNA transcripts at a single molecular level (Wang et al., 2012; Xu et al., 2013), was used to detect transcripts in the brain (Advanced Cell Diagnostics). Matched LH section from Vgatflox/flox and Pdx1-Cre:Vgatflox/flox mice (n = 3 each) were used to estimate percentage of LH neurons with Vgat deletion by Pdx1-Cre.
Food intake measurements.
Weekly body weight was monitored in all genotypes fed standard mouse chow (Teklad F6 Rodent Diet 8664, 4.05 kcal/g, 3.3 kcal/g metabolizable energy, 12.5% kcal from fat, Harlan Teklad) from 4- to 5- week-old mice. For 24 h feeding pattern measurements, food intake was determined every hour for 24 h and averaged for comparison between genotypes.
Immunohistochemistry assays.
For immunohistochemistry studies, we used a previously described protocol (Xu et al., 2012). Primary antibody against γ2 (NB300-190, Novus Biologicals) was used. Sections were visualized and photographed with a TCS SP5 confocal microscope (Leica). The images from brain sections incubated with the γ2 antibody were obtained using an Axioimager fluorescence microscope with Axiocam digital camera (Zeiss) and then exported in grayscale.
Brain slice electrophysiological recordings.
Brain slices were prepared from mice (4–6 weeks of age) anesthetized with isoflurane. Coronal brain slices (350 μm) were cut in ice-cold artificial CSF (aCSF) containing the following (in mm): 124 NaCl, 5 KCl, 1.25 NaH2PO4, 1.3 MgSO4, 2 CaCl2, 26 NaHCO3, and 11 glucose and adjusted to pH 7.4 by bubbling with 95% O2/5% CO2. Slices containing the PVH and/or LH were immediately transferred to a holding chamber and submerged in oxygenated aCSF. Slices were maintained for recovery for at least 1 h at 34°C before transferring to a recording chamber. aCSF at 32°C was perfused into slice chambers with ∼2 ml/min flow rate regulated by miniplus3 peristaltic pump (Gilson).
Sim1-Cre-expressing neurons in brain slices from reporter mice were identified by ds-Red fluorescence emission using a custom filter set and then visually targeted with infrared differential interference optics. Evoked IPSCs in Sim1 neurons were also recorded in voltage-clamp using the same internal solution. Stimuli (0.1 ms duration) were applied through bipolar stimulating electrodes (FHC) placed into the surrounding area of the PVH. The stimulus intensity was adjusted to the level (ranging from 100 μA to 1 mA) at which an evoked current was ∼90% of the amplitude of the maximal response. Kynurenic acid (1 mm) was used to block glutamate-mediated EPSCs. At the end of experiments, 20 μm bicuculline was applied to the bath to confirm the nature of GABA-A receptor-mediated currents. All recordings were made using a Multiclamp 700B amplifier (Molecular Devices), and data were digitized at 10 kHz and filtered at 2 kHz using pClamp 10.3 (Molecular Devices). Data were analyzed off-line with Clampfit (Molecular Devices) and MiniAnalysis software (Snaptosoft).
Channelrhodopsin 2 (ChR2)-assisted circuitry mapping and feeding.
The ChR2-assisted circuitry mapping was performed similarly as previously described (Kong et al., 2012). Adeno-associated vectors with Cre-dependent expression of ChR2 and yellow fluorescent protein (YFP), AAV-FLEX-ChR2-YFP (generated by Dr. Ben Arenkiel) (Herman et al., 2014) were delivered to bilateral LH of 6- to 7-week-old male Pdx1-Cre and Pdx1-Cre:Vgatflox/flox mice, and the recordings were performed 2–3 weeks after viral delivery. The same coordinates as described above for AAV-Cre-GFP delivery were used for the viral delivery. IPSCs were recorded from neurons in the posterior PVH with high Cl− internal solution (in mm: 140 CsCl, 1 BAPTA, 10 HEPES, 5 MgCl2, 5 Mg-ATP, 0.3 Na2GTP, and 10 QX-314, pH 7.35). To photostimulate the ChR2-expressing fibers, a laser source (473 nm; Opto Engine) was used with 1 Hz frequency stimulation. A previous estimate shows that all PVH neurons are Sim1-Cre positive (Kublaoui et al., 2008); thus, recording experiments on field stimulation-evoked IPSCs in Sim1 neurons and photostimulation-evoked IPSCs in PVH neurons target the same group of neurons.
For optogenetic feeding studies, an optic fiber cannula was implanted immediately after the completion of AAV-FLEX-ChR2-YFP delivery. The optic fiber cannula (200 μm in diameter and 0.39 NA, Thorlabs) was implanted ∼0.4 mm above the posterior PVH with the following coordinates: bregma: 1.0 mm; midline: 0 mm; dorsal surface: 4.8 mm. Targeted expression of AAV-FLEX-ChR2-YFP in LH Pdx1-Cre neurons and correct implantation of optic fibers were verified by post hoc examination. Light power was measured by a PM 100D meter with an S121C sensor (Thorlabs). An online program provided by Dr. Deisseroth laboratory at Stanford University (http://web.stanford.edu/group/dlab/cgi-bin/graph/chart.php) was used for estimating the actual light power on local fibers. After a 2 week recovery from surgery, mice were individually housed and regular chow diet was provided in a Petri dish. Fiber optic cables (1.5 m long, 200 μm diameter; Thorlabs) were firmly attached to the implanted fiber optic cannula with an opaque mating sleeve. Mice were allowed at least 3–4 d to acclimate before experimental sessions. Blue laser stimulation (473 nm, Opto Engine, light power exiting the fiber tip 3 mW) at 20 Hz with 40 ms duration for 10 min generated by Master 8 (AMPI) was used to test feeding levels in early morning (9:00 to 11:00 A.M.), which were compared with the feeding levels during periods of 10 min before and that after laser stimulation.
Statistical analysis.
Data are mean ± SEM, and comparison between means was performed with an appropriate statistical analysis specified in figure legends, using Prism 6.0 with p < 0.05 indicating statistical significance. Tukey multiple comparison was used for two-way ANOVA tests.
Results
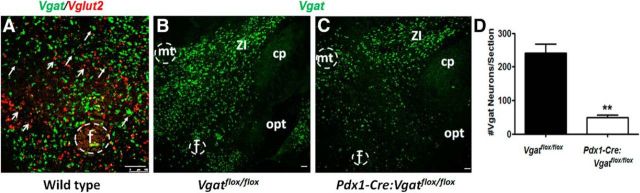
To examine the expression pattern of both Vgat and Vglut2 (also known as Slc32a1 and Slc17a6, respectively, required for presynaptic respective GABA and glutamate release) in the LH, we performed dual-color in situ hybridization for both genes. We showed that both mRNAs were abundantly expressed in the LH (Fig. 1A). Importantly, none to few LH neurons coexpressed Vgat and Vglut2, indicating that GABAergic neurons and glutamatergic neurons are largely segregated in the LH (Fig. 1A). Although the role of glutamatergic LH neurons has been illustrated (Jennings et al., 2013), the function of GABAergic LH neurons in feeding regulation is, however, unclear.
Figure 1.
Vgat and Vglut2 expression in the LH. A, Vgat (green) and Vglut2 (red) mRNA expression by dual fluorescent in situ hybridization in the LH. Open arrows indicate Vglut2-expressing neurons. Solid arrows indicate Vgat-expressing neurons. B, C, Vgat in situ hybridization in the LH of Vgatflox/flox (B) and Pdx1-Cre:Vgatflox/flox mice (C). D, Average number of Vgat-expressing neurons in Vgatflox/flox and Pdx1-Cre:Vgatflox/flox mice (n = 3 each). cp, Cerebral peduncle; f, fornix; mt, mammilothalamic tract; opt, optic tract; ZI, zona incerta. **p < 0.01, Student's t test. Scale bars, 100 μm.
We previously reported that Cre recombinase driven by the pancreas duodenum homeobox 1 promoter (Pdx1-Cre) is abundant in the LH, the DMH, the arcuate (Arc) and the preoptic area in the hypothalamus (Song et al., 2010). Importantly, in Pdx1-Cre:Vgatflox/flox mice, Vgat was largely deleted in throughout the LH (Fig. 1B,C) and was also reduced in other Pdx1-Cre expressing brain sites (data not shown). Based on the comparison of the Vgat expression pattern between controls and Pdx1-Cre:Vgatflox/flox mice, it appears that the LH regions with higher levels of Vgat expression showed more Vgat deletion (Fig. 1B,C). An estimate from matched LH sections from Vgatflox/flox and Pdx1-Cre:Vgatflox/flox mice (n = 3 each) showed that ∼80% LH GABAergic neurons expressed Pdx1-Cre (241 ± 27 vs 49 ± 7 GABAergic neurons in the LH of Vgatflox/flox and Pdx1-Cre:Vgatflox/flox mice, respectively) (Fig. 1D). These data indicate that Pdx1-Cre targets a major subset of GABAergic neurons in the LH. Thus, Pdx1-Cre mice, when combined with stereotaxic LH injections, allowed us to specifically target a major subset of GABAergic LH neurons.
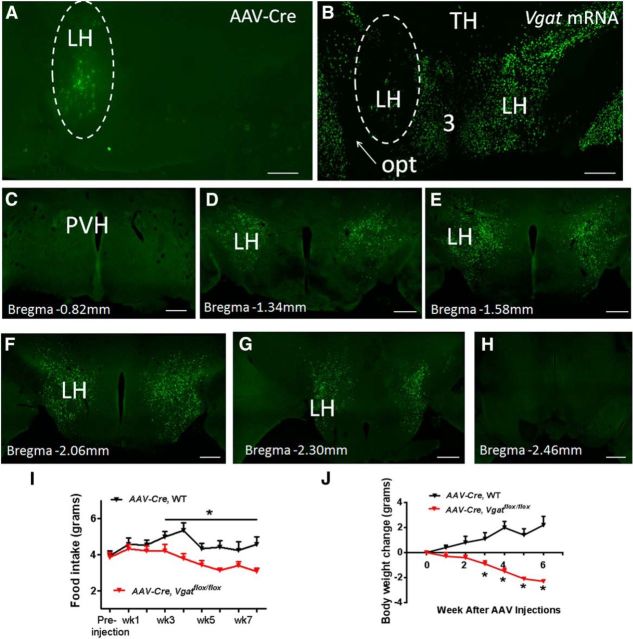
To examine the physiological role of GABA release from LH GABAergic neurons, we aimed to disrupt GABA release from LH neurons by stereotaxically injecting adeno-associated viral vectors that express Cre (AAV-Cre-GFP) to the LH of Vgatflox/flox mice. To examine the efficiency of AAV-Cre-GFP in deleting the Vgat gene, we stereotaxically delivered the Cre vector to one side of LH. Efficient Vgat deletion was validated by specific loss of Vgat mRNAs in the AAV-Cre-GFP infected area, compared with the other side of LH with intact Vgat expression (Fig. 2A,B). Based on post hoc analysis and the scoring criterion (see Materials and Methods), 5 Vgatflox/flox mice that received injections were scored as hit (Fig. 2C–H). Vgat deletion in LH significantly reduced feeding and body weight at 3 weeks after injection (Fig. 2I,J), indicating that GABA release from LH neurons is required to maintain normal feeding.
Figure 2.
Deletion of Vgat in adult LH neurons leads to reduced feeding. A, C–H, GFP expression after AAV-Cre-GFP vector delivery to the brain. B, In situ hybridization for Vgat in the hypothalamus. A, B, Two adjacent sections from one animal showing delivery of AAV-Cre-GFP vectors only to one side LH of Vgatflox/flox mice (A) and expression of Vgat mRNA (B). A, Micrograph was taken directly from sections freshly cut from frozen brains. White dashed line circle indicates the injected area. B, Micrograph showing sparsely distributed Vgat mRNA on the injected side LH (white dashed line circle) with AAV vector delivery, which was in contrast with the abundant Vgat mRNA on the opposite side, suggesting effective deletion of Vgat by AAV-Cre vectors. C–H, A representative case showing the expression profile of AAV-Cre-GFP vectors, from rostral to caudal levels as indicated by bregma levels, in the LH after stereotaxic delivery of the vectors to bilateral LH of Vgatflox/flox mice. A similar AAV-Cre injection profile was found in 4 other cases that were also scored as hit and peer-reviewed. I, J, Weekly food intake (I) and changes in body weight (J) measured for 8 weeks after AAV-vector injections. I, J, Data are mean ± SEM; n = 5–8. *p < 0.05 (unpaired Student's t tests). LH, Lateral hypothalamus; Opt, optic tract; PVH, paraventricular hypothalamic nucleus; TH thalamus. Scale bar, 250 μm.
To further illustrate the neural circuits that GABAergic LH neurons act upon to regulate feeding, we aimed to optogenetically stimulate these neurons using Pdx1-Cre expression in the LH. To selectively stimulate LH Pdx1-Cre neurons, we delivered Cre-dependent AAV-FLEX-ChR2-YFP vectors to the bilateral LH of Pdx1-Cre mice (Fig. 3A). As expected, YFP-expressing neurons were found selectively in the LH (Fig. 3B–D). Interestingly, YFP-expressing fibers were not found in the anterior PVH, but abundant in the posterior PVH (Fig. 3E,F). Upon laser stimulation of YFP-expressing fibers in the PVH, 10 of 12 posterior PVH neurons in control Vgatflox/flox mice showed IPSCs (Fig. 3G). In contrast, 0 of 12 neurons in Pdx1-Cre:Vgatflox/flox mice exhibited IPSCs (Fig. 3G). These results support the existence of a GABAergic LH → PVH circuit.
Figure 3.
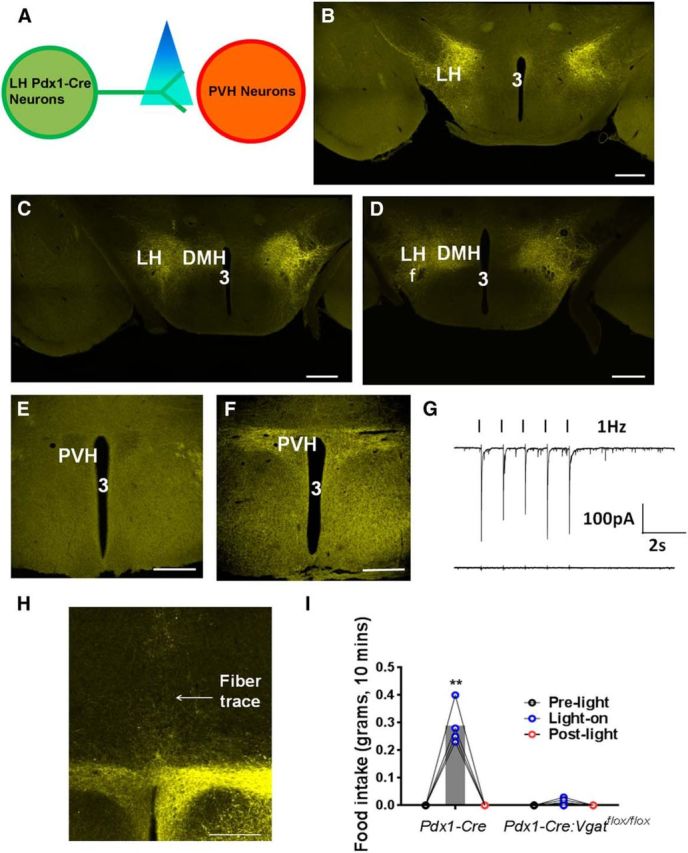
Optogenetic stimulation of GABAergic fibers from LH Pdx1-Cre neurons in the PVH promotes feeding in well-fed animals. A, Diagram showing ChR2-assisted circuitry mapping. B–D, AAV-FLEX-ChR2-YFP expression pattern in bilateral LH of Pdx1-Cre mice at 2 weeks after viral delivery at the levels of bregma −1.34 mm (B), −1.46 mm (C), and −1.58 mm (D). E, F, YFP-expressing fibers in the anterior (E) and the posterior PVH (F). G, IPSCs elicited by blue laser (black ticks) at 1 Hz in Pdx1-Cre (top) and Pdx1-Cre:Vgatflox/flox mice (bottom). H, A representative implantation of optic fiber cannula above the posterior PVH. I, Feeding response to laser stimulation during 10 min period in well-fed Pdx1-Cre and Pdx1-Cre:Vgatflox/flox mice. Data are mean ± SEM; n = 4–6. **p < 0.01 (two-way ANOVA tests). 3, Third ventricle; DMH, ventromedial hypothalamus; LH, lateral hypothalamus; PVH, paraventricular hypothalamic nucleus. Scale bar, 250 μm.
To examine the effect of photostimulation of YFP-expressing fibers in the PVH on feeding behavior, we implanted a fiberoptic directly above the posterior PVH (Fig. 3H). Low-intensity blue laser stimulation (3 mW) potently increased feeding during a 10 min photostimulation period in well-fed Pdx1-Cre mice (early morning) but had no obvious effect on feeding behavior in Pdx1-Cre:Vgatflox/flox mice (Fig. 3I). Notably, the distance between the tip of the fiberoptic and the closest LH Pdx1-Cre neurons was ∼1 mm, and 3 mW laser intensity used for feeding behavior, according to the estimation (the Deisseroth laboratory at Stanford University), will be reduced to 0.81 mW/mm2 at LH Pdx1-Cre neuronal soma, which is not sufficient to activate the neurons (Betley et al., 2013). These results, in conjunction with the previously observed hyperphagic GABA action in the PVH (Pu et al., 1999; Atasoy et al., 2012), indicate that activation of the GABAergic LH → PVH circuit promotes feeding behavior.
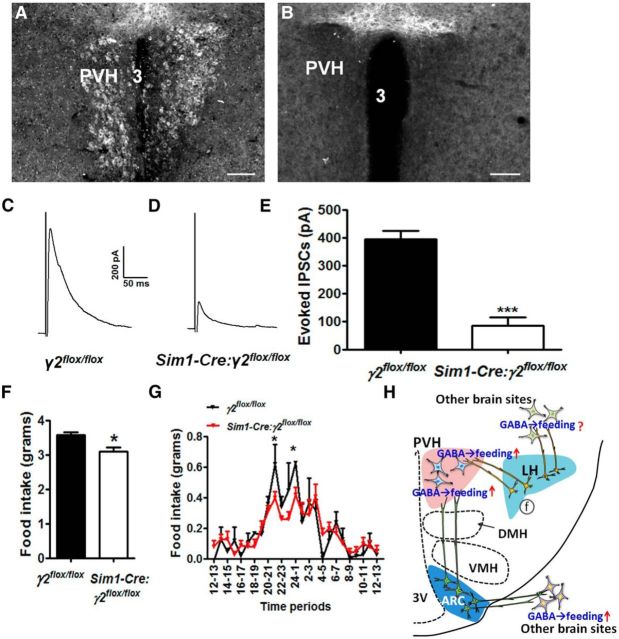
Despite extensive studies on GABAergic input to the PVH (Cowley et al., 2001; Cole and Sawchenko, 2002; Kalra and Kalra, 2004; Atasoy et al., 2012), its function in feeding, especially in physiological conditions, remains unclear. For example, reduced GABAergic tone to PVH does not contribute significantly to hypophagia following AgRP lesions (Wu et al., 2009). In addition, loss of GABA release from AgRP neurons has no significant impact on the feeding behavior induced by AgRP neuron activation (Krashes et al., 2013), suggesting that GABA release from AgRP neurons is not the sole transmitter that mediates feeding behavior by AgRP neuron activation. To more precisely examine GABAergic input to the PVH, we disrupted GABAergic input to the PVH by deleting γ2 subunit of GABA-A receptors. Toward this end, we crossed Sim1-Cre mice with γ2flox/flox mice to generate Sim1-Cre:γ2flox/flox mice (Wulff et al., 2007; Xu et al., 2013). In these mice, γ2 was largely deleted in the PVH (Fig. 4A,B), in the supraoptic nucleus and the nucleus of olfactory tract but was not obviously altered in other Sim1-Cre-expressing regions due to undetectable endogenous γ2 expression levels (data not shown). To examine the consequence of γ2 deletion on GABA-A receptors on PVH neurons, we performed whole-cell recording on PVH neurons in brain slices for GABA-A receptor-mediated currents IPSCs. Compared with control mice, evoked IPSCs in PVH neurons of Sim1-Cre:γ2flox/flox mice were diminished (Fig. 4C–E), confirming that the function of GABA-A receptors was disrupted. Interestingly, Sim1-Cre:γ2flox/flox mice showed reduced feeding (Fig. 4F), which was largely due to a specific reduction in nocturnal feeding (Fig. 4G). Thus, GABAergic input to PVH neurons promotes feeding, especially during the nocturnal period.
Figure 4.
Deletion of GABA-A receptor γ2 subunit in PVH neurons impairs GABAergic input and reduces feeding. A, B, Immunostaining of GABA-A receptor γ2 subunit in γ2flox/flox mice (A) and Sim1-Cre:γ2flox/flox mice (B). C, D, Representative traces showing evoked IPSCs in PVH neurons of γ2flox/flox mice (C) and Sim1-Cre:γ2flox/flox mice (D). E, Statistical comparison of evoked IPSC amplitude between γ2flox/flox mice and Sim1-Cre:γ2flox/flox mice. F, Average daily food intake for each week during ages of 4–5 weeks in males. G, Hourly food intake measured for a period of 24 h at the age 6–7 weeks. Data are mean ± SEM; n = 19 or 20 (E) or n = 7 or 8 (F, G). *p < 0.05 (unpaired Student's t tests). ***p < 0.001 (unpaired Student's t tests). H, A summary diagram showing the role of GABAergic projections from LH to PVH in feeding promotion along with the known GABAergic projections from the arcuate nucleus to the PVH and other brain sites that also promote feeding. Whether LH GABAergic projection to other brain sites also promotes feeding awaits further tests, as indicated by the question mark. 3V, Third ventricle; ↑, increased feeding; ARC, arcuate nucleus; DMH, ventromedial hypothalamus; f, fornix; LH, lateral hypothalamus; PVH, paraventricular hypothalamic nucleus; VMH, ventromedial hypothalamus.
Discussion
Here we identify a novel direct GABAergic projection from the LH to the PVH that promotes feeding. This is the second group of hypothalamic GABAergic neurons, alongside AgRP neurons, that have been identified to elicit feeding (Fig. 4H). GABA release is not required for mediating AgRP neurons in promoting feeding (Krashes et al., 2013), whereas it is required for LH GABA neurons. Thus, unlike AgRP neurons, GABA is the sole transmitter that mediates the feeding behavior of the LH → PVH projection.
LH GABAergic neurons promote feeding, whereas glutamatergic ones inhibit feeding (Jennings et al., 2013), suggesting divergent roles for LH neurons in feeding regulation. Lesions of LH cause hypophagia (Bernardis and Bellinger, 1996), suggesting that the effect of LH GABAergic neurons on feeding dominates over glutamatergic ones. Neurotensin and melanin-concentrating hormone (MCH) neurons represent two known groups of LH GABAergic neurons. Because LH neurotensin neurons are activated by leptin to reduce food intake (Leinninger et al., 2011), it is unlikely that these neurons contribute significantly to the observed feeding promotion. Lesions of MCH neurons cause hypophagia and reduced body weight (Alon and Friedman, 2006), and MCH partially colocalizes with Pdx1-Cre expression in the LH (data not shown), suggesting that GABA release from MCH neurons might contribute to the feeding behavior. However, two recent studies with optogenetic stimulation of MCH neurons did not report any changes in feeding behavior (Domingos et al., 2013; Tsunematsu et al., 2014). Alternatively, a novel group of LH GABAergic neurons that are nonpeptdiergic is responsible for the observed feeding effects.
Disruption of GABA-A receptors in the PVH reduced feeding, which was largely due to reduced nocturnal feeding, suggesting an important role for GABAergic input to PVH in feeding regulation. Among various sources of GABAergic input to PVH neurons that are known to regulate feeding, GABAergic input from the Arc unlikely contributes significantly to the feeding defect because nearly complete deletion of Vgat in the Arc by Rip-Cre or Vgat deletion by AgRP-Cre causes no obvious feeding defects (Tong et al., 2008; Kong et al., 2012). Notably, GABA release from AgRP neurons is sufficient, but not required for feeding induced by AgRP neuron activation (Krashes et al., 2013). In addition, our preliminary data showed that AAV-Cre mediated deletion of Vgat in the DMH led to no obvious changes in feeding (data not shown). Although it could not be ruled out that the GABAergic neurons outside LH contributed to the feeding defect observed in Sim1-Cre:γ2flox/flox mice, given our results showing that disrupted GABA release from LH reduced feeding, it is likely that LH GABAergic neurons contribute significantly to the reduced feeding. Furthermore, a role for GABAergic LH → PVH pathway in nocturnal feeding is supported by the known role of the LH in arousal (Adamantidis and de Lecea, 2008), a state when mice normally feed. Together, our observations that LH GABAergic neurons provide monosynaptic inputs to PVH neurons, that photostimulation of LH GABAergic fibers in the PVH invokes GABA release-dependent feeding behavior, and that disruption of GABA-A receptors in the PVH reduced feeding strongly support a role for GABAergic LH → PVH projections in promoting feeding. In summary, we have identified a novel GABAergic projection from the LH to the PVH that is critical for feeding regulation, and our data support a physiologically important role for this circuit in nocturnal feeding.
Footnotes
This work was supported by National Institutes of Health R01DK092605 and University of Texas Health Brain Initiative and CTSA UL1 TR000371 to Q.T., National Institutes of Health R01DK093587, R00DK085330, P30DK079638-03 to Yong Xu, and National Institutes of Health R01HL077400 to H.-L.P. We thank Dr. Peer Wulff for providing γ2flox/flox mice.
The authors declare no competing financial interests.
References
- Adamantidis A, de Lecea L. Physiological arousal: a role for hypothalamic systems. Cell Mol Life Sci. 2008;65:1475–1488. doi: 10.1007/s00018-008-7521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon T, Friedman JM. Late-onset leanness in mice with targeted ablation of melanin concentrating hormone neurons. J Neurosci. 2006;26:389–397. doi: 10.1523/JNEUROSCI.1203-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Bernardis LL, Bellinger LL. The lateral hypothalamic area revisited: ingestive behavior. Neurosci Biobehav Rev. 1996;20:189–287. doi: 10.1016/0149-7634(95)00015-1. [DOI] [PubMed] [Google Scholar]
- Betley JN, Cao ZF, Ritola KD, Sternson SM. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155:1337–1350. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole RL, Sawchenko PE. Neurotransmitter regulation of cellular activation and neuropeptide gene expression in the paraventricular nucleus of the hypothalamus. J Neurosci. 2002;22:959–969. doi: 10.1523/JNEUROSCI.22-03-00959.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Domingos AI, Sordillo A, Dietrich MO, Liu ZW, Tellez LA, Vaynshteyn J, Ferreira JG, Ekstrand MI, Horvath TL, de Araujo IE, Friedman JM. Hypothalamic melanin concentrating hormone neurons communicate the nutrient value of sugar. eLife. 2013;2:e01462. doi: 10.7554/eLife.01462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman AM, Huang L, Murphey DK, Garcia I, Arenkiel BR. Cell type-specific and time-dependent light exposure contribute to silencing in neurons expressing Channelrhodopsin-2. eLife. 2014;3:e01481. doi: 10.7554/eLife.01481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig G, Liou A, Berger M, German MS, Tecott LH. Precise pattern of recombination in serotonergic and hypothalamic neurons in a Pdx1-cre transgenic mouse line. J Biomed Sci. 2010;17:82. doi: 10.1186/1423-0127-17-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Rizzi G, Stamatakis AM, Ung RL, Stuber GD. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science. 2013;341:1517–1521. doi: 10.1126/science.1241812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra SP, Kalra PS. NPY and cohorts in regulating appetite, obesity and metabolic syndrome: beneficial effects of gene therapy. Neuropeptides. 2004;38:201–211. doi: 10.1016/j.npep.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Kong D, Tong Q, Ye C, Koda S, Fuller PM, Krashes MJ, Vong L, Ray RS, Olson DP, Lowell BB. GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell. 2012;151:645–657. doi: 10.1016/j.cell.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Shah BP, Koda S, Lowell BB. Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell Metab. 2013;18:588–595. doi: 10.1016/j.cmet.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H, Watabe-Uchida M, Uchida N, Liberles SD, Lowell BB. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507:238–242. doi: 10.1038/nature12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kublaoui BM, Gemelli T, Tolson KP, Wang Y, Zinn AR. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol Endocrinol. 2008;22:1723–1734. doi: 10.1210/me.2008-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinninger GM, Opland DM, Jo YH, Faouzi M, Christensen L, Cappellucci LA, Rhodes CJ, Gnegy ME, Becker JB, Pothos EN, Seasholtz AF, Thompson RC, Myers MG., Jr Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 2011;14:313–323. doi: 10.1016/j.cmet.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick I, Pronchuk N, Cowley MA, Grove KL, Colmers WF. Developmental switch in neuropeptide Y and melanocortin effects in the paraventricular nucleus of the hypothalamus. Neuron. 2007;56:1103–1115. doi: 10.1016/j.neuron.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KB. The mouse brain in stereotaxic coordinates. Ed 2. San Diego: Elsevier Science; 2004. [Google Scholar]
- Pu S, Jain MR, Horvath TL, Diano S, Kalra PS, Kalra SP. Interactions between neuropeptide Y and gamma-aminobutyric acid in stimulation of feeding: a morphological and pharmacological analysis. Endocrinology. 1999;140:933–940. doi: 10.1210/endo.140.2.6495. [DOI] [PubMed] [Google Scholar]
- Song J, Xu Y, Hu X, Choi B, Tong Q. Brain expression of Cre recombinase driven by pancreas-specific promoters. Genesis. 2010;48:628–634. doi: 10.1002/dvg.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley BG, Urstadt KR, Charles JR, Kee T. Glutamate and GABA in lateral hypothalamic mechanisms controlling food intake. Physiol Behav. 2011;104:40–46. doi: 10.1016/j.physbeh.2011.04.046. [DOI] [PubMed] [Google Scholar]
- Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunematsu T, Ueno T, Tabuchi S, Inutsuka A, Tanaka KF, Hasuwa H, Kilduff TS, Terao A, Yamanaka A. Optogenetic manipulation of activity and temporally controlled cell-specific ablation reveal a role for MCH neurons in sleep/wake regulation. J Neurosci. 2014;34:6896–6909. doi: 10.1523/JNEUROSCI.5344-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, Wu X, Vo HT, Ma XJ, Luo Y. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14:22–29. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff P, Goetz T, Leppä E, Linden AM, Renzi M, Swinny JD, Vekovischeva OY, Sieghart W, Somogyi P, Korpi ER, Farrant M, Wisden W. From synapse to behavior: rapid modulation of defined neuronal types with engineered GABAA receptors. Nat Neurosci. 2007;10:923–929. doi: 10.1038/nn1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Tong Q. Expanding neurotransmitters in the hypothalamic neurocircuitry for energy balance regulation. Protein Cell. 2011;2:800–813. doi: 10.1007/s13238-011-1112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Wu Z, Sun H, Zhu Y, Kim ER, Lowell BB, Arenkiel BR, Xu Y, Tong Q. Glutamate mediates the function of melanocortin receptor 4 on Sim1 neurons in body weight regulation. Cell Metab. 2013;18:860–870. doi: 10.1016/j.cmet.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, O'Brien WG, 3rd, Lee CC, Myers MG, Jr, Tong Q. Role of GABA release from leptin receptor-expressing neurons in body weight regulation. Endocrinology. 2012;153:2223–2233. doi: 10.1210/en.2011-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]