Abstract
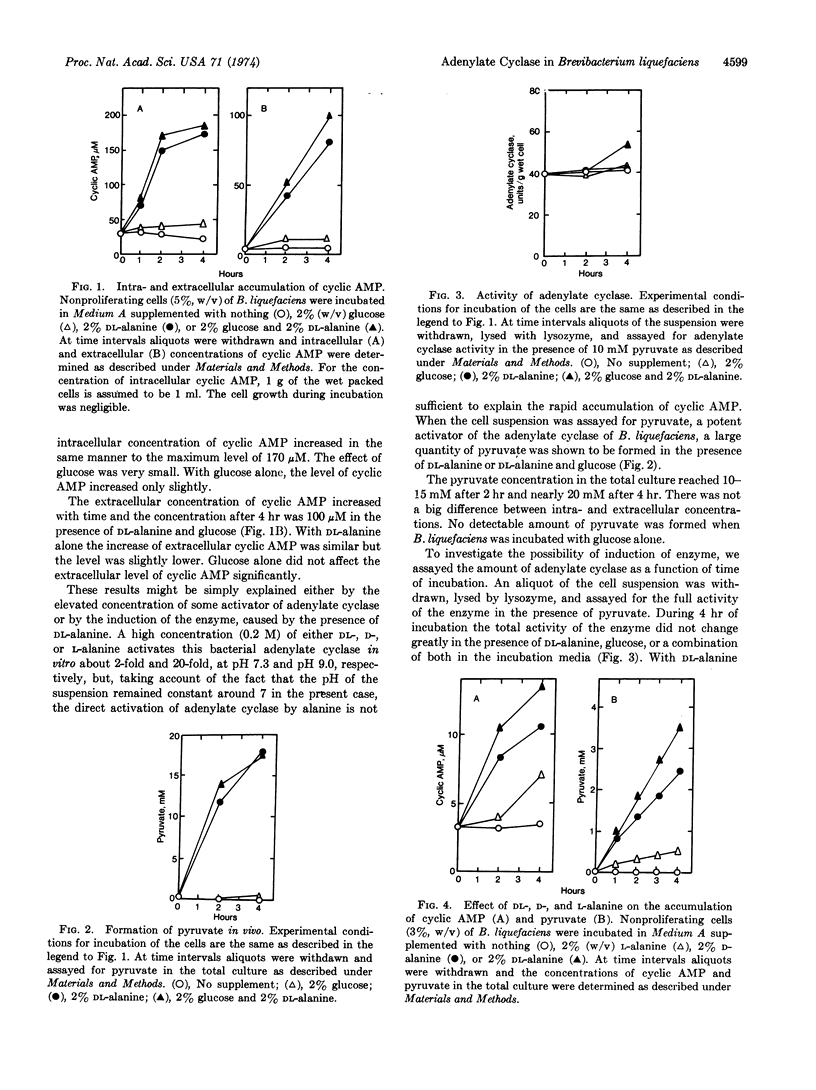
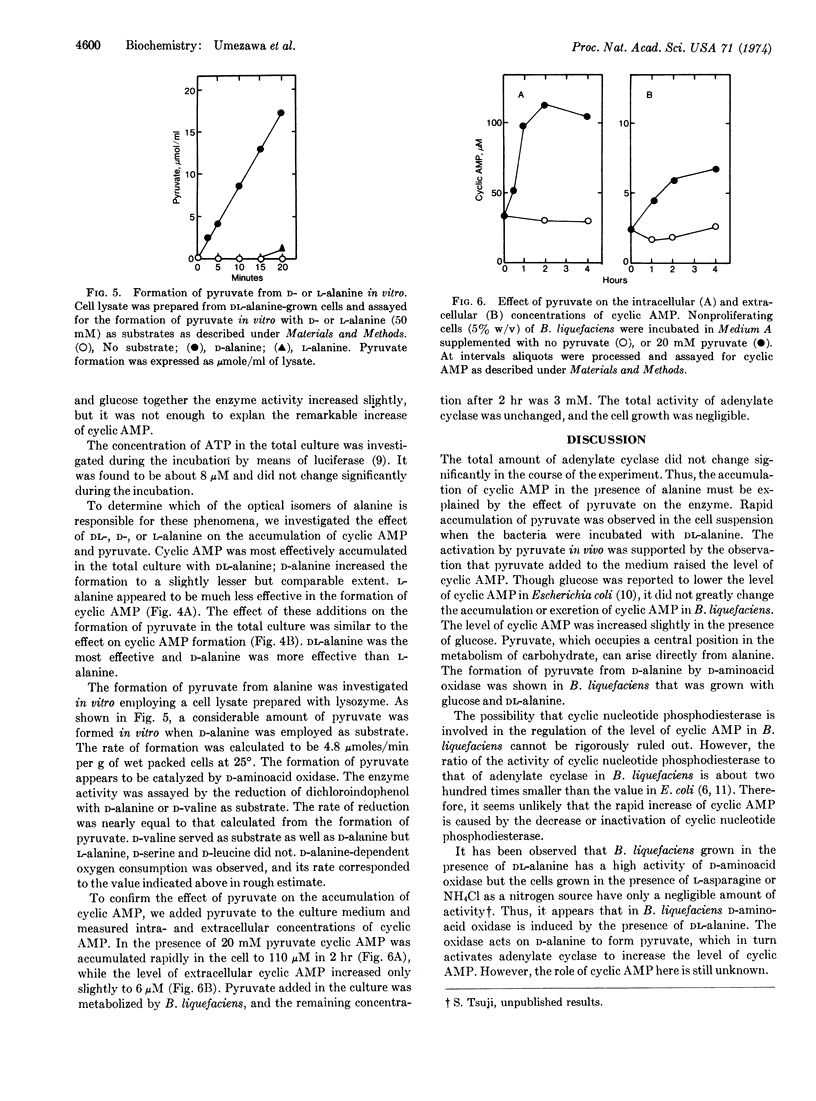
In the presence of DL-alanine intracellular cyclic AMP in nonproliferating cells of Brevibacterium liquefaciens increased rapidly to the maximum level of approximately 180 μM, and extracellular cyclic AMP increased to 100 μM within 4 hr at 25°. Adenylate cyclase (EC 4.6.1.1) induction was not observed during this incubation. The concentration of pyruvate in the total culture increased concomitantly with that of cyclic AMP and reached approximately 20 mM after 4 hr of incubation. Since the activity of cyclic nucleotide phosphodiesterase is extremely low in this bacterium, the accumulation of cyclic AMP with DL-alanine appeared to be due to the activation of adenylate cyclase by pyruvate. D-alanine was more effective than L-alanine in producing pyruvate, and a high activity of D-alanine oxidation was detected in the cell lysate of B. liquefaciens.
Thus, adenylate cyclase in this bacterium appeared to be regulated in vivo by pyruvate which was formed, in this case, predominantly from D-alanine through the action of D-aminoacid oxidase (EC 1.4.3.3). Pyruvate, added extracellularly, also caused a rapid accumulation of intracellular cyclic AMP. Glucose did not change the level of cyclic AMP significantly. It also did not affect the intracellular accumulation of cyclic AMP with DL-alanine.
Keywords: cyclic AMP, DL-alanine, D-aminoacid oxidase
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata M., Hayaishi O. Adenyl cyclase of Brevibacterium liquefaciens. Biochim Biophys Acta. 1967 Nov 21;149(1):1–11. doi: 10.1016/0005-2787(67)90685-5. [DOI] [PubMed] [Google Scholar]
- Ide M., Yoshimoto A., Okabayashi T. Formation of adenosine cyclic 3',5'-phosphate by nonproliferating cells and cell-free extract of Brevibacterium liquefaciens. J Bacteriol. 1967 Aug;94(2):317–322. doi: 10.1128/jb.94.2.317-322.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAKMAN R. S., SUTHERLAND E. W. ADENOSINE 3',5'-PHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1965 Mar;240:1309–1314. [PubMed] [Google Scholar]
- OKABAYASHI T., IDE M., YOSHIMOTO A. Excretion of adenosine-3',5'-phosphate in the culture broth of Brevibacterium liquefaciens. Arch Biochem Biophys. 1963 Jan;100:158–159. doi: 10.1016/0003-9861(63)90046-8. [DOI] [PubMed] [Google Scholar]
- OKABAYASHI T., YOSHIMOTO A., IDE M. OCCURRENCE OF NUCLEOTIDES IN CULTURE FLUIDS OF MICROORGANISMS. V. EXCRETION OF ADENOSINE CYCLIC 3',5'-PHOSPHATE BY BREVIBACTERIUM LIQUEFACIENS SP. N. J Bacteriol. 1963 Nov;86:930–936. doi: 10.1128/jb.86.5.930-936.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Glucose and the metabolism of adenosine 3':5'-cyclic monophosphate in Escherichia coli. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2794–2798. doi: 10.1073/pnas.68.11.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P. E., Williams S. G. Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Anal Biochem. 1969 Jun;29(3):381–392. doi: 10.1016/0003-2697(69)90323-6. [DOI] [PubMed] [Google Scholar]
- Takai K., Kurashina Y., Suzuki-Hori C., Okamoto H., Hayaishi O. Adenylate cyclase from Brevibacterium liquefaciens. I. Purification, crystallization, and some properties. J Biol Chem. 1974 Mar 25;249(6):1965–1972. [PubMed] [Google Scholar]



