Abstract
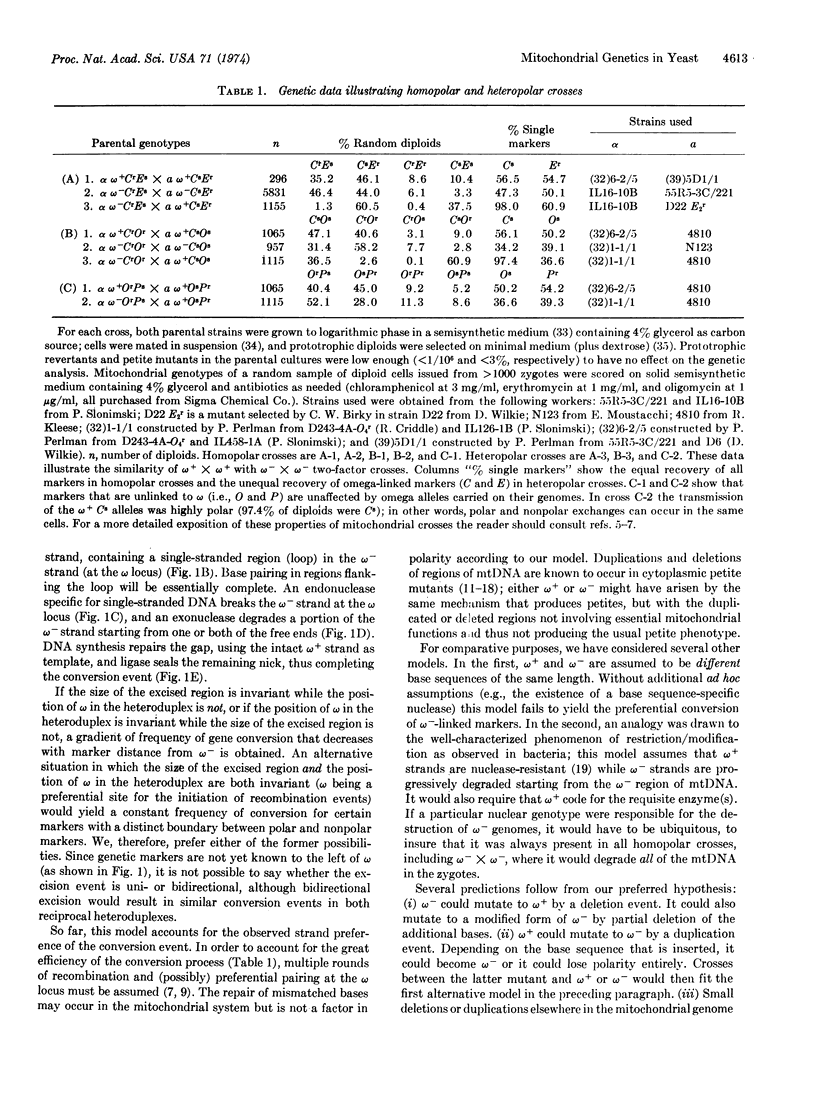
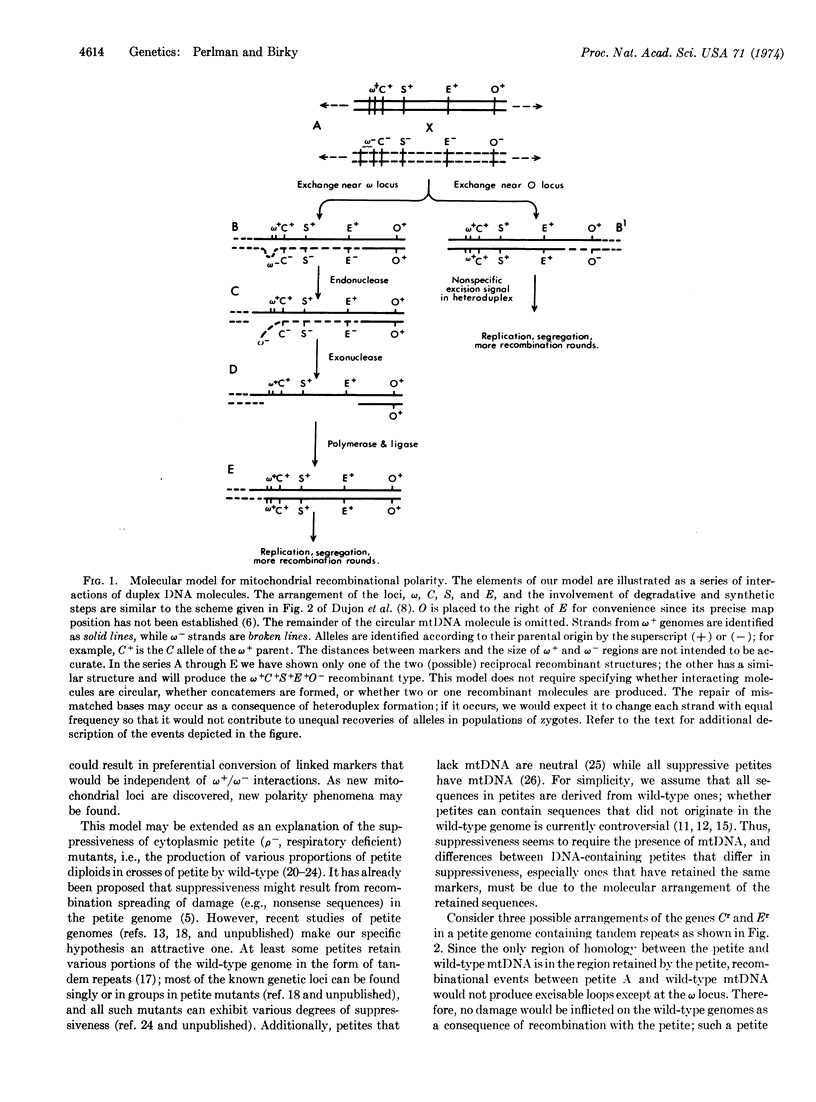
Recombinational polarity and suppressiveness are two well-known but puzzling cytoplasmic genetic phenomena in bakers' yeast, Saccharomyces cerevisiae. Little progress has been made in characterizing the underlying molecular mechanisms of these phenomena. In this paper we describe a molecular model for recombinational polarity that is compatible with the available genetic evidence. The model stresses the role of small deletions and excision/repair processes in otherwise canonical recombinational events. According to the model, both phenomena require recombination and may share mechanistic elements.
Keywords: mitochondrial DNA, Saccharomyces cerevisiae
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avner P. R., Coen D., Dujon B., Slonimski P. P. Mitochondrial genetics. IV. Allelism and mapping studies of oligomycin resistant mutants in S. cerevisiae. Mol Gen Genet. 1973 Sep 5;125(1):9–52. doi: 10.1007/BF00292982. [DOI] [PubMed] [Google Scholar]
- Benz W. C., Berger H. Selective allele loss in mixed infections with T4 bacteriophage. Genetics. 1973 Jan;73(1):1–11. doi: 10.1093/genetics/73.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H., Warren A. J. Effects of deletion mutations on high negative interference in T4D bacteriophage. Genetics. 1969 Sep;63(1):1–5. doi: 10.1093/genetics/63.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D., Deutsch J., Netter P., Petrochilo E., Slonimski P. P. Mitochondrial genetics. I. Methodology and phenomenology. Symp Soc Exp Biol. 1970;24:449–496. [PubMed] [Google Scholar]
- Deutsch J., Dujon B., Netter P., Petrochilo E., Slonimski P. P., Bolotin-Fukuhara M., Coen D. Mitochondrial genetics. VI. The petite mutation in Saccharomyces cerevisiae: interrelations between the loss of the p+ factor and the loss of the drug resistance mitochondrial genetic markers. Genetics. 1974 Feb;76(2):195–219. doi: 10.1093/genetics/76.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPHRUSS B., GRANDCHAMP S. ETUDES SUR LA SUPPRESSIVIT'E DES MUTANTS 'A DEFICIENCE RESPIRATOIRE DE LA LEVURE. I. EXISTENCE AU NIVEAU CELLULAIRE DE DIVERS "DEGR'ES DE SUPPRESSIVIT'E". Heredity (Edinb) 1965 Feb;20:1–7. doi: 10.1038/hdy.1965.1. [DOI] [PubMed] [Google Scholar]
- Ephrussi B., Jakob H., Grandchamp S. Etudes Sur La SuppressivitE Des Mutants a Deficience Respiratoire De La Levure. II. Etapes De La Mutation Grande En Petite Provoquee Par Le Facteur Suppressif. Genetics. 1966 Jul;54(1):1–29. doi: 10.1093/genetics/54.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi B., de Margerie-Hottinguer H., Roman H. SUPPRESSIVENESS: A NEW FACTOR IN THE GENETIC DETERMINISM OF THE SYNTHESIS OF RESPIRATORY ENZYMES IN YEAST. Proc Natl Acad Sci U S A. 1955 Dec 15;41(12):1065–1071. doi: 10.1073/pnas.41.12.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faye G., Fukuhara H., Grandchamp C., Lazowska J., Michel F., Casey J., Getz G. S., Locker J., Rabinowitz M., Bolotin-Fukuhara M. Mitochondrial nucleic acids in the petite colonie mutants: deletions and repetition of genes. Biochimie. 1973;55(6):779–792. doi: 10.1016/s0300-9084(73)80030-6. [DOI] [PubMed] [Google Scholar]
- Fink G. R., Styles C. A. Gene conversion of deletions in the his4 region of yeast. Genetics. 1974 Jun;77(2):231–244. doi: 10.1093/genetics/77.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara H., Faye G., Michel F., Lazowska J., Deutsch J., Bolotin-Fukuhara M., slonimski P. P. Physical and genetic organization of petite and grande yeast mitochondrial DNA.I. Studies by RNA-DNA hybridization. Mol Gen Genet. 1974 May 31;130(3):215–238. doi: 10.1007/BF00268801. [DOI] [PubMed] [Google Scholar]
- Gordon P., Rabinowitz M. Evidence for deletion and changed sequence in the mitochondrial deoxyribonucleic acid of a spontaneously generated petite mutant of Saccharomyces cerevisiae. Biochemistry. 1973 Jan 2;12(1):116–123. doi: 10.1021/bi00725a019. [DOI] [PubMed] [Google Scholar]
- LINN S., LEHMAN I. R. AN ENDONUCLEASE FROM NEUROSPORA CRASSA SPECIFIC FOR POLYNUCLEOTIDES LACKING AN ORDERED STRUCTURE. II. STUDIES OF ENZYME SPECIFICITY. J Biol Chem. 1965 Mar;240:1294–1304. [PubMed] [Google Scholar]
- Leblon G. Mechanism of gene conversion in Ascobolus immersus. II. The relationships between the genetic alterations in b 1 or b 2 mutants and their conversion spectrum. Mol Gen Genet. 1972;116(4):322–335. doi: 10.1007/BF00270089. [DOI] [PubMed] [Google Scholar]
- Linnane A. W., Saunders G. W., Gingold E. B., Lukins H. B. The biogenesis of mitochondria. V. Cytoplasmic inheritance of erythromycin resistance in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1968 Mar;59(3):903–910. doi: 10.1073/pnas.59.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locker J., Rabinowitz M., Getz G. S. Tandem inverted repeats in mitochondrial DNA of petite mutants of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1366–1370. doi: 10.1073/pnas.71.4.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler H. R., Perlman P. S. Induction of respiration deficient mutants in Saccharomyces cerevisiae by berenil. I. Berenil, a novel, non-intercalating mutagen. Mol Gen Genet. 1973 Mar 19;121(4):285–294. doi: 10.1007/BF00433228. [DOI] [PubMed] [Google Scholar]
- Meselson M., Yuan R., Heywood J. Restriction and modification of DNA. Annu Rev Biochem. 1972;41:447–466. doi: 10.1146/annurev.bi.41.070172.002311. [DOI] [PubMed] [Google Scholar]
- Michaelis G., Douglass S., Tsai M. J., Criddle R. S. Mitochondrial DNA and suppressiveness of petite mutants in Saccharomyces cerevisiae. Biochem Genet. 1971 Oct;5(5):487–495. doi: 10.1007/BF00487138. [DOI] [PubMed] [Google Scholar]
- NOMURA M., BENZER S. The nature of the "deletion" mutants in the rII region of phage T4. J Mol Biol. 1961 Oct;3:684–692. doi: 10.1016/s0022-2836(61)80031-4. [DOI] [PubMed] [Google Scholar]
- Nagley P., Linnane A. W. Mitochondrial DNA deficient petite mutants of yeast. Biochem Biophys Res Commun. 1970 Jun 5;39(5):989–996. doi: 10.1016/0006-291x(70)90422-5. [DOI] [PubMed] [Google Scholar]
- Rank G. H. Genetic evidence for 'Darwinian' selection at the molecular level. II. Genetic analysis of cytoplasmically-inherited high and low suppressitivity in Saccharomyces cervisiae. Can J Genet Cytol. 1970 Jun;12(2):340–346. doi: 10.1139/g70-050. [DOI] [PubMed] [Google Scholar]
- Sanders J. P., Flavell R. A., Borst P., Mol J. N. Nature of the base sequence conserved in the mitochondrial DNA of a low-density petite. Biochim Biophys Acta. 1973 Jul 13;312(3):441–457. doi: 10.1016/0005-2787(73)90443-7. [DOI] [PubMed] [Google Scholar]
- Sena E. P., Radin D. N., Fogel S. Synchronous mating in yeast. Proc Natl Acad Sci U S A. 1973 May;70(5):1373–1377. doi: 10.1073/pnas.70.5.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. Y., Wilkie D. Inhibition of mitochondrial synthesis in yeast by erythromycin: cytoplasmic and nuclear factors controlling resistance. Genet Res. 1968 Feb;11(1):33–41. doi: 10.1017/s0016672300011174. [DOI] [PubMed] [Google Scholar]
- Thomas D. Y., Wilkie D. Recombination of mitochondrial drug-resistance factors in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1968 Feb 26;30(4):368–372. doi: 10.1016/0006-291x(68)90753-5. [DOI] [PubMed] [Google Scholar]
- Wickerham L. J. A Critical Evaluation of the Nitrogen Assimilation Tests Commonly Used in the Classification of Yeasts. J Bacteriol. 1946 Sep;52(3):293–301. [PMC free article] [PubMed] [Google Scholar]
- Wolf K., Dujon B., Slonimski P. P. Mitochondrial genetics. V. Multifactorial mitochondrial crosses involving a mutation conferring paromomycin-resistance in Saccharomyces cerevisiae. Mol Gen Genet. 1973 Sep 5;125(1):53–90. doi: 10.1007/BF00292983. [DOI] [PubMed] [Google Scholar]



