Abstract
Being overweight or obese might be a risk factor for developing depression. It is also possible that low cardiorespiratory fitness, rather than overweight or obesity, is the better predictor of depressive symptom onset. Adults in the Aerobics Center Longitudinal Study (Dallas, Texas) underwent fitness and fatness assessments between 1979 and 1998 and later completed a questionnaire about depressive symptoms in 1990, 1995, or 1999. Separate logistic regression models were used to test the associations between 3 fatness measures (body mass index, waist circumference, and percentage of body fat) and the onset of depressive symptoms. Analyses were repeated using fitness as the predictor variable. Additional analyses were performed to study the joint association of fatness and fitness with the onset of depressive symptoms. After controlling for fitness, no measure of fatness was associated with the onset of depressive symptoms. In joint analyses, low fitness was more strongly associated with the onset of elevated depressive symptoms than was fatness, regardless of the measure of fatness used. Overall, results from the present study suggest that low fitness is more strongly associated with the onset of elevated depressive symptoms than is fatness. To reduce the risk of developing depression, individuals should be encouraged to improve their fitness regardless of body fatness.
Keywords: body fat, depression, fitness, obesity, overweight, physical activity
Editor's note: An invited commentary on this article appears on page 321, and the authors' response appears on page 325.
It is estimated that 16.6% of Americans will experience a major depressive episode at some point in their lifetimes (1). Depression is associated with functional impairment (2), cognitive impairment (3), poor quality of life (4), and increased risks of coronary heart disease (5) and type 2 diabetes mellitus (6). Persons who are depressed also are more likely to abuse drugs and alcohol (7) and to commit suicide (8). Considering the high prevalence and debilitating effects of depression, as well as the challenges in providing effective treatment to depressed individuals, it is critical to identify modifiable risk factors. Because more than two-thirds of American adults (68.0%) are overweight or obese (9), researchers have begun to question whether being overweight or obese might be 1 such risk factor.
Previous research in this area has yielded unclear conclusions (10). Much of the research has been cross-sectional (10, 11), making it impossible to determine whether overweight/obesity causes depression or vice versa. In terms of prospective studies, some researchers have investigated whether depression precedes obesity (12, 13) (often hypothesizing that depressed persons gain weight because they eat more and move less than their healthy peers), whereas others have investigated whether obesity precedes depression. In a recent meta-analysis, Luppino et al. (13) found significant associations in both directions. In 9 prospective studies that examined the potential effects of depression on obesity, the pooled odds ratio was 1.58, whereas in 8 prospective studies that examined the relationship in the other direction (the potential effects of obesity on depression), the pooled odds ratio was 1.55.
Importantly, most studies have not considered the relationships between percentage of body fat or level of abdominal obesity (i.e., waist circumference) and depression but rather have relied solely on body mass index (BMI) to categorize levels of overweight/obesity (10, 13). Fat tissue, particularly visceral abdominal fat tissue, is closely associated with metabolic health status (14, 15), and thus use of these measures might provide a clearer picture of which aspects of obesity (i.e., the extra weight itself or the common metabolic comorbid conditions) are most threatening to mental health. Most prospective studies also have not adequately considered physical activity level or cardiorespiratory fitness (CRF) (10), both of which might be protective against the development of depressive symptoms (16, 17).
Previous longitudinal analyses of data from the Aerobics Center Longitudinal Study (ACLS) showed that higher CRF level was associated with a lower incidence of elevated depressive symptoms (16) and that greater odds of incident depression complaints resulted from greater declines in CRF in late middle age (18). In the present article, we build on these previous findings (16, 18) by examining the independent and combined associations of overweight/obesity and CRF with the development of elevated depressive symptoms in the ACLS.
METHODS
Study population
The ACLS is an open cohort study in which participants enter and undergo health examinations at the Cooper Clinic (Dallas, Texas) at different time points and complete periodic follow-up health surveys by mail (19). Participants are primarily self-referred or sent by their employer for preventive medical examinations.
Eligible participants for this particular study included men and women 20–100 years of age who had a baseline health examination between 1979 and 1998 (n = 35,392) and who returned at least 1 mail-back health survey in 1990, 1995, or 1999 (19,521 were excluded because they did not return any surveys). If individuals returned multiple surveys, only their first survey response was used. Participants were excluded if they did not achieve at least 85% of their age-predicted maximum heart rate during treadmill fitness testing (n =391) or if they had cardiovascular disease, cancer, or abnormal resting or exercise electrocardiogram results at baseline (n =1,602). Individuals also were excluded if they had any previously diagnosed mental disorder, such as depression or anxiety, or if they had a nervous disorder, had thoughts of suicide, or had undergone psychiatric counseling in the past (n = 1,279). This mental health information was gathered using the standardized Cooper Clinic medical history questionnaire, which included the question, “Please indicate whether you have ever had a significant problem with any of the symptoms or conditions listed below (yes/no).” Final analyses included 12,599 adults who met the above criteria. All subjects provided written informed consent to participate in the ACLS. The Cooper Institute's institutional review board approved the ACLS protocol each year.
Clinical baseline assessment
Trained clinical personnel conducted all baseline examinations according to the Cooper Clinic's standardized manual of operations (19). Participants provided demographic information, personal and family health histories, and information on personal health habits (e.g., alcohol intake, smoking habits, physical activity level) via standardized Cooper Clinic questionnaires.
Measurement of fatness
Clinical personnel collected height and weight measurements with participants wearing light-weight clothing and no shoes. BMI was then calculated as weight in kilograms divided by height in meters squared. Waist circumference was measured at the level of the umbilicus with a plastic tape measure. Percentage of body fat was assessed using hydrostatic weighing, the sum of 7 skinfold measurements, or both measurements following standardized protocols. Standard clinical definitions were used for BMI (overweight: 25.0–29.9; obese: ≥30), waist circumference (for women, abdominal obesity was defined as a waist circumference >88.0 cm; for men, it was >102.0 cm), and percentage of body fat (for women, obesity was defined as a percentage ≥30%; for men, it was ≥25%).
Measurement of CRF
Maximal exercise treadmill testing was used to determine CRF (20). CRF was defined as total symptom-limited time on the treadmill, with the test endpoint being volitional exhaustion or termination by a supervising physician. In our primary analyses, CRF level was grouped using quintiles of the sex-specific distribution of maximal exercise duration in the overall ACLS population (21).
Measurement of depressive symptoms
Participants were asked to complete the 10-item Center for Epidemiological Studies Depression Scale (CES-D) questionnaire as part of a mail-back survey in 1990 (n = 6,657 participants), 1995 (n = 6,475), or 1999 (n = 7,579) (22, 23). The aggregate survey response rate was approximately 65% (16, 21). Nonresponse bias is a concern in epidemiologic surveillance. This issue has been investigated in the ACLS previously (24). Baseline health histories and clinical measures were similar between responders and nonresponders and between early and late responders (24). A score of 8 or higher indicated the presence of elevated symptoms (which corresponds to a cutpoint of 16 on the 20-item CES-D). This measure has been shown to be reliable and valid (22, 23, 25), and a cutpoint of 8 has been used in multiple previous studies (26, 27).
Statistical analyses
All analyses were sex-specific. Separate logistic regressions tested the associations between each measure of overweight/obesity and the onset of elevated depressive symptoms (CES-D score ≥8). To account for differences in survey response patterns among study participants and for the possibility that external events might have differentially affected responses to the CES-D during the 3 survey periods, dummy variables were created to indicate whether the outcome measurement was from 1990, 1995, or 1999. Covariates in model 1 included age and baseline examination year; covariates in model 2 included those in model 1 plus survey response year (1990, 1995, or 1999), current smoking (yes or no), heavy alcohol intake (for men, >14 vs. ≤14 drinks per week; for women, >7 vs. ≤7 drinks per week), physical activity level (active or sedentary), and personal history of hypertension, diabetes, and hypercholesterolemia; and covariates in model 3 included those in models 1 and 2 plus CRF (maximal treadmill time). Models were repeated using CRF as the predictor variable, substituting percentage of body fat for CRF as a covariate in the full model (model 3). Additional logistic regression analyses were used to determine the joint association of CRF and overweight/obesity with onset of depressive symptoms (separate interaction analyses were run for each fatness measure). In the joint analyses, CRF was grouped into a binary variable of unfit (quintile 1, the lowest 20%) and fit (quintiles 2–5, the remaining 80%). Although no consensus clinical definition of unfit currently exists, the approach we used for defining unfit is a standardized method in the ACLS. Previous reports from the ACLS have shown that unfit by this definition is an independent predictor of morbidity and mortality (21, 28–30). In addition, we collapsed BMI into categories of <25 and ≥25 in order to maintain a good number of participants in the cross-tabulation cells. Additionally, we conducted sensitivity analyses to examine the influence of missing CES-D responses at follow-up. We used the data set with complete CES-D scores from 1990 to impute the missing CES-D scores using the predicted value from the linear regression, which was adjusted for all variables in model 2. We then combined the reported data with imputed data and repeated the main analyses. Because the magnitude and direction of the associations from the sensitivity analyses were very similar to those from the initially proposed analyses, we did not report them in this article. Please see the Web Tables based on imputed analyses. All P values are 2-sided, with an α level of 0.05. All analyses were performed using SAS, version 9.2 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Participant characteristics
The mean baseline age for the study sample was 44.9 (standard deviation, 9.5) years, and 18.4% of participants (n =2,315) were women (Table 1). Table 1 also presents sample characteristics, including average BMI, waist circumference, and body fat percentage, by sex and presence of elevated symptoms. The prevalence of CRF was 7.9% (n = 992), 14.8% (n =1,864), 18.6% (n = 2,342), 25.1% (n = 3,165), and 33.6% (n =4,236) for quintiles 1–5, respectively. The low percentage of persons categorized as having a low fitness level was largely due to the exclusion of participants with cardiovascular disease, cancer, abnormal exercise electrocardiogram results, or mental disorders. After an average of 9.9 (standard deviation, 5.7) years of follow-up, 20.2% of women (n = 467) and 16.3% of men (n = 1,675) reported elevated depressive symptoms (CES-D score ≥ 8). To demonstrate which depressive symptoms were most common in the sample, Table 2 displays the distribution of responses to the CES-D questions for each survey response year (1990, 1995, or 1999).
Table 1.
Baseline Participant Characteristics According to Sex and Depressive Symptoms, Aerobics Center Longitudinal Study, Dallas, Texas, 1979–1998
| Characteristic | Sex |
Depressive Symptoms (CES-D Score) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men (n = 10,284) |
Women (n = 2,315) |
<8 (n = 10,457) |
≥8 (n = 2,142) |
|||||||||||||
| Mean | SE | No. | % | Mean | SE | No. | % | Mean | SE | No. | % | Mean | SE | No. | % | |
| Age, years | 44.9 | 9.3 | 44.5 | 10.2 | 45.0 | 9.4 | 44.0 | 9.9 | ||||||||
| Body mass indexa | 25.9 | 3.4 | 22.3 | 3.4 | 25.2 | 3.6 | 25.3 | 3.9 | ||||||||
| Waist circumference, cm | 92.5 | 10.2 | 71.0 | 9.1 | 88.5 | 12.8 | 88.5 | 13.8 | ||||||||
| % Body fat | 20.8 | 6.1 | 26.2 | 6.4 | 21.6 | 6.5 | 22.4 | 6.7 | ||||||||
| Max treadmill time, minutes | 19.4 | 4.9 | 14.5 | 4.5 | 18.7 | 5.1 | 17.7 | 5.5 | ||||||||
| Total cholesterol, mg/dL | 209.5 | 39.8 | 199.9 | 37.1 | 207.7 | 39.4 | 207.8 | 39.8 | ||||||||
| Blood pressure, mm Hg | ||||||||||||||||
| Systolic | 120.2 | 12.9 | 111.5 | 13.5 | 118.7 | 13.5 | 117.9 | 13.3 | ||||||||
| Diastolic | 80.4 | 9.3 | 75.2 | 9.2 | 79.5 | 9.5 | 79.3 | 9.3 | ||||||||
| Fasting plasma glucose, mg/dL | 99.8 | 14.8 | 94.4 | 13.5 | 98.8 | 14.7 | 98.5 | 14.7 | ||||||||
| Sedentary lifestyleb | 2,119 | 20.6 | 420 | 18.1 | 2,046 | 19.6 | 493 | 23.0 | ||||||||
| Current smoking | 1,357 | 13.2 | 186 | 8.0 | 1,241 | 11.9 | 302 | 14.1 | ||||||||
| Heavy drinkingc | 1,751 | 17.0 | 487 | 21.0 | 1,827 | 17.5 | 411 | 19.2 | ||||||||
| Baseline conditions | ||||||||||||||||
| Hypercholesterolemiad | 2,657 | 25.8 | 418 | 18.1 | 2,538 | 24.3 | 537 | 25.1 | ||||||||
| Diabetes mellituse | 260 | 2.5 | 38 | 1.6 | 241 | 2.3 | 57 | 2.7 | ||||||||
| Hypertensionf | 2,826 | 27.5 | 302 | 13.1 | 2,604 | 24.9 | 524 | 24.5 | ||||||||
Abbreviations: CES-D, Center for Epidemiological Studies-Depression Scale; SD, standard deviation.
a Weight (kg)/height (m)2.
b Defined as no leisure-time physical activity in the 3 months before the examination as reported on the medical questionnaire.
c Defined as more than 14 and more than 7 drinks/week for men and women, respectively.
d Defined as a total cholesterol concentration of 240 mg/dL or higher or a history of physician diagnosis.
e Defined as a fasting plasma glucose concentration of 126 mg/dL or higher, previous physician diagnosis of diabetes, or use of insulin.
f Defined as resting blood pressure of 140/90 mm Hg or higher or previous physician diagnosis of hypertension.
Table 2.
Distribution of Center for Epidemiological Studies-Depression Scale Responses by Year, Aerobics Center Longitudinal Study, Dallas, Texas
| Questionnaire Item | Year |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1990 |
1995 |
1999 |
|||||||
| Total No. | No. ≥1a | % | Total No. | No. ≥1a | % | Total No. | No. ≥1a | % | |
| Unusually bothered | 6,612 | 1,575 | 23.8 | 6,437 | 1,404 | 21.8 | 7,517 | 961 | 12.8 |
| Trouble concentrating | 6,638 | 1,995 | 30.5 | 6,403 | 1,903 | 29.7 | 7,533 | 1,485 | 19.7 |
| Felt depressed | 6,641 | 1,337 | 20.1 | 6,445 | 1,333 | 20.7 | 7,538 | 1,142 | 15.2 |
| Everything was an effort | 6,639 | 1,488 | 22.4 | 6,427 | 1,491 | 23.2 | 7,545 | 1,792 | 23.8 |
| Felt hopefulb | 6,639 | 2,120 | 31.9 | 6,409 | 1,779 | 27.8 | 7,520 | 2,133 | 28.4 |
| Felt fearful | 6,619 | 984 | 14.9 | 6,429 | 876 | 13.6 | 7,496 | 704 | 9.4 |
| Restless sleep | 6,641 | 2,953 | 44.5 | 6,428 | 3,248 | 50.5 | 7,540 | 3,694 | 49.0 |
| Was happyb | 6,639 | 2,101 | 31.7 | 6,422 | 1,782 | 27.8 | 7,526 | 2,229 | 29.6 |
| Felt lonely | 6,635 | 1,159 | 17.5 | 6,433 | 1,178 | 18.3 | 7,542 | 1,094 | 14.5 |
| Could not get “going” | 6,605 | 1,637 | 24.8 | 6,440 | 1,657 | 25.7 | 7,551 | 1,871 | 24.8 |
a Responses ranged from 0–3, with 0 = rarely or none of the time, 1 = some or a little of the time, 2 = occasionally or a moderate amount of the time, and 3 = most or all of the time.
b These items were reverse coded.
BMI, waist circumference, and percentage of body fat
BMI
For women (Table 3), BMI was not predictive of elevated depressive symptom onset in any of the 3 models (P > 0.32). For men (Table 4), the results of model 1 indicated that the odds of elevated depressive symptom onset were 17% greater for overweight participants and 40% greater for obese participants than for normal-weight participants. In model 2, the odds of reporting elevated depressive symptoms at follow-up were 16% and 35% higher, respectively. BMI was not significant in model 3 (which additionally controlled for CRF) for men (P = 0.82).
Table 3.
Odds of Developing Elevated Depressive Symptoms in Women by Indicator of Fatness, Aerobics Center Longitudinal Study, Dallas, Texas, 1990, 1995, or 1999
| Indicator | No. | Model 1a |
Model 2b |
Model 3c |
|||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Body mass indexd | |||||||
| <25.0 | 1,932 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 25.0–29.9 | 306 | 0.96 | 0.69, 1.32 | 0.94 | 0.67, 1.30 | 0.78 | 0.55, 1.09 |
| ≥30.0 | 77 | 1.47 | 0.83, 2.58 | 1.42 | 0.81, 2.59 | 1.05 | 0.58, 1.91 |
| Waist circumference, cm | |||||||
| ≤88 | 2,193 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| >88 | 122 | 1.54 | 0.99, 2.41 | 1.56 | 0.99, 2.49 | 1.32 | 0.82, 2.12 |
| % Body fat | |||||||
| <30 | 1,658 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| ≥30 | 657 | 1.38 | 1.10, 1.73 | 1.36 | 1.07, 1.71 | 1.14 | 0.89, 1.47 |
Abbreviations: CI, confidence interval; OR, odds ratio.
a Adjusted for age and baseline examination year.
b Adjusted for the above covariates plus survey response year(s), current smoking (yes or no), heavy alcohol intake (yes or no), physical activity level (active or sedentary), and personal history of hypertension, diabetes, and hypercholesterolemia.
c Adjusted for the above covariates plus cardiorespiratory fitness (maximal treadmill time).
d Weight (kg)/height (m)2.
Table 4.
Odds of Developing Elevated Depressive Symptoms in Men by Indicator of Fatness, Aerobics Center Longitudinal Study, Dallas, Texas, 1990, 1995, or 1999
| Indicator | No. | Model 1a |
Model 2b |
Model 3c |
|||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Body mass indexd | |||||||
| <25.0 | 4,622 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 25.0–29.9 | 4,517 | 1.17 | 1.05, 1.31 | 1.16 | 1.03, 1.30 | 1.04 | 0.92, 1.17 |
| ≥30.0 | 1,145 | 1.40 | 1.18, 1.67 | 1.35 | 1.12, 1.61 | 1.05 | 0.86, 1.28 |
| Waist circumference, cm | |||||||
| ≤102 | 8,734 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| >102 | 1,550 | 1.32 | 1.15, 1.52 | 1.27 | 1.10, 1.47 | 1.06 | 0.90, 1.24 |
| % Body fat | |||||||
| <25 | 7,786 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| ≥25 | 2,498 | 1.28 | 1.13, 1.44 | 1.23 | 1.08, 1.40 | 1.03 | 0.90, 1.18 |
Abbreviations: CI, confidence interval; OR, odds ratio.
a Adjusted for age and baseline examination year.
b Adjusted for the above covariates plus survey response year(s), current smoking (yes or no), heavy alcohol intake (yes or no), physical activity level (active or sedentary), and personal history of hypertension, diabetes, and hypercholesterolemia.
c Adjusted for the above covariates plus cardiorespiratory fitness (maximal treadmill time).
d Weight (kg)/height (m)2.
Waist circumference
For women (Table 3), waist circumference was not predictive of elevated depressive symptom onset in any of the 3 models (P > 0.06). For men (Table 4), in models 1 and 2, the odds of reporting elevated depressive symptoms at follow up were 32% and 27% higher, respectively, for those with a waist circumference greater than 102 cm than for those with a waist circumference of 102 cm or less. Results of model 3 were not significant for men (P = 0.48).
Percentage of body fat
Results from models 1 and 2 show that women with a body fat percentage of 30% or higher had 38% and 36% higher odds, respectively, of developing elevated depressive symptoms than did women with a body fat percentage below 30% (Table 3). For men (Table 4), results from models 1 and 2 show that those with a body fat percentage of 25% or higher had 28% and 23% higher odds, respectively, of developing elevated depressive symptoms than did those with a body fat percentage less than 25%. Model 3 showed no significant association between percentage of body fat and onset of depressive symptoms for women or men (P = 0.29 and 0.69, respectively).
Cardiorespiratory fitness
CRF level was significantly associated with the onset of elevated depressive symptoms in all models (Table 5), and thus only results from the full model (model 3) will be reported here. Compared with women with the lowest levels of CRF (quintile 1), women in the 2 highest CRF groups (quintiles 4 and 5) had 59% and 52% lower odds, respectively, of reporting elevated depressive symptoms at follow-up. Compared with men with the lowest levels of CRF (quintile 1), men in the 2 highest CRF groups (quintiles 4 and 5) had 40% and 46% lower odds, respectively, of reporting elevated depressive symptoms at follow-up.
Table 5.
Odds of Developing Elevated Depressive Symptoms in Women and Men by Quintile of Cardiorespiratory Fitness, Aerobics Center Longitudinal Study, Dallas, Texas, 1990, 1995, or 1999
| Sex and Quintile of CRF | No. | No. Cases | Model 1a |
Model 2b |
Model 3c |
|||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |||
| Women | ||||||||
| 1 (low) | 154 | 52 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 2 | 324 | 84 | 0.68 | 0.45, 1.04 | 0.66 | 0.44, 1.03 | 0.69 | 0.45, 1.06 |
| 3 | 401 | 90 | 0.56 | 0.37, 0.85 | 0.56 | 0.37, 0.85 | 0.59 | 0.38, 0.90 |
| 4 | 590 | 94 | 0.39 | 0.26, 0.59 | 0.38 | 0.25, 0.58 | 0.41 | 0.26, 0.63 |
| 5 (high) | 846 | 147 | 0.47 | 0.32, 0.69 | 0.43 | 0.29, 0.65 | 0.48 | 0.31, 0.75 |
| Men | ||||||||
| 1 (low) | 838 | 199 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 2 | 1,540 | 297 | 0.77 | 0.63, 0.94 | 0.77 | 0.63, 0.95 | 0.78 | 0.63, 0.96 |
| 3 | 1,941 | 329 | 0.66 | 0.54, 0.81 | 0.66 | 0.54, 0.81 | 0.68 | 0.55, 0.84 |
| 4 | 2,575 | 389 | 0.58 | 0.48, 0.70 | 0.59 | 0.48, 0.72 | 0.60 | 0.48, 0.75 |
| 5 (high) | 3,390 | 461 | 0.51 | 0.43, 0.62 | 0.52 | 0.42, 0.64 | 0.54 | 0.42, 0.69 |
Abbreviations: CI, confidence interval; CRF, cardiorespiratory fitness; OR, odds ratio.
a Adjusted for age and baseline examination year.
b Adjusted for the above covariates plus survey response year(s), current smoking (yes or no), alcohol intake (≥5 drinks/week or no), physical activity level (active or sedentary), and personal history of hypertension, diabetes, and hypercholesterolemia.
c Adjusted for the above covariates plus percentage of body fat.
Joint association of fitness and fatness
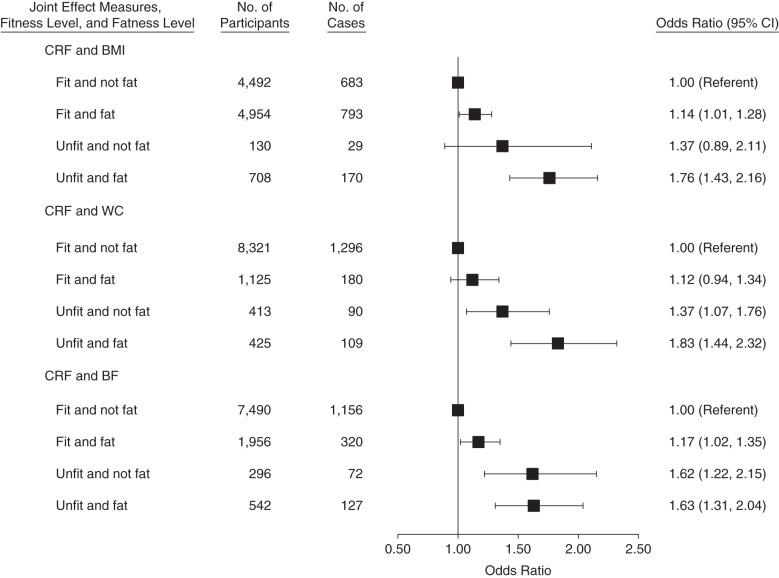
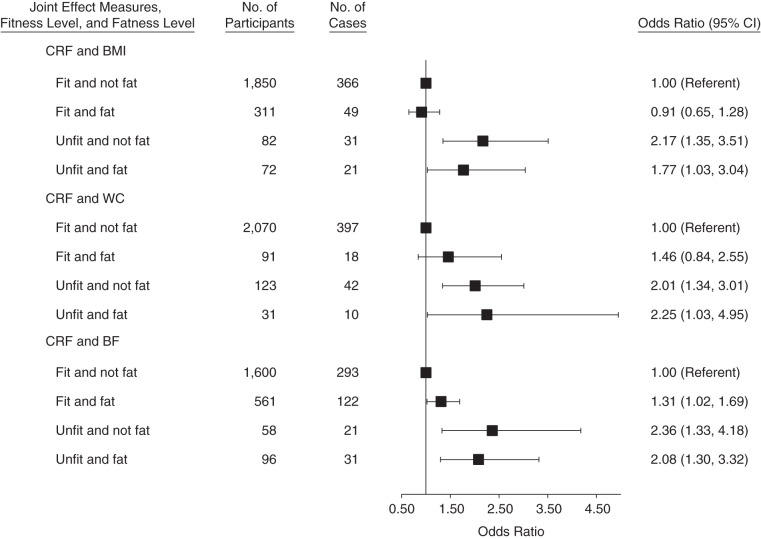
Figures 1 and 2 show the joint association of fitness and fatness with the risk of developing elevated depressive symptoms. In interaction analyses using BMI as the fatness measure, men (n = 708) and women (n = 72) who were both unfit and overweight or obese had 76% and 77% higher odds, respectively, of developing elevated depressive symptoms compared with the normal-weight and fit reference groups. Using waist circumference as the fatness measure, men (n = 425) and women (n =31) who were both unfit and overweight or obese had 83% and 125% higher odds, respectively, of developing elevated depressive symptoms. Using percentage of body fat, men (n = 542) and women (n = 96) who were both unfit and overweight or obese had 63% and 108% higher odds, respectively, of developing elevated depressive symptoms.
Figure 1.
Joint association of cardiorespiratory fitness (CRF) and fatness with the odds of developing elevated depressive symptoms in men, Aerobics Center Longitudinal Study, Dallas, Texas, 1990, 1995 or 1999. Fit was defined as the top 80% of the overall study population; unfit is defined as the bottom 20% of the overall study population. Fat was defined as having a body mass index (weight (kg)/height (m)2; BMI) ≥25, a waist circumference (WC) >102 cm, or percentage of body fat (BF) ≥25%. Not fat was defined as having a BMI <25, a WC ≤102 cm, and percentage of BF <25%, respectively. Odds ratios (ORs) were adjusted for age, baseline examination year, survey response year(s), current smoking (yes or no), alcohol intake (≥5 drinks/week or no), physical activity level (active or sedentary), and personal history of hypertension, diabetes, and hypercholesterolemia. CI, confidence interval.
Figure 2.
Joint association of cardiorespiratory fitness (CRF) and fatness with the odds of developing elevated depressive symptoms in women, Aerobics Center Longitudinal Study, Dallas, Texas, 1990, 1995, or 1999. Fit is defined as the top 80% of the overall study population; unfit is defined as the bottom 20% of the overall study population. Fat was defined as having a body mass index (weight (kg)/height (m)2; BMI) ≥25, a waist circumference (WC) >88 cm, or percentage of body fat (BF) ≥30%. Not fat was defined as having a BMI <25, a WC ≤88 cm, or percentage of BF <30%, respectively. Odds ratios (ORs) were adjusted for age, baseline examination year, survey response year(s), current smoking (yes or no), alcohol intake (≥5 drinks/week or no), physical activity level (active or sedentary), and personal history of hypertension, diabetes, and hypercholesterolemia. CI, confidence interval.
When using waist circumference as the fatness measure, there was no significant difference in the odds of developing elevated depressive symptoms in fit men and women, regardless of fatness level (i.e., lean and overweight/obese women who were fit had the same odds of developing depressive symptoms). However, among overweight and obese men, there was a significant difference in the odds of developing elevated depressive symptoms, with unfit/fat men having 63% higher odds than fit/fat men. There was also no significant difference in the odds of developing elevated depressive symptoms in fit women, regardless of fatness level, when using BMI as the fatness measure. Conversely, unfit/fat women had 94% higher odds of developing elevated depressive symptoms than did fit/fat women.
In additional comparisons using the fit/fat group as the reference (data not shown), unfit women with BMI less than 25 had 139% higher odds of developing elevated depressive symptoms than did fit women with a BMI of 25 or higher (odds ratio =2.39; 95% confidence interval: 1.05, 3.58). Unfit men with percentage of body fat less than 25% had 38% higher odds than did fit men with body fat percent of 25% or higher (odds ratio =1.38; 95% confidence interval: 1.02, 1.87).
DISCUSSION
Principal findings
In the present study, we demonstrated that being overweight or obese is not predictive of the onset of elevated depressive symptoms when CRF level is considered. This was the case regardless of whether BMI, waist circumference, or percentage of body fat was used to determine adiposity. Importantly, fatness was often predictive of the onset of depressive symptoms when CRF level was not considered. Because most previous studies that linked fatness and depression did not adequately consider physical activity or CRF (10), the relationship between fatness and depression might often be overestimated.
Consistent with a previous ACLS investigation (16), we found that higher CRF was associated with lower odds of elevated symptom onset in overweight and obese individuals. In some (but not all) analyses, fit/fat individuals appeared to have no higher odds of developing elevated depressive symptoms than did fit, normal weight-individuals, whereas unfit/fat individuals (i.e., overweight and obese individuals with low CRF) were at significantly higher risk (increased odds ranging from 63% to 125%). Interestingly, fit/fat individuals also had a lower risk of depression than did unfit/not fat individuals in multiple analyses, which reiterates that CRF might be more essential to positive mental health than is low fatness.
Possible mechanisms
It is important to clarify that these findings do not indicate that there is no link between obesity and depression; rather, they suggest that CRF might be a better predictor of depressive symptom onset and might even be protective against the potential depressive effects of obesity. Indeed, severely obese individuals might suffer functional impairment because of their excess weight and have more negative thoughts about their health status (31). Obese women and persons with a high socioeconomic status might also be at a higher risk for depression because of social stigma associated with obesity, body image dissatisfaction, and repeated dieting (31). Obesity is also associated with increased inflammation, possible hypothalamic pituitary adrenal axis dysregulation, and increased risk of diabetes mellitus and insulin resistance, all of which might contribute to a link with depression (13).
Many psychosocial and physiological mechanisms could explain how CRF might negate or buffer against the possible multilevel, prodepressive effects of obesity. Individuals who attain high CRF through regular physical activity might experience increased self-efficacy, self-esteem, and social reinforcement (32, 33). From a physiological standpoint, regular physical activity might be associated with increased quantity and activity of key neurotransmitters (e.g., serotonin, norepinephrine, dopamine) (34), attenuation of the stress response (e.g., lower cortisol levels, lower cardiovascular reactivity to mental stressors) (35, 36), and reduced inflammation (37). Antidepressant properties of physical activity might also be the result of increased levels of key growth factors (e.g., brain-derived neurotrophic factor) and hippocampal neurogenesis (38–40).
Strengths
A strength of the present study is the use of fatness measures that are indicative of metabolic health (i.e., waist circumference and percentage of body fat in addition to the standard BMI), because in a recent study, Hamer et al. (41) showed that poor metabolic health, rather than obesity, was associated with an increased risk of depression. Other major strengths of the present study include its longitudinal design, large sample size, and use of maximal exercise testing to quantify CRF, which is a better marker of regular physical activity participation than are self-reported activity questionnaires. Additionally, this study followed participants for an extensive period of time (mean = 9.9 years).
Limitations
A limitation of this study is its generalizability to other populations, given that the ACLS is composed largely of well-educated, white individuals with a middle-to-high socioeconomic status. Further, a large number of individuals who underwent examinations at the Cooper Clinic between 1979 and 1998 did not return any mail-back health surveys in 1990, 1995, or 1999 and were therefore excluded from analyses. Although the nonresponse issue has been studied in the overall ACLS population (24), the fact that it might have affected our results remains a limitation. Importantly, the magnitude and direction of the associations between fitness, fatness, and depressive symptoms did not change when we used imputed data to account for missing CES-D responses.
An additional limitation is the use of a self-reported, nondiagnostic measure of depression (i.e., the CES-D). However, in a recent meta-analysis of studies that investigated the potential antidepressant effects of exercise, Rethorst et al. (42) showed that effect sizes did not differ between studies that conducted a clinical interview and those that did not. Additionally, depressive symptoms have been linked to poor health and impaired functioning regardless of whether clinical diagnostic criteria for depression are met (43). Regardless, we did not use a structured clinical interview to diagnose depression, and interpretations should be made accordingly.
It should be noted that physical symptoms of depression might overlap with symptoms related to low fitness levels. For example, 2 items on the CES-D might be interpreted as reflecting physical fatigue (i.e., “Did you feel that everything you did was an effort?” and “Did you feel you could not get ‘going’?”) rather than apathy or low motivation. The relationship between low fitness and depressive symptoms, as assessed using the CES-D, could theoretically be driven by only the questions that reflect fitness itself; indeed, in a sensitivity analysis, the prevalence of elevated depressive symptoms dropped substantially in our sample when these 2 items were omitted. Future research should investigate whether composite measures of depression, such as the CES-D, are most appropriate for studying the fitness-depression relationship. Because CES-D data were not available at baseline, only a standardized Cooper Clinic question regarding mental health history was used to exclude individuals with previous mental health concerns. Thus, it is possible that some individuals with high depressive symptoms at baseline might have been inadvertently included in analyses, and therefore reverse causation cannot be entirely ruled out.
Implications and future research
Having a low CRF level might be more strongly associated with an increased risk of elevated depressive symptoms than is being overweight or obese; as such, promoting physical activity to improve CRF rather than weight loss, per se, might be most effective for reducing the risk of developing elevated depressive symptoms. It will be important to determine whether gains in CRF are necessary or whether other physiological or psychosocial factors are actually more critical mediators of the antidepressant effects of physical activity. If improvements in CRF are not necessary, activities at lower intensities (which might be more comfortable for overweight and obese individuals) might lower the risk and/or reduce depressive symptoms. Indeed, results from a 2008 review suggested that relatively low levels of physical activity might protect against depression (44).
Conclusions
Fit/fat individuals might be less likely to develop elevated depressive symptoms than unfit/fat and even unfit/not fat individuals, which suggests that fitness might have antidepressant effects strong enough to buffer against general depressive symptoms, as well as any prodepressive effects of being overweight or obese. These findings contribute to the established fit versus fat literature (29, 30, 45) by demonstrating that mental health, like physical health, might also be more greatly influenced by CRF than by adiposity. It should be emphasized that physical activity, the primary determinant of CRF, is firmly established as an essential feature of weight-maintenance and weight-loss strategies. Thus, in addition to all the plausible pathways linking physical activity directly to improved mental health (irrespective of weight loss), maintaining a healthy body weight or losing weight through a physically active lifestyle might also improve mental health.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Exercise Science, Arnold School of Public Health, University of South Carolina, Columbia, South Carolina (Katie M. Becofsky, Xuemei Sui, Sara Wilcox, Steven N. Blair); Department of Kinesiology, College of Human Sciences, Iowa State University, Ames, Iowa (Duck-chul Lee); Prevention Research Center, Arnold School of Public Health, University of South Carolina, Columbia, South Carolina (Sara Wilcox, Steven N. Blair); and Department of Epidemiology/Biostatistics, Arnold School of Public Health, University of South Carolina, Columbia, South Carolina (Jiajia Zhang, Steven N. Blair).
This work was supported by National Institutes of Health grants AG06945, HL62508, R21DK088195, and T32-GM081740 and in part by an unrestricted research grant from The Coca-Cola Company.
Conflict of interest: S.N.B. has received research funding from the following organizations/companies: National Institutes of Health, Department of Defense, Body Media, and The Coca-Cola Company. He is on scientific/medical advisory boards for the following organizations/companies: Technogym, Santech, Clarity, International Council on Active Aging, and Cancer Fit Steps for Life. K.M.B., X.S., D.-c.L., S.W., and J.Z. have no conflict of interest to report.
REFERENCES
- 1.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.McKnight PE, Kashdan TB. The importance of functional impairment to mental health outcomes: a case for reassessing our goals in depression treatment research. Clin Psychol Rev. 2009;29(3):243–259. doi: 10.1016/j.cpr.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murrough JW, Iacoviello B, Neumeister A, et al. Cognitive dysfunction in depression: neurocircuitry and new therapeutic strategies. Neurobiol Learn Mem. 2011;96(4):553–563. doi: 10.1016/j.nlm.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Papakostas GI, Petersen T, Mahal Y, et al. Quality of life assessments in major depressive disorder: a review of the literature. Gen Hosp Psychiatry. 2004;26(1):13–17. doi: 10.1016/j.genhosppsych.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Rugulies R. Depression as a predictor for coronary heart disease. A review and meta-analysis. Am J Prev Med. 2002;23(1):51–61. doi: 10.1016/s0749-3797(02)00439-7. [DOI] [PubMed] [Google Scholar]
- 6.Mezuk B, Eaton WW, Albrecht S, et al. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008;31(12):2383–2390. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant BF, Stinson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61(8):807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- 8.Nock MK, Hwang I, Sampson NA, et al. Mental disorders, comorbidity and suicidal behavior: results from the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15(8):868–876. doi: 10.1038/mp.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 10.Atlantis E, Baker M. Obesity effects on depression: systematic review of epidemiological studies. Int J Obes (Lond) 2008;32(6):881–891. doi: 10.1038/ijo.2008.54. [DOI] [PubMed] [Google Scholar]
- 11.de Wit L, Luppino F, van Straten A, et al. Depression and obesity: a meta-analysis of community-based studies. Psychiatry Res. 2010;178(2):230–235. doi: 10.1016/j.psychres.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Blaine B. Does depression cause obesity?: A meta-analysis of longitudinal studies of depression and weight control. J Health Psychol. 2008;13(8):1190–1197. doi: 10.1177/1359105308095977. [DOI] [PubMed] [Google Scholar]
- 13.Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67(3):220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 14.Matsuzawa Y, Shimomura I, Nakamura T, et al. Pathophysiology and pathogenesis of visceral fat obesity. Obes Res. 1995;3(suppl 2):187S–194S. doi: 10.1002/j.1550-8528.1995.tb00462.x. [DOI] [PubMed] [Google Scholar]
- 15.Després JP, Moorjani S, Lupien PJ, et al. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis. 1990;10(4):497–511. doi: 10.1161/01.atv.10.4.497. [DOI] [PubMed] [Google Scholar]
- 16.Sui X, Laditka JN, Church TS, et al. Prospective study of cardiorespiratory fitness and depressive symptoms in women and men. J Psychiatr Res. 2009;43(5):546–552. doi: 10.1016/j.jpsychires.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Physical Activity Guidelines Advisory Committee report, 2008. To the Secretary of Health and Human Services. Part A: executive summary. Nutr Rev. 2009;67(2):114–120. doi: 10.1111/j.1753-4887.2008.00136.x. [DOI] [PubMed] [Google Scholar]
- 18.Dishman RK, Sui X, Church TS, et al. Decline in cardiorespiratory fitness and odds of incident depression. Am J Prev Med. 2012;43(4):361–368. doi: 10.1016/j.amepre.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blair SN, Kampert JB, Kohl HW, 3rd, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276(3):205–210. [PubMed] [Google Scholar]
- 20.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, et al. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262(17):2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 21.Sui X, LaMonte MJ, Blair SN. Cardiorespiratory fitness as a predictor of nonfatal cardiovascular events in asymptomatic women and men. Am J Epidemiol. 2007;165(12):1413–1423. doi: 10.1093/aje/kwm031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 23.Kohout FJ, Berkman LF, Evans DA, et al. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5(2):179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 24.Macera CA, Jackson KL, Davis DR, et al. Patterns of non-response to a mail survey. J Clin Epidemiol. 1990;43(12):1427–1430. doi: 10.1016/0895-4356(90)90112-3. [DOI] [PubMed] [Google Scholar]
- 25.Andresen EM, Malmgren JA, Carter WB, et al. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression scale) Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- 26.Carnethon MR, Biggs ML, Barzilay JI, et al. Longitudinal association between depressive symptoms and incident type 2 diabetes mellitus in older adults: the cardiovascular health study. Arch Intern Med. 2007;167(8):802–807. doi: 10.1001/archinte.167.8.802. [DOI] [PubMed] [Google Scholar]
- 27.Win S, Parakh K, Eze-Nliam CM, et al. Depressive symptoms, physical inactivity and risk of cardiovascular mortality in older adults: the cardiovascular health study. Heart. 2011;97(6):500–505. doi: 10.1136/hrt.2010.209767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blair SN, Kohl HW, 3rd, Barlow CE, et al. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA. 1995;273(14):1093–1098. [PubMed] [Google Scholar]
- 29.Wei M, Kampert JB, Barlow CE, et al. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA. 1999;282(16):1547–1553. doi: 10.1001/jama.282.16.1547. [DOI] [PubMed] [Google Scholar]
- 30.Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nutr. 1999;69(3):373–380. doi: 10.1093/ajcn/69.3.373. [DOI] [PubMed] [Google Scholar]
- 31.Markowitz S, Friedman MA, Arent SM. Understanding the relation between obesity and depression: causal mechanisms and implications for treatment. Clin Psychol Sci Pract. 2008;15(1):1–20. [Google Scholar]
- 32.Blumenthal JA, Babyak MA, Doraiswamy PM, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69(7):587–596. doi: 10.1097/PSY.0b013e318148c19a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolff E, Gaudlitz K, von Lindenberger BL, et al. Exercise and physical activity in mental disorders. Eur Arch Psychiatry Clin Neurosci. 2011;261(suppl 2):S186–S191. doi: 10.1007/s00406-011-0254-y. [DOI] [PubMed] [Google Scholar]
- 34.Deslandes A, Moraes H, Ferreira C, et al. Exercise and mental health: many reasons to move. Neuropsychobiology. 2009;59(4):191–198. doi: 10.1159/000223730. [DOI] [PubMed] [Google Scholar]
- 35.Dishman RK, Berthoud HR, Booth FW, et al. Neurobiology of exercise. Obesity (Silver Spring) 2006;14(3):345–356. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- 36.Hamer M. Psychosocial stress and cardiovascular disease risk: the role of physical activity. Psychosom Med. 2012;74(9):896–903. doi: 10.1097/PSY.0b013e31827457f4. [DOI] [PubMed] [Google Scholar]
- 37.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Ernst C, Olson AK, Pinel JP, et al. Antidepressant effects of exercise: evidence for an adult-neurogenesis hypothesis? J Psychiatry Neurosci. 2006;31(2):84–92. [PMC free article] [PubMed] [Google Scholar]
- 39.van Praag H, Shubert T, Zhao C, et al. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38):8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 41.Hamer M, Batty GD, Kivimaki M. Risk of future depression in people who are obese but metabolically healthy: the English Longitudinal Study of Ageing. Mol Psychiatry. 2012;17(9):940–945. doi: 10.1038/mp.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rethorst CD, Wipfli BM, Landers DM. The antidepressive effects of exercise: a meta-analysis of randomized trials. Sports Med. 2009;39(6):491–511. doi: 10.2165/00007256-200939060-00004. [DOI] [PubMed] [Google Scholar]
- 43.Reeves WC, Strine TW, Pratt LA, et al. Mental illness surveillance among adults in the United States. MMWR Surveill Summ. 2011;60(Suppl 3):1–29. [PubMed] [Google Scholar]
- 44.Teychenne M, Ball K, Salmon J. Physical activity and likelihood of depression in adults: a review. Prev Med. 2008;46(5):397–411. doi: 10.1016/j.ypmed.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 45.Blair SN, Brodney S. Effects of physical inactivity and obesity on morbidity and mortality: current evidence and research issues. Med Sci Sports Exerc. 1999;31(11 suppl):S646–S662. doi: 10.1097/00005768-199911001-00025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.