Abstract
The tissue microenvironment shapes the characteristics and functions of dendritic cells (DCs), which are important players in HIV infection and dissemination. Notably, DCs in the gut have the daunting task of orchestrating the balance between immune response and tolerance. They produce retinoic acid (RA), which imprints a gut homing phenotype and influences surrounding DCs. To investigate how the gut microenvironment impacts the ability of DCs to drive HIV infection, we conditioned human immature monocyte derived DCs (moDCs) with RA (RA-DCs), before pulsing them with HIV and mixing them with autologous T cells. RA-DCs showed a semi-mature, mucosal-like phenotype and released higher amounts of TGF-β1 and CCL2. Using flow cytometry, western blot and microscopy, we determined that moDCs express the cell adhesion molecule MAdCAM-1 and that RA increases its expression. MAdCAM-1 was also detected on a small population of DCs in rhesus macaque (Macaca mulata) mesenteric lymph node. RA-DCs formed more DC-T cell conjugates and promoted significantly higher HIV replication in DC-T cell mixtures compared to moDCs. This correlated with the increase in MAdCAM-1 expression. Blocking MAdCAM-1 partially inhibited the enhanced HIV replication. In summary, RA influences DC phenotype increasing their ability to exacerbate HIV infection. We describe a previously unknown mechanism that may contribute to rapid HIV spread in the gut, a major site of HIV replication after mucosal exposure.
Introduction
Since the 1990s it is known that DCs mediate HIV trans-infection of CD4+ T cells (1, 2). This results in a burst of virus replication that is much greater than that resulting from direct, cis-infection of either DCs or T cells, or trans-infection between T cells (1, 3). Such DC-to-T cell trans infection involves a complex sequence of steps: attachment, entry and replication patterns that may have similarities among antigen presenting cells (APCs), but also differences in the receptors involved, intracellular trafficking, and productive and nonproductive replication pathways (4–6). Importantly, because DCs reside in submucosal tissues, they are thought to be among the first cells that encounter HIV following sexual transmission (7, 8). However, DC phenotype and function are determined by the microenvironment and their ability to fuel HIV infection may change according to the type of DC and their anatomical location (9). How the mucosal environment shapes a DC’s ability to transfer the virus to T cells and their susceptibility to HIV has never been addressed.
The gut and the gut inductive sites, mesenteric lymph nodes (MLNs) and Peyers patches (PPs), are major sites of early HIV and SIV replication and amplification, resulting in profound and rapid CD4+ T cell loss (10, 11). CD4+ T cells in the intestinal tract are infected 10-fold more frequently than those in blood (12, 13). Moreover, the depth of CD4+ T cell depletion in the gut mucosa is associated with progression to AIDS (14, 15). The underlying mechanisms that render the gut and the gut associated lymphoid tissue (GALT) especially receptive to HIV replication are not fully understood.
Due to the continuous exposure to dietary antigens and commensal microbes, the gut represents a unique immunological environment, requiring a balance between prompt, effective responses to pathogens and tolerance to innocuous antigens. Appropriate mucosal immune responses depend on specialized DCs (16) and the gut microenvironment has a key role in imprinting on DCs the unique ability to mediate both T cell priming/activation and tolerance (17). One of the major players in the maintenance of the balance between immunity and tolerogenicity is retinoic acid (RA). Depending on the presence of other soluble factors in the gut microenvironment, including transforming growth factor beta 1 (TGF-β1), IL-6 and IL-10, RA is able to sway the T cell responses towards a Treg or a Th17 phenotype (18–20). RA released by intestinal epithelial cells and lamina propria (LP) stromal cells primes LP gut-DCs to become CD103+ mucosal DCs (21–24). A distinctive characteristic of CD103+ mucosal DCs, not possessed by DCs from other anatomical sites, is the ability to metabolize Vitamin A into RA (25). Through their ability to produce RA, mucosal DCs modulate T and B cell phenotype and induce the expression of integrin α4β7 (α4β7), the gut homing receptor (26). Notably, α4β7high CD4+ T cells are highly susceptible to HIV infection and are preferentially depleted during the early, acute stages after mucosal transmission (27, 28). Recently we showed that their frequency in the rectal mucosa is associated with susceptibility to rectal SIV infection (29) and in vivo blocking of α4β7 reduces susceptibility to vaginal SIV transmission (30).
The α4β7 ligand, mucosal vascular addressin cell adhesion molecule-1 (MAdCAM-1), is predominantly expressed on high endothelial venules (HEV) of the GALT and on venules at chronically inflamed mucosal sites (31). However, MAdCAM-1 has the potential to be expressed outside the endothelial cell lineage, e.g. by fibroblasts, melanoma cells and mesenchymal follicular dendritic cells (FDCs) (32). MAdCAM-1 expression by DCs of monocyte lineage has never been reported.
Herein we describe how the gut microenvironment can shape the ability of DCs to promote and respond to HIV infection. We define the mucosal-like phenotype of RA conditioned human monocyte derived DCs (RA-DCs) and we reveal their increased capacity to form DC-T cell conjugates and release TGF-β1 and CCL2 (monocyte chemotactic protein 1, MCP-1). Notably, we report for the first time MAdCAM-1 detection on DCs and its upregulation by RA. Finally, we found that RA treatment of DCs enhances their ability to drive HIV replication in the DC-T cell milieu compared to immature moDCs and this is partially mediated by MAdCAM-1 interaction with α4β7 on the CD4+ T cells.
Methods
Ethics Statement
Tissues from 15 healthy SIV uninfected adult female Indian rhesus macaques (Macaca mulatta) being used for a separate study were used to detect MAdCAM-1 in DCs in vivo. The animals were housed in compliance with the regulations under the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals, at Tulane National Primate Research Center (TNPRC; Covington, LA). Animals were socially housed, indoors in climate controlled conditions with a 12/12-light/dark cycle. Animals were monitored continuously by veterinarians to ensure their welfare and fed commercially prepared monkey chow twice daily. Water was available at all times. The TNPRC environmental enrichment program is reviewed and approved by the IACUC semiannually. All the animals were euthanized using methods consistent with recommendations of the American Veterinary Medical Association (AVMA) Panel on Euthanasia and per the recommendations of the IACUC. Specifically, the animals were anesthetized with tiletamine/zolazepam (8 mg/kg IM) and given buprenorphine (0.01 mg/kg IM) followed by an overdose of pentobarbital sodium. Death was confirmed by auscultation of the heart and pupillary dilation. All studies were approved by the Animal Care and Use Committee of the TNPRC (OLAW assurance #A4499-01) and in compliance with animal care procedures. TNPRC is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC #000594).
Cell isolation and culture
Macaque iliac LNs and MLNs were obtained at necropsy, cut in small pieces and passed through a 40 μm cell strainer. PBMCs were isolated from macaque blood and human leukopacks (New York Blood Center, New York) using Ficoll-Hypaque density gradient centrifugation (Amersham Pharmacia Biotech GE Healthcare, Little Chalfont, UK). Human CD14+ monocytes were isolated using the CD14 magnetic cell sorting system (Miltenyi Biotec, Auburn, CA) and moDCs generated by culturing monocytes in 100 U/mL IL-4 (Gibco, Life Technologies) and 1000 U/mL GM-CSF (Biosource, Life Technologies) added every 2 days. Cells were cultured for 6 days at 106 cells/ml in 6-well plates in R1 (RPMI-1640 1% human plasma + 2 mM L-glutamine, 10 mM HEPES, Pen/Strep). On day 4, cells were also treated with different concentrations of RA (Sigma, St. Louis, MO; RA-DCs) or mock treated (DMSO, Sigma; moDCs). On day 6, their phenotype was analyzed by multicolor flow cytometry. Autologous CD14− cells were kept in culture in R10 (RPMI-1640 10% FBS + L-glutamine, HEPES, Pen/Strep) with 1 U/ml IL-2 (NCI Preclinical Repository). On day 5 CD4+ T cells were isolated from CD14− cell cultures using the CD4 T cell isolation kit (Miltenyi Biotech). CD4+ T cells were cultured for one day in R5 (RPMI-1640 5% human serum + 2 mM L-glutamine, 10 mM HEPES, Pen/Strep) with 1 U/ml IL-2.
HIV-loaded DCs and DC-T cell cultures
HIV-BaL was kindly provided by the AIDS and Cancer Virus Program (SAIC Frederick, NCI, Frederick, MD). moDCs and RA-DCs were pulsed with HIV (8×104 TCID50/106 DCs) for 2h at 37°C in a 15 ml conical tube at a concentration of 107 DCs/ml. Cells were washed three times before recounting them and mixing them with T cells in R5 (1 U/ml IL-2). 105 DCs were added to 3x105 cells CD4+ T cells (per well of a 96 flat well plate) in triplicates in the absence or presence of 500 nM of αRAR (LE540, Wako Chemical USA, Inc., Richmond, VI; Ro 41-5253, Enzo Life Sciences, Inc. Farmingdale, NY). For the MAdCAM-1 blocking experiments, 105 HIV-loaded moDCs and RA-DCs were incubated with 5 μg/ml of anti-human MAdCAM-1 mAb (clone 314G8, Bio-Rad AbD Serotec, Raleigh, NC) or 5 μg/ml of IgG1 control (Bio-Rad AbD Serotec) in a 96 flat well plate for 20 min at 4°C before the addition of 3x105 cells CD4+ T cells (1.25 μg/ml final concentration). No additional anti-MAdCAM-1 was added during the culture. After 9 days, the cells were harvested and HIV-qPCR was performed on cell lysates as previously described (33). For the analysis of HIV capture by DCs, HIV-loaded moDCs and RA-DCs were permeabilized and stained, immediately after the HIV pulse, with FITC-anti-p24 mAb (Beckman Coulter Inc., Brea, CA). For the infection of moDCs and RA-DCs, HIV-loaded DCs were seeded in a 96-well flat bottom plate (3x105 DCs/well) in the absence or presence of αRAR. Samples were set up in triplicate in R1 (IL-4/GM-CSF supplemented). 6 days later DCs were harvested and HIV-qPCR was performed on the cell lysates. For the analysis of moDC-T cell and RA-DC-T cell conjugates and T cell phenotype, 105 DCs, without HIV, were seeded in triplicate in R5 (1 U/ml IL-2) in a 96-well flat bottom plate with 3x105 cells CD4+ T cells in the absence or presence of αRAR and in the absence or presence of 10μg/ml the anti-MAdCAM-1 mAb (314G8; AbD Serotec) or of an anti-CD54 (AbD Serotec) or both. 5 days later DC-T cell cultures were harvested and the cells stained for flow cytometry.
Flow cytometry
Cells were incubated with the Live/Dead Aqua dye (Molecular probes, Life Technologies, Carlsbad, CA) before surface staining. mAbs included in the human DC panel were anti-: CD11c-AF700 (eBioscience, San Diego, CA), CD1c-APC (Miltenyi), β7-APC (Biolegend, San Diego, CA), CD29-APC, CD103-APC (eBioscience), CD4-APCH7, CD80-APCH7, CD103-FITC (eBioscience), CD206-FITC, CD209-FITC, CD11b-PCPCy5.5 (Biolegend), CXCR4-PCPCy5.5, MAdCAM-1-PE (AbD Serotec), dimeric α4β7-PE (clone Act1; NHP Reagent Resource, MassBiologics, University of Massachusetts, Boston, MA), CD25-PeCy7 (Biolegend), CD206-PeCy7 (Biolegend), anti-HLA-DR-QDot ™605 (Invitrogen, Life Technologies) and CD14-V450. The following mAbs CCR5-PeCy7, CD103-PeCy7, CD141-PeCy7, CD54-PCPCy5.5 were directly conjugated using the Lightning-Link kits (Innova Biosciences, Cambridge, UK). For the T cell phenotype in uninfected DC-T cell cultures the panel included anti-: CD69-AF700, CD45RO-APC, CD62L-APC, CD4-APCH7, CD45RA-FITC, PD-1-PCP-efluor710 (eBioscience), α4β7-PE, anti-HLA-DR-QDot ™605, CD3-V450 (all the mAbs were from BD Biosciences, San Jose, CA, unless otherwise indicated). After surface staining, the cells were fixed and permeabilized with fix/perm buffer and incubated with anti-FOXP3-FITC (eBioscience) for 45 min at RT. For macaque cells, the following mAbs were used: CD11c-AF700 (eBioscience), CD14-V450, CD3-V450, CD20-V450, HLA-DR BV605, CD4-APCH7, CD45RA-FITC, CD103-APC and MAdCAM-1-PE. The CCR7-PeCy7 and dimeric α4β7-PeCy7 mAbs were directly conjugated using Lightning-Link labeling kits (Innova Biosciences). For the binding of recombinant α4β7 (R&D Systems, Minneapolis, MN) to moDCs and T cells, biotinylated (E-Z Link biotinylation reagent; Pierce Thermo Scientific, Rockford, IL) α4β7 was incubated with the cells 20 min at 4°C, washed and detected with PE-Neutravidine (Pierce Thermo Scientific). The buffer was HEPES-buffered saline with 100 mM CaCl2 and 1 mM MnCl2. At least 200,000 events were acquired using the LSRII (BD Biosciences) and FlowJo-V9 (Tree Star, Ashland, OR) was used to analyze the data.
Western Blot
Day 6 moDCs and RA-DCs (2×106 cells) were lysed with lysis buffer (NuPAGE® LDs; Life Technologies). Total cell lysates were subjected to electrophoresis under manufacturer’s conditions (NuPAGE® Bis-Tris Mini Gels; Life Technologies) and transferred onto PVDF membranes (Invitrogen). Membranes were blocked for 2h with 5% nonfat dry milk in PBS 0.05% Tween-20 (PBS-T) and incubated in 1% dry milk with primary mAbs (anti-MAdCAM-1 clones 17F5, 314G8 (AbD Serotec) and CA2.2 (ebioscience) and anti-β-Actin (Abcam, Cambridge, MA)) at a 1:1000 dilution overnight at 4°C. The membranes were washed with PBS-T, incubated with HRP-secondary Abs diluted 1:3000 in 1% PBS-T at RT for 1h and washed with PBS-T. The immunoreactive bands were detected by chemiluminescence reagent (Thermo Scientific Waltham, MA), visualized on SuperRX film (Thermo Scientific) and quantified (after normalization on β-Actin) using ImageJ (NIH, Bethesda, MD).
Detection of soluble proteins
The concentration of TGF-β1 in the supernatants was measured by ELISA (R&D Systems). Samples were measured with and without the chemical activation step suggested by the manufacturer and analyzed for the presence of cytokines and chemokines using the Cytokine Human 25-Plex Panel kit (Invitrogen) according to the manufacturer’s protocol. The kit allows for the measurement of GM-CSF, IL-1β, IL-1RA, IL-6, CXCL8, TNFα, IFNγ, IL-2, IL-2R, IL-4, IL-5, IL-10, IFNα, IL-7, IL-12, IL-13, IL-15, IL-17, CXCL10, CCL2, CXCL9, CCL5, CCL3 and CCL4. The data were detected with the Luminex® 200 ™ System and analyzed using the xPONENT® v3.1 Software (Life Technologies).
Microscopy
Cells were adhered to glass slides coated with Alcian blue (Sigma) and fixed with 3.7% PFA 10 min at 4°C. PBS-BSA 1% was used to dilute Abs and rinse the cells between incubation steps. All steps were performed at RT. Nonspecific staining was blocked with 10% sheep serum (Sigma) in PBS for 20 min before being immunostained with an anti-MAdCAM-1 mAb (Bio-Rad AbD Serotec, clone 314G8; 20 μg/ml) or the isotype control (mIgG1; 20 μg/ml) for 30 min. Bound Abs were detected by adding a goat anti-mouse AF488 (2.5 μg/ml) for 30 min. For the detection of MAdCAM-1 on HEV of macaque MLNs, formalin-fixed paraffin-embedded 5 μm slices of tissue were prepared at the TNPRC. Deparaffinization was achieved by incubation 3x in xylene 5 min, 3x in 100% EtOH for 2 min, once in 95% EtOH 2 min, once in 80% EtOH for 2 min, twice in distilled water for 2 min. For antigen retrieval slides were incubated 20 min in Diva Decloaker (Biocare Medical, Concord, CA) at 98°C. Tissues were incubated 40 min in blocking buffer (PBS, 0.2% Fish Skin Gelatin, 10% normal goat serum, 1% BSA), washed in washing buffer (PBS, 0.2% Fish Skin Gelatin, 0.1% Triton X-100) and incubated 1h at RT in the dark with AF568 anti-MAdCAM-1 (clone 314G8; AbD Serotec) or Mouse IgG1 Negative Control diluted 1:100 in blocking buffer. Slides were mounted with ProLong gold with DAPI mounting media (Molecular probes, Life Technologies). Images were captured on a wide-field fluorescence microscope (motorized Z-drive; Zeiss) equipped with a Hamamatsu Orca ER B/W digital camera and the MetaVue acquisition Software (Molecular Devices, Sunnyvale, CA) using a plan apo 40x or 100x oil immersion objective. The images were processed with ImageJ software (NIH, Bethesda, MD).
Statistics
The different conditions were compared using non-parametric Wilcoxon Signed-Ranked test. The correlation between the fold increase in mean fluorescence intensities (MFIs) and fold increase in HIV replication were analyzed with linear regression analysis and the p-value of the Spearman rank coefficient analysis was used to determine significance. The analysis was performed using GraphPad Prism 5.03 (GraphPad InStat Inc., San Diego, CA).
Results
RA promotes a semi-mature mucosal-like phenotype on DCs
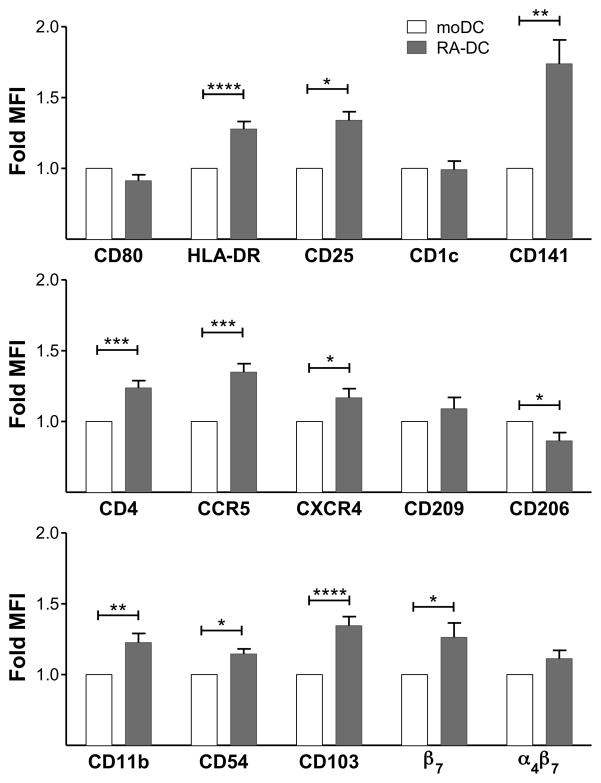
To examine how the RA-rich gut microenvironment influences the phenotype and function of DCs, we conditioned human moDCs with RA (RA-DCs) adapting a protocol for the generation of mucosal DCs from murine bone marrow cells (21). After generating immature moDCs by culturing CD14+ monocytes with GM-CSF and IL-4 for 4 days, the cells were cultured for additional 2 days in presence of RA or a mock solution (to generate RA-DCs vs. moDCs side-by-side). Multicolor flow cytometry at day 6 revealed that RA treatment significantly increased the expression of HLA-DR, CD25, CD141, CD11b, β7, but reduced expression of CD206, and had limited or no effect on CD80, CD1c, CD209 and α4β7 (relative to moDC controls) (Fig. 1 and supplemental S1A). Remarkably, the HIV receptors CD4, CCR5 and CXCR4 were all significantly upregulated by RA treatment. Moreover, the expression of CD103, a marker of mucosal DCs (16, 17, 34), and CD54, involved in the formation of DC-T cell synapses (35), were also significantly higher on RA-DCs than on mock-treated moDCs. Initial experiments where moDCs were treated with different concentrations of RA revealed that treatment with 0.1 μM RA consistently resulted in more significant changes than treatment with the other concentrations of RA tested (Supplemental Fig. S1B). Thus, we decided to explore further the biology of RA-DCs treated with 0.1 μM RA. Addition of RA to moDC cultures at day 3 instead of day 4 did not substantially change its effect on the moDC phenotype (not shown). We did not test the addition of RA to the CD14+ monocytes at the beginning of the culture, as published in a previous report (36), because that would not mimic the effect of RA on developed immature DCs as it occurs in the gut microenvironment.
Figure 1. RA-DCs show a semi-mature mucosal-like phenotype.
moDCs and RA-DCs were gated on live, single, CD11c+ cells. The fold increase (mean ± SEM, n=4–41) of the MFI of each marker on RA-DCs are shown compared to the control moDCs (set as 1) (*p<0.05 is considered significant; **p<0.01; ***p<0.001; ****p<0.0001).
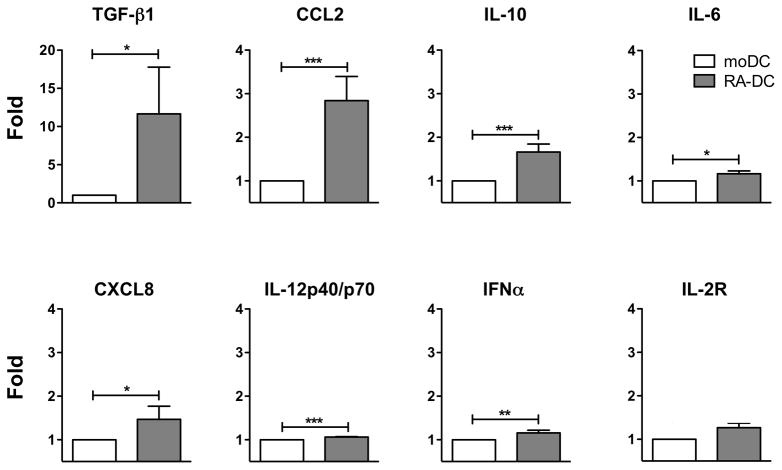
We found that RA-DCs released more total TGF-β1 than moDCs (Fig. 2 and Supplemental Fig. S2A) in all the samples tested with the exception of one donor (out of 8) that had 4 times less TGF-β1 in RA-DCs than moDCs. The donor was excluded from the analysis and the data reported. We measured also the bioactive form of TGF-β1, testing the samples without the chemical activation step required by the assay. The donor with the highest amount of total TGF-β1 had a detectable amount of bioactive TGF-β1 in the supernatant of the RA-DCs (15.3 pg/ml) but none in the moDCs. All the other RA-DC and moDC samples had no detectable bioactive TGF-β1. Notably, RA-DCs released significantly more CCL2, a Th2 response-inducing factor (37), than control moDCs (Fig. 2 and Supplemental Fig. S2A). RA-DCs released also small amounts of the anti-inflammatory cytokine IL-10, compared to almost undetectable amounts produced by moDCs. In line with their semi-mature phenotype, RA-DCs released also slightly more inflammatory cytokines: IL-6, CXCL8 (IL-8), IL-12 (IL-12p40/p70) and IFNα (Fig. 2 and Supplemental Fig. S2A). RA-DCs consistently produced more IL-2R than moDCs, but this was not significant (p=0.07).
Figure 2. RA-DCs release tolerogenic cytokines.
The fold increase (mean ± SEM, n=8) in the concentration of the indicated soluble factors in the supernatants of RA-DCs are shown compared to the control moDCs (set as 1) at day 6 of culture. The data from 1 donor were excluded for TGF-β1, because this donor had 4-fold less TGF-β1 in RA-DCs than in moDCs. This was in contrast with all other donors showing the opposite trend (*p<0.05; **p<0.01; ***p<0.001).
MAdCAM-1 is detected on moDCs and on DCs in MLNs
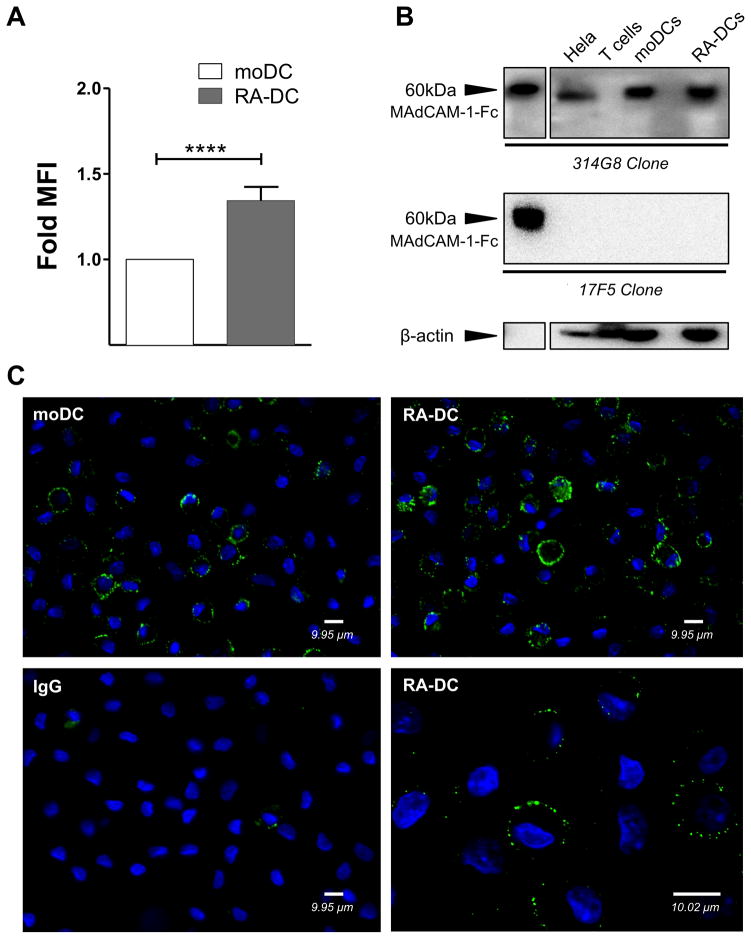
MAdCAM-1 is constitutively expressed by HEVs of PPs and MLNs as well as on postcapillary venules of gut LP, but has the potential to be expressed outside the endothelial cell lineage (32). MAdCAM-1 has been detected also on fibroblastic like cells and FDCs (38). Remarkably, using flow cytometry, western blot and microscopy, we detected MAdCAM-1 on moDCs (Fig. 3 and supplemental S1A). Interestingly, MAdCAM-1 was recognized by the 314G8 and CA2.2 clones (Fig. 3A–C and not shown), which bind the Ig-domain of the protein, but not by the 17F5 clone that binds the mucin-domain (Fig. 3B). By microscopy, MAdCAM-1 appears to be similarly distributed in small clusters on the moDC and RA-DC surfaces (Fig. 3C). By flow cytometry we found that surface expression of MAdCAM-1 was higher on RA-DCs than on moDCs and this was supported by the microscopy. However, the increase in total MAdCAM-1 detected by western blot was less pronounced. Finally, we also verified binding of PE-conjugated recombinant α4β7 to moDCs. In contrast binding to T cells was not detected (Supplemental Fig. S2B).
Figure 3. MAdCAM-1 is detected on moDCs and RA treatment increases its expression.
(A) moDCs and RA-DCs were gated on live, single, CD11c+ cells. The fold increase (mean ± SEM, n=39) of MAdCAM-1 MFI on RA-DCs is shown compared to the control moDCs (set as 1). p<0.05 is considered significant (****p<0.0001). (B) Representative blots of MAdCAM-1 detection in total lysates of moDCs, RA-DCs, CD4+ T cells (negative control) and HeLa cells (cell lysate positive control) with anti-MAdCAM-1 mAbs (clones 314G8 upper membrane and 17F5 middle membrane). Soluble MAdCAM-Fc (MAdCAM-1) was used as positive control. Anti-β-Actin mAb was used for normalization (lower membrane). RA-DCs expressed slightly more total MAdCAM-1, 1.18±0.06 fold (mean ± SEM, n=7), when comparing the fold intensity of normalized MAdCAM-1 content in RA-DCs compared to the control moDCs (set as 1). (C) moDCs (upper and lower left) and RA-DCs (upper and lower right) were immunostained for surface MAdCAM-1 (green) or the IgG control (lower left). DAPI-stained nuclei are in blue. Representative results of 1 out of 10 different donors are shown. Magnifications: 40x (upper row and lower left) and 100x (lower right). Scale bars correspond to 9.95μm (40x) and 10.02μm (100x).
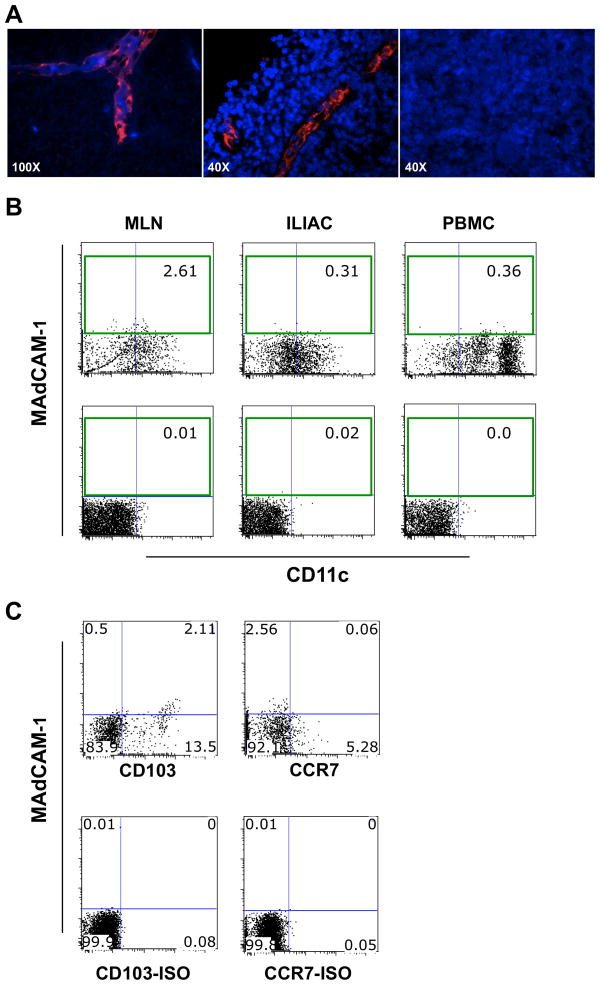
In order to explore if MAdCAM-1 can be expressed by DCs in vivo, we tested the binding of the anti-MAdCAM-1 mAb clone 314G8 on DCs from different rhesus macaque tissues. Once confirmed that the 314G8 clone recognizes MAdCAM-1 on the HEV of macaque MLNs (Fig. 4A), we performed multicolor flow cytometry on cells from MLNs, iliac LNs and blood to search for MAdCAM-1 positive cells with a clear DC phenotype. We detected a small, but distinct population of Lin− HLA-DR+ DCs that expressed MAdCAM-1 in 15 out of 15 MLNs tested (Figure 4B; Range: 0.58% – 2.98%; mean = 1.1%). In contrast, reactivity to the anti-MAdCAM-1 mAb could barely be detected (above the isotype controls) in iliac DCs (Range: 0.00% – 0.67%; mean = 0.29%) or blood DCs (Range: 0.04% – 0.79%; mean = 0.24%). The majority of MAdCAM-1+ DCs in the MLNs were CD11clow, CD103+ and CCR7− (Figure 4C).
Figure 4. MAdCAM-1 on a small population of MLNs DCs.
(A) MLNs from uninfected healthy rhesus macaques were stained with the anti-MAdCAM-1 mAb clone 314G8 (Red; left and center) or an isotype control (right). Nuclei were detected with DAPI (blue). (B) A representative plot from MLN, iliac lymph nodes (ILIAC) and blood (PBMC) of 1 out of 15 macaques (upper level) and isotype control (lower level). Cells were gated on live, singlets, Lin− and HLA-DR+. (C) Representative plots of Lin− HLA-DR+ cells in MLN from the same animal as in (B) showing the CD103+ CCR7− phenotype of MAdCAM-1+ DCs.
RA-DCs form more DC-T cell conjugates and induce a regulatory CD4+ T cell phenotype
In order to determine the impact that the RA treatment has on the DC’s ability to drive phenotypic changes in CD4+ T cells, we co-cultured RA-DCs and moDCs with autologous CD4+ T cells for 5 days. Before mixing RA-DCs with the T cells, the DCs were extensively washed to remove exogenous RA. However, RA treatment is known to induce RA-producing capability on DCs (21, 36). Thus, to distinguish the effect of RA-DC-derived RA from the effect of other RA-DC-specific characteristics on the CD4+ T cells, we treated half of the CD4+ T cells with RA receptor (RAR) antagonists (RARα and RARβ antagonists Ro 41- 5253 and LE540; here together: αRAR) prior to mixing them with the DCs. All the DC-T cell experiments were performed in presence of αRAR or a mock solution in parallel.
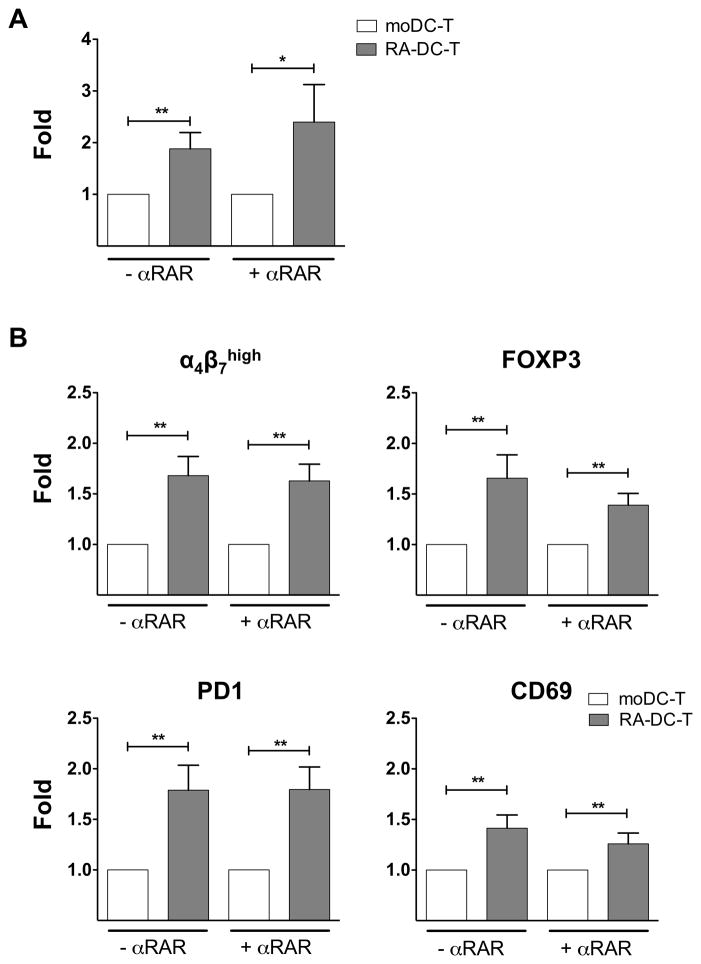
Interestingly, we found a higher frequency of DC-T cell conjugates in the RA-DC-T cell co-cultures than in the moDC-T cell mixtures both in the presence and absence of αRAR (Fig. 5A). The frequency of conjugates was not affected by treatment with the αRAR (Supplemental Fig. S2C). Moreover, in agreement with other in vitro models of mucosal DCs (21), we found that the RA-DCs increase the expression of α4β7 on co-cultured CD4+ T cells. Specifically, we found a higher frequency of α4β7high memory CD4+ T cells (Fig. 5B and Supplemental S3) in RA-DC-T cell mixtures than in the moDC-T cell mixtures. We also observed higher expression of FOXP3, PD1 and CD69, markers of induced regulatory T cells (iTreg) (39, 40) on the CD4+ T cells co-cultured with the RA-DCs (Fig. 5B and Supplemental S3). Notably, these increases occurred also in presence of the αRAR, suggesting they were not exclusively dependent on the RA produced by the RA-DCs as it was reported for T cells co-cultured with TLR-ligands stimulated RA-DCs (21, 36).
Figure 5. RA treatment of moDCs increases DC-T cell conjugate formation and induces a Treg phenotype.
(A) The fold increase (mean ± SEM, n=9) in the frequency of DC-T cell conjugates (% of events positive for CD3 staining within the large DC gate) in RA-DC-T cell co-cultures in the absence and in presence of αRAR compared with moDC-T cell co-cultures (set as 1) are shown. (B) The fold increase (mean ± SEM, n=9) in the frequency of α4β7high, FOXP3+, PD1+ and CD69+ CD4+ T cells in RA-DC-T cell vs. moDC-T cell mixtures are shown (without or with the addition of αRAR). *p<0.05 is considered significant; **p<0.01.
RA-DCs promote greater HIV replication than moDCs in DC-T cell mixtures
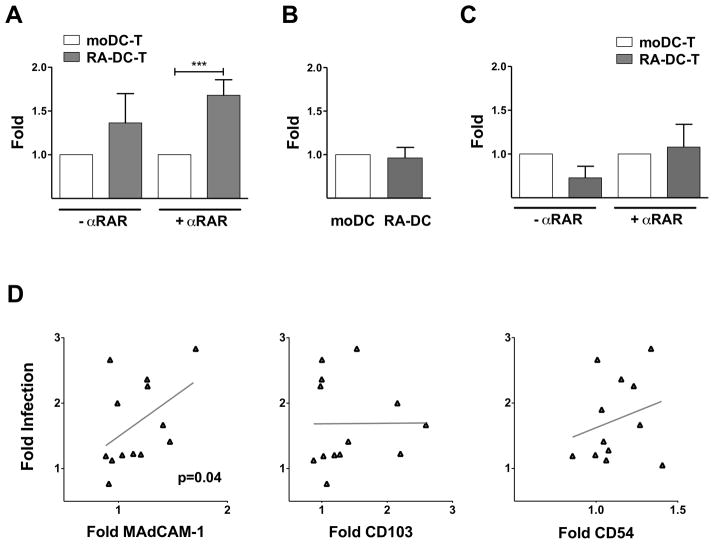
Considering the impact of RA on the DC phenotype and the effect of the RA-DCs on the T cells, we hypothesized that RA may change the ability of DCs to spread HIV infection. To demonstrate this, we co-cultured HIV-loaded RA-DCs and moDCs with autologous CD4+ T cells. Since RA can induce T cell activation and modulate HIV replication (41–46), we cultured the infected moDC-T cell and RA-DC-T cell mixtures in presence of αRAR or a mock solution. Remarkably, HIV replication was significantly higher in the RA-DC-T cell mixtures in presence of αRAR (Fig. 6A) and it was also higher, but not significantly, in the absence of the αRAR. This indicates that changes induced in the DCs by RA, other than the induction of RA-producing capabilities in the DCs, are responsible for driving HIV replication in the RA-DC-T cell milieu. HIV replication in the co-cultures treated with αRAR was lower than in their absence (Supplemental Fig. S4A) and this was likely due to blocking the effect of serum-derived RA and RA released by the RA-DCs on the T cells. The RA-DC-driven increase in HIV infection in the DC-T cell mixtures was not due to an enhanced ability of RA-DCs to capture the virions (Fig. 6B) nor to increased HIV replication in the RA-DCs (Fig. 6C).
Figure 6. RA-DCs drive greater HIV replication than moDCs in DC-T cell cultures.
(A) The fold increase in HIV copies/cell (mean ± SEM, n= 7–10) for the RA-DC-T cell cultures are shown compared to the control moDC-T cell mixtures (set as 1) in the absence and presence of αRAR (***p<0.001). The fold increase in the MFI of the anti-p24 staining of HIV-pulsed DCs (B) and in HIV copies/cell of day 6-infected DC cultures (C) (mean ± SEM, n= 8) are shown for RA-DCs vs. moDCs (set as 1). (D) The fold increases in the MFI of MAdCAM-1, CD54 and CD103 on RA-DCs compared with moDCs (set as 1) are plotted against the fold increases in infection (HIV copies/cell) in the RA-DC-T cell mixtures (over moDC-T cell infections) with αRAR (each dot represents 1 donor run in triplicate; n=9–12). The linear regression fitting line and the spearman rank correlation p values are shown (*p<0.05 is considered significant).
Since RA modulated the expression of specific receptors on the DCs, but not others, we investigated if any of the changes in the expression of these surface proteins could be correlated with the increase in HIV infection in the RA-DC-T cell co-cultures. Among all the receptors impacted by RA, only the increase in the expression of MAdCAM-1 correlated with the increase in HIV replication in the co-cultures in the presence of αRAR (Fig. 6D). Interestingly, neither the increased expression of CD103, marker of mucosal DCs, nor of CD54, known to impact the formation of virological synapses, correlated with the increase in HIV infection in the co-cultures (Fig. 6D).
To further explore the biology of RA-DC-T cell infection, we investigated if the HIV-infected RA-DC-T cell cultures released different soluble factors than the infected moDC-T cell cultures. Supernatants from day 3 and day 6 of the co-cultures were analyzed by 25-Plex luminex, but no significant differences were noted (not shown). Similar to the RA-DC cultures (Fig. 2), more CCL2 was detected in the RA-DC-T cell mixtures, but the difference was not significant. RA-DC-T cell co-cultures released slightly lower amounts of the inflammatory cytokines CXCL-10 (IP-10) and IFNγ, but this was also not significant (not shown).
Blocking MAdCAM-1 partially decreases the enhanced ability of RA-DCs to fuel HIV infection
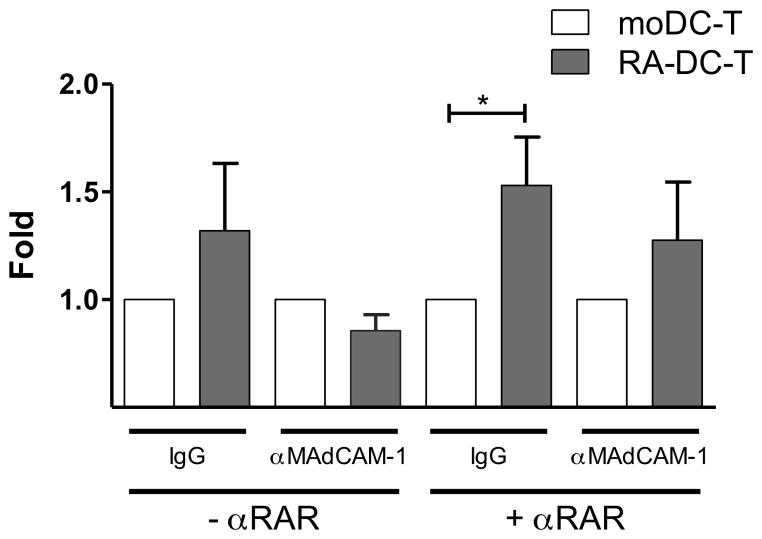
Since we determined that the RA-driven increase in MAdCAM-1 expression on RA-DCs correlated with the increase in HIV replication in the RA-DC-T cell cultures, we hypothesized that MAdCAM-1 could be involved, at least partially, in the enhancement of viral replication. First we investigated weather the presence of the anti-MAdCAM-1 mAb clone 314G8 had an impact on DC-T conjugates formation. We found that blocking MAdCAM-1 did not change the frequency of conjugates, while, as previously reported (47), blocking of CD54 reduced conjugate formation of about 50%. However, this was independent of the DC treatment and of presence of αRAR (Supplemental Fig. S4C). Subsequently, we compared the HIV infection levels in presence or absence of the anti-MAdCAM-1 mAb. When the anti-MAdCAM-1 mAb was added, the increase in HIV infection seen in the RA-DC-T cell co-cultures in presence of the αRAR was no longer significant (relative to the respective moDC controls; Fig. 7). In absence of the αRAR, the infection in the RA-DC-T cell mixtures was reduced to the levels seen in the moDC-T cells. No difference in HIV replication was noted between moDC-T cell co-cultures in presence or absence of the anti-MAdCAM-1 mAb (Supplemental Fig. S4B).
Figure 7. Blocking MAdCAM-1 protein reduced the RA-DC’s enhanced ability to increase HIV infection.
DC-T cell co-cultures were set up (in the presence or absence of αRAR) with RA-DCs or moDCs pre-treated with IgG or anti-MAdCAM-1 (αMAdCAM-1). The fold increases in HIV infection (HIV copies/cell) in RA-DC-T cell vs. moDC-T cell cultures (set as 1) are shown (mean ± SEM, n=7). *p<0.05 is considered significant.
Discussion
The GALT is a primary site of HIV expansion and dissemination after mucosal exposure and its disruption correlates with HIV disease progression (11, 13, 48). Moreover, loss of CD103+ mucosal DCs has been associated with damage in the gut tissue (49). Hence, DCs in this anatomical location play a decisive role in HIV transmission and pathogenesis. However, how the intestinal environment shapes the ability of DCs to fuel HIV infection has never been investigated. DCs in the LP, PPs, and MLNs, but not in the skin or peripheral LNs, have the unique ability to produce RA. Gut-DCs gain this hallmark feature during their education and maturation in the intestinal compartment and RA itself has a key role in shaping the DC phenotype (16, 23, 50, 51).
Here we describe in detail the effect of RA on specific markers of DC maturation, on receptors involved in HIV attachment, internalization and infection, and on adhesion molecules important for DC trafficking. After extensive phenotyping of human RA-DCs, we found that RA conditioning increased the expression of some maturation markers, not affecting or reducing the expression of others such as CD80 and CD206. Thus, RA-DCs appear to have a semi-mature phenotype. Notably, our results confirm other reports that RA increases the expression of CD103 (36), a marker of mucosal DCs in both mice and humans (50). We also found a substantial increase in the expression of CD141. High levels of this receptor are expressed by cross-presenting human DCs with functional homology to mouse CD103 and LN DCs that express high levels of CD103 co-express high levels of CD141 (52, 53). Moreover, human gut CD103+ Sirpα− (hSP, for human single positive) DCs share significant similarities with human blood CD141+ DCs (50). Although the monocytic origin of LP CD103+ DCs is disputed (54), CD103 pairing with β7 forms integrin αEβ7, which is essential for cell trafficking and retention within the epithelium of mucosal tissues. Moreover, we found that RA-DCs secrete higher amounts of total TGF-β1, which is an important factor in the maintenance of the tolerogenic gut environment. TGF-β1 is critical to the differentiation of iTreg acting in synergy with RA (18). In line with this, we showed that RA-DCs induced the expression of FOXP3, PD1 and CD69 on co-cultured T cells, all three important markers of iTreg phenotype (39, 55). This was also true when the T cells were pretreated with αRAR, suggesting that the effect of RA-DCs in T cells is mediated either by TGF-β1 alone or by additional RA-driven changes in DCs. Notably, the increase in the frequency of α4β7high CD4+ T cells was still present after blocking the ability of CD4+ T cells to respond to RA. The meaning and mechanism of this finding need further investigation. We could not detect the activated form of TGF-β1 in most of the samples probably because the sensitivity of the assay was too low. Nonetheless, an increased amount of total TGF-β1 in the gut microenvironment could be transformed in bioactive TGF-β1 by additional state-specific stimuli. In fact, upon stimulation with anti-CD40 agonist murine bone marrow, RA-DCs were shown to release more bioactive TGF-β1 (21). Our data on FOXP3 expression by CD4+ T cells agree with the murine model of RA-DCs described by Feng et al. (21), but differ from those of Bakdash et al. (36). This may be due to the several differences between our models of human RA-DCs and RA-DC-T cell cultures. The differences include the use in Bakdash’s work of allogeneic naïve CD4+ T cells and generation of RA-DCs with the addition of RA from day 0. Moreover, most of their conclusions are drawn by data on LPS-activated RA-DCs. In line with the RA-DCs mostly tolerogenic phenotype, in our model, RA-DCs released more IL-10, while, although significant, the increase in inflammatory cytokines was very small. RA treatment also substantially increased the release of the Th2-skewing chemokine CCL2, suggesting that RA-conditioned DCs may also regulate a Th2 type of response (37).
One of the most intriguing and possibly controversial findings, of our work was the detection of MAdCAM-1 on moDCs, which seems to be increased by RA conditioning. In vivo, we detected MAdCAM-1 on the surface of a small population of DCs in rhesus macaque MLNs. Notably, only clones against the Ig domain, but not the mucin domain, recognized MAdCAM-1 on both human and macaque DCs. This may be explained by the existence of alternatively spliced variants that lack either parts of the second Ig domain, all or part of the mucin domain (56, 57). These findings indicate that the expression of MAdCAM-1 may be highly specific and its function may be regulated by extensive modifications to its multi-domain structure (56). Considering that the primary focus of our work was to investigate the effect of RA on the ability of DCs to spread HIV, a more thorough investigation of the expression of MAdCAM-1 by DCs will be part of future work.
Remarkably, we demonstrated that, compared to immature moDCs, RA-DCs have an enhanced ability to augment HIV infection in DC-T cell cultures. This increase was significant when the RA receptors were blocked. It is likely that the presence of the antagonists reduced the intra-assay variability due to the effect of serum-derived and DC-derived RA, which can directly modulate CD4+ T cells activation and HIV replication (41–43, 45) and possibly mask the enhancing effect due to RA-DCs. Thus, we found that RA treatment modulates the DC phenotype in a way that increases their ability to fuel HIV replication independent of the presence of RA or other gut-specific factors. Ultimately, the influence of the gut microenvironment on HIV replication will be driven by the sum of the many effects of RA, TGF-β1 and other gut-specific factors and will require further exploration in more complex systems. RA-DCs expressed significantly more CD4, CCR5, and CXCR4, they were not more susceptible to HIV infection and they did not have an increased ability to capture/internalize the virus. On the other hand, the higher frequency of DC-T cell conjugates, as well as an increased availability of highly susceptible α4β7high CD4+ T cells may play a role in the enhanced HIV replication in the RA-DC-T cell co-cultures by increasing the efficiency of viral transfer from DCs to T cells. Although the RA-driven increase in MAdCAM-1 expression on DCs suggests a role for MAdCAM-1 in the RA-DC-mediated increase in HIV replication, blocking this receptor only partially reduced the infection in the RA-DC-T cell co-cultures and had little effect on the moDC-T cell mixtures. Thus, MAdCAM-1 increased expression alone does not explain the RA-DC-driven increase in HIV replication. Nonetheless, enhanced MAdCAM-1 interaction with α4β7 on the CD4+ T cells may induce a stronger costimulatory signal increasing cell activation and HIV replication (58). This could explain why blocking MAdCAM-1 reduced the RA-DC-driven enhancement of HIV replication, while having no effect on conjugate formation. The αMAdCAM-1 inhibitory effect appears stronger in absence of αRAR. This might be due to the fact that the initial increase in HIV replication was lower (non-significant) or to a specific effect created by blocking MAdCAM-1 in presence of RA.
Finally, we found that RA increases the expression of CD54, but this did not correlate with the increase in HIV replication and that blocking CD54 inhibited DC-T cell conjugate formation, but this was independent of RA treatment of DCs. Blockage of CD54 would disrupt HIV transfer between DCs and CD4+ T cells independent of RA treatment of DCs and cannot explain the increase in HIV replication specifically due to RA treatment of DCs. Other not yet identified DC receptors could be influenced by RA treatment and play a role in the RA-DCs’ increased ability to drive HIV infection. In conclusion, herein we describe the effect of RA, a key mediator of the intestinal immunological response, on an array of DC surface receptors and soluble factors. We report for the first time the detection of MAdCAM-1 on DCs and its increase by RA treatment. Our results suggest that in an RA-rich microenvironment, such as the small intestine and the GALT, DCs interact with CD4+ T cells in a way that supports higher levels of HIV replication. Considering the importance of the GALT in HIV transmission, pathogenesis and in the establishment and maintenance of the HIV reservoir (12, 13), our results suggest that the mechanisms of HIV expansion in this anatomical site may be different from those known in blood, genital submucosa and skin. An increased awareness of these mechanisms is key to the development of effective means of HIV eradication.
Supplementary Material
Acknowledgments
We would like to thank: Drs. Jeffrey Lifson and Julian Bess for kindly providing the HIV-BaL used for these study; the staff of the Population Council Cell Biology and Flow Cytometry Facility; the staff of the TNPRC for their continued support.
Footnotes
This work was funded with the support of the NIH base grants R37 AI040877-15, R01 AI098456-01 and the United States Agency for International Development (USAID) Cooperative Agreement GPO-A-00-04-00019-00. Additional support was provided by the Tulane National Primate Research Center Base Grant OD011104. NGP is a F. M. Kirby Foundation fellow and MR is a 2002 Elizabeth Glaser scientist.
Disclosure
The authors declare no conflict of interest.
References
- 1.Pope M, Betjes MGH, Romani N, Hirmand H, Cameron PU, Hoffman L, Gezelter S, Schuler G, Steinman RM. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell. 1994;78:389–398. doi: 10.1016/0092-8674(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 2.Derby N, Martinelli E, Robbiani M. Myeloid dendritic cells in HIV-1 infection. Current opinion in HIV and AIDS. 2011;6:379–384. doi: 10.1097/COH.0b013e3283499d63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peressin M, Proust A, Schmidt S, Su B, Lambotin M, Biedma ME, Laumond G, Decoville T, Holl V, Moog C. Efficient transfer of HIV-1 in trans and in cis from Langerhans dendritic cells and macrophages to autologous T lymphocytes. AIDS. 2014;28:667–677. doi: 10.1097/QAD.0000000000000193. [DOI] [PubMed] [Google Scholar]
- 4.Turville SG, Aravantinou M, Stossel H, Romani N, Robbiani M. Resolution of de novo HIV production and trafficking in immature dendritic cells. Nature methods. 2008;5:75–85. doi: 10.1038/nmeth1137. [DOI] [PubMed] [Google Scholar]
- 5.McDonald D. Dendritic Cells and HIV-1 Trans-Infection. Viruses. 2010;2:1704–1717. doi: 10.3390/v2081704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rinaldo CR. HIV-1 Trans Infection of CD4(+) T Cells by Professional Antigen Presenting Cells. Scientifica. 2013;2013:164203. doi: 10.1155/2013/164203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilkinson J, Cunningham AL. Mucosal transmission of HIV-1: first stop dendritic cells. Current drug targets. 2006;7:1563–1569. doi: 10.2174/138945006779025482. [DOI] [PubMed] [Google Scholar]
- 8.Harman AN, Kim M, Nasr N, Sandgren KJ, Cameron PU. Tissue dendritic cells as portals for HIV entry. Reviews in medical virology. 2013;23:319–333. doi: 10.1002/rmv.1753. [DOI] [PubMed] [Google Scholar]
- 9.Boltjes A, van Wijk F. Human dendritic cell functional specialization in steady-state and inflammation. Frontiers in immunology. 2014;5:131. doi: 10.3389/fimmu.2014.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 11.Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. The Journal of experimental medicine. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 13.Mehandru S, Poles MA, Tenner-Racz K, Manuelli V, Jean-Pierre P, Lopez P, Shet A, Low A, Mohri H, Boden D, Racz P, Markowitz M. Mechanisms of gastrointestinal CD4+ T-cell depletion during acute and early human immunodeficiency virus type 1 infection. Journal of virology. 2007;81:599–612. doi: 10.1128/JVI.01739-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenchley JM, Douek DC. HIV infection and the gastrointestinal immune system. Mucosal immunology. 2008;1:23–30. doi: 10.1038/mi.2007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nature reviews. Immunology. 2005;5:783–792. doi: 10.1038/nri1706. [DOI] [PubMed] [Google Scholar]
- 16.Scott CL, Aumeunier AM, Mowat AM. Intestinal CD103+ dendritic cells: master regulators of tolerance? Trends in immunology. 2011;32:412–419. doi: 10.1016/j.it.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, Coombes JL, Berg PL, Davidsson T, Powrie F, Johansson-Lindbom B, Agace WW. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. The Journal of experimental medicine. 2008;205:2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mucida D, Pino-Lagos K, Kim G, Nowak E, Benson MJ, Kronenberg M, Noelle RJ, Cheroutre H. Retinoic acid can directly promote TGF-beta-mediated Foxp3(+) Treg cell conversion of naive T cells. Immunity. 2009;30:471–472. doi: 10.1016/j.immuni.2009.03.008. author reply 472–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moro JR, Iwata M, von Andriano UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nature reviews. Immunology. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cassani B, Villablanca EJ, De Calisto J, Wang S, Mora JR. Vitamin A and immune regulation: role of retinoic acid in gut-associated dendritic cell education, immune protection and tolerance. Molecular aspects of medicine. 2012;33:63–76. doi: 10.1016/j.mam.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng T, Cong Y, Qin H, Benveniste EN, Elson CO. Generation of mucosal dendritic cells from bone marrow reveals a critical role of retinoic acid. J Immunol. 2010;185:5915–5925. doi: 10.4049/jimmunol.1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iliev ID, Mileti E, Matteoli G, Chieppa M, Rescigno M. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal immunology. 2009;2:340–350. doi: 10.1038/mi.2009.13. [DOI] [PubMed] [Google Scholar]
- 23.Vicente-Suarez I, Larange A, Reardon C, Matho M, Feau S, Chodaczek G, Park Y, Obata Y, Gold R, Wang-Zhu Y, Lena C, Zajonc DM, Schoenberger SP, Kronenberg M, Cheroutre H. Unique lamina propria stromal cells imprint the functional phenotype of mucosal dendritic cells. Mucosal immunology. 2014 doi: 10.1038/mi.2014.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klebanoff CA, Spencer SP, Torabi-Parizi P, Grainger JR, Roychoudhuri R, Ji Y, Sukumar M, Muranski P, Scott CD, Hall JA, Ferreyra GA, Leonardi AJ, Borman ZA, Wang J, Palmer DC, Wilhelm C, Cai R, Sun J, Napoli JL, Danner RL, Gattinoni L, Belkaid Y, Restifo NP. Retinoic acid controls the homeostasis of pre-cDC-derived splenic and intestinal dendritic cells. The Journal of experimental medicine. 2013;210:1961–1976. doi: 10.1084/jem.20122508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tao Y, Yang Y, Wang W. Effect of all-trans-retinoic acid on the differentiation, maturation and functions of dendritic cells derived from cord blood monocytes. FEMS immunology and medical microbiology. 2006;47:444–450. doi: 10.1111/j.1574-695X.2006.00108.x. [DOI] [PubMed] [Google Scholar]
- 26.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Cicala C, Martinelli E, McNally JP, Goode DJ, Gopaul R, Hiatt J, Jelicic K, Kottilil S, Macleod K, O’Shea A, Patel N, Van Ryk D, Wei D, Pascuccio M, Yi L, McKinnon L, Izulla P, Kimani J, Kaul R, Fauci AS, Arthos J. The integrin alpha4beta7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20877–20882. doi: 10.1073/pnas.0911796106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kader M, Bixler S, Roederer M, Veazey R, Mattapallil JJ. CD4 T cell subsets in the mucosa are CD28+Ki-67-HLA-DR-CD69+ but show differential infection based on alpha4beta7 receptor expression during acute SIV infection. Journal of medical primatology. 2009;38(Suppl 1):24–31. doi: 10.1111/j.1600-0684.2009.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinelli E, Veglia F, Goode D, Guerra-Perez N, Aravantinou M, Arthos J, Piatak M, Jr, Lifson JD, Blanchard J, Gettie A, Robbiani M. The frequency of alpha4beta7high memory CD4+ T cells correlates with susceptibility to rectal SIV infection. J Acquir Immune Defic Syndr. 2013 doi: 10.1097/QAI.0b013e31829f6e1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Byrareddy SN, Kallam B, Arthos J, Cicala C, Nawaz F, Hiatt J, Kersh EN, McNicholl JM, Hanson D, Reimann KA, Brameier M, Walter L, Rogers K, Mayne AE, Dunbar P, Villinger T, Little D, Parslow TG, Santangelo PJ, Villinger F, Fauci AS, Ansari AA. Targeting alpha4beta7 integrin reduces mucosal transmission of simian immunodeficiency virus and protects gut-associated lymphoid tissue from infection. Nature medicine. 2014;20:1397–1400. doi: 10.1038/nm.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobayashi M, Hoshino H, Suzawa K, Sakai Y, Nakayama J, Fukuda M. Two distinct lymphocyte homing systems involved in the pathogenesis of chronic inflammatory gastrointestinal diseases. Seminars in immunopathology. 2012;34:401–413. doi: 10.1007/s00281-012-0302-3. [DOI] [PubMed] [Google Scholar]
- 32.Leung E, Kanwar RK, Kanwar JR, Krissansen GW. Mucosal vascular addressin cell adhesion molecule-1 is expressed outside the endothelial lineage on fibroblasts and melanoma cells. Immunology and cell biology. 2003;81:320–327. doi: 10.1046/j.1440-1711.2003.t01-1-01175.x. [DOI] [PubMed] [Google Scholar]
- 33.Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, Casazza JP, Kuruppu J, Kunstman K, Wolinsky S, Grossman Z, Dybul M, Oxenius A, Price DA, Connors M, Koup RA. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 34.Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Forster R, Agace WW. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. The Journal of experimental medicine. 2005;202:1063–1073. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Springer TA, Dustin ML. Integrin inside-out signaling and the immunological synapse. Current opinion in cell biology. 2012;24:107–115. doi: 10.1016/j.ceb.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bakdash G, Vogelpoel LT, van Capel TM, Kapsenberg ML, de Jong EC. Retinoic acid primes human dendritic cells to induce gut-homing, IL-10-producing regulatory T cells. Mucosal immunology. 2014 doi: 10.1038/mi.2014.64. [DOI] [PubMed] [Google Scholar]
- 37.Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404:407–411. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- 38.Szabo MC, Butcher EC, McEvoy LM. Specialization of mucosal follicular dendritic cells revealed by mucosal addressin-cell adhesion molecule-1 display. J Immunol. 1997;158:5584–5588. [PubMed] [Google Scholar]
- 39.Cortes JR, Sanchez-Diaz R, Bovolenta ER, Barreiro O, Lasarte S, Matesanz-Marin A, Toribio ML, Sanchez-Madrid F, Martin P. Maintenance of immune tolerance by Foxp3 regulatory T cells requires CD69 expression. Journal of autoimmunity. 2014 doi: 10.1016/j.jaut.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amarnath S, Mangus CW, Wang JC, Wei F, He A, Kapoor V, Foley JE, Massey PR, Felizardo TC, Riley JL, Levine BL, June CH, Medin JA, Fowler DH. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Science translational medicine. 2011;3:111ra120. doi: 10.1126/scitranslmed.3003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hall JA, Cannons JL, Grainger JR, Dos Santos LM, Hand TW, Naik S, Wohlfert EA, Chou DB, Oldenhove G, Robinson M, Grigg ME, Kastenmayer R, Schwartzberg PL, Belkaid Y. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity. 2011;34:435–447. doi: 10.1016/j.immuni.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bidad K, Salehi E, Oraei M, Saboor-Yaraghi AA, Nicknam MH. Effect of all-trans retinoic acid (ATRA) on viability, proliferation, activation and lineage-specific transcription factors of CD4+ T cells. Iranian journal of allergy, asthma, and immunology. 2011;10:243–249. [PubMed] [Google Scholar]
- 43.Turpin JA, Vargo M, Meltzer MS. Enhanced HIV-1 replication in retinoid-treated monocytes. Retinoid effects mediated through mechanisms related to cell differentiation and to a direct transcriptional action on viral gene expression. J Immunol. 1992;148:2539–2546. [PubMed] [Google Scholar]
- 44.Yamaguchi K, Groopman JE, Byrn RA. The regulation of HIV by retinoic acid correlates with cellular expression of the retinoic acid receptors. AIDS. 1994;8:1675–1682. doi: 10.1097/00002030-199412000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Towers G, Harris J, Lang G, Collins MK, Latchman DS. Retinoic acid inhibits both the basal activity and phorbol ester-mediated activation of the HIV long terminal repeat promoter. AIDS. 1995;9:129–136. [PubMed] [Google Scholar]
- 46.Zoumpourlis V, Ergazaki M, Spandidos DA. Transcriptional activation of the human immunodeficiency virus long terminal repeat sequences by retinoic acid in human epithelial and fibroblast tumor cell lines. The International journal of biological markers. 1996;11:153–158. doi: 10.1177/172460089601100303. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez-Plata MT, Puigdomenech I, Izquierdo-Useros N, Puertas MC, Carrillo J, Erkizia I, Clotet B, Blanco J, Martinez-Picado J. The infectious synapse formed between mature dendritic cells and CD4(+) T cells is independent of the presence of the HIV-1 envelope glycoprotein. Retrovirology. 2013;10:42. doi: 10.1186/1742-4690-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hofer U, Speck RF. Disturbance of the gut-associated lymphoid tissue is associated with disease progression in chronic HIV infection. Seminars in immunopathology. 2009;31:257–266. doi: 10.1007/s00281-009-0158-3. [DOI] [PubMed] [Google Scholar]
- 49.Klatt NR, Estes JD, Sun X, Ortiz AM, Barber JS, Harris LD, Cervasi B, Yokomizo LK, Pan L, Vinton CL, Tabb B, Canary LA, Dang Q, Hirsch VM, Alter G, Belkaid Y, Lifson JD, Silvestri G, Milner JD, Paiardini M, Haddad EK, Brenchley JM. Loss of mucosal CD103+ DCs and IL-17+ and IL-22+ lymphocytes is associated with mucosal damage in SIV infection. Mucosal immunology. 2012;5:646–657. doi: 10.1038/mi.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watchmaker PB, Lahl K, Lee M, Baumjohann D, Morton J, Kim SJ, Zeng R, Dent A, Ansel KM, Diamond B, Hadeiba H, Butcher EC. Comparative transcriptional and functional profiling defines conserved programs of intestinal DC differentiation in humans and mice. Nature immunology. 2014;15:98–108. doi: 10.1038/ni.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iliev ID, Spadoni I, Mileti E, Matteoli G, Sonzogni A, Sampietro GM, Foschi D, Caprioli F, Viale G, Rescigno M. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut. 2009;58:1481–1489. doi: 10.1136/gut.2008.175166. [DOI] [PubMed] [Google Scholar]
- 52.Haniffa M, Shin A, Bigley V, McGovern N, Teo P, See P, Wasan PS, Wang XN, Malinarich F, Malleret B, Larbi A, Tan P, Zhao H, Poidinger M, Pagan S, Cookson S, Dickinson R, Dimmick I, Jarrett RF, Renia L, Tam J, Song C, Connolly J, Chan JK, Gehring A, Bertoletti A, Collin M, Ginhoux F. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity. 2012;37:60–73. doi: 10.1016/j.immuni.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van de Ven R, van den Hout MF, Lindenberg JJ, Sluijter BJ, van Leeuwen PA, Lougheed SM, Meijer S, van den Tol MP, Scheper RJ, de Gruijl TD. Characterization of four conventional dendritic cell subsets in human skin-draining lymph nodes in relation to T-cell activation. Blood. 2011;118:2502–2510. doi: 10.1182/blood-2011-03-344838. [DOI] [PubMed] [Google Scholar]
- 54.Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, Stanley ER, Nussenzweig M, Lira SA, Randolph GJ, Merad M. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gianchecchi E, Delfino DV, Fierabracci A. Recent insights into the role of the PD-1/PD-L1 pathway in immunological tolerance and autoimmunity. Autoimmunity reviews. 2013;12:1091–1100. doi: 10.1016/j.autrev.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 56.Leung E, Greene J, Ni J, Raymond LG, Lehnert K, Langley R, Krissansen GW. Cloning of the mucosal addressin MAdCAM-1 from human brain: identification of novel alternatively spliced transcripts. Immunology and cell biology. 1996;74:490–496. doi: 10.1038/icb.1996.81. [DOI] [PubMed] [Google Scholar]
- 57.Sampaio SO, Li X, Takeuchi M, Mei C, Francke U, Butcher EC, Briskin MJ. Organization, regulatory sequences, and alternatively spliced transcripts of the mucosal addressin cell adhesion molecule-1 (MAdCAM-1) gene. J Immunol. 1995;155:2477–2486. [PubMed] [Google Scholar]
- 58.Teague TK, Lazarovits AI, McIntyre BW. Integrin alpha 4 beta 7 co-stimulation of human peripheral blood T cell proliferation. Cell adhesion and communication. 1994;2:539–547. doi: 10.3109/15419069409014217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.