Abstract
The administration of estradiol-17β (E) to animal models after loss of ovarian steroid production has many beneficial effects on neural functions, particularly in the serotonin system in nonhuman primates (NHPs). E also has anorexic effects, although the mechanism of action is not well defined. In the US, obesity has reached epidemic proportions, and blame is partially directed at the Western style diet, which is high in fat and sugar. This study examined the interaction of E and diet in surgically menopausal nonhuman primates with a 2 × 2 block design. Marmosets (Callithrix jacchus; n=4/group) were placed on control-low fat diet (LFD; 14%kcal from fat) or high fat diet (HFD; 28%kcal from fat) 1 month prior to ovariectomy (Ovx). Empty (placebo) or E-filled Silastic capsules were implanted immediately following Ovx surgery. Treatments extended 6 months. The established groups were: placebo+LFD; E+LFD; placebo+HFD, or E+HFD. At necropsy, the brain was flushed with saline and harvested. The midbrain was dissected and a small block containing the dorsal raphe nucleus was processed for qRT-PCR using Evagreen (Biotinum). Genes previously found to impact serotonin neural functions were examined. Results were compared with 2-way ANOVA followed by Bonferroni post-hoc tests or Cohen’s D analysis. There was a significant effect of treatment on tryptophan hydroxylase 2 (TPH2) across the groups (p=0.019). E stimulated TPH2 expression and HFD prevented E-stimulated TPH2 expression (p<0.01). Treatment differentially affected monoamine oxidase B (MAO-B) across the groups (p=0.05). E increased MAO-B with LFD, and this stimulatory effect was prevented by HFD (p<0.05). There was a significant difference between treatments in corticotrophin releasing factor-receptor 2 (CRF-R2) expression (p=0.012). E increased CRF-R2 and this stimulatory effect was blocked by HFD (p<0.01). Regardless of diet, E increased Fev mRNA (p=0.028) and decreased CRF-receptor 1 (CRF-R1) mRNA (p=0.04). HFD suppressed urocortin 1 (UCN1; stresscopin) expression (p=0.045) but E treatment had no effect. Monoamine oxidase A (MAO-A) was different due to treatment across the groups (p=0.028). MAO-A was increased in the E+HFD group (p<0.01) whereas previous studies showed E suppressed MAO-A in macaques. The serotonin reuptake transporter (SERT), the serotonin 1A receptor (5HT1A), estrogen receptor beta (ERβ) and progestin receptor (PR) expressions were not different between groups. Estrogen receptor alpha (ERα) was undetectable. In summary, the data indicate that important actions of hormone therapy in the serotonin system may be lost in the context of a HFD.
Keywords: Non-human primate, marmosets, ovariectomy, estrogen, estrogen receptors, diet, high fat, serotonin, CRF receptors, tryptophan hydroxylase, serotonin reuptake transporter, serotonin autoreceptor, monoamine oxidase
Introduction
Optimal function of the serotonin neural system is critical for numerous and wide-ranging outcomes such as mood or affect (Halbreich and Tworek, 1993), anxiety (Linthorst, 2005, Reimold et al., 2008), cognition (Merens et al., 2008), metabolic syndrome (Muldoon et al., 2004), sleep (Dugovic, 2001), pituitary hormone secretion (Van de Kar et al., 2001) and vulnerability to stress (Bethea et al., 2008, Bethea et al., 2005, Maier and Watkins, 2005). In women, and female animal models, ovarian steroids support serotonin neural function; and loss of ovarian hormones leads to suboptimal serotonin neurotransmission with an accompanying deterioration of supported functions. (Bethea et al., 2002, Frey et al., 2008, Halbreich et al., 1995, Hiroi and Neumaier, 2006, Oxenkrug, 2010).
We have devoted significant effort to understanding the cellular effects of ovarian steroids on the serotonin system in a macaque model of surgical menopause. Ovarian hormone replacement to ovariectomized (Ovx) macaques changed the expression of serotonin related genes and proteins in a manner that would cause an increase in serotonin neurotransmission (Bethea, Lu, 2002, Centeno et al., 2007, Sanchez et al., 2005, Smith et al., 2004). In a similar macaque model, E increased serotonin in several compartments of the basal ganglia and in frontal and cingulate cortex, supporting the concept that E increased serotonin neurotransmission (Sanchez et al., 2013). Also, E decreased raphe 5HT1A receptors of hemi-parkinsonian macaques, thereby removing the ‘brakes’ on serotonin neuronal activity (Sanchez, Morissette, 2013). An important component of improved serotonin function was the ability of E to decrease expression of CRF-R1 and increase expression of CRF-R2 in dorsal raphe (Sanchez et al., 2010). These receptors decrease or increase serotonin neurotransmission, respectively (Forster et al., 2008, Keck et al., 2005, Lukkes et al., 2008, Pernar et al., 2004). Furthermore, E appeared to increase the axonal transport of urocortin 1 (UCN1), also in the CRF family of peptides (Bethea and Reddy, 2012, Sanchez, Reddy, 2010). UCN1 binds to CRF-R2, thereby promoting serotonin transmission (Amat et al., 2004, Skelton et al., 2000).
In addition to improvements in serotonergic function, we found that 1-month of estradiol (E) plus progesterone (P) administration to Ovx macaques decreased gene and protein expression of pivotal proteins in the caspase-independent apoptosis pathway (Bethea and Reddy, 2008, Tokuyama et al., 2008). Moreover, 1- month of E+P treatment significantly decreased DNA fragmentation in serotonin neurons of Ovx macaques, as determined with a TUNEL assay (Lima and Bethea, 2009). Recently, we found that ovarian steroid administration increased gene expression related to DNA repair, protein folding, ubiquinases, and transport as well (data submitted for publication). Altogether, the data indicate that ovarian steroids not only promote serotonin neuronal function, but also maintain cell health and viability. Thus, the effects of E on the serotonin neural system in macaques were robustly positive, suggesting that in women, E therapy should increase aspects of cognition and executive function as well as decrease anxiety, depression and vulnerability to stress while increasing coping mechanisms. That is, E therapy should render a postmenopausal woman more capable of executive decisions and resilient to stress.
One of the major problems with hormone therapy for postmenopausal women has been the inconsistency in results from trial to trial depending on the time of administration relative to menopause, the formulation of the hormones administered, the endpoints examined and the length of treatment times. Moreover, hormone replacement in women has often lacked the expected robust outcomes observed in the neurobiology of animal models. However, more recent human trials have found consistent improvements in mood and cognition when women are administered bioidentical estradiol in the perimenopausal period (Schmidt et al., 2004, Schmidt et al., 2013, Schmidt and Rubinow, 2009; Amin et al., 2005, Epperson et al., 2012)
Our previous studies utilized a cost effective adult macaque model of surgical menopause with brief hormone replacement. Importantly, the animals were always maintained on a diet of normal monkey chow (LabDiet 5000), which is low in sugar and fat and high in micronutrients. More recently, scientists are recognizing that lab animal chow differs significantly from the average American diet. Postmenopausal women in the US eat a Western style diet, which is high in fat and sugar. Often weight gain accompanies menopause with detectable changes in the distribution of body fat and muscle.
Therefore, we questioned the effect of a high-fat and -sugar diet on the beneficial effects of E in the serotonin system, which were previously observed with a low-fat and -sugar diet. To approach this question, we used a small, new world primate of the Callithrix genus, the common marmoset. Marmosets are social monkeys with a 28-day ovulatory cycle similar to rhesus macaques and women (Abbott et al., 2003, Jull et al., 2014, Tardif et al., 2011).
Materials and Methods
This study was approved by the IACUC of the Wisconsin National Primate Research Center (WNPRC) and conducted in accordance with the 2011 Eight Edition of the National Institute of Health Guide for the Care and Use of Laboratory Animals
Animals
The interaction of E and diet in surgically menopausal nonhuman primates was examined with a 2 × 2 block design. Sixteen adult female marmosets (Callithrix jacchus; n=4/group) were used. All animals were born at the WNPRC, were aged between 3–4 years, weighed between 350 and 400 gm and were in good health. They were placed on control-low fat diet (LFD) or high fat diet (HFD) ad libitum 1 month prior to ovariectomy. Age and weight were documented prior to experimental diet initiation and at necropsy.
The marmoset model of estrogen replacement therapy has been previously described (Kendrick and Dixson, 1985). For bilateral ovariectomy, females were anesthetized with ketamine (15 mg/kg, im) and maintained on isoflurane (2%; 0.6 liter/min oxygen). Atropine (0.02 – 0.04 mg/kg i.m.) and buprenorphine (0.01 mg/kg i.m.) were also administered at the time of ketamine administration. Each ovary was isolated through a ventral midline incision and exteriorized for visualization of the fallopian tube and ovarian pedicle. Subsequent histological examination confirmed complete ovarian removal. At the end of the surgery while the animals were still under anesthesia, two Silastic™ capsules (inner diameter, 0.058 in.; outer diameter, 0.077 in.; length, 11-mm; Dow Corning, Midland, MI) were subcutaneously implanted into each female via a small dorsal midline incision between the scapulae. A 2% lidocaine (2 mg/kg) sc injection was also used to anesthetize the skin. Four females on each diet were implanted with E-filled capsules (1,3,5(10)-estratrien-3,17-b-diol; Sigma, St. Louis, MO) previously shown to maintain approximately pre-ovulatory-phase levels of E (Abbott, D. H., unpublished observations), whereas the remaining females were implanted with empty Silastic™ capsules. The treatments continued for 6 months.
Diets
The diets were lactalbumin, dextrin, and sucrose based from Teklad Custom Research Diets (Madison, WI). LFD consists by weight of 14.0% protein, 64.3% carbohydrate and 5.6% fat. HFD consists of 15.4% protein, 56.5% carbohydrate and 12.7% fat. HFD has 2 times more sucrose and 2.4 times more fat than LFD. Diet and E treatments continued for 6 months. Hence, the established groups were: placebo+LFD; E+LFD; placebo+HFD, or E+HFD.
Hormone Assays
Serum E concentrations were measured at WNPRC with liquid chromatography-tandem mass spectrometry (LC/MS/MS). Briefly, samples were liquid extracted with methyl-tert-butyl- ether (MTBE) followed by dichloromethane. Samples were then derivitized with dansyl chloride before injection. Within-assay coefficients of variation were less than 6% and between-assay coefficients of variation were less than 13%.
Euthanasia and Tissue Retrieval
The monkeys were euthanized at the end of the treatment periods according to procedures recommended by the Panel on Euthanasia of the American Veterinary Association. Each animal was sedated with intramuscular administered ketamine within 1–3 minutes of cage entry and given an intravenous overdose of pentobarbital (50 mg/kg, i.v.) within 7–11 minutes of cage entry.
The upper body was flushed with 700 ml of saline and the brain was harvested. After freezing in isopentane (−78.5°C), the brain was stored at −80°C until it was shipped to ONPRC on dry ice. Using a warmed scalpel, the pontine midbrain was removed from the frozen brain and further micro-dissected to obtain a small block of tissue that contained the serotonergic dorsal raphe nucleus.
qRT-PCR
RNA was obtained from the microdisected block containing the dorsal raphe nucleus using TriReagent and further cleaned with a Qiagen RNAeasy column (Velencia, CA). The quality of the RNA from the Qiagen column was examined on an Agilent Bioanalyzer and found acceptable and of equal quality.
Complementary DNA (cDNA) synthesis was performed using Oligo-dT 15 primer (Invitrogen Life Technologies, Carlsbad, CA) and Superscript III reverse transcriptase (200 U/μg of RNA, Invitrogen Life Technologies) at 42°C for 1 hr. A pool of RNAs from different rhesus tissues was used as the standard.
Quantitative polymerase chain reaction (qRT-PCR) was conducted with Fast EvaGreen qPCR mix (Biotium #31003-1). This mix contains Evagreen® qPCR dye and Cheetah™ Taq hotstart DNA polymerase. There is a linear increase in fluorescence detected as the concentration of amplified double stranded product cDNA increases during the reaction. The fluorescence was detected with an ABI 7900 thermal cycler during forty cycles. The reaction (final volume 20 μl) contained dilutions of a standard pool containing 1 – 1000 ng of total cDNA or samples containing 50ng of total cDNA, 100 nM of forward and reverse primers and 1X Invitrogen PCR mix. The amount of cDNA added to the reaction mix was measured as sscDNA with the Nanodrop Spectrophotometer, and from the amount of RNA used for reverse transcriptase. A standard curve was generated for all of the primer sets. The slope of the curve was used to calculate the relative pg of each transcript in the RNA extracted from the raphe blocks. Then, the ratio of each transcript to GAPDH was calculated. Genes previously found to impact serotonin neural functions were examined. and monkey specific gene sequences were used in primer selection. The genes coded for tryptophan hydroxylase (TPH2), the serotonin reuptake transporter (SERT), the serotonin 1A autoreceptor (5HT1A), monoamine oxidases A and B (MAO-A, MAO-B), corticotropin releasing factor receptors 1 and 2 (CRF-R1, CRF-R2), urocortin 1 (UCN1), estrogen receptor alpha (ERα), estrogen receptor beta (ERβ), and progestin receptor (PR). Housekeeping genes 18S and GAPDH were also measured. GAPDH was used for normalization of gene expression. Cycle times (CT) for GAPDH were 26.2±0.32, 26.03±0.57, 25.92±0.17 and 26.21±0.32 in placebo+LFD, E+LFD, placebo+HFD and E+HFD groups, respectively (F [3,41]=0.167; p=0.918). These values fell on the linear portion of the standard curve and there was no difference between the groups.
Primer selection
At the time of the study, marmoset sequences were not available for the genes of interest. Therefore, we designed primers using Macaca mulatta specific gene sequences. Despite a divergence time of 25 million years between the Old World and New World monkeys, the primers amplified excellently in Callithrix jaccus. In evolutionary terms, positive selection occurred after divergence in select pathways of marmosets largely relating to twinning and body size, which did not include the genes in this study (Marmoset Genome et al., 2014).
The coding regions of each gene in macaques were compared to related genes and a unique region was identified with no homology. The target sequence was then loaded into Primer Express software, which chooses the primers for optimum qRT-PCR. The primers were obtained from Invitrogen Life Technologies. Direct comparison of homology between macaques and marmosets was not found. However, macaques have 97.5% and marmosets have 93.8% exonic sequence homology with humans, so the sequence homology between macaques and marmosets should be quite high (Sierens et al., 2004). The primers utilized are shown in Table 1.
Table 1.
Primer sequences used in qRT-PCR reactions with related information. The primers were derived from rhesus macaque or human sequences depending on availability.
| Gene name | Gene Symbol | NCBI Gene reference | Primer sequences | Amplicon size |
|---|---|---|---|---|
| Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | XR_013566 | F CTGACACTGAGTACGTCGTGGA R TGGACTGTGGTCATGAGTCCTT |
268 |
| Tryptophan Hydroxylase 2 | TPH2 | AY_098914 | F CTGACACTGAGTACGTCGTGGA R TCCCAGTGACAGGAAGAG |
253 |
| FEV1 Oncogene similar to PET1 | FEV1 | XM_001095962 | F_CAGAAAGGCAGCGGACAGAT R_CCTGGAAGTCGAAGCGGTAG |
268 |
| SLC6A4(SERT)rhesus | SERT | EF126285 | F_CAGAGGTGGTGTGGGTGACAG R_GACAAATCCCGAAACGAAGCT |
305 |
| 5HT1A receptor rhesus | 5HTR1A | XM_001083407 | F_ACCGGGTCCCTTTGAGACC R_ACGTCCAGGGCGATAAACAG |
301 |
| Monoamine Oxidase A | MAO-A | XM_001096840 | F_CCTCCTGGGATCATGACTCAA R_AGGTTCCTCTCCCAGAAGGTG |
251 |
| Monoamine Oxidase B | MAO-B | XM_001096953 | F_TGTGTGTCACTGCAGAGACCC R_TTCATGCCCAGAGTAGGAGGA |
301 |
| ER alpha estrogen receptor 1 | ESR1 | XM_001097228 | F_ACCAACCAGTGCACCATTGA R_GGCCGGGCTGTTCTTCTTA |
252 |
| ER beta estrogen receptor 2 | ESR2 | XM_001101433 | F_TGCGCTGTCTGCAGTGATTT R_TGCCACTCCTCTTGGCCTT |
301 |
| Progesterone receptor | PR | XM_001095317 | F_AGGGCCTGCAGCAGGTCTA R_TGCGGATTTTATCAACGATGC |
251 |
| Corticotropin releasing factor receptor 1 | CRFR1 | NM_001145148.1 | F_ TTTCAACATCGTCCGCATC R_ATGGCAGAACGGACCTCACT |
262 |
| Corticotropin releasing factor receptor 2 | CRFR2 | Xm_001085887.2 | F_CACAGTGTGAGCCCATTTTGG R_TTCGCAGGATAAGGTGGTGA |
192 |
| Urocortin1 (UCN1) | UCN1 | NM_001265661.1 | F_CCTGGGAACAGCCAGAGGA R_TATGATGCGGTTCTGCTCGG |
294 |
Statistical analysis
The average relative expression of each gene across the 4 groups (n=4 animals/group) from the qRT-PCR assays were compared with 2-way ANOVA followed by Bonferroni’s posthoc pairwise comparison. Variance between animals is not unusual for this type of preparation, and more animals would reduce the chance of making a type 2 error. Therefore, negative results need further confirmation. Comparisons were considered significantly different when there was a 95% or greater chance that the groups were different (p≤0.05). Posthoc pairwise comparisons were performed with Bonferroni’s test and p<0.05 was accepted as different. Prism 5.0 from Graph Pad (San Diego, CA) was used for ANOVA comparisons. Cohen’s D analysis was applied to determine effect size when trends were observed (http://www.uccs.edu/~lbecker/).
Results
The average age and weights of the marmosets during the experimental period are shown in Table 2. At the beginning of the experiment, the animals were matched by age and weight. The weights were in the normal range for adult marmosets. After 6 months of treatment, there was no statistical difference in weight due to treatment or time (2-way ANOVA). However, there was a 4.5% increase in weight in the combined HFD groups compared to a 1% increase in the combined LFD groups. Over time, Ovx per se will increase weight, but HFD exacerbated this effect. There was a 2% decrease in weight from baseline in the E+LFD group, whereas there was a 5% increase in weight from baseline in the E+HFD group. Serum estradiol concentrations (pg/ml) at 2 months after Silastic capsule implantation equaled 14.9±6.7, 6.6±1.5, 1556±552 and 1387±425 in the LFD+placebo, HFD+placebo, LFD+E and HFD+E, respectively. There was a significant difference between the groups for drug treatment (F [1,12]=17.58; p = 0.0012).
Table 2.
Age and weight of marmosets before and after experimental manipulations. Control (C) indicates the placebo treated groups.
| Baseline | ||||||
|---|---|---|---|---|---|---|
| Age (yr) | Weight (g) | |||||
| Mean | SEM | Range | Mean | SEM | Range | |
| C +LFD | 3.45 | 0.57 | 2.6–5.1 | 384.25 | 14.10 | 349–414 |
| E +LFD | 3.47 | 0.27 | 3.2–4.0 | 437.67 | 38.86 | 360–479 |
| C + HFD | 3.43 | 0.28 | 2.7–4.0 | 386.00 | 14.67 | 361–420 |
| E + HFD | 3.59 | 0.52 | 2.7–5.1 | 404.00 | 12.32 | 379–438 |
| At necropsy | ||||||
|---|---|---|---|---|---|---|
| Age (yr) | Weight (g) | |||||
| Mean | SEM | Range | Mean | SEM | Range | |
| C +LFD | 4.03 | 0.57 | 3.2–5.7 | 397.50 | 21.80 | 354–458 |
| E +LFD | 4.05 | 0.27 | 3.8–4.6 | 426.00 | 50.82 | 333–508 |
| C + HFD | 4.01 | 0.28 | 3.2–4.6 | 402.50 | 13.93 | 367–435 |
| E + HFD | 4.17 | 0.52 | 3.3–5.7 | 421.75 | 17.74 | 373–450 |
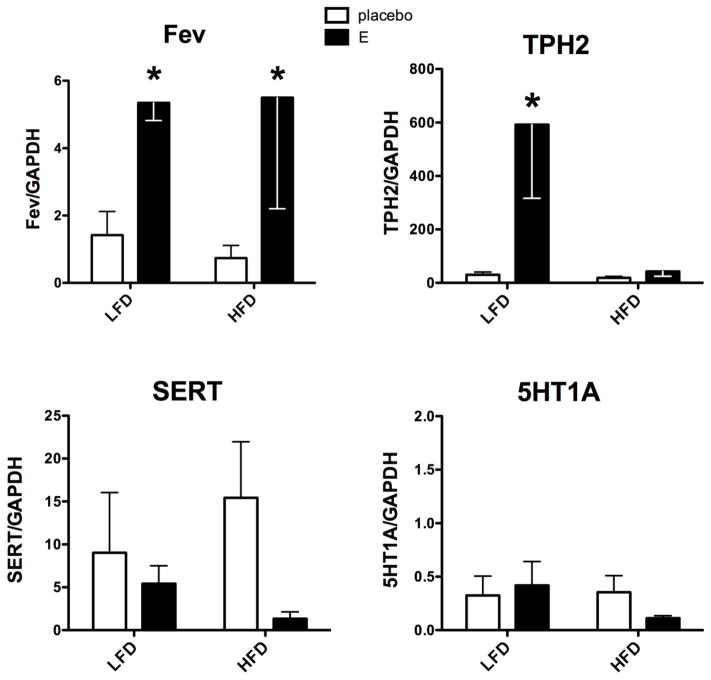
Figure 1 illustrates the relative mRNA expression of serotonergic specific genes, TPH2, SERT, 5HT1A autoreceptor and Fev, the serotonin master gene (Hendricks et al., 1999). The Fev gene, which determines the serotonergic phenotype of neurons and regulates TPH2, SERT and 5HT1A gene expression (Hendricks, Francis, 1999, Krueger and Deneris, 2008, Liu et al., 2010), exhibited a significant effect of treatment (F[1,11]=7.37; p=0.028). There was no effect of diet and no interaction. E treatment increased Fev mRNA expression on both diets. Of note, previous studies suggest that expression of downstream genes should reflect the expression of Fev (Hendricks, Francis, 1999). However, the downstream genes in this study had different patterns of expression compared to Fev.
Figure 1.
Histograms are shown that illustrate the effect of diet and E treatment on serotonin-related gene expression in Ovx marmosets. Fev expression was increased by E-treatment in both diet groups (2-way ANOVA treatment effect, F[1,11]=7.37; p=0.023). E significantly stimulated TPH2 on LFD, but not on HFD (Bonferroni p<0.01). There was no statistical difference in SERT between the groups, but the effect size of E treatment was greater with HFD (Cohen’s D =0.59) than with LFD (Cohen’s D= 0.199). 5HT1A expression was unchanged by diet or E treatment.
*statistically different, Bonferroni’s posthoc test.
TPH2 mRNA expression exhibited a significant effect of treatment (F[1,11]=7.55; p=0.02), a significant effect of diet (F[1,11]=6.92; p= 0.023) and a significant interaction (F[1,11]=6.34; p=0.028). TPH2 was significantly higher in E-treated than placebo-treated Ovx monkeys on LFD (p<0.01). In marked contrast, TPH2 mRNA expression did not respond to E in Ovx monkeys on HFD.
There was no effect of treatment or diet on SERT mRNA expression, largely due to the variance around a small number of animals. However, there was an interesting trend in the delta between placebo- and -E-treatments. That is, there was a modest reduction in SERT mRNA expression with E treatment on LFD. With HFD, the difference between placebo- and E-treated groups appeared larger. This ‘effect size’ can be calculated with Cohen’s D analysis, where 0.2 is a small effect, 0.6 is a medium effect and 0.8 is a large effect. In the LFD groups, the effect size of the treatments equaled 0.199, indicating a small effect. In the HFD groups, the effect size of the treatments equaled 0.59, indicating a medium effect. There was no change in expression of 5HT1A autoreceptor mRNA across the groups.
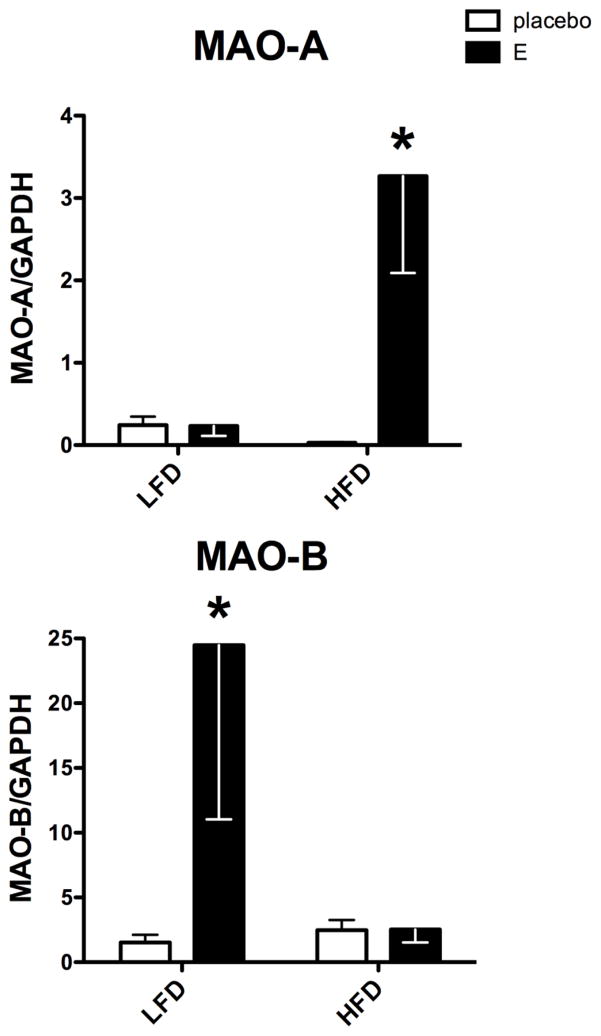
Figure 2 illustrates the mRNA expression of the MAO-A and MAO-B genes that code for biogenic amine degradation with preference for serotonin, norepinephrine (NE), and dopamine (DA) respectively. MAO-A mRNA expression exhibited a significant effect of treatment (F[1,11]=6.31; p=0.03), a significant effect of diet (F[1,11]=4.8; p=0.05), and a significant interaction (F[1,11]=6.38; p=0.028). MAO-A mRNA expression with LFD was low in the Ovx-placebo group and unchanged by E treatment. However, with HFD, MAO-A was low in the Ovx-placebo group, but significantly increased by E treatment (p<0.01).
Figure 2.
Histograms are shown that illustrate the effect of diet and E treatment on gene expression of amine degradation enzymes in Ovx marmosets. MAO-A expression exhibited a significant effect of E treatment, diet and an interaction (p= 0.23, 0.029 and 0.028, respectively). MAO-A was significantly higher in the E+HFD group than in the placebo+HFD group (Bonferroni p<0.01). MAO-B expression exhibited a significant effect of E treatment (p=0.05), and no effect of diet, with a potential interaction between the two (p=0.0508). MAO-B expression was significantly higher in the E+LFD group than the placebo+LFD group (Bonferroni p<0.05).
*statistically different, Bonferroni’s posthoc test.
MAO-B mRNA expression exhibited a significant effect of treatment (F[1,11]=4.84; p=0.05), but no effect of diet. However, there was a significant interaction between treatment and diet (F [1,11]=4.81; p=0.05). With LFD, E treatment significantly increased MAO-B mRNA expression over placebo treatment (p<0.05). However, with HFD, E failed to increase MAO-B over the Ovx-placebo group.
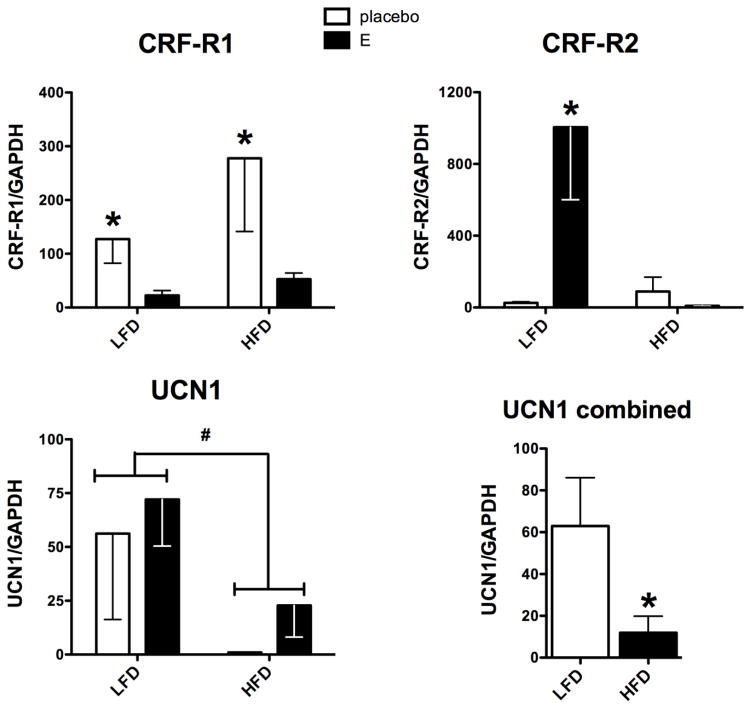
Figure 3 illustrates the mRNA expression of the two CRF receptors, R1 and R2, as well as the mRNA expression of UCN1. CRF-R1 is considered an anxiogenic receptor (Kirby et al., 2008, Kirby et al., 2000). CRF-R1 mRNA expression exhibited a significant effect of treatment (F[1,11]=5.73; p=0.04). However, there was no effect of diet on CRF-R1 and no interaction between treatment and diet. CRF-R1 was higher in the Ovx-placebo groups than in the E-treated groups on both diets. Of note, HFD elevated CRF-R1 in both control and E-treated groups ~2-fold compared to LFD. Cohen’s D analysis for placebo versus E-treatment in each diet group showed the effect size was 0.626 and 0.557, for LFD and HFD, respectively, where values ~0.6 are considered medium effects.
Figure 3.
Histograms are shown that illustrate the effect of diet and E treatment on gene expression of CRF receptors and UCN1 in Ovx marmosets. CRF-R1 exhibited a significant effect of E treatment (p=0.040), but no effect of diet and no interaction between E treatment and diet. Cohen’s D analysis of placebo versus E treatment in each diet group showed a large effect size equal to 0.626 and 0.557, for LFD and HFD, respectively. CRF-R2 expression showed significant effects of E treatment and diet with a significant interaction between the two (p=0.017, 0.014 and 0.007, respectively). There was a significant increase in CRF-R2 in the E treated group compared to the control group in the LFD cohort (Bonferroni p<0.01), but not in the HFD cohort. UCN1 expression was significantly suppressed by the HFD diet (t test p=0.045), but it was not affected by E treatment.
*statistically different, Bonferroni’s posthoc test or t-test
# 2-way ANOVA (F[1,11]=4.52; p=0.057)
CRF-R2 mediates anxiolytic actions (Bale and Vale, 2004, Valentino et al., 2010). CRF-R2 mRNA expression exhibited a significant effect of treatment (F [1,11]=7.76; p=0.017), a significant effect of diet (F[1,11]=8.34; p=0.015), and a significant interaction (F[1,11]=10.73; p=0.007). With LFD, CRF-R2 exhibited low mRNA expression in the Ovx-placebo group and E treatment significantly increased CRF-R2 mRNA expression (p<0.01). In contrast, with HFD, E treatment failed to increase CRF-R2 mRNA expression over the Ovx-placebo group.
The mRNA expression of UCN1, previously named ‘stresscopin’, appeared to change with diet, but it was not statistically different (F[1,11]=4.52; p=0.057). There was no effect of treatment and no interaction between diet and treatment. The diet effect was more apparent by combining the data from control and E treated animals, and then comparing by diet. There was a significant decrease in UCN1 mRNA in the HFD cohort compared to the LFD cohort (t-test, p=0.045).
Examination of 3 steroid receptors showed that ERα mRNA was undetectable. Figure 4 illustrates the mRNA expression of the two detectable steroid receptors, ERβ and PR. There was no effect of treatment or diet on ERβ, and due to the variance even Cohen’s D was negative. However, this needs to be repeated with more animals to completely discount the trends. PR expression was low and unchanged across the treatment groups.
Figure 4.
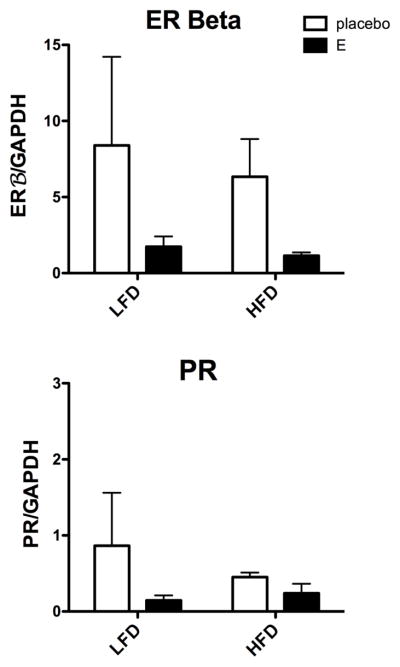
Histograms are shown that illustrate the effect of diet and E treatment on gene expression of ERβ and PR in Ovx marmosets. There was no statistical difference between the groups in the expression of ERβ or PR, although confirmation with more animals is needed.
Discussion
This study concerns two conundrums of health care today: hormone replacement therapy for postmenopausal women and the epidemic of obesity concomitant with metabolic syndrome. Important contributors to obesity are lack of exercise and consumption of a high-fat and -sugar Western-style diet. Two-thirds of the US population is overweight or obese, while 40% exhibit symptoms of pre- or frank diabetes. Thus, the average woman eats a Western style diet and has a very good chance of being overweight when she enters menopause. It is not possible to find the BMIs of all the women included in the numerous clinical trials of hormone replacement therapy, but it stands to reason that many subjects were probably overweight or obese. We hypothesized that obesity or a Western style diet per se could contribute to the different responses of animal models and women to hormone replacement therapy.
Hormone therapy has been under assault for treatment of menopausal women since release of the results of the Women’s Health Initiative (WHI), which administered conjugated equine estrogens and medroxyprogesterone acetate to women approximately 10 years after onset of menopause. Neither of these formulations are human hormones. Hence, the term ‘hormone replacement’ is a misnomer with regard to the WHI. Nonetheless, estrogenic compounds have been used in women after complete hysterectomy, and estrogenic plus progestogenic compounds should be used in women with a reproductive tract. We used immediate E-only replacement in marmosets with only ovarian removal for this 6-month study since development of endometrial cancer was not a concern within that time frame, and all animals were scheduled for euthanasia.
Many physiological endpoints have been measured in women on hormone therapy compared to women without hormone therapy. Differences that pertain to various functions of the brain, such as cognition and mood have been particularly difficult to document. However, careful studies using imaging techniques or studies working with younger, perimenopausal women are finding important beneficial effects on neural functions and outcomes between women that do take hormone therapy compared to controls (Amin et al., 2005, Amin et al., 2006a, Amin et al., 2006b, Epperson et al., 2012, Schmidt, Keenan, 2013, Schmidt and Rubinow, 2009, Steinberg et al., 2008). In addition, interaction between E and serotonin has emerged in tests of working memory and mood in women (Epperson, Amin, 2012). The beneficial effect of E in laboratory models on a large number of different neural outcomes is undisputed.
In contrast, HFD significantly impairs memory, working memory attention and inhibitory control in humans. Likewise, rodents on HFD exhibit significant impairment in spatial and non-spatial memory tasks, negative-feature discrimination problems, variable-interval delayed-alteration task and discrimination reversal task suggesting that HFD impairs inhibitory control [human and animal studies reviewed in (Francis and Stevenson, 2013)]. Careful parsing of behavior data indicated that HFD markedly decreased attention to testing (Reyes, 2014). HFD precipitates depressive behaviors in macaques, (Chilton et al., 2011, Shively et al., 2008), and cognitive impairment in rodents (Pistell et al., 2010, White et al., 2009).
To examine the interaction of diet on E action in the serotonin system, Ovx marmosets were maintained on specially formulated LFD or HFD, and individuals from each diet group were treated with E or placebo. HFD had various effects on gene expression. Notably, HFD prevented a number of beneficial actions of E on gene expression in the serotonin system. In addition, some genes responded to E in a manner that was independent of diet, and one gene appeared to respond to diet only. Several genes were not regulated by E or diet.
E potently stimulated TPH2, CRF-R2 and MAO-B with LFD in marmosets, but E was unable to stimulate TPH2, CRF-R2 or MAO-B in marmosets on HFD. TPH2 codes for the rate- limiting enzyme in serotonin synthesis, and it is pivotal for optimum serotonin neurotransmission (Walther and Bader, 2003). We have shown that E stimulates TPH2 mRNA and protein expression in Ovx macaques and in lazer captured serotonin neurons (Bethea and Reddy, 2008, Bethea et al., 2000, Sanchez, Reddy, 2005). The ability of HFD to block the stimulatory effect of E on TPH2 mRNA expression means that eating a diet high in fat would compromise serotonin production, which in turn could lead to depression and cognitive impairment.
CRF-R2 is considered an anxiolytic receptor and it stimulates serotonin neurotransmission (Bale and Vale, 2004, Forster, Pringle, 2008, Lukkes, Forster, 2008, Reul and Holsboer, 2002, Valentino, Lucki, 2010). We previously postulated that E-induced serotonin inhibits CRF production in the hypothalamus of macaques (Bethea and Centeno, 2008, Sanchez, Reddy, 2010) forming a beneficial feedback loop for regulation of the stress response. CRF-R2 also plays an important role in homeostasis. While on a high-fat diet, CRF-R2 knock out mice consumed substantially more food (Bale et al., 2003). We showed that E stimulated CRF-R2 expression in macaques (Sanchez, Reddy, 2010). E also stimulated CRF-R2 in marmosets on LFD, but not with HFD. Thus, the block of E-induced CRF-R2 mRNA expression could remove an important mechanism for terminating the stress response and may drive further feeding.
MAO-B gene expression adds to the problems generated by HFD. MAO-B degrades norepinephrine and dopamine more readily than serotonin in primates (Garrick and Murphy, 1982), and it is robustly expressed in terminal regions like the raphe (Gundlah et al., 2001). NE inhibits serotonin neurons (Clement et al., 1992, Haddjeri et al., 1996) and elevated NE is found in anxiety disorders (Abelson et al., 1991, Siever et al., 1982). In addition, NE increases vigilance in nonhuman primates (Aston-Jones et al., 1991). Removing NE can ameliorate anxiety, which means that elevated MAO-B could act to restrain anxiety. We previously found that MAO-B protein was elevated in the raphe of macaques with 1- or 5-mo of E+P treatment (Smith et al., 2004). In this study, E stimulated MAO-B mRNA with LFD, but not HFD. Therefore with LFD, one mechanism by which E could decrease anxiety is through elevated MAO-B. Clearly this response was blocked by HFD. In women on HFD, this could compound the loss of E-induced TPH2 and CRF-R2 gene expression, altogether potentially promoting both depression and anxiety as well as cognitive decline.
The expression of 2 genes, Fev and CRF-R1, responded to E in a manner that may have been independent of diet. E increased Fev expression in both diets. Fev, also called Pet1, has been studied intensely in rodents. It is pivotal in neural development and determines serotonergic phenotype (Hendricks et al., 1999; Beck et al., 2010). Data in humans and macaques on LFD largely agree with the rodent studies (Iyo et al., 2005, Krueger and Deneris, 2008, Lima et al., 2009). Fev also contributes to the regulation of TPH2, SERT and 5HT1A genes in rodents on lab chow (Hendricks, Francis, 1999, Krueger and Deneris, 2008, Liu, Maejima, 2010). We previously reported that Ovx significantly reduced Fev gene expression in Japanese macaques (Bethea et al., 2012). Consistently, in this study Fev expression was significantly lower in the Ovx placebo groups compared to the E-treated groups, but diet did not affect the response. However, Fev expression trended lower in the placebo group on HFD compared to the placebo group on LFD. More animals are needed to determine the significance of this small trend, but if validated it would mean that HFD alone could decrease Fev. In this study, TPH2 expression reflected Fev expression on LFD, but not HFD, while SERT and 5HT1A did not reflect Fev on either diet. Interpretation of the different patterns probably requires better understanding of Fev expression, regulation and downstream effects in marmosets. Nonetheless, it is attractive to speculate that HFD prevents Fev from interacting with its promoter on downstream genes in an unknown manner.
CRF-R1 is the anxiogenic receptor, and it inhibits serotonin neurotransmission (Bailey et al., 2011, Kirby, Rice, 2000). E inhibited CRF-R1 on both diets, which is consistent with our previous observations in rhesus monkeys on low fat chow (Sanchez, Reddy, 2010). CRF-R1 knock out mice also exhibited resistance to diet-induced obestity (Sakamoto et al., 2013) suggesting that the E-induced decrease in CRF-R1 would have a similar beneficial action. However, the overall expression of CRF-R1 was two-fold higher in both treatment groups on HFD compared to the same treatments on LFD. In contrast, mice on HFD exhibited a reduction in CRF-R1 in the amygdala (Sharma et al., 2013). We have previously observed a difference in the hypothalamic-adrenal-axis between the response of monkeys and mice (Bethea and Centeno, 2008). If HFD does increase CRF-R1 in higher primates then a woman on HFD, with or without E replacement, might retain enough CRF-R1 will have more inhibition of serotonin than a woman on LFD.
UCN1 mRNA expression appeared to be dependent on diet alone and E did not appear to affect UCN1 expression. Rather, HFD significantly reduced UCN1 compared to LFD. UCN1, is important for terminating the stress response (Bale and Vale, 2004, Dautzenberg and Hauger, 2002, Reul and Holsboer, 2002). Moreover, peripherally administered UCN1 had a potent inhibitory effect on food intake and weight gain of high-fat-fed obese mice (Tanaka et al., 2009) indicating that UCN1 is also an important factor in satiety. UCN1 has higher affinity for CRF-R2 receptors than CRF-R1 receptors and it stimulates serotonin neurons (Amat, Tamblyn, 2004). Hence, the decrease in both UCN1 and lack of E-induced CRF-R2 mRNA expression with HFD indicates that HFD interferes with 2 important mechanisms for termination of the stress response and satiety.
Several genes did not respond significantly to either diet or E including SERT, 5HT1A, ERβ or PR. The response of the SERT gene to E and diet in our marmosets was not different between the groups due to high ‘within group’ variance. However, the trend points to higher SERT mRNA with placebo compared to E in both diets. SERT performs reuptake duties in the synapse, and theoretically elevated SERT should remove more serotonin, thereby decreasing serotonin neurotransmission and in turn, precipitating depression, Studies with various PET ligands in humans have confounded this notion (Cannon et al., 2007, Dahlstrom et al., 2000, Malison et al., 1998, Meyer et al., 2004, Parsey et al., 2006, Willeit et al., 2000). Further complicating our understanding of SERT are species differences in the response of gene expression to E administration, and lack of translation in accordance with gene expression (McQueen et al., 1996, Pecins-Thompson et al., 1998, Lu et al., 2003). In the marmosets, E reduced SERT mRNA expression with both diets, which could increase serotonin in the synapse, leading to resilience. However, it is premature to speculate how SERT gene expression translates to transporter function in marmosets and more animals are needed to confirm verify the trend. In addition, SERT mRNA expression did not reflect Fev mRNA expression and further investigation on the mechanism of this action is warranted.
There was no effect of diet or treatment on ERβ or PR mRNAs. We previously observed no difference in ERβ mRNA in most brain areas when Ovx rhesus were treated with E or E+P and thus, proposed that ERβ was constitutively expressed in serotonin neurons (Gundlah et al., 2000). The lack of significant difference in ERβ mRNA between the diet groups in this study would support that contention. However, the trend toward suppression of ERβ by E treatment raises the issue of power in the analysis. There could be a difference between marmosets and rhesus in the regulation of ERβ, or not. This examination needs to be repeated with more animals. We did not examine the expression of the membrane ER-1, also known as the orphan receptor GPCR30, and lately referred to as GPER (Filardo and Thomas, 2012). There is now evidence that previously reported activities of GPER in response to E were through its ability to induce ER-alpha36 expression. Hence, the ER-alpha variant, ER-alpha36, not GPER, is involved in non-genomic estrogen signaling (Kang et al., 2010). In our model, the monkeys are treated for months. During that time frame, the nuclear ER would be highly activated although additional actions through a membrane receptor cannot be ruled out.
The expression of MAO-A mRNA on control LFD diverged from previous observations in macaques, which decreases cogency. In previous studies with macaques on normal monkey chow, MAO-A was elevated in OVX animals and significantly suppressed by E administration (Gundlah, Lu, 2001). This would be beneficial because MAO-A degrades serotonin and E would prevent the degradation. In the marmosets, no such regulation was found in LFD groups. With HFD, MAO-A was increased by E treatment, which is the reverse of what was previously observed in macaques on normal monkey chow. This could translate to greater serotonin degradation with E replacement and HFD. Since all of the PCR reactions were conducted on the same samples, we wonder if marmosets are different from macaques in MAO-A regulation. Our Postmenopausal Resource is maintaining older, menopausal rhesus macaques on HFD, with and without E, so future results may shed light on the marmoset results. Although further speculation is reserved, an E-induced increase in MAO-A expression would degrade what little serotonin is made in individuals on HFD.
The absolute change in weight between the groups did not reach statistical significance. However, there was a trend toward weight gain in the animals maintained on HFD. The high metabolic activity of the marmosets may have played a role in the small differences in weight. It should be noted that Ovx per se causes weight gain. The fact that the E+LFD showed a slight decrease in weight while the E+HFD showed a modest increase in weight suggests that E may have lost its anorexic effect in individuals eating a HFD. On the positive side, the relatively minor changes in weight means that the diet itself was playing a larger role than weight gain.
HFD is highly inflammatory (Posey et al., 2009, Reyes, 2014), which points to the possibility that immune signals prevent the normal serotonergic response to E. We showed that E reduces NFκB translocation to the nucleus in serotonin neurons of animals on LFD, which means that serotonin neurons are responsive to cytokines (Bethea et al., 2006). It is attractive to speculate that HFD-induced cytokines and other immune ligands may offset the action of E in the serotonin system. Indeed, HFD in rodents induces cytokine expression in microglia and circumventricular organs of the brain (Reyes, 2014, Serrats et al., 2010).
At this time, we had to choose between examination of mRNA or protein expression since the two procedures require different extraction buffers (qRT-PCR versus westerns) or different processing (qRT-PCR versus immunohistochemistry). With the limited number of animals in this initial study, qRT-PCR was deemed to provide the most information. Funding for expanding this study is sought.
In summary, HFD interferes with the ability of E to positively alter mRNA expression in the dorsal raphe of marmosets. Other studies have shown that HFD leads to depression in macaques and impairs various aspects of cognition in humans and lab animal models as discussed above. Translating our marmoset data and other behavioral data to women eating LFD, E action would support serotonin neurotransmission and degrade NE, thereby decreasing depression and anxiety while increasing verbal memory and executive function. In women consuming HFD, these actions would be blocked and hormone replacement therapy would be ineffective. Loosely assuming that obese people eat a diet high in fat and sugar, then an obese population may find no relief of depression, anxiety or executive function with hormone therapy. If obese women who eat HFD, are represented in clinical trials at the same frequency as the general population, the variance around the treatment group outcomes would make it difficult to detect the beneficial effects of hormone therapy that have been found in animal models on laboratory chow. Altogether, these data suggest that in a large proportion of women in the US, there may be little improvement in serotonin function, mood or cognition following hormone replacement therapy. It will be important to discover if sensitivity to E returns in the serotonin system when animals on HFD are switched back to LFD.
Highlights.
High fat diet blocked the stimulatory effect of estrogen on TPH2 gene expression.
High fat diet blocked the stimulatory effect of estrogen on CRF-R2 receptor expression.
High fat diet blocked the stimulatory effect of estrogen on MAO-B expression.
High fat diet suppressed UCN1 gene expression.
Acknowledgments
The authors are grateful to the WNPRC animal care and veterinary staff, including Dr. Kevin G. Brunner and Victoria R. Carter; as well as to Amber K. Edwards, Megan Sosa and Scott Baum for technical assistance. This study was funded, in part, by NIH grants MH86542 to CLB, 8P51 OD1192, 2P50 HD44405, T32 HD041921 and R25 GM083252.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Abbott DH, Barnett DK, Colman RJ, Yamamoto ME, Schultz-Darken NJ. Aspects of common marmoset basic biology and life history important for biomedical research. Comp Med. 2003;53:339–50. [PubMed] [Google Scholar]
- Abelson JL, Glitz D, Cameron OG, Lee MA, Bronzo M, Curtis GC. Blunted growth hormone response to clonidine in patients with generalized anxiety disorder. Arch Gen Psychiatry. 1991;48:157–62. doi: 10.1001/archpsyc.1991.01810260065010. [DOI] [PubMed] [Google Scholar]
- Amat J, Tamblyn JP, Paul ED, Bland ST, Amat P, Foster AC, et al. Microinjection of urocortin 2 into the dorsal raphe nucleus activates serotonergic neurons and increases extracellular serotonin in the basolateral amygdala. Neuroscience. 2004;129:509–19. doi: 10.1016/j.neuroscience.2004.07.052. [DOI] [PubMed] [Google Scholar]
- Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. Behav Cognitive Neurosci Rev. 2005;4:43–58. doi: 10.1177/1534582305277152. [DOI] [PubMed] [Google Scholar]
- Amin Z, Epperson CN, Constable RT, Canli T. Effects of estrogen variation on neural correlates of emotional response inhibition. Neuroimage. 2006a;32:457–64. doi: 10.1016/j.neuroimage.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Amin Z, Gueorguieva R, Cappiello A, Czarkowski KA, Stiklus S, Anderson GM, et al. Estradiol and tryptophan depletion interact to modulate cognition in menopausal women. Neuropsychopharmacology. 2006b;31:2489–97. doi: 10.1038/sj.npp.1301114. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Chiang C, Alexinsky T. Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Prog Brain Res. 1991;88:501–20. doi: 10.1016/s0079-6123(08)63830-3. [DOI] [PubMed] [Google Scholar]
- Bailey JE, Papadopoulos A, Diaper A, Phillips S, Schmidt ME, van der Ark P, et al. Preliminary evidence of anxiolytic effects of the CRF1 receptor antagonist R317573 in the 7.5% CO2 proof-of-concept experimental model of human anxiety. J Psychopharmacol. 2011;25:1199–206. doi: 10.1177/0269881111400650. [DOI] [PubMed] [Google Scholar]
- Bale TL, Anderson KR, Roberts AJ, Lee KF, Nagy TR, Vale WW. Corticotropin-releasing factor receptor-2-deficient mice display abnormal homeostatic responses to challenges of increased dietary fat and cold. Endocrinology. 2003;144:2580–7. doi: 10.1210/en.2002-0091. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–57. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Beck S, Craige C, Piel D, Krueger K, Deneris E, Calizo L, et al. Abnormal physiology and morphology of raphe serotonin neurons early in development and in those lacking the Pet-1 transcriptional factor. Annual meeting of the American College of Neuropsychopharmacology; Miami, FL. 2010. p. 257. [Google Scholar]
- Bethea C, Reddy A. Effect of ovarian hormones on survival genes in laser captured serotonin neurons from macaques. J Neurochem. 2008;105:1129–43. doi: 10.1111/j.1471-4159.2008.05213.x. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Centeno ML. Ovarian steroid treatment decreases corticotropin-releasing hormone (CRH) mRNA and protein in the hypothalamic paraventricular nucleus of ovariectomized monkeys. Neuropsychopharmacology. 2008;33:546–56. doi: 10.1038/sj.npp.1301442. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Centeno ML, Cameron JL. Neurobiology of stress-induced reproductive dysfunction in female macaques. Mol Neurobiol. 2008;38:199–230. doi: 10.1007/s12035-008-8042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Frontiers in Neuroendocrinology. 2002;23:41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Mirkes SJ, Shively CA, Adams MR. Steroid regulation of tryptophan hydroxylase protein in the dorsal raphe of macaques. Biol Psychiatry. 2000;47:562–76. doi: 10.1016/s0006-3223(99)00156-0. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Pau FK, Fox S, Hess DL, Berga SL, Cameron JL. Sensitivity to stress-induced reproductive dysfunction linked to activity of the serotonin system. Fertil Steril. 2005;83:148–55. doi: 10.1016/j.fertnstert.2004.06.051. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Reddy AP. The effect of long-term ovariectomy on midbrain stress systems in free ranging macaques. Brain Res. 2012;1488:24–37. doi: 10.1016/j.brainres.2012.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Reddy AP, Smith LJ. Nuclear factor kappa B in the dorsal raphe of macaques: an anatomical link for steroids, cytokines and serotonin. J Psychiatry Neurosci. 2006;31:105–14. doi: 10.1016/j.yfrne.2006.03.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Smith AW, Centeno ML, Reddy AP. Long-term ovariectomy decreases serotonin neuron number and gene expression in free ranging macaques. Neuroscience. 2012;49:251–62. doi: 10.1016/j.neuroscience.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon DM, Ichise M, Rollis D, Klaver JM, Gandhi SK, Charney DS, et al. Elevated serotonin transporter binding in major depressive disorder assessed using positron emission tomography and [11C]DASB; comparison with bipolar disorder. Biol Psychiatry. 2007;62:870–7. doi: 10.1016/j.biopsych.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Centeno ML, Reddy AP, Smith LJ, Sanchez RL, Henderson JA, Salli NC, et al. Serotonin in microdialysate from the mediobasal hypothalamus increases after progesterone administration to estrogen primed macaques. Eur J Pharmacol. 2007;555:67–75. doi: 10.1016/j.ejphar.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton FH, Lee TC, Willard SL, Ivester P, Sergeant S, Register TC, et al. Depression and altered serum lipids in cynomolgus monkeys consuming a Western diet. Physiol Behav. 2011;104:222–7. doi: 10.1016/j.physbeh.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement HW, Gemsa D, Wesemann W. The effect of adrenergic drugs on serotonin metabolism in the nucleus raphe dorsalis of the rat, studied by in vivo voltammetry. Eur J Pharmacol. 1992;217:43–8. doi: 10.1016/0014-2999(92)90509-3. [DOI] [PubMed] [Google Scholar]
- Dahlstrom M, Ahonen A, Ebeling H, Torniainen P, Heikkila J, Moilanen I. Elevated hypothalamic/midbrain serotonin (monoamine) transporter availability in depressive drug-naive children and adolescents. Mol Psychiatry. 2000;5:514–22. doi: 10.1038/sj.mp.4000766. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Hauger RL. The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol Sci. 2002;23:71–7. doi: 10.1016/s0165-6147(02)01946-6. [DOI] [PubMed] [Google Scholar]
- Dugovic C. Role of serotonin in sleep mechanisms. Rev Neurol (Paris) 2001;157:S16–9. [PubMed] [Google Scholar]
- Epperson CN, Amin Z, Ruparel K, Gur R, Loughead J. Interactive effects of estrogen and serotonin on brain activation during working memory and affective processing in menopausal women. Psychoneuroendocrinology. 2012;37:372–82. doi: 10.1016/j.psyneuen.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo EJ, Thomas P. Minireview: G protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology. 2012;153:2953–62. doi: 10.1210/en.2012-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster GL, Pringle RB, Mouw NJ, Vuong SM, Watt MJ, Burke AR, et al. Corticotropin-releasing factor in the dorsal raphe nucleus increases medial prefrontal cortical serotonin via type 2 receptors and median raphe nucleus activity. Eur J Neurosci. 2008;28:299–310. doi: 10.1111/j.1460-9568.2008.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis H, Stevenson R. The longer-term impacts of Western diet on human cognition and the brain. Appetite. 2013;63:119–28. doi: 10.1016/j.appet.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Frey BN, Lord C, Soares CN. Depression during menopausal transition: a review of treatment strategies and pathophysiological correlates. Menopause Int. 2008;14:123–8. doi: 10.1258/mi.2008.008019. [DOI] [PubMed] [Google Scholar]
- Garrick NA, Murphy DL. Monoamine oxidase type A: differences in selectivity towards l-norepinephrine compared to serotonin. Biochem Pharmacol. 1982;31:4061–6. doi: 10.1016/0006-2952(82)90656-6. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Kohama SG, Mirkes SJ, Garyfallou VT, Urbanski HF, Bethea CL. Distribution of estrogen receptor beta (ERb) mRNA in hypothalamus, midbrain and temporal lobe of spayed macaque: continued expression with hormone replacement. Mol Brain Res. 2000;76:191–204. doi: 10.1016/s0006-8993(99)02475-0. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Lu NZ, Bethea CL. Ovarian steroid regulation of monoamine oxidase-A and -B mRNAs in the macaque dorsal raphe and hypothalamic nuclei. Psychopharmacology. 2001;160:271–82. doi: 10.1007/s00213-001-0959-0. [DOI] [PubMed] [Google Scholar]
- Haddjeri N, Blier P, de Montigny C. Effect of the alpha-2 adrenoceptor antagonist mirtazapine on the 5-hydroxytryptamine system in the rat brain. J Pharmacol Exp Ther. 1996;277:861–71. [PubMed] [Google Scholar]
- Halbreich U, Rojansky N, Palter S, Tworek H, Hissin P, Wang K. Estrogen augments serotonergic activity in postmenopausal women. Biol Psychiatry. 1995;37:434–41. doi: 10.1016/0006-3223(94)00181-2. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Tworek H. Altered serotonergic activity in women with dysphoric premenstrual syndromes. Int J Psychiatry Med. 1993;23:1–27. doi: 10.2190/J2W0-RTGD-NYKK-FF77. [DOI] [PubMed] [Google Scholar]
- Hendricks T, Francis N, Fyodorov D, Deneris ES. The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes. J Neurosci. 1999;19:10348–56. doi: 10.1523/JNEUROSCI.19-23-10348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi R, Neumaier JF. Differential effects of ovarian steroids on anxiety versus fear as measured by open field test and fear-potentiated startle. Behav Brain Res. 2006;166:93–100. doi: 10.1016/j.bbr.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Iyo AH, Porter B, Deneris ES, Austin MC. Regional distribution and cellular localization of the ETS-domain transcription factor, FEV, mRNA in the human postmortem brain. Synapse. 2005;57:223–8. doi: 10.1002/syn.20178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jull J, Stacey D, Beach S, Dumas A, Strychar I, Ufholz LA, et al. Lifestyle interventions targeting body weight changes during the menopause transition: a systematic review. J Obesity. 2014;2014:824310. doi: 10.1155/2014/824310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Zhang X, Xie Y, Tu Y, Wang D, Liu Z, et al. Involvement of estrogen receptor variant ER-alpha36, not GPR30, in nongenomic estrogen signaling. Mol Endocrinol. 2010;24:709–21. doi: 10.1210/me.2009-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck ME, Ohl F, Holsboer F, Muller MB. Listening to mutant mice: a spotlight on the role of CRF/CRF receptor systems in affective disorders. Neurosci Biobehav Rev. 2005;29:867–89. doi: 10.1016/j.neubiorev.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Dixson AF. Effects of oestradiol 17B, progesterone and testosterone upon proceptivity and receptivity in ovariectomized common marmosets (Callithrix jacchus) Physiol Behav. 1985;34:123–8. doi: 10.1016/0031-9384(85)90089-7. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Freeman-Daniels E, Lemos JC, Nunan JD, Lamy C, Akanwa A, et al. Corticotropin-releasing factor increases GABA synaptic activity and induces inward current in 5-hydroxytryptamine dorsal raphe neurons. J Neurosci. 2008;28:12927–37. doi: 10.1523/JNEUROSCI.2887-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–62. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Krueger KC, Deneris ES. Serotonergic transcription of human FEV reveals direct GATA factor interactions and fate of Pet-1-deficient serotonin neuron precursors. J Neurosci. 2008;28:12748–58. doi: 10.1523/JNEUROSCI.4349-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima FB, Bethea CL. Ovarian steroids decrease DNA fragmentation in the serotonin neurons of non-injured rhesus macaques. Mol Psychiatry. 2009 doi: 10.1038/mp.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima FB, Centeno ML, Costa ME, Reddy AP, Cameron JL, Bethea CL. Stress sensitive female macaques have decreased fifth Ewing variant (Fev) and serotonin-related gene expression that is not reversed by citalopram. Neuroscience. 2009;164:676–91. doi: 10.1016/j.neuroscience.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linthorst AC. Interactions between corticotropin-releasing hormone and serotonin: implications for the aetiology and treatment of anxiety disorders. Handb Exp Pharmacol. 2005:181–204. doi: 10.1007/3-540-28082-0_7. [DOI] [PubMed] [Google Scholar]
- Liu C, Maejima T, Wyler SC, Casadesus G, Herlitze S, Deneris ES. Pet-1 is required across different stages of life to regulate serotonergic function. Nat Neurosci. 2010;13:1190–8. doi: 10.1038/nn.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu NZ, Eshleman AJ, Janowsky A, Bethea CL. Ovarian steroid regulation of serotonin reuptake transporter (SERT) binding, distribution and function in female macaques. Molecular Psychiatry. 2003;8:353–60. doi: 10.1038/sj.mp.4001243. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Forster GL, Renner KJ, Summers CH. Corticotropin-releasing factor 1 and 2 receptors in the dorsal raphe differentially affect serotonin release in the nucleus accumbens. Eur J Pharmacol. 2008;578:185–93. doi: 10.1016/j.ejphar.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–41. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Malison RT, Price LH, Berman R, van Dyck CH, Pelton GH, Carpenter L, et al. Reduced brain serotonin transporter availability in major depression as measured by [123I]-2beta-carbomethoxy-3beta-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry. 1998;44:1090–8. doi: 10.1016/s0006-3223(98)00272-8. [DOI] [PubMed] [Google Scholar]
- Marmoset Genome, Sequence Analysis, Consortium. The common marmoset genome provides insight into primate biology and evolution. Nat Genet. 2014;46:850–7. doi: 10.1038/ng.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen JK, Wilson H, Dow RC, Fink G. Oestradiol-17B increases serotonin transporter (SERT) binding sites and SERT mRNA expression in discrete regions of female rat brain. J Physiology. 1996;495:114. [Google Scholar]
- Merens W, Booij L, Haffmans PM, Van der Does AJ. The effects of experimentally lowered serotonin function on emotional information processing and memory in remitted depressed patients. J Psychopharmacol. 2008 doi: 10.1177/0269881107081531. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Houle S, Sagrati S, Carella A, Hussey DF, Ginovart N, et al. Brain serotonin transporter binding potential measured with carbon 11-labeled DASB positron emission tomography: effects of major depressive episodes and severity of dysfunctional attitudes. Arch Gen Psychiatry. 2004;61:1271–9. doi: 10.1001/archpsyc.61.12.1271. [DOI] [PubMed] [Google Scholar]
- Muldoon MF, Mackey RH, Williams KV, Korytkowski MT, Flory JD, Manuck SB. Low central nervous system serotonergic responsivity is associated with the metabolic syndrome and physical inactivity. J Clin Endocrinol Metab. 2004;89:266–71. doi: 10.1210/jc.2003-031295. [DOI] [PubMed] [Google Scholar]
- Oxenkrug GF. Metabolic syndrome, age-associated neuroendocrine disorders, and dysregulation of tryptophan-kynurenine metabolism. Ann N Y Acad Sci. 2010;1199:1–14. doi: 10.1111/j.1749-6632.2009.05356.x. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Hastings RS, Oquendo MA, Huang YY, Simpson N, Arcement J, et al. Lower serotonin transporter binding potential in the human brain during major depressive episodes. Am J Psychiatry. 2006;163:52–8. doi: 10.1176/appi.ajp.163.1.52. [DOI] [PubMed] [Google Scholar]
- Pecins-Thompson M, Brown NA, Bethea CL. Regulation of serotonin re-uptake transporter mRNA expression by ovarian steroids in rhesus macaques. Mol Brain Res. 1998;53:120–9. doi: 10.1016/s0169-328x(97)00286-6. [DOI] [PubMed] [Google Scholar]
- Pernar L, Curtis AL, Vale WW, Rivier JE, Valentino RJ. Selective activation of corticotropin-releasing factor-2 receptors on neurochemically identified neurons in the rat dorsal raphe nucleus reveals dual actions. J Neurosci. 2004;24:1305–11. doi: 10.1523/JNEUROSCI.2885-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, et al. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol. 2010;219:25–32. doi: 10.1016/j.jneuroim.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posey KA, Clegg DJ, Printz RL, Byun J, Morton GJ, Vivekanandan-Giri A, et al. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab. 2009;296:E1003–12. doi: 10.1152/ajpendo.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimold M, Batra A, Knobel A, Smolka MN, Zimmer A, Mann K, et al. Anxiety is associated with reduced central serotonin transporter availability in unmedicated patients with unipolar major depression: a [11C]DASB PET study. Mol Psychiatry. 2008;13:606–13. 557. doi: 10.1038/sj.mp.4002149. [DOI] [PubMed] [Google Scholar]
- Reul JM, Holsboer F. Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr Opin Pharmacol. 2002;2:23–33. doi: 10.1016/s1471-4892(01)00117-5. [DOI] [PubMed] [Google Scholar]
- Reyes TM. Diet, Inflammation and the Brain: Commentary on the 2014 Named Series. Brain Behav Immun. 2014 doi: 10.1016/j.bbi.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Sakamoto R, Matsubara E, Nomura M, Wang L, Kawahara Y, Yanase T, et al. Roles for corticotropin-releasing factor receptor type 1 in energy homeostasis in mice. Metabolism. 2013;62:1739–48. doi: 10.1016/j.metabol.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Sanchez MG, Morissette M, Di Paolo T. Oestradiol modulation of serotonin reuptake transporter and serotonin metabolism in the brain of monkeys. J Neuroendocrinol. 2013;25:560–9. doi: 10.1111/jne.12034. [DOI] [PubMed] [Google Scholar]
- Sanchez RL, Reddy AP, Bethea CL. Ovarian steroid regulation of the midbrain corticotropin releasing factor and urocortin systems in macaques. Neuroscience. 2010;171:893–909. doi: 10.1016/j.neuroscience.2010.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez RL, Reddy AP, Centeno ML, Henderson JA, Bethea CL. A second tryptophan hydroxylase isoform, TPH-2 mRNA, is increased by ovarian steroids in the raphe region of macaques. Brain Res Mol Brain Res. 2005;135:194–203. doi: 10.1016/j.molbrainres.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Haq N, Rubinow DR. A longitudinal evaluation of the relationship between reproductive status and mood in perimenopausal women. Am J Psychiatry. 2004;161:2238–44. doi: 10.1176/appi.ajp.161.12.2238. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Keenan PA, Schenkel LA, Berlin K, Gibson C, Rubinow DR. Cognitive performance in healthy women during induced hypogonadism and ovarian steroid addback. Arch Womens Ment Health. 2013;16:47–58. doi: 10.1007/s00737-012-0316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Rubinow DR. Sex hormones and mood in the perimenopause. Ann N Y Acad Sci. 2009;1179:70–85. doi: 10.1111/j.1749-6632.2009.04982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrats J, Schiltz JC, Garcia-Bueno B, van Rooijen N, Reyes TM, Sawchenko PE. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron. 2010;65:94–106. doi: 10.1016/j.neuron.2009.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Fernandes MF, Fulton S. Adaptations in brain reward circuitry underlie palatable food cravings and anxiety induced by high-fat diet withdrawal. Int J Obes (Lond) 2013;37:1183–91. doi: 10.1038/ijo.2012.197. [DOI] [PubMed] [Google Scholar]
- Shively CA, Register TC, Adams MR, Golden DL, Willard SL, Clarkson TB. Depressive behavior and coronary artery atherogenesis in adult female cynomolgus monkeys. Psychosom Med. 2008;70:637–45. doi: 10.1097/PSY.0b013e31817eaf0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierens JE, Scobie GA, Wilson J, Saunders PT. Cloning of oestrogen receptor beta from Old and New World primates: identification of splice variants and functional analysis. J Mol Endocrinol. 2004;32:703–18. doi: 10.1677/jme.0.0320703. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Uhde TW, Silberman EK, Jimerson DC, Aloi JA, Post RM, et al. Growth hormone response to clonidine as a probe of noradrenergic receptor responsiveness in affective disorder patients and controls. Psychiatry Res. 1982;6:171–83. doi: 10.1016/0165-1781(82)90005-1. [DOI] [PubMed] [Google Scholar]
- Skelton KH, Owens MJ, Nemeroff CB. The neurobiology of urocortin. Regul Pept. 2000;93:85–92. doi: 10.1016/s0167-0115(00)00180-4. [DOI] [PubMed] [Google Scholar]
- Smith LJ, Henderson JA, Abell CW, Bethea CL. Effects of ovarian steroids and raloxifene on proteins that synthesize, transport and degrade serotonin in the raphe region of macaques. Neuropsychopharmacology. 2004;29:2035–45. doi: 10.1038/sj.npp.1300510. [DOI] [PubMed] [Google Scholar]
- Steinberg EM, Rubinow DR, Bartko JJ, Fortinsky PM, Haq N, Thompson K, et al. A cross-sectional evaluation of perimenopausal depression. J Clin Psychiatry. 2008;69:973–80. doi: 10.4088/jcp.v69n0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka C, Asakawa A, Ushikai M, Sakoguchi T, Amitani H, Terashi M, et al. Comparison of the anorexigenic activity of CRF family peptides. Biochem Biophys Res Commun. 2009;390:887–91. doi: 10.1016/j.bbrc.2009.10.069. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Mansfield KG, Ratnam R, Ross CN, Ziegler TE. The marmoset as a model of aging and age-related diseases. ILAR Journal. 2011;52:54–65. doi: 10.1093/ilar.52.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyama Y, Reddy AP, Bethea CL. Neuroprotective actions of ovarian hormones without insult in the raphe region of rhesus macaques. Neuroscience. 2008;154:720–31. doi: 10.1016/j.neuroscience.2008.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Lucki I, Van Bockstaele E. Corticotropin-releasing factor in the dorsal raphe nucleus: Linking stress coping and addiction. Brain Res. 2010;1314:29–37. doi: 10.1016/j.brainres.2009.09.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Kar LD, Javed A, Zhang Y, Serres F, Raap DK, Gray TS. 5-HT2A receptors stimulate ACTH, corticosterone, oxytocin, renin, and prolactin release and activate hypothalamic CRF and oxytocin-expressing cells. J Neurosci. 2001;21:3572–9. doi: 10.1523/JNEUROSCI.21-10-03572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 2003;66:1673–80. doi: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- White CL, Pistell PJ, Purpera MN, Gupta S, Fernandez-Kim SO, Hise TL, et al. Effects of high fat diet on Morris maze performance, oxidative stress, and inflammation in rats: contributions of maternal diet. Neurobiol Dis. 2009;35:3–13. doi: 10.1016/j.nbd.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willeit M, Praschak-Rieder N, Neumeister A, Pirker W, Asenbaum S, Vitouch O, et al. [123I]-beta-CIT SPECT imaging shows reduced brain serotonin transporter availability in drug-free depressed patients with seasonal affective disorder. Biol Psychiatry. 2000;47:482–9. doi: 10.1016/s0006-3223(99)00293-0. [DOI] [PubMed] [Google Scholar]