Abstract
BACKGROUND
Aerobic exercise and the attention-deficit/hyperactivity disorder medication, atomoxetine (ATO), are two monotherapies that have been shown to suppress reinstatement of cocaine seeking in an animal model of relapse. The present study investigated the effects of combining wheel running and ATO vs. each treatment alone on cocaine seeking precipitated by cocaine and cocaine-paired cues in rats with differing susceptibility to drug abuse (i.e., high vs. low impulsive).
METHODS
Rats were screened for high (HiI) or low impulsivity (LoI) based on their performance on a delay-discounting task and then trained to self-administer cocaine (0.4 mg/kg/inf) for 10 days. Following 14 days of extinction, both groups were tested for reinstatement of cocaine seeking precipitated by cocaine or cocaine-paired cues in the presence of concurrent running wheel access (W), pretreatment with ATO, or both (W+ATO).
RESULTS
HiI rats acquired cocaine self-administration more quickly than LoI rats. While both individual treatments and W+ATO significantly attenuated cue-induced cocaine seeking in HiI and LoI rats, only W+ATO was effective in reducing cocaine-induced reinstatement compared to vehicle treatment. There were dose-dependent and phenotype-specific effects of ATO with HiI rats responsive to the low but not high ATO dose. Floor effects of ATO and W on cue-induced reinstatement prevented the assessment of combined treatment effects.
CONCLUSIONS
These findings demonstrated greater attenuation of cue- vs. cocaine-induced reinstatement by ATO and W alone and recapitulate impulsivity phenotype differences in both acquisition of cocaine self-administration and receptivity to treatment.
Keywords: Atomoxetine, cocaine, exercise, impulsivity, relapse, reinstatement, wheel running
Introduction
Relapse to drug use is a major challenge to the treatment of addiction, and the development of novel treatment approaches requires greater understanding of the psychological and neurobiological mechanisms underlying vulnerability to substance use and relapse. Accumulating evidence suggests that the most successful treatment strategies involve a combination of behavioral and pharmacotherapies (Potenza et al. 2011, McLellan et al. 1993, Peirce et al. 2006, Ball and Ross et al. 1991, Carroll et al. 1998, Carroll et al. 2004, Bickel et al. 1997), and recent animal work demonstrated that multiple treatments may be combined to generate customized treatment approaches for individuals with certain risk factors for addiction (Zlebnik et al. 2014). Among the major vulnerability factors for addiction and relapse is impulsivity (Perry and Carroll 2008, Carroll et al. 2009). Clinical studies have demonstrated that more impulsive individuals have greater drug use, display more severe withdrawal symptoms (Moeller et al. 2001), experience heightened cravings (Doran et al. 2007), and maintain shorter abstinence periods than lower impulsive individuals (Moeller et al. 2001). Rats screened for high trait impulsivity acquired drug self-administration more quickly (Perry et al. 2005, 2008), self-administered greater amounts of drug (Dalley et al. 2007, Belin et al. 2008), and exhibited higher drug-seeking behavior in an animal model of relapse compared to their low impulsive counterparts (Perry et al. 2008, Economidou et al. 2009, Broos et al. 2012, Diergaarde et al. 2008). Therefore, impulsive behavior not only predisposes an individual to develop substance use problems (de Wit 2009, Jentsch et al. 2014), but it also influences the likelihood of relapse after a period of abstinence (Doran et al. 2007, Moeller et al. 2001, Perry et al. 2008, Economidou et al. 2009, Broos et al. 2012, Diergaarde et al. 2008).
A current candidate pharmacotherapy for psychostimulant addiction is atomoxetine (ATO) (Sofuoglu and Sewell 2009, Somaini et al. 2011), an FDA-approved nonstimulant medication to reduce symptoms of attention-deficit/hyperactivity disorder (ADHD) such as inattention, hyperactivity, and impulsivity. Atomoxetine is a selective norepinephrine (NE) reuptake inhibitor that primarily increases NE and dopamine (DA) in the prefrontal cortex (PFC) (Bymaster et al. 2002). Using ATO, studies have demonstrated reduced impulsivity across a range of behavioral tasks such as the 5-choice serial reaction time (Baarendse and Vanderschuren 2012, Robinson et al. 2008), stop-signal reaction time (Robinson et al. 2008), and delay-discounting (Bizot et al. 2011, Robinson et al. 2008) tasks (but see Baarendse & Vanderschuren, 2012, Broos et al. 2012, Sun et al. 2012). Regarding atomoxetine’s effects on stimulant addiction, some clinical investigations have found no therapeutic effect on cocaine use (Levin et al. 2009, Walsh et al. 2013) and the subjective effects of methamphetamine (Rush et al. 2011), while others found reduced physiological and subjective effects of d-amphetamine (Sofuoglu et al. 2009), significant attenuation of alcohol cravings (Wilens et al. 2011), longer abstinence from alcohol use (Benegal et al. 2013), and fewer days of heavy alcohol drinking (Wilens et al. 2008). Similarly equivocal findings were demonstrated in rodents. Treatment with ATO did not affect cocaine self-administration (Economidou et al. 2009), but it did decrease cue-primed cocaine (Economidou et al. 2009, 2011) and heroin (Economidou et al. 2011) seeking in addition to reducing the conditioned stimulus effects of nicotine (Reichel et al. 2007) and nicotine withdrawal symptoms (Davis and Gould 2007). Together, these results argue for a potentially useful role for ATO in decreasing relapse-related behaviors vs. attenuating the acute rewarding effects of drugs of abuse, and further investigations will be needed to better characterize the therapeutic effects of ATO.
However, promising research aimed at quantifying combined treatment effects has shown that using ATO as an adjunctive treatment to the standard treatment for ADHD has yielded better outcomes than standard treatment alone (Treuer et al. 2013, Holzer et al. 2013). Additionally, other research found that substance abuse relapse prevention counseling supplemented with ATO maintained longer abstinence than counseling by itself (Benegal et al. 2013). These results contribute to the body of literature supporting enhanced treatment by a combination of behavioral and pharmacological treatments (Potenza et al. 2011) and identify a role for ATO as an adjunctive treatment strategy.
In addition to ATO, it has been suggested that substance abuse treatment can be augmented by the addition of aerobic exercise (USDHHS 1996, Ussher et al. 2012, Zlebnik et al. 2014). Like ATO, exercise increases brain catecholamines like NE and DA in the PFC (Ma 2008, Meeusen and de Meirleir 1995, Paluska and Schwenk 2000). Exercise has been shown to reduce cravings for alcohol (Ussher et al. 2004), cigarettes (Daniel et al. 2004), and cannabis (Buchowski et al. 2011), and it also alleviates symptoms of tobacco withdrawal (Ussher et al. 2001, Daniel et al. 2004, Williams et al. 2011). Further, animal models of relapse have demonstrated a reduction in cue- (Lynch et al. 2010, Smith et al. 2012, Zlebnik et al. 2014), stress- (Zlebnik et al. 2014), and cocaine- (Zlebnik et al. 2010, 2014, Smith et al. 2012) induced reinstatement of cocaine seeking by wheel running. As a supplemental treatment, exercise enhanced the effectiveness of behavioral counseling in traditional tobacco cessation programs (Martin et al. 1997) and had an additive effect with contingency management for the treatment of substance use disorders in an outpatient setting (Weinstock et al. 2008). Moreover, it has been suggested that exercise may enhance standard treatment of ADHD symptoms (Kim et l. 2011, Robinson et al. 2012, Wigal et al. 2013). While some have found no additive treatment effects of exercise and methylphenidate (Medina et al. 2010), recent work in humans found greater attenuation of clinical ADHD symptoms when exercise was administered along with methylphenidate compared to methylphenidate alone (Choi et al. 2014). However, to date, the combination of exercise and ATO has not been investigated for its effects on addiction and relapse, but given prior results, its examination is warranted.
In the present experiment, effects of dual treatment with ATO and exercise were examined in high vs. low impulsive rats in an animal model of cocaine relapse. First, rats were screened for high (HiI) or low (LoI) impulsivity based on performance on a delay-discounting task and were subsequently trained to self-administer cocaine. Cocaine access was then discontinued, and animals were allowed to extinguish operant responding. Reinstatement of cocaine-seeking behavior was precipitated by cocaine priming injections or the presentation of cocaine-paired cues during sessions when rats were treated with ATO and/or given concurrent access to a locked or unlocked running wheel. Given previous work, it was hypothesized that the combination of ATO and wheel running would have a greater treatment effect than either treatment alone. Further, based on the effects of ATO and exercise on ADHD symptoms, it was hypothesized that W + ATO would more effectively reduce relapse-related behavior in HiI vs. LoI rats.
Materials and methods
Animals
Twenty-three adult female Wistar rats were used in this experiment (Harlan, Inc., Madison, WI), as prior work has shown that female rats demonstrated greater receptivity to treatment of cocaine seeking by wheel running than male rats (Cosgrove et al. 2002, Smith et al. 2011). Estrous cycle was not monitored to prevent disruption of cocaine- and exercise-maintained behavior by repeated vaginal lavage (Walker et al. 2002).
All rats began behavioral testing around postnatal day 90. Before behavioral testing, rats were group-housed in polycarbonate cages with ad libitum access to food and chow (Harlan-Teklad 2018, Harlan, Inc.) in temperature (21-23°C)- and humidity (65%)-controlled colony rooms under a 12-h light-dark cycle (lights on at 6:00 am). All procedures conformed to the eighth edition of the National Institutes of Health Guide for Care and Use of Laboratory Animals (National Research Council 2011) and were approved by the University of Minnesota Institutional Animal Care and Use Committee. Laboratory facilities were certified by the Association for Assessment and Accreditation of Laboratory Animal Care.
Apparatus
Rats were housed and tested in custom-build operant conditioning chambers as previously described (Zlebnik et al. 2010, 2012). Data collection and programming were conducted using PC computers with a Med-PC interface (MedAssociates, Inc., St. Albans, VT).
Drugs
Cocaine HCl (National Institute on Drug Abuse, Research Triangle Institute, Research Triangle Park, NC, USA) was dissolved in sterile saline at a concentration of 1.6 mg/ml, and heparin (5 USP/ml) was added to the cocaine solution to prevent catheter occlusion from thrombin accumulation. The flow rate of each cocaine infusion was 0.025 ml/s, and the duration of pump activation (1 s/100 g of body weight) was adjusted weekly to provide a 0.4 mg/kg unit dose throughout self-administration testing. Atomoxetine HCl (ATO) was purchased from Tocris Biosciences (Bristol, UK) and dissolved in sterile saline at a concentration of 3 mg/ml.
Catheterization surgery
Rats were implanted with chronic indwelling jugular catheters following methods previously described (Carroll and Boe 1982, Zlebnik et al. 2010). Following the surgical procedure, rats were allowed 3 days to recover while antibiotics (enrofloxacin, 10 mg/kg, sc) and analgesics (buprenorphine, 0.05 mg/kg, sc; ibuprofen, 15 mg/kg, po) were administered. After surgery until the remainder of the experiment, rats wore an infusion harness (CIH95AB, Instech, Plymouth Meeting, PA, USA) and tether (C313CS-MN, PlasticsOne, Roanoke, VA, USA). Catheters were flushed with a solution (0.3 ml, iv) of heparinized saline (20 USP/ml) and cefazolin (10.0 mg/ml) daily to prevent catheter blockage and infection. Rats were weighed, and catheter patency was checked weekly by injecting a 0.1-ml solution containing ketamine (60 mg/kg), midazolam (3 mg/kg), and saline. If loss of the righting reflex did not result from an iv infusion of this solution, a second catheter was implanted in the left jugular vein, and the experiment was resumed following a 3-day recovery period.
Procedure
Table 1 outlines the experimental phases: impulsivity screening by delay discounting, running wheel training, and maintenance, extinction, and reinstatement of cocaine-maintained behavior.
Table 1.
Experimental timeline
| Phase | Delay Discounting | Wheel Training | Maintenance | Extinction | Reinstatement |
|
|---|---|---|---|---|---|---|
| Coc | Cue | |||||
|
|
||||||
| Days | 5 | 3 | 10 | 14 | 6 | 6 |
|
|
||||||
| Sessions | 2 h/day | 6 h/day | ||||
Delay Discounting
Following acclimation to the laboratory, rats were singly-housed in polycarbonate cages before they began daily behavioral testing on a delay-discounting task in operant conditioning chambers (Perry et al. 2005, 2008; Perry and Carroll 2008). Briefly, sessions were divided into 15 blocks of 4 trials each, and within each block there were 2 forced-choice trials followed by 2 free-choice trials. In all trials, a lever-press response on one lever resulted in the immediate delivery of one 45-mg pellet (Bio-Serv, Frenchtown, NJ); whereas, a response on the other lever resulted in three 45-mg pellets delivered after a delay. Initially, the delay on the delayed reward lever was set at 6 s, but the length of this delay changed based on the animal’s performance during the free-choice trials. A response on the small, immediate reward lever yielded a 1-s decrease in the delay to the larger, delayed reward; conversely, a response on the larger, delayed reward lever yielded a 1-s increase in the delay. Each day’s session began with the final delay from the previous day’s session. These procedures were repeated until the MAD (mean adjusted delay of all free-choice trials) stabilized (differed by < 5 s for 5 consecutive days with no increasing or decreasing trend). The MAD served as a quantitative measure of impulsive choice; rats with MADs < 9 s or > 13 s were considered HiI or LoI, respectively.
Wheel training
Once rats were screened for high or low impulsivity, they were moved to housing in operant conditioning chambers with attached running wheels where they were trained to run following previously-published methods (Zlebnik et al. 2012). Briefly, rats were exposed to an unlocked running wheel for 6-h sessions/day, and acquisition of wheel running was defined as 3 days of > 100 revolutions/session. After meeting this criterion, rats underwent catheter implantation surgery, and the doors to the running wheels were closed until the reinstatement phase commenced.
Cocaine self-administration acquisition and maintenance
Rats were implanted with jugular catheters following acquisition of wheel running and then trained to lever press for iv infusions of cocaine (0.4 mg/kg) under a fixed-ratio 1 (FR 1) schedule of reinforcement during daily 6-h sessions. Sessions began with illumination of the house light, and responses on the active/drug-paired lever started the infusion pump and illuminated the stimulus lights located directly above the lever for the duration of the infusion. Responses on the active lever during the length of the infusion (2-4 seconds) were recorded but had no programmed consequences. Responses on the inactive lever illuminated the stimulus lights above that lever for the same duration as an infusion, but they did not activate the infusion pump. Initially, 3 experimenter-delivered priming infusions of 0.4 mg/kg cocaine were administered periodically every 2 hours during each training session followed by placement of a small amount of ground food on the active/drug-paired lever. Acquisition was complete when rats earned at least 60 infusions during a single session in the absence of experimenter-delivered priming infusions. Following acquisition, rats were allowed to maintain unlimited self-administration of cocaine for 10 additional 6-h sessions. The door leading to the wheel remained closed throughout the self-administration training and maintenance (no wheel access) phases.
Extinction
Following maintenance, access to cocaine self-administration was discontinued for the remainder of the experiment. Presentation of cocaine-paired stimuli such as the house light, stimulus lights, and infusion pump was also suspended, and rats were given 14 days to extinguish lever pressing.
Reinstatement
In a within-subjects design, reinstatement of cocaine-seeking behavior was induced by experimenter-delivered cocaine priming injections or the presentation of cocaine-paired cues (e.g., house light, stimulus lights, infusion pump). The block of cocaine-primed reinstatement sessions was counterbalanced with the block of cue-primed reinstatement sessions across rats. The treatment sequence within each priming condition block was nonsystematic and included pretreatment with either ATO (1.5 mg/kg, ATO1.5; 3 mg/kg, ATO3, ip) or saline (S) 30-min prior to the start of session and concurrent access to an unlocked (W) or locked running wheel. Rats were exposed to the priming condition (e.g., cocaine-paired cues, cocaine) every other day, and saline or no cue conditions were administered on intervening days to allow for extinction of responding prior to the next cocaine seeking assessment. An example of a priming sequence for an individual rat follows: S+Coc, S, A3+Coc, S, W+Coc, S, A3+W+Coc, S, A3+S, S, A1.5+Coc, S, A1.5+W+Coc, S, A1.5+S, no cue, S+cues, no cue, A3+cues, no cues, W+cues, no cues, A3+W+cues, no cues, A1.5+cues, no cues, A1.5+W+cues.
Data Analysis
The primary dependent measures were MADs, infusions during acquisition and maintenance, wheel revolutions during reinstatement, and responses during maintenance, extinction, and reinstatement. Time to acquire cocaine self-administration was analyzed with a Kaplan-Meier survival analysis and log-rank test. For maintenance and extinction, data were grouped into 2-day blocks to reduce daily variability and the number of post-hoc contrasts. Mean adjusted delays were compared with a 2-tailed Student’s t-test, and the other measures were analyzed with 2-factor mixed analyses of variance (ANOVA) with phenotype (HiI vs. LoI) as the between-subjects factors and blocks of sessions as the repeated measure. Separate 3-factor ANOVA were performed for each priming condition (e.g., cocaine, cues): phenotype X W access X ATO dose. Comparisons were made using the Newman-Keuls posthoc test, and results were considered significant if p<0.05. Statistical analyses were performed using GB Stat (Dynamic Microsystems, Inc., Silver Spring, MD, USA).
Results
Delay discounting, acquisition, maintenance, and extinction
In accordance with the impulsivity screening criteria, MADs on the delay-discounting task differed significantly between the HiI (4.97 ± 0.44) and LoI (29.13 ± 3.16) rats (t21 = 9.44, p < 0.0001). Time to acquire cocaine self-administration was significantly shorter in HiI vs. LoI rats (Fig. 1, X1 = 15.57, p < 0.001); all HiI rats met acquisition criteria by session 5, while all LoI rats did not meet acquisition criteria until session 15. However, there were no phenotype differences in responses (Fig. 2A) or infusions (data not shown) during maintenance. When data were collapsed across groups, there was a significant main effect of session block (F4,114 = 5.65, p < 0.001), and post-hoc analyses revealed an increase in infusions from sessions 1-2 to sessions 9-10 (p < 0.01), indicating an escalation of cocaine intake over the maintenance period. Similarly, there were no phenotype differences in responses during extinction (Fig. 2B), but there was a main effect of session block (F6,153 = 4.12, p < 0.001). Once groups were collapsed, there was a significant decrease in responses from sessions 1-2 to 13-14 (p < 0.01).
Figure 1.
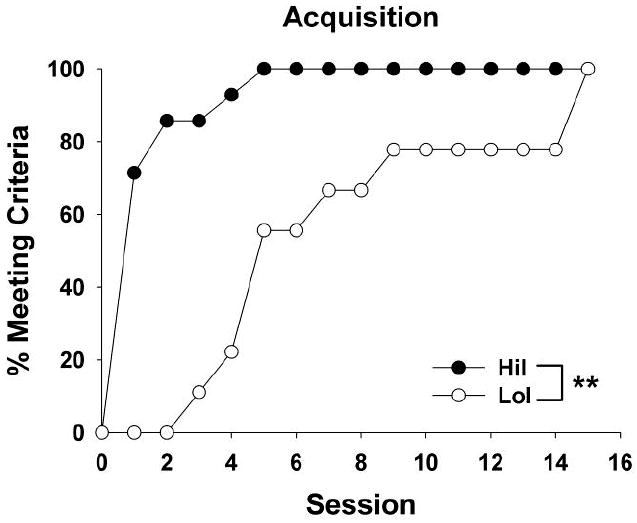
Time to meet cocaine self-administration acquisition criteria. HiI rats acquired cocaine self-administration in fewer sessions than LoI rats (** p < 0.001).
Figure 2.
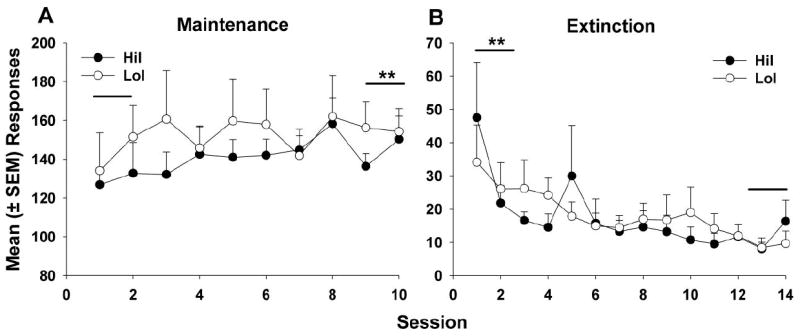
Mean (± SEM) responses during the maintenance and extinction periods. (A) There were no group differences in mean (±SEM) daily responses for cocaine (0.4 mg/kg/inf) during the 10-day maintenance period. Both HiI and LoI rats significantly increased their responding for cocaine from days 1-2 to days 9-10 (** p < 0.01).(B) There were no group differences in mean (±SEM) daily unreinforced responses during the 14-day extinction period. Both HiI and LoI rats significantly decreased their responding for cocaine from days 1-2 to days 13-14 (** p < 0.01).
Cue-primed reinstatement
Reinstatement responding precipitated by cocaine-paired cues (Fig. 3A) was analyzed by 3-factor ANOVA, and results indicated a significant main effect of W (F1,183 = 26.14, p < 0.0001), main effect of ATO (F3,183 = 17.11, p < 0.0001), phenotype X ATO interaction (F3,183 = 3.04, p < 0.05), W X ATO interaction (F3,183 = 15.69, p < 0.0001), and phenotype X W X ATO interaction (F3,183 = 3.76, p < 0.05). Post-hoc analyses showed significantly greater cue-induced reinstatement responding in the LoI rats compared to the HiI rats (p < 0.01). However, there were no other phenotype differences. Treatment with ATO1.5 or ATO3 alone significantly attenuated responding compared to vehicle treatment (p < 0.01) in the LoI rats, while only ATO1.5 reduced responding compared to vehicle in the HiI rats (p < 0.05). Access to W alone (p < 0.01) and the combination treatments W+ATO1.5 (p < 0.05) and W+ATO3 (p < 0.01) decreased cue-primed reinstatement responding in both HiI and LoI rats, and W+ATO3 decreased cue-induced responding more than ATO3 alone (p < 0.05) in HiI rats. Therefore, both treatment with ATO and the combination of W+ATO were effective in reducing cue-induced cocaine seeking in both HiI and LoI rats.
Figure 3.
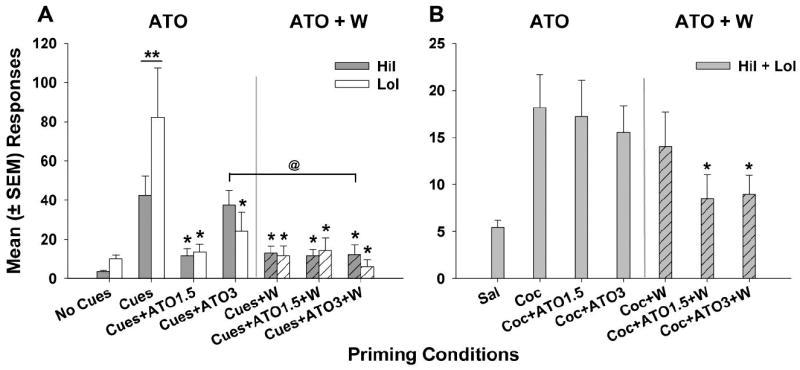
Mean (± SEM) responses per treatment session over both reinstatement priming conditions. (A) LoI rats responded significantly more for cocaine-paired cues under control conditions than HiI rats (** p < 0.01). However, both HiI and LoI rats had attenuated responding following treatment with W, ATO1.5, W+ATO1.5, W+ATO3 (* p < 0.01), and LoI rats also had reduced responding following treatment with ATO3 (* p < 0.01). The combination treatment W+ATO3 was more effective than ATO3 alone in HiI rats (@ p < 0.01). (B) There were significant main effects of wheel access and atomoxetine dose. While there were no phenotype differences and none of the individual treatments were effective in reducing cocaine-primed reinstatement, W+ATO1.5 and W+ATO3 both significantly attenuated responding compared to vehicle treatment conditions (* p < 0.05).
Wheel revolutions during W, W+ATO1.5, and W+ATO3 treatment conditions (Table 2) were analyzed in a 2-factor ANOVA resulting in a significant main effect of phenotype (F1,68 = 4.63, p < 0.05), but there was no main effect of treatment condition or a phenotype X treatment condition interaction. The treatment conditions were collapsed, and a t-test revealed that LoI rats made significantly more wheel revolutions compared to HiI rats (t62 = 2.71, p < 0.01) during cue-induced reinstatement sessions.
Table 2.
Mean (SEM) wheel revolutions during reinstatement
| Reinstatement |
||||||
|---|---|---|---|---|---|---|
| Cue | Cocaine | |||||
|
|
||||||
| Sal | ATO1.5 | ATO3 | Sal | ATO1.5 | ATO3 | |
|
|
||||||
| HiI | 91 (31) | 207 (63) | 288 (128) | 373 (179) | 479 (173) | 414 (101) |
| LoI | 372 (188)† | 645 (290)† | 505 (188)† | 498 (175) | 591 (226) | 421 (177) |
Main effect: LoI > HiI
Cocaine-primed reinstatement
Reinstatement of cocaine seeking precipitated by a cocaine priming injection (Fig. 3B) was analyzed by 3-factor ANOVA. While there were main effects of W (F1,175 = 8.39, p < 0.01) and ATO (F3,175 = 6.78, p < 0.01), there was no main effect of phenotype or any significant interactions. Data from HiI and LoI rats were collapsed, and subsequent ANOVA [main effects of W (F1,175 = 4.34, p < 0.05) and ATO (F3,175 = 6.38, p < 0.01)] and post hoc analyses did not reveal significant treatment effects from ATO1.5, ATO3, or W alone. However, W+ATO1.5 (p < 0.05) and W+ATO3 (p < 0.05) were more effective in decreasing cocaine-induced reinstatement than vehicle treatment. Wheel revolutions during cocaine-primed reinstatement sessions did not differ between HiI and LoI rats or across treatment conditions. Thus, despite no differences in wheel revolutions during W treatment sessions, the combination treatment W+ATO attenuated cocaine-primed cocaine seeking when each individual treatment (e.g., W, ATO1.5, ATO3) did not.
Discussion
Results of the present study demonstrated phenotype differences in acquisition of drug self-administration and significant treatment effects of ATO and W on relapse-related behavior in HiI and LoI rats. Confirming earlier work (Perry et al. 2005, 2008), HiI rats acquired cocaine self-administration more rapidly than LoI rats. However, across both phenotypes, ATO and W alone selectively attenuated cue- but not cocaine-primed reinstatement of cocaine seeking, and in some instances, these treatment effects were dose-dependent and phenotype-specific. However, regardless of phenotype, the combination of W + ATO markedly reduced both cue- and cocaine-primed reinstatement. Together, these findings suggest that combinatorial treatment approaches may help to overcome a broad range of relapse triggers (e.g., cocaine, cocaine-paired cues) in individuals with differential susceptibility to drug use.
Selective reduction of cue- but not cocaine-primed reinstatement of cocaine-seeking behavior by ATO is consistent with previous research demonstrating attenuation of responding for drug-associated stimuli but not suppression of cocaine self-administration following pretreatment with ATO (Economidou et al. 2009, 2011). While these results suggest little role for ATO in modulating the acute priming effects of cocaine, ATO, when combined with W, was sufficient to reduce cocaine-primed reinstatement responding compared to vehicle treatment. Contrary to earlier work with rats not selected for trait impulsivity (Zlebnik et al. 2010, 2014), W alone did not suppress cocaine-primed reinstatement in HiI and LoI rats in the present experiment. This may have been due to the age of the rats; prior to the self-administration procedure in the current study, the rats were trained on a delay-discounting task and may have been at a slightly older age during reinstatement testing compared to earlier work.
Whereas there were no HiI vs. LoI differences during cocaine-primed reinstatement, phenotype differences were represented in cue-primed reinstatement as the LoI rats had greater cocaine seeking and reached higher levels of wheel running than the HiI rats. Our earlier work suggests that these results are not due to differential locomotor behavior between the HiI and LoI rats (Perry et al. 2005). However, the discrepancy between the present results and prior work (Diergaarde et al. 2008, Broos et al. 2012) in reinstatement responding among the phenotypes could be due to differences in the delay-discounting procedures used to screen rats for high vs. low impulsivity. Diergaarde et al. (2008) and Broos et al. (2012) both used a fixed increasing delay procedure whereas our work used a self-adjusting delay procedure. The LoI rats that were selected based on our screening criteria were accustomed to very long delays (range: 18-42 sec; average: 29 sec) on the delayed lever, and the HiI rats that we selected were accustomed to very short delays (range: 0-9 sec; average: 5 sec) on the delayed lever, resulting in a short delay to reinforcement on both levers for these rats. Therefore, by definition, the LoI rats may be tolerant of waiting for reinforcement and more persistent than HiI rats in their pursuit of reinforcement when it is not readily available, making them more resistant to extinction. Conversely, given their history of near-immediate reinforcement on both levers, the HiI rats may not persistently seek reinforcement when it is not readily available, making their extinction more rapid than their LoI counterparts. In support of this, we previously found that female LoI rats had higher extinction responding following termination of cocaine self-administration than HiI rats (Perry et al. 2008). In that study, rats were allowed to extinguish responding to cocaine-paired stimuli during the extinction period, and in the present study, they were not. Consequently, the absence of cocaine-paired stimuli during extinction may account for the lack of extinction responding differences among the phenotypes, and then the presence of cocaine-paired stimuli during cue-induced reinstatement may stimulate cocaine seeking in the LoI vs. HiI rats under control treatment conditions.
There were additional phenotype differences in treatment response as LoI rats showed overall better attenuation of responding by treatment with ATO compared to HiI rats. Specifically, cue-primed reinstatement was reduced by both ATO1.5 and ATO3 in the LoI rats, whereas only ATO1.5 reduced cue-primed reinstatement in the HiI rats. However, consequently, in the HiI rats, W+ATO3 was more effective than ATO3 alone in suppressing cue-induced reinstatement responding, indicating a phenotype-specific combinatorial treatment effect as the efficacy of ATO1.5, ATO3, W+ATO1.5, and W+ATO3 was approximately equivalent in LoI rats. In fact, reinstatement remained at very low levels for all conditions incorporating ATO1.5 and W, suggesting floor effects of these individual treatment conditions. However, overall results are consistent with previous reports of treatment effects in other models of individual differences in drug abuse vulnerability. For example, as in the present study, the low vulnerable phenotype showed greater reduction of binge-like cocaine intake by treatment with progesterone (Anker et al. 2012) or baclofen (Holtz and Carroll 2011) compared to the high vulnerable phenotype. Additionally, a recent experiment in our laboratory using LoI and HiI selected using the same criteria as a the present experiment demonstrated greater attenuation of cocaine- and caffeine-primed reinstatement of cocaine seeking by allopreganolone in LoI compared to HiI rats (Regier et al. 2014). While more work is needed to fully characterize treatment effects in these models, results suggest better treatment receptivity in low vs. high susceptible phenotypes.
Differential reinstatement of cocaine seeking and response to ATO treatment between the HiI and LoI rats may be indicative of a possible underlying dissimilarity in neurobiological substrates of addiction and/or impulsivity among these phenotypes. Examining rats selected for HiI and LoI on a delay-discounting task, Regier et al. (2012) found differences in cocaine-induced activation of executive control areas such as the orbitofrontal cortex and cingulate area 1 but not other areas of the PFC such as the prelimbic or infralimbic areas. To date, no one has examined activation of these areas by cocaine-paired cues in HiI and LoI rats, but dose-dependent effects of ATO treatment on cue-induced reinstatement suggest differences in monoaminergic transmission. Atomoxetine is a selective NET inhibitor but also has a very low affinity for DAT and the serotonin transporter, SERT. At the systemic doses tested in the present experiment (1.5 and 3 mg/kg, ip), ATO has been shown to increase both NE and DA selectively in the PFC but not the striatum (Bymaster et al. 2002). Norepinephrine in the PFC modulates cognitive functioning and has been associated with attention regulation, working memory, and behavioral inhibition (Arnsten 2000, Arnsten and Casey 2011). While results examining the effects of DA receptor agonists and antagonists on delay discounting have been mixed (Hamidovic et al. 2008, Wade et al. 2000, van Gaalen et al. 2006), previous work found decreased impulsive choice during a delay-discounting task with NET inhibition by ATO (Bizot et al. 2011, Robinson et al. 2008, but see Baarendse and Vanderschuren 2012, Broos et al 2012, Sun et al 2012). Exercise also increases extracellular levels of NE and DA in the PFC (Ma 2008, Meeusen and De Meirleir 1995, Paluska and Schwenk 2000), and it may be by this mechanism that wheel running augmented the treatment effects of ATO and resulted in differential levels of wheel running in LoI vs. HiI rats. Additional investigations will be required to outline the mechanism whereby ATO and wheel running attenuate cocaine seeking in HiI and LoI rats, but existing data support a role of targeting NE and/or DA transmission in executive control areas.
In the present study, wheel running may also have reduced drug-seeking behavior and augmented the treatment effects of ATO by serving as an alternative nondrug reinforcer. Many experiments have demonstrated that wheel running is reinforcing and will maintain lever pressing under a range of experimental conditions (Iversen et al. 1993; Belke et al. 1997, 2000; Belke and Dunbar 2001). Access to alternative nondrug reinforcers has been shown to reduce drug-motivated behaviors in both animals (Nader and Woolverton 1991, 1992; Carroll et al. 1989; Carroll and Lac 1993; Comer et al. 1996) and humans (Foltin et al. 1994; Hatsukami et al. 1994; Higgins et al. 1994, 1996), and it has been suggested by us and others that when wheel running is concurrently available in the operant chamber it may serve as an effective alternative reinforcer to drug reward (Cosgrove et al. 2002; Zlebnik et al. 2010, 2012; Smith and Lynch 2012; Miller et al. 2012). Human studies also support the notion that exercise and other nondrug rewards have an attenuating influence on drug use. Substance use often occurs at the expense of other rewarding activities, and drug users reported engaging in fewer pleasant and enjoyable activities, such as exercise-related activities, than their control counterparts (Van Etten et al. 1998). Additionally, incorporating exercise into a contingency management treatment program resulted in longer abstinence than contingency management alone (Weinstock et al. 2008), suggesting that enriching the environment with alternative nondrug reinforcers may enhance treatment outcomes. The present results support these findings and warrant additional investigations of combinatorial treatment effects that incorporate exercise as a nondrug alternative reinforcer.
Together, current and prior work demonstrates promising treatment effects of ATO and W on relapse-related behavior. Importantly, ATO is well-tolerated (Spencer et al. 2001, Quintana et al. 2007, Jasinski et al. 2008), has low abuse potential (Jasinski et al. 2008), and is already approved by the FDA for treatment of ADHD. Likewise, exercise is a low-cost behavioral intervention that conveys psychological and physiological benefits (Garber et al. 2011) that may help protect against relapse such as perceived coping ability (Steptoe et al. 1989), increased self-esteem (Spence et al. 2005), and prevention of cessation-induced weight gain (Gritz et al. 1989, Klesges et al. 1992). Dual treatment with ATO and exercise may help former addicts overcome frequent threats to recovery, including both cue- and drug-induced cravings, and may also facilitate the adoption of healthier patterns of behavior. Overall, the present results using combined behavioral (W) and pharmacotherapies (ATO) support a customized treatment approach for relapse in individuals with differing vulnerability to addiction (i.e., high vs. low impulsive) and may help to inform substance abuse treatment in a clinical setting.
Acknowledgments
The authors would like to thank Vanessa Adamson, Yosef Amrami, Cole Batty, Luke Bushman, Clare Chamberlain, Nathan Holtz, Seth Johnson, Sarah Korthauer, Nathan Omdalen, Amy Saykao, Heather Veglahn, and Ashley Xiong for technical assistance and Krista Walkowiak, DVM, for veterinary care. Funding for this study was provided by the National Institute on Drug Abuse grants T32 DA007097 (PI: Thomas W. Molitor), F31 DA036248 (NEZ), R01 DA003240 (MEC), and K05 DA015267 (MEC).
References
- Anker JJ, Holtz NA, Carroll ME. Effects of progesterone on escalation of intravenous cocaine self-administration in rats selectively bred for high or low saccharin intake. Behav Pharmacol. 2012;23(2):205–10. doi: 10.1097/FBP.0b013e32834f9e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Through the looking glass: differential noradenergic modulation of prefrontal cortical function. Neural Plast. 2000;7(1-2):133–46. doi: 10.1155/NP.2000.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Casey BJ. Prefrontal cortical organization and function: implications for externalizing disorders. Biol Psychiatry. 2011;69(12):1131–2. doi: 10.1016/j.biopsych.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Baarendse PJ, Vanderschuren LJ. Dissociable effects of monoamine reuptake inhibitors on distinct forms of impulsive behavior in rats. Psychopharmacology (Berl) 2012;219(2):313–26. doi: 10.1007/s00213-011-2576-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball JC, Ross A. The Effectiveness of Methadone Maintenance Treatment. New York, NY: Springer Verlag; 1991. [Google Scholar]
- Belke TW. Running and responding reinforced by the opportunity to run: effect of reinforcer duration. J Exp Anal Behav. 1997;67(3):337–51. doi: 10.1901/jeab.1997.67-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belke TW. Varying wheel-running reinforcer duration within a session: effect on the revolution-postreinforcement pause relation. J Exp Anal Behav. 2000;73(2):225–39. doi: 10.1901/jeab.2000.73-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belke TW, Dunbar MJ. Effects of cocaine on fixed-interval responding reinforced by the opportunity to run. J Exp Anal Behav. 2001;75(1):77–91. doi: 10.1901/jeab.2001.75-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benegal V, Viswanath B, Narayanaswamy JC, Jose SP, Chakraborty V, Sankar D, Kandavel T, Kesavan M. The efficacy of atomoxetine as adjunctive treatment for co-morbid substance use disorders and externalizing symptoms. Asian J Psychiatr. 2013;6(6):544–7. doi: 10.1016/j.ajp.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Madden GJ, DeGrandpre RJ. Modeling the effects of combined behavioral and pharmacological treatment on cigarette smoking: Behavioral –economic analyses. Exp Clin Psychopharm. 1997;5:334–343. doi: 10.1037//1064-1297.5.4.334. [DOI] [PubMed] [Google Scholar]
- Bizot JC, David S, Trovero F. Effects of atomoxetine, desipramine, d-amphetamine and methylphenidate on impulsivity in juvenile rats, measured in a T-maze procedure. Neurosci Lett. 2011;489(1):20–4. doi: 10.1016/j.neulet.2010.11.058. [DOI] [PubMed] [Google Scholar]
- Broos N, Diergaarde L, Schoffelmeer AN, Pattij T, De Vries TJ. Trait impulsive choice predicts resistance to extinction and propensity to relapse to cocaine seeking: a bidirectional investigation. Neuropsychopharmacology. 2012;37(6):1377–86. doi: 10.1038/npp.2011.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broos N, Schmaal L, Wiskerke J, Kostelijk L, Lam T, Stoop N, Weierink L, Ham J, de Geus EJ, Schoffelmeer AN, van den Brink W, Veltman DJ, de Vries TJ, Pattij T, Goudriaan AE. The relationship between impulsive choice and impulsive action: a cross-species translational study. PLoS One. 2012;7(5):e36781. doi: 10.1371/journal.pone.0036781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchowski MS, Meade NN, Charboneau E, Park S, Dietrich MS, Cowan RL, Martin PR. Aerobic exercise training reduces cannabis craving and use in non-treatment seeking cannabis-dependent adults. PLoS One. 2011;6(3):e17465. doi: 10.1371/journal.pone.0017465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: A potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Carroll K, Connors GJ, Cooney NL, DiClemente CC, Donovan DM, Kadden RR, Longabaugh RL, Rounsaville BJ, Wirtz PW, Zweben A. A cognitive behavioral approach: Treating cocaine addiction. Rockville, MD: NIDA; 1998. [DOI] [PubMed] [Google Scholar]
- Carroll K, Rounsaville BJ, Kosten TR. Choosing a behavioral therapy platform for pharmacotherapy of drug users. Drug Alcohol Depend. 2004;75:123–134. doi: 10.1016/j.drugalcdep.2004.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ, Perry JL. Modeling risk factors for nicotine and other drug abuse in the preclinical laboratory. Drug Alcohol Depend. 2009;104(Suppl 1):S70–8. doi: 10.1016/j.drugalcdep.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Boe IN. Increased intravenous drug self-administration during deprivation of other reinforcers. Pharmacol Biochem Behav. 1982;17(3):563–7. doi: 10.1016/0091-3057(82)90319-7. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac S. Authoshaping iv cocaine self-administration in rats: effects of nondrug alternative reinforcers on acquisition. Psychopharmacology. 1993;110:5–12. doi: 10.1007/BF02246944. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST, Nygaard SL. A concurrently available nondrug reinforcer prevents the acquisition or decreases the maintenance of cocaine-reinforced behavior. Psychopharmacology. 1989;97:23–29. doi: 10.1007/BF00443407. [DOI] [PubMed] [Google Scholar]
- Choi JW, Han DH, Kang KD, Jung HY, Renshaw PF. Aerobic Exercise and Attention Deficit Hyperactivity Disorder: Brain Research. Med Sci Sports Exerc. 2014 May 12; doi: 10.1249/MSS.0000000000000373. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Lac ST, Wvell CL, Carroll ME. Combined effects of buprenorphine and a nondrug alternative reinforcer on iv cocaine self-administration in rats maintained under FR schedules. Psychopharmacology. 1996;125(4):355–60. doi: 10.1007/BF02246018. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Hunter R, Carroll ME. Wheel-running attenuates intravenous cocaine self-administration in rats: sex differences. Pharm Biochem Behav. 2002;73:663–67. doi: 10.1016/s0091-3057(02)00853-5. [DOI] [PubMed] [Google Scholar]
- Daniel J, Cropley M, Ussher M, West R. Acute effects of a short bout of moderate versus light intensity exercise versus inactivity on tobacco withdrawal symptoms in sedentary smokers. Psychopharmacology. 2004;174(3):320–6. doi: 10.1007/s00213-003-1762-x. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. Atomoxetine reverses nicotine withdrawal-associated deficits in contextual fear conditioning. Neuropsychopharmacology. 2007;32(9):2011–9. doi: 10.1038/sj.npp.1301315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14(1):22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, De Vries TJ. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63(3):301–8. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Doran N, Spring B, McChargue D. Effect of impulsivity on craving and behavioral reactivity to smoking cues. Psychopharmacology (Berl) 2007;194(2):279–88. doi: 10.1007/s00213-007-0832-x. [DOI] [PubMed] [Google Scholar]
- Economidou D, Dalley JW, Everitt BJ. Selective norepinephrine reuptake inhibition by atomoxetine prevents cue-induced heroin and cocaine seeking. Biol Psychiatry. 2011;69(3):266–74. doi: 10.1016/j.biopsych.2010.09.040. [DOI] [PubMed] [Google Scholar]
- Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ. High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol Psychiatry. 2009;65(10):851–6. doi: 10.1016/j.biopsych.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Effects of buprenorphine on the self-administration of cocaine by humans. Behav Pharmacol. 1994;5:79–89. doi: 10.1097/00008877-199402000-00009. [DOI] [PubMed] [Google Scholar]
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Medicine and Science in Sports and Exercise. 2008;43:1334–59. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- Gritz ER, Klesges RC, Meyers AW. The smoking and body weight relationship: Implications for intervention and postcessation weight control. Annals of Behavioral Medicine. 1989;11:144–53. [Google Scholar]
- Hamidovic A, Kang UJ, de Wit H. Effects of low to moderate acute doses of pramipexole on impulsivity and cognition in healthy volunteers. J Clin Psychopharmacol. 2008;28:45–51. doi: 10.1097/jcp.0b013e3181602fab. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Thomson TN, Pentel PR, Flygare BK, Carroll ME. Self-administration of smoked cocaine. Psychopharmacol. 1994;2:115–125. [Google Scholar]
- Higgins ST, Bickel WK, Hughes JR. Influence of an alternative reinforcer on human cocaine self-administration. Life Sci. 1994;55:179–187. doi: 10.1016/0024-3205(94)00878-7. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Roll JM, Bickel WK. Alcohol pretreatment increases preference for cocaine over monetary reinforcement. Psychopharmacology. 1996;123:1–8. doi: 10.1007/BF02246274. [DOI] [PubMed] [Google Scholar]
- Holtz NA, Carroll ME. Baclofen has opposite effects on escalation of cocaine self-administration: increased intake in rats selectively bred for high (HiS) saccharin intake and decreased intake in those selected for low (LoS) saccharin intake. Pharmacol Biochem Behav. 2011;100(2):275–83. doi: 10.1016/j.pbb.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer B, Lopes V, Lehman R. Combination use of atomoxetine hydrochloride and olanzapine in the treatment of attention-deficit/hyperactivity disorder with comorbid disruptive behavior disorder in children and adolescents 10-18 years of age. J Child Adolesc Psychopharmacol. 2013;23(6):415–8. doi: 10.1089/cap.2013.0029. [DOI] [PubMed] [Google Scholar]
- Iverson IH. Techniques for establishing schedules with wheel running as reinforcement in rats. J Exp Anal Behavior. 1993;60:219–238. doi: 10.1901/jeab.1993.60-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski DR, Faries DE, Moore RJ, Schuh LM, Allen AJ. Abuse liability assessment of atomoxetine in a drug-abusing population. Drug Alcohol Depend. 2008;95:140–146. doi: 10.1016/j.drugalcdep.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Ashenhurst JR, Cervantes MC, Groman SM, James AS, Pennington ZT. Dissecting impulsivity and its relationships to drug addictions. Ann N Y Acad Sci. 2014 Mar 21; doi: 10.1111/nyas.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Heo HI, Kim DH, Ko IG, Lee SS, Kim SE, Kim BK, Kim TW, Ji ES, Kim JD, Shin MS, Choi YW, Kim CJ. Treadmill exercise and methylphenidate ameliorate symptoms of attention deficit/hyperactivity disorder through enhancing dopamine synthesis and brain-derived neurotrophic factor expression in spontaneous hypertensive rats. Neurosci Lett. 2010;504(1):35–9. doi: 10.1016/j.neulet.2011.08.052. [DOI] [PubMed] [Google Scholar]
- Klesges RC, Schumaker SA. Understanding the relations between smoking and body weight and their importance to smoking cessation and relapse. Health Psychology. 1992;11:1–3. doi: 10.1037/h0090339. [DOI] [PubMed] [Google Scholar]
- Ko IG, Kim SE, Kim TW, Ji ES, Shin MS, Kim CJ, Hong MH, Bahn GH. Swimming exercise alleviates the symptoms of attention-deficit hyperactivity disorder in spontaneous hypertensive rats. Mol Med Rep. 2013;8(2):393–400. doi: 10.3892/mmr.2013.1531. [DOI] [PubMed] [Google Scholar]
- Levin FR, Mariani JJ, Secora A, Brooks D, Cheng WY, Bisaga A, Nunes E, Aharonovich E, Raby W, Hennessy G. Atomoxetine Treatment for Cocaine Abuse and Adult Attention-Deficit Hyperactivity Disorder (ADHD): A Preliminary Open Trial. J Dual Diagn. 2009;5(1):41–56. doi: 10.1080/15504260802628767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Piehl KB, Acosta G, Peterson AB, Hemby SE. Aerobic exercise attenuates reinstatement of cocaine-seeking behavior and associated neuroadaptations in the prefrontal cortex. Biol Psychiatry. 2010;68(8):774–7. doi: 10.1016/j.biopsych.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. Beneficial effects of moderate voluntary physical exercise and its biological mechanisms on brain health. Neuroscience Bulletin. 2008;24:265–270. doi: 10.1007/s12264-008-0402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JE, Kalfas KJ, Patten CA. Prospective evaluation of three smoking interventions in 205 recovering alcoholics: one-year results of project SCRAP-Tobacco. Journal of Consulting and Clinical Psychology. 1997;65:190–4. doi: 10.1037//0022-006x.65.1.190. [DOI] [PubMed] [Google Scholar]
- McLellan A, Arndt IO, Metzger D, Woody GE, O’Brien CP. The effects of psychosocial services in substance abuse treatment. JAMA. 1993;269:1953–1959. [PubMed] [Google Scholar]
- Meeusen R, De Meirleir K. Exercise and brain neurotransmission. Sports Medicine. 1995;20:160–188. doi: 10.2165/00007256-199520030-00004. [DOI] [PubMed] [Google Scholar]
- Miller ML, Vaillancourt BD, Wright MJ, Jr, Aarde SM, Vandewater SA, Creehan KM, Taffe MA. Reciprocal inhibitory effects of intravenous d-methamphetamine self-administration and wheel activity in rats. Drug Alcohol Depend. 2012;121(1-2):90–6. doi: 10.1016/j.drugalcdep.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. J Subst Abuse Treat. 2001;21(4):193–8. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Nader MA, Woolverton WL. Effects of increasing the magnitude of an alternative reinforcer on drug choise in a discrete-trials choice procedure. Psychopharmacology. 1991;105:169–174. doi: 10.1007/BF02244304. [DOI] [PubMed] [Google Scholar]
- Nader MA, Woolverton WL. Choice between cocaine and food by rhesus monkeys: effects of conditions of food availability. Behav Pharmacol. 1992;3:635–638. [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Animals. 8. The National Academies Press; Washington, DC: 2011. [Google Scholar]
- Paluska SA, Schwenk TL. Physical activity and mental health: Current concepts. Sports Medicine. 2000;29:167–180. doi: 10.2165/00007256-200029030-00003. [DOI] [PubMed] [Google Scholar]
- Peirce JM, Petry NM, Stitzer ML, Blaine JD, Kellog S, Satterfield F, Schwartz M, Krasnansky J, Pencer E, Silva-Vazquez L, et al. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: A National Drug Abuse Treatment Clinical Trials Network study. Arch Gen Psychiatry. 2006;63:201–208. doi: 10.1001/archpsyc.63.2.201. [DOI] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008;200(1):1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl) 2005;178(2-3):193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Carroll ME. Impulsive choice as a predictor of acquisition of IV cocaine self- administration and reinstatement of cocaine-seeking behavior in male and female rats. Exp Clin Psychopharmacol. 2008;16(2):165–77. doi: 10.1037/1064-1297.16.2.165. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Sofuoglu M, Carroll KM, Rounsaville BJ. Neuroscience of behavioral and pharmacological treatments for addictions. Neuron. 2011;69(4):695–712. doi: 10.1016/j.neuron.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier PS, Carroll ME, Meisel RL. Cocaine-induced c-Fos expression in rats selectively bred for high or low saccharin intake and in rats selected for high or low impulsivity. Behav Brain Res. 2012;233(2):271–9. doi: 10.1016/j.bbr.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier PS, Claxton AB, Zlebnik NE, Carroll ME. Cocaine-, caffeine-, and stress-evoked cocaine reinstatement in high vs. low impulsive rats: Treatment with allopregnanolone. Drug and Alcohol Dependence. 2014 doi: 10.1016/j.drugalcdep.2014.07.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Linkugel JD, Bevins RA. Nicotine as a conditioned stimulus: impact of attention-deficit/hyperactivity disorder medications. Exp Clin Psychopharmacol. 2007;15(5):501–9. doi: 10.1037/1064-1297.15.5.501. [DOI] [PubMed] [Google Scholar]
- Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, Dalley JW, Robbins TW. Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology. 2008;33(5):1028–37. doi: 10.1038/sj.npp.1301487. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Lile JA, Glaser PE, Hays LR. Physiological and subjective effects of acute intranasal methamphetamine during atomoxetine maintenance. Pharmacol Biochem Behav. 2011;100(1):40–7. doi: 10.1016/j.pbb.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Lynch WJ. Exercise as a potential treatment for drug abuse: evidence from preclinical studies. Front Psychiatry. 2012;2:82. doi: 10.3389/fpsyt.2011.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Pennock MM, Walker KL, Lang KC. Access to a running wheel decreases cocaine-primed and cue-induced reinstatement in male and female rats. Drug Alcohol Depend. 2012;121(1-2):54–61. doi: 10.1016/j.drugalcdep.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Walker KL, Cole KT, Lang KC. The effects of aerobic exercise on cocaine self-administration in male and female rats. Psychopharmacology. 2011;218(2):357–69. doi: 10.1007/s00213-011-2321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Poling J, Hill K, Kosten T. Atomoxetine attenuates dextroamphetamine effects in humans. Am J Drug Alcohol Abuse. 2009;35(6):412–6. doi: 10.3109/00952990903383961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Sewell RA. Norepinephrine and stimulant addiction. Addict Biol. 2009;1410(2):119–29. doi: 10.1111/j.1369-1600.2008.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somaini L, Donnini C, Raggi MA, Amore M, Ciccocioppo R, Saracino MA, Kalluppi M, Malagoli M, Gerra ML, Gerra G. Promising medications for cocaine dependence treatment. Recent Pat CNS Drug Discov. 2011;6(2):146–60. doi: 10.2174/157488911795933893. [DOI] [PubMed] [Google Scholar]
- Spence JC, McGannon KR, Poon P. The effect of exercise on global self-esteem: a quantitative review. Journal of Sport & Exercise Psychology. 2005;27:311–334. [Google Scholar]
- Spencer T, Biederman J, Heiligenstein J, Wilens T, Faries D, Prince J, et al. An open-label, dose-ranging study of atomoxetine in children with attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol. 2001;11:251–265. doi: 10.1089/10445460152595577. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Edwards S, Moses J, Mattews A. The effects of exercise training on mood and perceived coping ability in anxious adults from the general population. Journal of Psychosomatic Research. 1989;33:537–47. doi: 10.1016/0022-3999(89)90061-5. [DOI] [PubMed] [Google Scholar]
- Sun H, Cocker PJ, Zeeb FD, Winstanley CA. Chronic atomoxetine treatment during adolescence decreases impulsive choice, but not impulsive action, in adult rats and alters markers of synaptic plasticity in the orbitofrontal cortex. Psychopharmacology. 2012;219(2):285–301. doi: 10.1007/s00213-011-2419-9. [DOI] [PubMed] [Google Scholar]
- Treuer T, Gau SS, Méndez L, Montgomery W, Monk JA, Altin M, Wu S, Lin CC, Dueñas HJ. A systematic review of combination therapy with stimulants and atomoxetine for attention-deficit/hyperactivity disorder, including patient characteristics, treatment strategies, effectiveness, and tolerability. J Child Adolesc Psychopharmacol. 2013;23(3):179–93. doi: 10.1089/cap.2012.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana H, Cherlin EA, Duesenberg DA, Bangs ME, Ramsey JL, Feldman PD, Allen AJ, Kelsey DK. Transition from methylphenidate or amphetamine to atomoxetine in children and adolescents with attention-deficit/hyperactivity disorder—a preliminary tolerability and efficacy study. Clin Ther. 2007;29:1168–1177. doi: 10.1016/j.clinthera.2007.06.017. [DOI] [PubMed] [Google Scholar]
- USDHHS. Physical activity and health: A report of the surgeon general. Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; Atlanta, GA: 1996. [Google Scholar]
- Ussher M, Nunziata P, Cropley M, West R. Effect of a short bout of exercise on tobacco withdrawal symptoms and desire to smoke. Psychopharmacology. 2001;158(1):66–72. doi: 10.1007/s002130100846. [DOI] [PubMed] [Google Scholar]
- Ussher M, Sampuran AK, Doshi R, West R, Drummond DC. Acute effect of a brief bout of exercise on alcohol urges. Addiction. 2004;99(12):1542–7. doi: 10.1111/j.1360-0443.2004.00919.x. [DOI] [PubMed] [Google Scholar]
- Ussher MH, Taylor A, Faulkner G. Exercise interventions for smoking cessation. Cochrane Database Syst Rev. 2012 Jan 18;1:CD002295. doi: 10.1002/14651858.CD002295.pub4. [DOI] [PubMed] [Google Scholar]
- Van Etten ML, Higgins ST, Budney AJ, Badger GJ. Comparison of the frequency and enjoyability of pleasant events in cocaine abusers using a standardized behavioral inventory. Addiction. 1998;93:1669–1680. doi: 10.1046/j.1360-0443.1998.931116695.x. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, van Koten R, Schoffelmeer AN, Vanderschuren LJ. Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biol Psychiat. 2006;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Wade TR, de Wit H, Richards JB. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology. 2000;150:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- Walker QD, Nelson CJ, Smith D, Kuhn CM. Vaginal lavage attenuates cocaine-stimulated activity and establishes place preference in rats. Pharmacol Biochem Behav. 2002;73:743–752. doi: 10.1016/s0091-3057(02)00883-3. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Middleton LS, Wong CJ, Nuzzo PA, Campbell CL, Rush CR, Lofwall MR. Atomoxetine does not alter cocaine use in cocaine dependent individuals: double blind randomized trial. Drug Alcohol Depend. 2013;130(1-3):150–7. doi: 10.1016/j.drugalcdep.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock J, Barry D, Petry NM. Exercise-related activities are associated with positive outcome in contingency management treatment for substance use disorders. Addict Behav. 2008;33:1072–1075. doi: 10.1016/j.addbeh.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigal SB, Emmerson N, Gehricke JG, Galassetti P. Exercise: applications to childhood ADHD. J Atten Disord. 2013;17(4):279–90. doi: 10.1177/1087054712454192. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Adler LA, Tanaka Y, Xiao F, D’Souza DN, Gutkin SW, Upadhyaya HP. Correlates of alcohol use in adults with ADHD and comorbid alcohol use disorders: exploratory analysis of a placebo-controlled trial of atomoxetine. Curr Med Res Opin. 2011;27(12):2309–20. doi: 10.1185/03007995.2011.628648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DM, Dunsiger S, Whiteley JA, Ussher MH, Ciccolo JT, Jennings EG. Acute effects of moderate intensity aerobic exercise on affective withdrawal symptoms and cravings among women smokers. Addict Behav. 2011;36(8):894–7. doi: 10.1016/j.addbeh.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Anker JJ, Carroll ME. Exercise to reduce the escalation of cocaine self-administration in adolescent and adult rats. Psychopharmacology. 2012;224(3):387–400. doi: 10.1007/s00213-012-2760-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Anker JJ, Gliddon LA, Carroll ME. Reduction of extinction and reinstatement of cocaine seeking by wheel running in female rats. Psychopharmacology. 2010;209(1):113–25. doi: 10.1007/s00213-010-1776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Saykao AT, Carroll ME. Effects of combined exercise and progesterone treatments on cocaine seeking in male and female rats. Psychopharmacology (Berl) 2014 doi: 10.1007/s00213-014-3513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


