Abstract
Rationale
2-([2-(4-cyano-2,5-dimethoxyphenyl)ethylamino]methyl)phenol (25CN-NBOH) is structurally similar to N-benzyl substituted phenethylamine hallucinogens currently emerging as drugs of abuse. 25CN-NBOH exhibits dramatic selectivity for 5-HT2A receptors in vitro, but has not been behaviorally characterized.
Objective
25CN-NBOH was compared to the traditional phenethylamine hallucinogen R(−)-2,5-dimethoxy-4-iodoamphetamine (DOI) using mouse models of drug-elicited head twitch behavior and drug discrimination.
Methods
Drug-elicited head twitches were quantified for 10 min following administration of various doses of either DOI or 25CN-NBOH, with and without pretreatments of 0.01 mg/kg 5-HT2A antagonist M100907 or 3.0 mg/kg 5-HT2C antagonist RS102221. The capacity of 25CN-NBOH to attenuate DOI-elicited head twitch was also investigated. Mice were trained to discriminate DOI or M100907 from saline, and 25CN-NBOH was tested for generalization.
Results
25CN-NBOH induced a head twitch response in the mouse that was lower in magnitude than that of DOI, blocked by M100907, but not altered by RS102221. DOI-elicited head twitch was dose-dependently attenuated by 25CN-NBOH pretreatment. 25CN-NBOH produced an intermediate degree of generalization (55%) for the DOI training dose, and these interoceptive effects were attenuated by M100907. Finally, 25CN-NBOH did not generalize to M100907 at any dose, but ketanserin fully substituted in these animals.
Conclusions
25CN-NBOH was behaviorally active, but less effective than DOI in two mouse models of hallucinogenic effects. The effectiveness with which M100907 antagonized the behavioral actions of 25CN-NBOH strongly suggests that the 5-HT2A receptor is an important site of agonist action for this compound in vivo.
Introduction
Drugs binding as agonists to serotonin 5-HT2A receptors (5-HT2AR) have typically been referred to as “psychedelics” or “hallucinogens” and are generally derived from three distinct structural backbones: the lysergic acid diethylamide-like (LSD) ergolines, the psilocybin-like tryptamines, and the mescaline-like phenethylamines (Nichols, 2004; Fantegrossi et al., 2008). Although 5-HT2AR appears to be the primary site of hallucinogenic action for these compounds, all three structural classes also bind several of the other 5-HT receptors with varying degrees of selectivity and intrinsic efficacies, complicating the study of these compounds following systemic administration in intact organisms expressing all of the native 5-HT receptors. Because the hallucinogenic phenethylamines display reasonable selectivity for 5-HT2 receptor subtypes (but typically lack selectivity among the 2A, 2B and 2C subtypes) (Glennon et al., 1992; Roth et al., 1998), extensive medicinal chemistry efforts have focused on this scaffold and have produced numerous highly potent agonists (Parker et al., 1998; McLean et al., 2006).
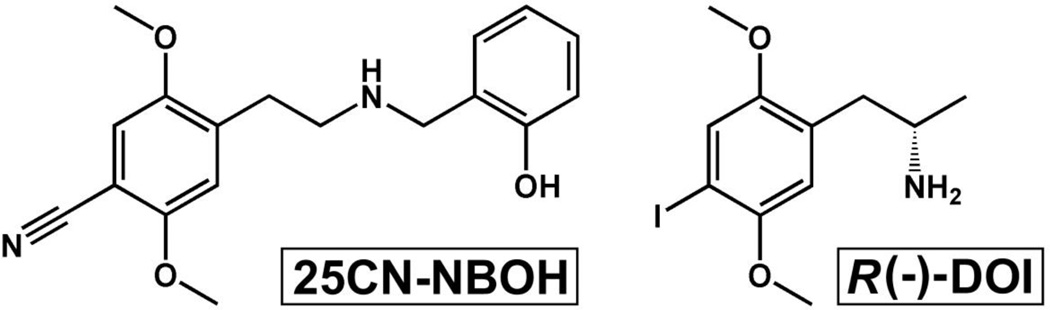
In this regard, N-benzyl and N-(2-methoxy)benzyl substitution has been used to generate novel phenethylamines with further improved 5-HT2AR binding affinity and functional activity in vitro (Braden et al., 2006) and in vivo (Halberstadt and Geyer, 2014), and further efforts with conformationally restricted N-benzylphenethylamines have resulted in the synthesis of an agonist compound with greater than 100-fold selectivity for 5-HT2AR over 5-HT2CR (Juncosa et al., 2013). Most recently, as part of larger efforts towards developing selective 5-HT2AR agonists as PET ligands, 48 closely-related N-benzyl phenethylamines were evaluated as 5-HT2A/2CR agonists (Hansen et al., 2014). Several of these compounds displayed affinities and potencies in the picomolar range with varying levels of selectivity for the 5-HT2AR, with the most selective compound, 2-([2-(4-cyano-2,5-dimethoxyphenyl)ethylamino]methyl)phenol (25CN-NBOH, Figure 1), exhibiting a 100-fold greater binding affinity for 5-HT2AR over 5-HT2CR (Hansen et al., 2014), and a 46-fold selectivity for 5-HT2AR over 5-HT2BR (Hansen, 2011). The structure of 25CN-NBOH is similar to that of other N-benzyl substituted phenethylamines currently emerging as drugs of abuse, including 25B-NBOMe, 25C-NBOMe, and 25I-NBOMe (Lawn et al., 2014), all of which are potent hallucinogens in man, consistent with the finding that N-benzyl substitutions dramatically increase affinity for 5-HT2AR in vitro and in vivo (Blaazer et al., 2008; Nichols et al., 2008; Advisory Council on the Misuse of Drugs, 2013; Halberstadt and Geyer, 2014).
Figure 1.
Structures of 25CN-NBOH (left) and R(−)-DOI (right).
The present experiments were thus designed to evaluate the in vivo hallucinogen-like effects of 25CN-NBOH in male NIH Swiss mice, in comparison with those of the traditional phenethylamine hallucinogen R(−)-2,5-dimethoxy-4-iodoamphetamine (DOI, Figure 1, >10-fold selective for 5-HT2AR over 5-HT2CR [Knight et al., 2004]), using complementary assays of drug discrimination and drug-elicited head twitch behavior. Because 25CN-NBOH has previously been shown to elicit low efficacy agonist effects at 5-HT2AR (Hansen, 2011; Hansen et al., 2014), we speculated that it would substitute – at least partially – for the discriminative cue elicited by DOI, but not by that elicited by the selective 5-HT2AR antagonist (+)-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenylethyl)]-4-piperidine-methanol (M100907) (16-fold selective for 5-HT2AR over 5-HT2CR [Knight et al., 2004]). Similarly, because DOI-elicited head twitch behavior is limited by its activation of 5-HT2CR at higher doses (Fantegrossi et al., 2010), we expected that 25CN-NBOH would produce head twitch behavior over a wider range of doses than DOI, and that it would dose-dependently attenuate DOI-elicited head twitches when the two drugs were coadministered. Thus, we established dose-effect functions for DOI and M100907 using 2-lever, food-reinforced drug discrimination in mice, and then substituted 25CN-NBOH in both groups of animals. In separate experiments, we generated dose-effect curves for DOI and 25CN-NBOH in the head twitch assay, then re-determined the effects of selected 25CN-NBOH doses in the presence of M100907 or the selective 5-HT2CR antagonist 8-[5-(2,4-dimethoxy-5-(4-trifluoromethylphenylsulfonamido) phenyl-5-oxopentyl]1,3,8-triazaspiro[4,5] decane-2,4-dione hydrochloride (RS 102221) (over 200-fold selective for 5-HT2CR over 5-HT2AR [Knight et al., 2004]). Finally, the effects of 25CN-NBOH pretreatment on DOI-elicited head twitches were also determined. The hypotheses tested were that 1) the discriminative stimulus effects of 25CN-NBOH would be DOI-like, but not M100907-like, and would be mediated via 5-HT2AR activation; 2) head twitches elicited by 25CN-NBOH would be mediated by 5-HT2AR but not by 5-HT2CR; and 3) pretreatment with 25CN-NBOH would produce a dose-dependent attenuation of DOI-elicited head twitch behavior.
Methods
Animals
Male NIH Swiss mice (Harlan Sprague Dawley, Inc.) weighing 20–25g on delivery were housed 3 animals per cage in temperature-controlled rooms maintained at an ambient temperature of 22±2°C at 45–50% humidity. Lights were set to a 12-h light/dark cycle (experiments were conducted 3–5 hours into the light phase), and animals were fed ad libitum with standard rodent chow (Laboratory Rodent Diet #5001) and water for at least 5 days after arrival. Mice were randomly assigned to experimental groups; animals allocated for head twitch experiments (n=5 per group) were free fed for the duration of all experimental manipulations, while mice assigned to drug discrimination studies (n=6 per group) were food-restricted to maintain weights at 30±2g throughout testing, with supplemental daily feedings provided after behavioral testing. For head twitch studies, each animal was used in only one experimental observation.
Procedure
Drug-elicited Head Twitch
On experimental days, mice were weighed, marked, and returned to the home cage. Doses were then calculated and prepared for intraperitoneal (ip) injection. To generate dose-effect functions, individual animals were removed from the home cage, injected with saline or various doses of DOI or 25CN-NBOH and placed into an observation cage containing fresh bedding. Ten minutes following this injection, an overhead camera was activated and behavior was recorded for 10 min. To evaluate mechanism of 25CN-NBOH-elicited head twitch, a 15 minute pretreatment with 0.01 mg/kg of the selective 5-HT2AR antagonist M100907 was administered before several effective doses of 25CN-NBOH, or 3.0 mg/kg of the selective 5-HT2CR antagonist RS102221 was administered prior to the highest dose of 25CN-NBOH. In a final cohort of animals, various doses of 25CN-NBOH were administered 15 min before 1.0 mg/kg DOI. In all cases, videotapes were later scored for drug-elicited head twitches (defined as a rapid rotational jerk of the head which can be distinguished from species-appropriate grooming or scratching behaviors) by one blind observer. Neither food nor water was available during the experiments.
Drug discrimination
Studies were conducted in operant-conditioning chambers for mice (MED Associates, St. Albans, VT) that were individually enclosed in larger lightproof Malaguard sound-attenuating cubicles (MED Associates). The side wall of each chamber used in these studies was equipped with an aperture through which liquid reinforcement was delivered, driven by a dipper mounted outside the chamber but within the cubicle. The reinforcement aperture was centered between two retractable levers and contained an amber stimulus light, which was illuminated during reinforcer delivery. Mice were trained five days per week under a fixed-ratio schedule of reinforcement wherein completion of the response requirement on either lever was reinforced by 2 sec of access to a palatable liquid reinforcer (approximately 0.02 ml evaporated milk diluted 1:1 with tap water) followed by a 10 sec time-out (TO) before programmed consequences were reinitiated. Once a response requirement was met on either lever, that lever was retracted and subjects were required to meet the response requirement on the other lever. When the response requirement was met on each of the levers, both levers were reintroduced following the TO. In this manner, mice received equivalent reinforcement following responding on each lever, and no subsequent biases for one lever or the other were noted. Animals initially acquired lever-pressing behavior on a fixed ratio (FR) 1 schedule of reinforcement in session lasting 60 min or until 60 reinforcers had been earned (whichever came first). The FR value increased by one every 20th reinforcer earned within a given session, and the FR value achieved was carried over between sessions until mice were responding under an FR5. This segment of the training was complete when mice performed stably over five consecutive sessions under the terminal FR5 schedule.
Next, mice were trained in thirty minute sessions five days per week to discriminate either 0.3 mg/kg DOI or 0.1 mg/kg M100907 from saline vehicle. Injections were administered ip 10 min before extension of the response levers, signaling the start of the behavioral session. During discrimination training, responses on the injection-inappropriate lever reset the FR on the injection-appropriate lever, but had no other programmed consequences. Completion of the FR5 on the injection-appropriate lever was reinforced as described above. Percent drug-appropriate responding was calculated as the number of responses emitted on the injection-appropriate lever divided by the total number of responses on both levers, multiplied by 100. Training was composed of an alternating schedule of drug or saline injection. Subjects were switched from saline to drug or vice versa for the next day of training if they achieved a criterion of greater than 80% injection-appropriate responding prior to delivery of the first reinforcer, and greater than 90% injection-appropriate responding for the overall session.
Drug-induced stimulus control was assumed to be present when, in five consecutive sessions, animals achieved 80% or better injection-appropriate responding prior to delivery of the first reinforcer. After stimulus control was established with the training drugs, tests were conducted once or twice per week in each animal so long as performance did not fall below the criterion level of 80% injection-appropriate responding in any one of the previous three training sessions. Approximately half of the test sessions were conducted the day after saline training sessions with the remainder following drug training sessions. During test sessions, a multiple component cumulative dosing procedure was used, and no responses were reinforced. Each component was terminated after the emission of five responses on either lever. Mice were then removed from the chamber, administered the next cumulative dose, and returned to the chamber. Ten minutes later, levers were re-extended into the experimental space. In this manner, multiple doses of drug could be tested in a single session, with approximately 10 min elapsing between each testing component. The distribution of responses between the two levers was expressed as a percentage of total responses emitted on the drug-appropriate lever. Response rate was calculated for each session by dividing the total number of responses emitted on both levers by the elapsed time prior to 5 responses on either lever.
Complete generalization of a training drug to a test drug occurs when (a) a mean of 80% or more of all test responses occurs on the drug-appropriate lever and (b) there is a statistically significant difference between the response distributions of the test drug and saline control sessions. An intermediate degree of generalization is defined as being present when response distributions after a test drug are less than 80% drug-appropriate, and are significantly different from saline control sessions. Finally, when the response distribution after a test drug is not statistically significantly different from that in saline control sessions, an absence of generalization of the training drug to the test drug is assumed. Failure to complete an FR5 on either lever within 5 min terminated the sessions and indicated disruption of schedule-controlled behavior.
Data Analysis
Data are presented as mean ± SEM. In all figures, points without error bars indicate instances where the variance is contained within the data point. For drug-elicited head twitch dose-effect determinations, data were not normally distributed and were therefore analyzed by one way analysis of variance (ANOVA) on ranks, followed by Dunn’s post-hoc test for all pairwise comparisons. Data from experiments involving effects of 25CN-NBOH pretreatment on DOI-elicited head twitch behavior were analyzed with a one way ANOVA followed by Tukey’s HSD test for all pairwise comparisons. Drug discrimination data were statistically analyzed using a repeated measures one way ANOVA followed by Tukey’s HSD test for all pairwise comparisons. In all cases, significance was judged at the level of p < 0.05.
Drugs
R(−)-DOI was purchased from Sigma Aldrich (St. Louis, MO). RS 102221 was purchased from Tocris Bioscience (Ellisville, MO). M100907 was synthesized in the Laboratory of Medicinal Chemistry at the NIH/NIDDK and was supplied as a generous gift from Kenner C. Rice, Ph.D. 25CN-NBOH was synthesized by one of us (MH) at the Department of Drug Design and Pharmacology at the University of Copenhagen. All drugs were dissolved in physiological saline, which, along with all other experimental supplies, was obtained from standard commercial sources. Sonication in warm water was required to dissolve 25CN-NBOH at the highest concentration (3.0 mg/ml) used in these studies.
Results
Drug-elicited head twitch
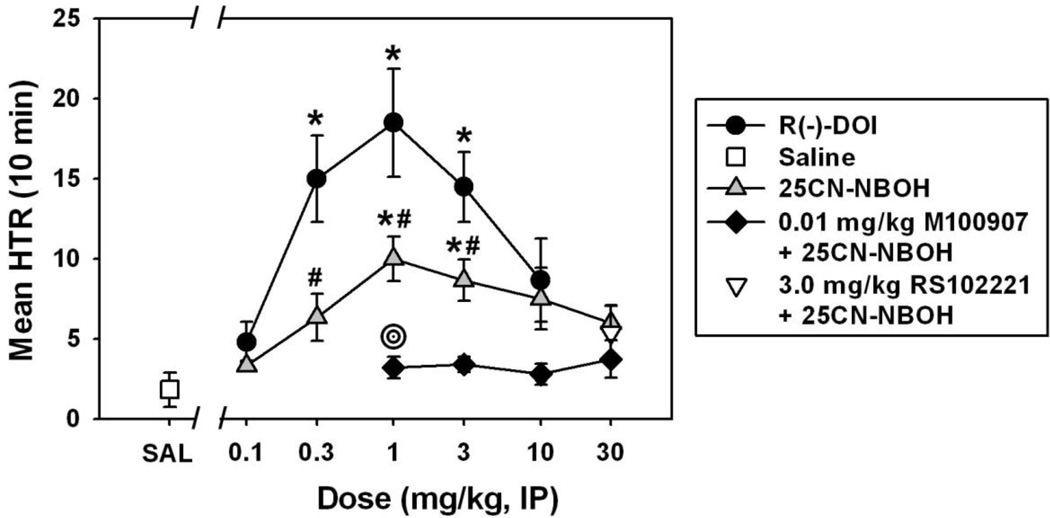
As expected, saline injection elicited very few head twitches. DOI elicited a characteristic biphasic dose-effect function (Figure 2, filled circles), similar to those we have previously generated in earlier experiments with this drug (Fantegrossi et al., 2010). DOI-elicited head twitch behavior was significantly different from that observed after saline administration at doses of 0.3 (q=4.059), 1.0 (q=4.351) and 3.0 mg/kg (q= 3.924, df=14 and p<0.05 in all cases). A maximum of approximately 20 head twitches was quantified in the 10 minute observation period at a dose of 1.0 mg/kg, which is typically the most effective DOI dose using our procedure (Fantegrossi et al., 2010). Interestingly, 25CN-NBOH did not engender a particularly strong head twitch response at any of the doses tested (Figure 2, grey triangles), and only doses of 1.0 (q=3.811) and 3.0 mg/kg (q=3.536, df=14 and p<0.05 for both comparisons) were significantly different from saline. Head twitch behavior elicited by 25CN-NBOH was significantly different from that induced by the same dose of DOI at doses of 0.3 (q=4.244), 1.0 (q= 3.745) and 3.0 mg/kg (q=3.329, df=14 and p<0.05 for all comparisons). The maximum number of twitches observed with 25CN-NBOH was approximately 10 at a dose of 1.0 mg/kg, which was approximately half the maximal effect observed with DOI. The slope of the “descending limb” of the 25CN-NBOH dose-effect curve was shallow when compared to that of the DOI dose-effect function. Head twitch behavior elicited by 1.0, 3.0 and 10.0 mg/kg 25CN-NBOH was attenuated by M100907 pretreatment (Figure 2, filled diamonds), but a statistical difference was only observed at the 1.0 mg/kg 25CN-NBOH dose (q=2.952, p<0.05). In contrast to what we have previously observed with DOI (Fantegrossi et al., 2010), pretreatment with RS102221 did not alter head twitch behavior elicited by a dose of 25CN-NBOH on the descending limb of the dose-effect curve (30.0 mg/kg, Figure 2, white inverted triangle) (q=0.166, p>0.05).
Figure 2.
Biphasic dose-dependent effects of R(−)-DOI (black circles) and 25CN-NBOH (grey triangles) on head twitch behavior in NIH Swiss mice. Black diamonds represent 25CN-NBOH-elicited head twitch behavior after pretreatment with 0.01 mg/kg M100907, while the white inverted triangle represents 25CN-NBOH-elicited head twitch behavior following pretreatment with 3.0 mg/kg RS102221. Asterisks indicate significant differences from saline, hash marks indicate significant differences from the same dose of DOI, and bullseyes indicate differences from the same dose of 25CN-NBOH (without antagonist pretreatment). Abscissa: Dose of DOI or 25CN-NBOH, expressed as mg/kg on a log scale. Ordinate: Mean head twitches recorded over a 10 minute observation period.
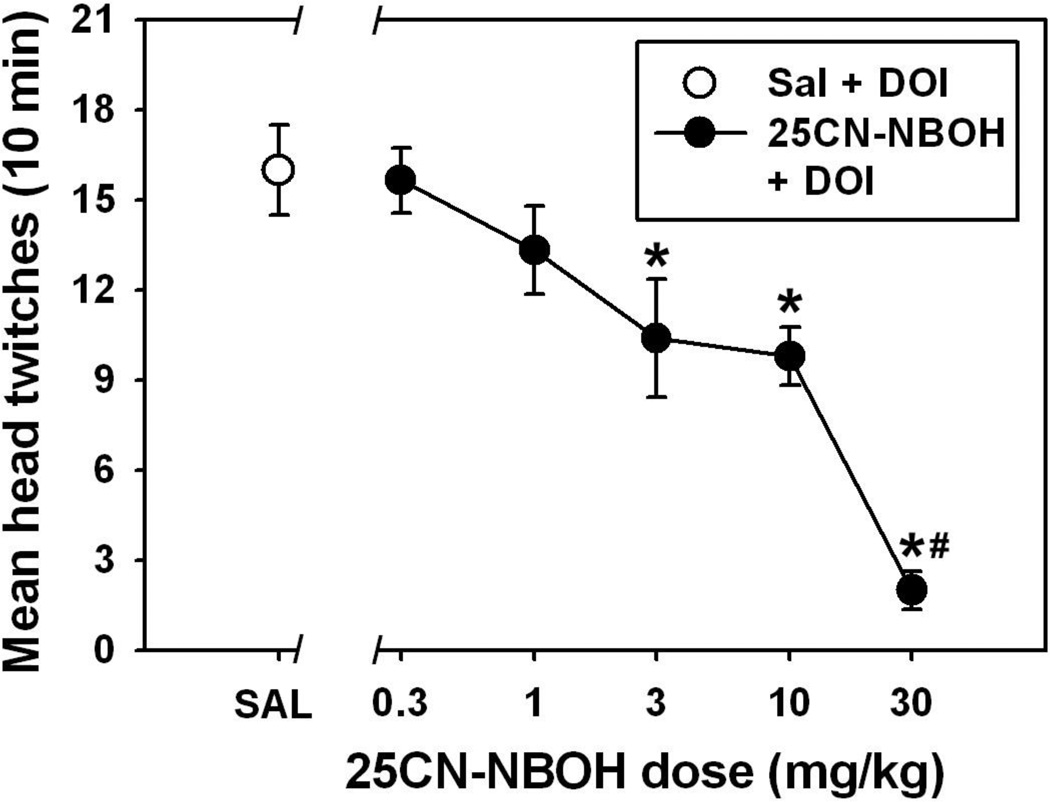
When administered as a pretreatment, 25CN-NBOH dose-dependently suppressed head twitch behavior elicited by subsequent injection of 1.0 mg/kg DOI (Figure 3, filled circles). Following 25CN-NBOH pretreatment doses of 0.3 and 1.0 mg/kg, DOI-elicited head twitch behavior was not significantly different from that observed following saline pretreatment (q=0.267 and 2.151, respectively, and p>0.05 in both cases), but 25CN-NBOH doses of 3.0, 10.0 and 30.0 mg/kg significantly blunted head twitch behavior induced by 1.0 mg/kg DOI (q=4.517, 5.001 and 11.292, respectively, df=5 and p<0.05 in all cases). There were no significant differences in DOI-elicited head twitch behavior following 25CN-NBOH doses of 3.0 and 10.0 mg/kg (q=1.882, p>0.05), but the 30 mg/kg pretreatment condition significantly differed from 25CN-NBOH doses of 0.3 (q=11.024), 1.0 (q=9.141), 3.0 (q=6.775) and 10.0 mg/kg (q=6.291, df=5 and p<0.05 in all cases). Importantly, the level of DOI-elicited head twitches observed after 25CN-NBOH pretreatment doses of 3.0 and 10.0 mg/kg was similar to that quantified after a maximally-effective dose of 25CN-NBOH on its own.
Figure 3.
Head twitch behavior elicited by 1.0 mg/kg R(−)-DOI following pretreatment with saline (white circle) or various doses of 25CN-NBOH (black circles.) Asterisks indicate significant differences from saline pretreatment, while hash marks indicate significant differences from lower pretreatment doses of 25CN-NBOH. Abscissa: Dose of 25CN-NBOH, expressed as mg/kg on a log scale, and administered as a pretreatment 15 min prior to R(−)-DOI injection. Ordinate: as described in Figure 2.
DOI discrimination
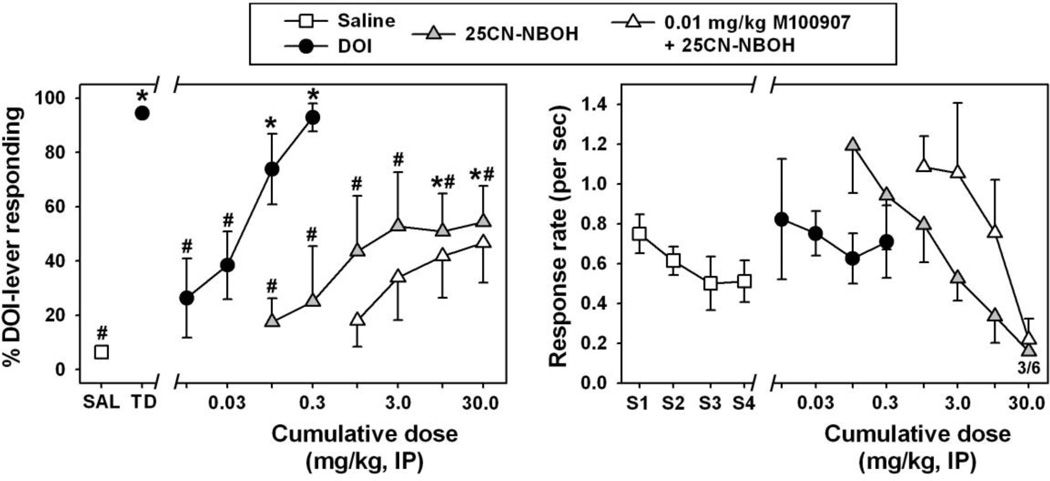
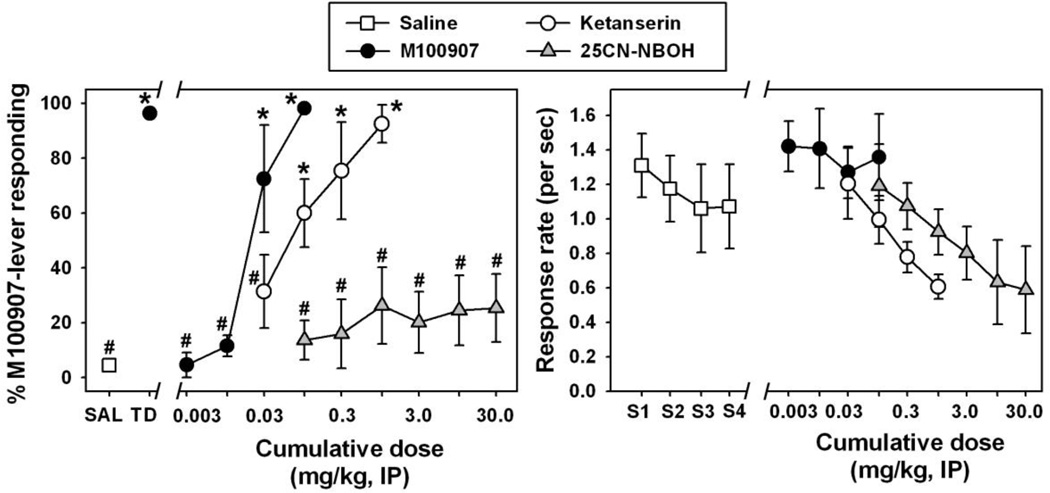
Mice were trained to reliably discriminate the DOI training dose (Figure 4, left, black circle at ‘TD’) from saline (Figure 4, left, white square at ‘SAL’) (q=9.205, p<0.05). When saline was administered in training sessions, mice primarily responded on the saline lever. Similarly, when the training dose of 0.3 mg/kg was administered, mice responded almost exclusively on the DOI lever. During substitution tests with cumulative doses of DOI (Figure 4, left, black circles), dose-dependent substitution for the training dose was observed such that cumulative doses of 0.1 and 0.3 mg/kg were significantly different from saline (q=7.591 and 9.297, respectively, df=12 and p<0.05 in both cases), and near exclusive choice of the drug lever was observed at a cumulative dose of 0.3 mg/kg. Interestingly, 25CN-NBOH (Figure 4, left, grey triangles) only partially substituted for the DOI training dose, eliciting no more than 55% DOI-appropriate responding at any dose tested. DOI-appropriate responding elicited by cumulative 25CN-NBOH doses of 10.0 and 30.0 mg/kg was significantly different from that observed following saline administration (q=5.233 and 5.332, respectively, df=12 and p<0.05 in both cases), as well as from that observed after administration of the DOI training dose (q=5.259 and 5.348, respectively, df=12 and p<0.05 in both cases). Despite only partially substituting for the DOI training dose, the dose effect curve for DOI-like interoceptive effects elicited by cumulative doses of 25CN-NBOH was shifted to the right by prior administration of M100907 (Figure 4, left, open triangles), although statistical significance was not obtained at any dose. Importantly, the partial substitution observed with 25CN-NBOH occurred at doses which suppressed response rates (Figure 4, right, grey triangles). At the highest cumulative dose of 25CN-NBOH, with or without M100907 pretreatment, response rates were extremely low, and 3/6 mice failed to complete the FR5 during testing, indicating behavioral suppression and defining the maximum testable dose using these procedures.
Figure 4.
Left panel - Discriminative stimulus effects of R(−)-DOI (black circles), 25CN-NBOH (grey triangles), and 25CN-NBOH following pretreatment with M100907 (white triangles) in mice trained to discriminate 0.3 mg/kg R(−)-DOI from saline. Asterisks indicate significant differences from saline, while hash marks indicate significant differences from the DOI training dose. Abscissa: Cumulative dose of DOI or 25CN-NBOH expressed as mg/kg on a log scale. Ordinate: percent of total responses emitted on the R(−)-DOI-appropriate lever. Right panel – Response rates (per second) obtained during substitution testing with cumulative doses of R(−)-DOI and 25CN-NBOH. Fractions represent number of animals completing the response requirement at a given dose, if less than 6. Abscissa: ‘S1–S4' represents four discrete saline injections administered across four components of a substitution session. Numbers refer to cumulative doses of drugs during substitution sessions, expressed as mg/kg on a log scale. Ordinate: response rates, expressed as lever presses per second.
M100907 discrimination
Mice were trained to reliably discriminate the M100907 training dose (Figure 5, left, black circle at ‘TD’) from saline (Figure 5, left, white square at ‘SAL’) (q=10.506, df=16 and p<0.05). When saline was administered in training sessions, mice primarily responded on the saline lever, and when the training dose of 0.1 mg/kg was administered, mice responded almost exclusively on the M100907 lever. During substitution tests with cumulative doses of M100907 (Figure 5, left, black circles), dose-dependent substitution for the training dose was observed such that cumulative doses of 0.03 and 0.1 mg/kg were significantly different from saline (q=7.448 and 10.331, respectively, df=16 and p<0.05 in both cases), and near exclusive choice of the drug lever was observed at a cumulative dose of 0.1 mg/kg. Substitution testing with cumulative doses of ketanserin (Figure 5, left, open circles) produced dose-dependent and complete generalization to the M100907 training dose, with cumulative doses of 0.1, 0.3 and 1.0 mg/kg eliciting significantly more M100907-lever responding than saline (q=6.484, 8.173, and 10.105, respectively, df=16 and p<0.05 in all cases), and 1.0 mg/kg ketanserin eliciting statistically indistinguishable M100907-lever responding from the M100907 training dose (q=0.401, p>0.05). In contrast, 25CN-NBOH (Figure 5, left, grey triangles) did not substitute for M100907, and elicited only saline-like responding across the cumulative doses tested, up to doses which significantly suppressed response rates (Figure 5, right, grey triangles). M100907-like responding elicited by cumulative 25CN-NBOH doses of 0.1, 0.3, 1.0, 3.0, 10.0 and 30.0 mg/kg was significantly different from that observed following administration of the M100907 training dose (q=9.438, 9.082, 7.882, 8.485, 8.205, and 8.107, respectively, df=16 and p<0.05 in all cases).
Figure 5.
Left panel - Discriminative stimulus effects of M100907 (black circles), ketanserin (white circles) and 25CN-NBOH (grey triangles) in mice trained to discriminate 0.1 mg/kg M100907 from saline. Asterisks indicate significant differences from saline, while hash marks indicate significant differences from the M100907 training dose. Abscissa and ordinate as described in Figure 4. Right panel – Response rates (per second) obtained during substitution testing with cumulative doses of M100907, ketanserin and 25CN-NBOH. Abscissa and ordinate as described in Figure 4.
Discussion
These experiments suggest that 25CN-NBOH is behaviorally active in two mouse assays which model hallucinogen effects. First, the capacity of this compound to induce the head twitch response, and the potent antagonism of this effect by prior injection of M100907, suggests that a primary site of agonist action for 25CN-NBOH is the 5-HT2AR. Moreover, the relatively “wide” dose-effect function for head twitch behavior elicited by 25CN-NBOH (compared to that induced by DOI), coupled with the lack of modification of 25CN-NBOH effects by the selective 5-HT2CR antagonist RS102221, strongly suggests that the high degree of 5-HT2AR selectivity observed with 25CN-NBOH in vitro (Hansen et al., 2014) is maintained in vivo. In previous studies with DOI, we demonstrated that the descending limb of the dose-effect curve for head twitch behavior is likely mediated by agonist actions of DOI at 5-HT2CR, because pretreatment with selective 5-HT2CR agonists insurmountably blocked DOI-elicited head twitch behavior, while pretreatment with selective 5-HT2CR antagonists induced parallel rightward shifts in head twitch behavior elicited by DOI doses on the descending limb only (Fantegrossi et al., 2010). Based on those findings, we would expect that a compound with much greater selectivity for 5-HT2AR than that displayed by DOI would elicit biological effects over a wide range of active doses, exhibit a shallow descending limb, and display no significantly different effects following pretreatment with a 5-HT2CR antagonist. That pattern of results is essentially what was observed here with 25CN-NBOH.
Despite this apparently greater 5-HT2AR selectivity, 25CN-NBOH was not as effective in the head twitch procedure as was DOI, eliciting only about half as many head twitches as the maximally-effective DOI dose. It is not immediately clear why this should be the case, especially since limiting effects of agonist actions at 5-HT2CR are not likely to be involved. It may be the case that pharmacokinetic factors of absorption, distribution, or metabolism differ significantly from those of DOI, such that the particulars of the presently-used assay were not sufficient to capture 25CN-NBOH effects. For example, if 25CN-NBOH has a slower onset of action, then the 10 min pretreatment period prior to initiating behavioral scoring may not have allowed significant concentrations to reach the site of action. Similarly, if 25CN-NBOH has a relatively short duration of action, then the 10 min scoring period might be too long and minimize the apparent effectiveness of the drug. Most recently, rapid N-debenzylation of 25CN-NBOH and related analogues was demonstrated in human liver microsomes, resulting in a higher intrinsic clearance for the N-benzylated phenethylamines as compared to the parent phenethylamines (Leth-Petersen et al., in press). However, it seems unlikely that any of these conditions would not be overcome by simply increasing the 25CN-NBOH dose. Therefore, it seems more likely that pharmacodynamic differences between DOI and 25CN-NBOH account for the presently observed behavioral differences, with a likely explanation being that 25CN-NBOH is a lower-efficacy agonist than DOI. It is a basic principle of pharmacology that a lower-efficacy agonist can function as an antagonist when coadministered with a higher-efficacy agonist (depending on the efficacy demand of the specific assay), but these antagonist-like effects will be limited by the intrinsic efficacy of the low efficacy agonist. Consistent with this notion, we observed that pretreatments with 25CN-NBOH dose-dependently suppressed head twitch behavior elicited by DOI to a level equivalent to the maximal effect elicited by 25CN-NBOH on its own, but then completely suppressed DOI-elicited head twitch behavior at the highest dose tested. This abrupt suppression of head twitch behavior is consistent with saturation of 5-HT2AR by 25CN-NBOH and DOI, resulting in free DOI binding and activating 5-HT2CR, which we have previously shown to attenuate head twitch behavior (Fantegrossi et al., 2010). Alternatively, DOI-elicited HTR is also modulated by numerous receptors outside the 5-HT2 family (reviewed in Canal and Morgan, 2012), so until a thorough screening of the binding affinity of 25CN-NBOH at various brain recognition sites is accomplished, it remains possible that the complete attenuation of DOI-induced HTR observed following treatment with 30 mg/kg 25CN-NBOH could be due to “off target” interactions with other receptors. Finally, functional selectivity could also be involved in the differential effects of DOI and 25CN-NBOH in the present experiments. The role of various intracellular signaling pathways in DOI-elicited HTR is incompletely understood (reviewed most recently by Canal and Morgan, 2012), but it may be the case that 25CN-NBOH induces an agonist-specific signaling cascade distinct from that of DOI.
However, the failure of 25CN-NBOH to substitute – even partially – in mice trained to discriminate M100907 from saline strongly suggests that 25CN-NBOH is not an antagonist at the 5-HT2AR despite its apparent antagonist-like effects against DOI-elicited head twitches. The initial report of M100907 as a discriminative stimulus described a remarkably specific interoceptive cue, where dose-dependent M100907-appropriate responding was elicited by administration of the selective 5-HT2AR antagonist SR46349 (eplivanserin, (Z,E)-1-(2-fluorophenyl)-3-(4-hydroxyphenyl)-2-propen-1-one O-[2-(dimethylamino)ethyl]oxime), but not the selective 5-HT2BR antagonist SB204,741 (N-(1-Methyl-1H-indol-5-yl)-N'-(3-methylisothiazol-5-yl)urea), the 5-HT2B/2CR antagonist SB206,553 (5-methyl-1-(3-pyridylcarbamoyl)-1,2,3,5-tetrahydropyrrolo[2,3-f]indole), or the selective 5-HT2CR antagonists SB242,084 (6-chloro-5-methyl-N-{6-[(2-methylpyridin-3-yl)oxy]pyridin-3-yl]indoline-1-carboxamide) or RS102221 (Dekeyne et al., 2002). The present generalization of the M100907 training dose to ketanserin suggests that the M100907 discrimination is likely mediated by antagonist actions at 5-HT2A receptors in the mouse as well, consistent with previous findings in the rat (Dekeyne et al., 2002; Li et al., 2009). Similarly, the partial DOI-like discriminative stimulus effects elicited by 25CN-NBOH and the attenuation of these effects by M100907 are also consistent with a 5-HT2AR-mediated mechanism of low efficacy agonist action for this compound. Alternatively, it may be the case that the DOI discrimination is mediated by a “compound cue” consisting of a cluster of agonist effects at the three 5-HT2 receptor subtypes, while the interoceptive effects of 25CN-NBOH are more selective, overlapping with those of DOI only in terms of 5-HT2AR agonist effects. This could also explain a partial substitution, and could be tested by attempting to replicate a more DOI-like interoceptive state by coadministration of 25CN-NBOH with selective 5-HT2BR and / or 5-HT2CR agonists. In either case, it is unlikely that simply testing higher doses of 25CN-NBOH would result in greater DOI-like effects, because the current studies used doses sufficient to dramatically suppress operant responding.
Although much of our information regarding the biological effects of novel N-benzylphenethylamine hallucinogens is largely anecdotal in nature, accounts posted online (for example, erowid.org and bluelight.org) strongly attest to the psychoactive properties of these substances. Additionally, a recent report (Halberstadt and Geyer, 2014) demonstrated dose-dependent 5-HT2AR-mediated head twitch behavior elicited by two structural analogues of 25CN-NBOH: 25I-NBOMe and 25I-NBMD. Reports of toxicity and lethality following recreational use of this class of compound continue to emerge (Nikolaou et al., in press; Tang et al., 2014; Walterscheid et al., 2014), highlighting the need for further basic science research with these compounds, and for education of emergency medical personnel who may encounter patients following ingestion of these substances.
Acknowledgements
We thank the UAMS Division of Laboratory Animal Medicine for expert husbandry services. This research was generously supported, in part, by an NIGMS IDeA Program award (GM110702), by the UAMS Translational Research Institute (RR029884), and by the Lundbeck Foundation. DS received a Summer Undergraduate Research Fellowship from the American Society of Pharmacology and Experimental Therapeutics which funded his time in the laboratory conducting the head twitch experiments described herein. The views expressed herein are those of the authors and do not necessarily represent the views of the University of Arkansas for Medical Sciences.
References
- Advisory Council on the Misuse of Drugs (ACMD) “NBOMe” compounds: A review of the evidence of use and harm. [Accessed 03 July 2014];2013 Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/204808/J_TCDO_report_on_5-6APB_and_NBOMe_compounds.pdf.
- Blaazer AR, Smid P, Kruse CG. Structure-activity relationships of phenylalkylamines as agonist ligands for 5-HT(2A) receptors. Chem Med Chem. 2008;3(9):1299–1309. doi: 10.1002/cmdc.200800133. [DOI] [PubMed] [Google Scholar]
- Braden MR, Parrish JC, Naylor JC, Nichols DE. Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists. Mol Pharmacol. 2006;70(6):1956–1964. doi: 10.1124/mol.106.028720. [DOI] [PubMed] [Google Scholar]
- Canal CE, Morgan D. Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Test Anal. 2012;4(7–8):556–576. doi: 10.1002/dta.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Murnane KS, Reissig CJ. The behavioral pharmacology of hallucinogens. Biochem Pharmacol. 2008;75(1):17–33. doi: 10.1016/j.bcp.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Simoneau J, Cohen MS, Zimmerman SM, Henson CM, Rice KC, Woods JH. Interaction of 5-HT2A and 5-HT2C receptors in R(−)-2,5-dimethoxy-4-iodoamphetamine-elicited head twitch behavior in mice. J Pharmacol Exp Ther. 2010;335(3):728–734. doi: 10.1124/jpet.110.172247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA, Raghupathi RK, Bartyzel P, Teitler M, Leonhardt S. Binding of phenylalkylamine derivatives at 5-HT1C and 5-HT2 serotonin receptors: evidence for a lack of selectivity. J Med Chem. 1992;35:734–740. doi: 10.1021/jm00082a014. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. Effects of the hallucinogen 2,5-dimethoxy-4-iodophenethylamine (2C-I) and superpotent N-benzyl derivatives on the head twitch response. Neuropharmacol. 2014;77:200–207. doi: 10.1016/j.neuropharm.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Phonekeo K, Paine JS, Leth-Petersen S, Begtrup M, Bräuner-Osborne H, Kristensen JL. Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists. ACS Chem Neurosci. 2014;5(3):243–249. doi: 10.1021/cn400216u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M. Ph.D. thesis. University of Copenhagen; 2011. [01 July 2014]. Design and Synthesis of Selective Serotonin Receptor Agonists for Positron Emission Tomography Imaging of the Brain. Accessed at https://docs.google.com/file/d/0BwXelgjm5BeEaEJJU0lPa1NnaGM. [Google Scholar]
- Juncosa JI, Jr, Hansen M, Bonner LA, Cueva JP, Maglathlin R, McCorvy JD, Marona-Lewicka D, Lill MA, Nichols DE. Extensive rigid analogue design maps the binding conformation of potent N-benzylphenethylamine 5-HT2A serotonin receptor agonist ligands. ACS Chem Neurosci. 2013;4(1):96–109. doi: 10.1021/cn3000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight AR, Misra A, Quirk K, Benwell K, Revell D, Kennett G, Bickerdike M. Pharmacological characterisation of the agonist radioligand binding site of 5-HT(2A), 5-HT(2B) and 5-HT(2C) receptors. Naunyn Schmiedebergs Arch Pharmacol. 2004;370(2):114–123. doi: 10.1007/s00210-004-0951-4. [DOI] [PubMed] [Google Scholar]
- Lawn W, Barratt M, Williams M, Horne A, Winstock A. The NBOMe hallucinogenic drug series: Patterns of use, characteristics of users and self-reported effects in a large international sample. J Psychopharmacol. 2014 doi: 10.1177/0269881114523866. in press. [DOI] [PubMed] [Google Scholar]
- Leth-Petersen S, Bundgaard C, Hansen M, Carnerup MA, Kehler J, Kristensen JL. Correlating the Metabolic Stability of Psychedelic 5-HT2A Agonists with Anecdotal Reports of Human Oral Bioavailability. Neurochem Res. 2014 doi: 10.1007/s11064-014-1253-y. in press. [DOI] [PubMed] [Google Scholar]
- Li JX, Unzeitig A, Javors MA, Rice KC, Koek W, France CP. Discriminative stimulus effects of 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane (DOM), ketanserin, and (R)-(+)-{alpha}-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-pipidinemethanol (MDL100907) in rats. J Pharmacol Exp Ther. 2009;331(2):671–679. doi: 10.1124/jpet.109.157560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean TH, Parrish JC, Braden MR, Marona-Lewicka D, Gallardo-Godoy A, Nichols DE. 1-Aminomethylbenzocycloalkanes: conformationally restricted hallucinogenic phenethylamine analogues as functionally selective 5-HT2A receptor agonists. J Med Chem. 2006;49(19):5794–5803. doi: 10.1021/jm060656o. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101(2):131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Frescas SP, Chemel BR, Rehder KS, Zhong D, Lewin AH. High specific activity tritium-labeled N-(2-methoxybenzyl)-2,5-dimethoxy-4-iodophenethylamine (INBMeO): A high-affinity 5-HT2A receptor-selective agonist radioligand. Bioorg Med Chem. 2008;16:6116–6123. doi: 10.1016/j.bmc.2008.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaou P, Papoutsis I, Stefanidou M, Spiliopoulou C, Athanaselis S. 2C-I-NBOMe, an "N-bomb" that kills with "Smiles". Toxicological and legislative aspects. Drug Chem Toxicol. 2014 doi: 10.3109/01480545.2014.911882. in press. [DOI] [PubMed] [Google Scholar]
- Parker MA, Marona-Lewicka D, Lucaites VL, Nelson DL, Nichols DE. A novel (benzodifuranyl)aminoalkane with extremely potent activity at the 5-HT2A receptor. J Med Chem. 1998;41(26):5148–5149. doi: 10.1021/jm9803525. [DOI] [PubMed] [Google Scholar]
- Roth BL, Willins DL, Kristiansen K, Kroeze WK. 5-Hydroxytryptamine2-family receptors (5-hydroxytryptamine2A) 5-hydroxytryptamine2B, 5-hydroxytryptamine2C): where structure meets function. Pharmacol Ther. 1998;79(3):231–257. doi: 10.1016/s0163-7258(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Tang MH, Ching CK, Tsui MS, Chu FK, Mak TW. Two cases of severe intoxication associated with analytically confirmed use of the novel psychoactive substances 25B-NBOMe and 25C-NBOMe. Clin Toxicol. 2014;52(5):561–565. doi: 10.3109/15563650.2014.909932. [DOI] [PubMed] [Google Scholar]
- Walterscheid JP, Phillips GT, Lopez AE, Gonsoulin ML, Chen HH, Sanchez LA. Pathological findings in 2 cases of fatal 25I-NBOMe toxicity. Am J Forensic Med Pathol. 2014;35(1):20–25. doi: 10.1097/PAF.0000000000000082. [DOI] [PubMed] [Google Scholar]