Abstract
Developmental exposure to polychlorinated biphenyls (PCBs) causes auditory deficits. Thus, we recently conducted a study to investigate if developmental PCB exposure would exacerbate noise-induced hearing loss in adulthood. Unexpectedly, some PCB-exposed rats exhibited seizure-like behaviors when exposed to loud noise. Therefore, we conducted the current experiment to determine if adult rats perinatally exposed to PCBs are more susceptible to audiogenic seizures when tested in a standard audiogenic seizure paradigm. Adult male and female rats exposed to PCBs during gestation and lactation (0, 1, 3 or 6 mg/kg/day) and previously tested in the noise-induced hearing loss study were presented with a 100dB noise stimulus. If they did not exhibit clonus in response to the 100dB noise, they were exposed to a 105dB stimulus 24-48 hours later. This was followed by an 110dB stimulus 24-48 hours later if they did not exhibit clonus at 105 dB. Female and male rats exposed to either 3 or 6 mg/kg PCBs exhibited a significantly higher incidence of audiogenic seizures, shorter latency to onset of seizures, and greater severity of seizures compared to controls. Thyroxine measured in littermates at weaning was significantly lower in all PCB groups compared to controls, suggesting a potential mechanism for the increased incidence of audiogenic seizures. This is the first study to show that developmental PCB exposure increases the susceptibility to audiogenic seizures in adulthood.
Keywords: PCBs, hearing loss, audiogenic seizures
1. INTRODUCTION
Polychlorinated biphenyls (PCBs) are industrial contaminants that were manufactured for use as dielectric fluids in transformers and capacitors (Crinnion, 2011). Their production has been banned since the late 1970s, but due to their chemical stability and lipophilicity, they continue to persist in the environment. PCBs have bio-accumulated and bio-magnified up the food chain, and human exposure is primarily via consumption of contaminated fish and seafood (Crinnion 2011). PCBs readily cross the mammalian placenta and are mobilized from body fat into breast milk during lactation, putting the developing offspring at risk (Jacobson et al. 1984). One cause for concern is that developmental exposure to PCBs has been associated with long-lasting hearing deficits in animal models and humans (Crofton et al. 2000; Goldey et al. 1995; Jusko et al., 2014; Poon et al. 2011; Powers et al. 2006; Trnovec et al. 2010).
In an early study, maternal exposure to Aroclor 1254 (a commercial PCB mixture) led to increased auditory thresholds at low frequencies in the offspring (Goldey et al. 1995). Later, Crofton (2000) reported loss of outer hair cells in the region of the cochlea responsible for low-frequency hearing in adult rats after perinatal exposure to Aroclor 1254. These findings led to additional experiments to further elucidate the cochlear site of action of PCBs using distortion product otoacoustic emissions (DPOAEs), which test the integrity of the outer hair cells, and auditory brainstem responses, which measure the integrity of the auditory neuronal pathway. Exposure to Aroclor 1254 or to an environmentally relevant PCB mixture (Fox River PCB Mix; Kostyniak et al. 2005) during development resulted in decreased DPOAE amplitudes that lasted into adulthood in the absence of any changes in amplitudes or latencies of auditory brainstem responses, further suggesting that PCBs act at a cochlear site to produce auditory deficits (Lasky et al. 2002; Powers et al. 2006, 2009).
Given the evidence that PCBs act at the level of cochlea outer hair cells, we investigated the interaction between PCB exposure during cochlear development and exposure to loud noise in adulthood (Poon et al. unpublished results). We hypothesized that early PCB exposure might increase the likelihood of noise-induced hearing loss later in life. Unexpectedly, when exposed to loud noise, many rats in the highest PCB dose group (6 mg/kg PCB) exhibited bouts of wild running followed by a period when they were unresponsive to stimuli. These behaviors appeared to be similar those that occur during audiogenic seizures (reviewed in Ross and Coleman 2000), and these preliminary results led to the current study to investigate this more directly.
Audiogenic seizures are elicited by exposure to loud noise, require the activation of the auditory brainstem and are initiated mainly in the inferior colliculus (Coleman et al. 1999; Eells et al. 2004; Faingold 2002; Ishida et al. 1995; Ross and Coleman 2000). These seizures present differently from forebrain induced seizures which start with facial and forelimb tremors and progress to a clonic or clonic-tonic state (Racine 1972). Interestingly, the Long-Evans rats used in our studies are generally less susceptible to seizures than other rat strains, but can be made more susceptible to audiogenic seizures by priming with damaging noise during the critical period of cochlear development (Ross and Coleman 1999; 2000).
One hypothesis for this induced sensitivity to audiogenic seizures is that priming to loud noise during cochlear development results in short-term hearing loss during this critical developmental window, resulting in a permanent down regulation of inhibitory circuits in the inferior colliculus. This serves to amplify the weaker afferent signals coming from the cochlea. This imbalance towards excitatory signaling leads to a hypersensitivity to intense stimulation later in life (reviewed in Ross and Coleman 2000). If hearing loss can increase the sensitivity to audiogenic seizures, then PCB-induced hair cell damage and the resultant hearing deficits (Poon et al. unpublished results) may underlie the apparent increase in seizure responsiveness we observed.
In this study, our goal was to directly investigate whether developmental exposure to the Fox River PCB mixture would make rats more susceptible to audiogenic seizures in adulthood. We used a classic audiogenic seizure testing paradigm in which the rats were exposed to a brief high intensity noise (Ross and Coleman 1999) and the incidence, severity and latency to onset of seizure behaviors were recorded.
2. METHODS
2.1 Animals
Primiparous female Long-Evans rats, approximately 8-10 weeks of age, were purchased from Harlan (Indianapolis, IN) in three cohorts. They were individually housed in standard polycarbonate plastic shoebox cages with corn-cob bedding, and fed rat chow (Harlan Teklad rodent diet (W) 8604) and water ad libitum. All rats were housed in a temperature- and humidity-controlled room (22°C, 40–55% humidity) on a 12/12-hr light cycle (lights on at 0830). Average sound pressure levels in the housing room were less than 55 dB, a level well below that which would be expected to lead to hearing loss. In addition, all animals were housed in the same room, and thus were exposed to the same daily noise exposure.
The rats were maintained in facilities accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Illinois at Urbana-Champaign and were in accordance with the guidelines of the National Institutes of Health (2002) and National Research Council (2003).
2.2 Exposure
Female rats were randomly assigned to exposure groups and treated daily with one of four dosing solutions consisting of corn oil vehicle or 1, 3, or 6 mg/kg PCBs in corn oil. Each exposure group was represented in each of the three cohorts. Exposure began 28 days prior to breeding and continued until weaning of the pups on postnatal day (PND) 21. The PCB mixture (Fox River PCB mixture) was formulated to mimic the congener profile found in walleye, a popular sport-caught fish from the Fox River in northeast Wisconsin. The mixture consisted of 35% Aroclor 1242 (Monsanto lot KB 05-415; St. Louis, MO), 35% Aroclor 1248 (AccuStandards lot F-110; New Haven, CT), 15% Aroclor 1254 (Monsanto lot KB 05-612), and 15% Aroclor 1260 (AccuStandards lot 021-020) (Kostyniak et al. 2005). The doses of the PCB mixture were selected based on the results of earlier studies assessing the in vivo developmental toxicity and auditory toxicity of this mixture in rats (see Kostyniak et al. 2005; Powers et al. 2006). The PCBs diluted in corn oil (Mazola) or the corn oil vehicle was pipetted onto one-half of a vanilla wafer cookie (Keebler Golden Vanilla Wafers) at a volume of 0.4 mL solution/kg body weight. The individual dosing solutions were mixed at concentrations of 2.25 mg/mL, 7.5 mg/mL and 15 mg/mL for the PCB doses of 1 mg/kg, 3 mg/kg and 6 mg/kg respectively. Females were weighed and fed the PCB or vehicle-treated cookies daily.
2.3 Breeding, pregnancy, and weaning
After the initial four weeks of PCB exposure, each female was paired with an unexposed male Long-Evans rat (Harlan, Indianapolis, IN) in a hanging wire cage for 8 consecutive days with food and water ad libitum. Females were returned to their home cages briefly each day for PCB dosing. Females were monitored daily for the presence of a sperm plug in order to establish gestational day 0.
On the day of parturition (PND 0), pups were examined for abnormalities, sexed and weighed. On PND 2, litters were culled to 10 pups (five males and five females when possible), and litters with at least 7 pups had extra pups cross-fostered into them from the same treatment group to bring the litters to 8–10 pups. Cross-fostered pups were ear-notched and not used for the experiment. There were 42 of 60 successful litters. Of the remaining dams, 9 were not pregnant and 9 had litters too small to be included in the study (≤7 pups). Overall, non-pregnant dams and dams with small litters were evenly distributed across the treatment groups.
Dosing of the dams continued until the pups were weaned on PND 21. Although the dams were not separated from the pups during the postnatal dosing, the researchers directly gave the cookie to the dam and the cookies were consumed by the dams in a few minutes without the offspring receiving any portion. One male and one female per litter were retained for a noise-induced hearing loss study beginning at approximately PND 200 (Poon et al. unpublished results) and the audiogenic seizure testing reported here was conducted in these same animals at approximately PND 400.
All animals were subjected to some noise during habituation. Habituation consisted of exposing the rats to noise increasing in intensity from 75 to 97dB over a period of 15 min. This occurred every day prior to exposure to 97dB of broadband noise centered around 8 KHz for 4 hours a day for 5 consecutive days. Based on previous research, this degree of noise exposure was expected to result in temporary hearing loss in control rats (Pouyatos et al. 2005). However, some animals exhibited seizures during the initial habituation period on the first day and were removed from the study without being subjected to the louder noise over an extended period. As a result, some of the rats (mainly the control and 1 mg/kg PCB animals) used in the audiogenic seizure study had previously been subjected to noise at a level expected to result in temporary hearing loss, whereas others (some of the 3 mg/kg rats and over half of the 6 mg/kg rats) were not exposed to this noise due to the seizure-like behaviors they displayed at the onset of noise exposure. Therefore, the control group that received noise exposure in the previous experiment was referred to as the noise-exposed (NE) control. Previous noise exposure could potentially sensitize the rats to be more prone to audiogenic seizures. Thus, to account for the fact that all of the NE control animals had previous noise exposure, whereas only a subset of the 3 and 6 mg/kg PCB-exposed animals did, we also included a noise-naïve (NN) control group in the current study. Eight Long-Evans females and eight Long-Evans males (Harlan, IN) aged 10-12 months were used as the NN control group.
2.4 Total Thyroxine Radioimmunoassay
Because developmental hypothyroxinemia could contribute to hearing loss and/or susceptibility to audiogenic seizures, serum collected at weaning from littermates of the rats tested in this study was assayed for total T4 concentrations using a Total T4 Coat-A-Count Kit (Siemens) and counted in a gamma counter (Packard Cobra Autogamma II).
2.5 Audiogenic Seizures
Approximately 4-5 months after the rats (one male and one female per litter) completed testing for the noise-induced hearing loss study, they were tested for audiogenic seizures. For testing, each rat was placed individually in a cylindrical plexiglass tube (29 cm diameter × 31 cm height) that was lined on the bottom with sound insulating material (Soundsoak, Armstrong World Industries, Lancaster, PA). The chamber had a removable top lined with four equally spaced tweeter speakers (3″ OEM cone tweeter, MCM Electronics, Dayton, OH). Using a sound pressure level meter (RadioShack SPL-meter 33-4050), the noise level was calibrated to 100dB, 105dB or 110dB when the chamber was closed. The tweeter speakers were connected to an amplifier (Techron 5507 power supply amplifier) which was connected to a music player (Sandisk Sansa Clip+ 4GB MP3 player) loaded with the octave band noise with a center frequency of 8 kHz and played on a loop. The noise was created using MATLAB software (The MathWorks, Inc., Natick, MA).
On the first audiogenic seizure testing day, each rat was placed in the plexiglass chamber, exposed to 2 min of noise at 100dB, and monitored for seizure behaviors during noise exposure. The severity of audiogenic seizures was rated using the following scale to calculate audiogenic response scores (ARS): 0 = no abnormal behavior, 1 = 1-2 wild running episodes, and 2 = clonus with full-body loss of posture (Ross and Coleman 2000). Wild running was defined as intense running mixed with high jumps. A rat was removed from the experiment and not tested further if it experienced clonus. If a rat did not experience clonus at 100dB, then it was exposed to 105dB 24-48 hours later and observed for seizure behaviors. If the rat still did not experience clonus, it was exposed to 110dB 24-48 hours later and observed. The dependent measures assessed at each decibel level (100, 105, 110) were: percentage of rats experiencing wild running or clonus, latency to onset and incidence of wild running, and seizure severity (audiogenic response score; ARS).
2.6 Statistical analysis
Statistical analyses were conducted using SPSS for MS Windows (version 20.0; IBM SPSS Statistics with Exact Tests Module) with statistical significance set at p< 0.05. For all analyses, all rats within a treatment group were included regardless of their previous noise exposure. The incidence of audiogenic seizures and wild running was analyzed using a 5×2 Pearson Chi-Square (χ2) test, and comparisons between the control groups and each treated group were conducted using a Fischer’s Exact Test with two-sided exact p-values. ARS and latency to onset of wild running scores had ceilings and required non-parametric statistics. Therefore, these scores were analyzed with a Kruskal Wallis test including all groups, and then Mann-Whitney tests were used as post doc tests to compare control and treated groups. Because animals that exhibited clonus at 100 dB were not tested again at 105 or 110 dB, the numbers in some experimental groups dropped considerably over days of testing. Therefore, latency to wild running, incidence of wild running, and ARS were only analyzed for the initial 100 dB noise exposures. The data for males and females were analyzed separately because there was not 1 male and 1 female available for testing from every litter. The alternative, an analysis using litter as the experimental unit and including sex as a variable nested within litter, would have necessitated dropping the data from litters that did not have both a male and a female available for testing.
Circulating T4 concentrations measured in littermates on the day of weaning were analyzed via mixed ANOVA with treatment as a between-subjects factor, and litter as a unit of variance with sex nested within litter. Post hoc Tukey’s tests were conducted to examine treatment effects.
3. RESULTS
There were no signs of clinical toxicity in the dams from any of the treatment groups. As we have previously reported for these PCB doses (Poon et al., 2011), liver weights were increased and thymus weights were decreased in the PCB-exposed pups at weaning. The 3 and 6 mg/kg PCB-exposed groups also had slightly lower body weights than the NE controls with a 6-7% decrease in the 3 mg/kg group and a 14-16% decrease in the 6 mg/kg group, on postnatal days 7, 14 and 21. No other developmental abnormalities were noted.
3.1 Audiogenic Seizure Incidence
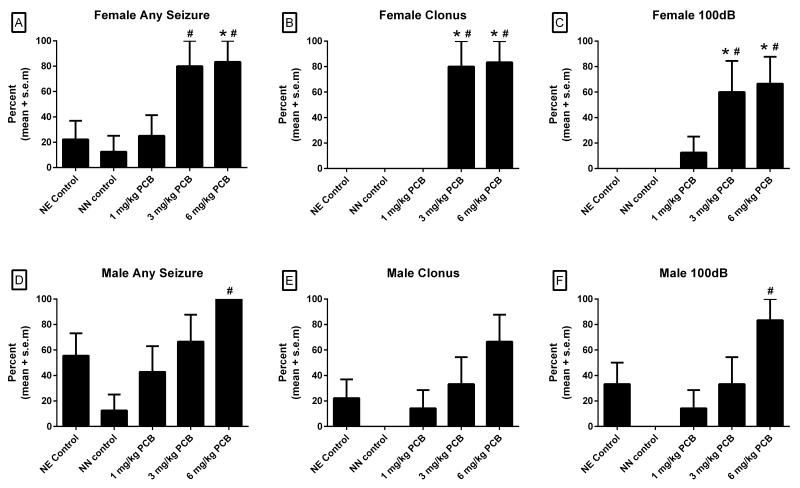
The numbers of females used in this analysis were 9, 8, 8, 5, and 6 for the NE control, NN control, 1, 3, and 6 mg/kg PCB groups, respectively. Analysis of the number of rats exhibiting any seizure behaviors (wild running or wild running progressing into clonus) across all three noise intensities (100dB, 105dB, and 110dB) revealed that there was a significant main effect of treatment (χ2=12.59, df=4, p=0.01). Shown in Figure 1A, group comparisons revealed that significantly more females in the 3 mg/kg group showed seizure behaviors compared to the NN control group (χ2=5.40, df=1, p<0.05), and more females in the 6 mg/kg PCB group exhibited seizure behaviors in comparison to females in the NE control group (χ2=5.92, df=1, p<0.05) and NN control group (χ2=7.02, df=1, p<0.05).
Figure 1.
Percentage of female rats with (A) any seizure behavior across all three noise intensities, (B) clonic seizures across all three noise intensities, (C) and any seizure behavior at 100 dB. Percentage of male rats with (D) any seizure behavior across all three noise intensities, (E) clonic seizures across all three noise intensities, and (F) any seizure behavior at 100 dB. *p<.05 vs NE controls, #p<.05 vs NN controls
Analysis of the number of female rats experiencing seizures that progressed to clonus (most severe form) across all three noise intensities also revealed a main effect of treatment (χ2=27.29, df=4, p<0.001). Shown in Figure 1B, comparisons between different treatment groups revealed that the 3 mg/kg PCB group had more female rats that exhibited clonic seizures than the NE control group (χ2=10.08, df=1, p=0.005) and the NN control group (χ2=9.24, df=1, p<0.01). Similarly, the 6 mg/kg PCB group had more females with clonic seizures than the NE control group (χ2=11.25, df=1, p<0.005) and the NN control group (χ2=10.37, df=1, p<0.005).
Analyses of the number of female rats exhibiting any seizure behaviors at the lowest noise intensity tested (100dB) were also conducted to determine the incidence of seizures at a noise intensity not typically associated with seizure activity in LE rats (Ross and Coleman, 2000). As shown in Figure 1C, this analysis also revealed a main effect of treatment (χ2=16.28, df=4, p<0.005) with the 3 mg/kg PCB group having significantly more females that exhibited seizure behaviors at 100 dB than the NE controls (χ2=6.87, df=1, p<0.05) and NN controls (χ2=6.24, df=1, p<0.05). More females in the 6 mg/kg PCB group also displayed seizure behavior at 100db compared to the NE control group (χ2=8.18, df=1, p=0.01) and the NN control group (χ2=7.47, df=1, p<0.05).
The numbers of males used in this analysis were 9, 8, 7, 6, and 6 for the NE control, NN control, 1, 3 and 6 mg/kg PCB groups respectively. Analyses of all groups revealed a significant effect of treatment in the number of males exhibiting any seizure behaviors across all three noise intensities (χ2=11.34, df=1, p<0.05; Figure 1D) and in the number of rats exhibiting any seizure behavior at the lowest noise intensity (100dB) (χ2=12.32, df=1, p<0.05; Figure 1F). There was also a trend for a treatment effect in the number of rats exhibiting clonic seizures across all three noise intensities (χ2=8.91, df=1, p=0.06; Figure 1E). Fisher’s Exact Tests revealed that the 6 mg/kg PCB-treated males had more seizures across all intensities (χ2=10.50, df=1, p=0.005) and more seizures at 100dB (χ2=10.37, df=1, p<0.005) relative to the NN controls. There were no significant differences on any seizure incidence measure between the NE controls and any PCB-treated groups in the males. Although the incidence measures differed somewhat in the NE and NN male controls, direct comparisons between these two groups failed to produce a significant effect.
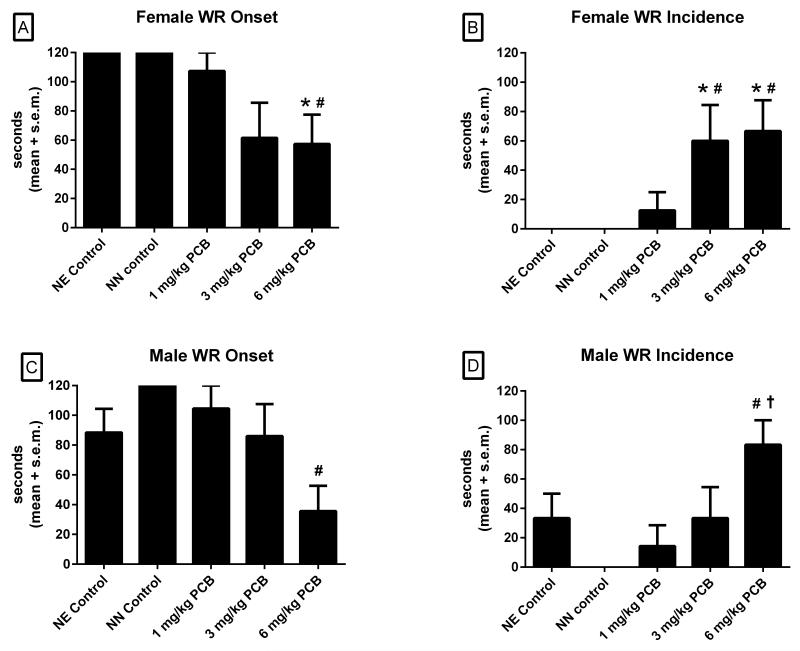
3.2 Latency to Onset and Incidence of Wild Running
The numbers of females used in these analyses were 9, 8, 8, 5, and 6 for the NE control, NN control, 1, 3, and 6 mg/kg PCB groups, respectively. There was a significant main effect of treatment in female rats in mean latencies to onset of wild running [χ2 (4,N=36)=15.04, p<.005]. Shown in Figure 2A, post hoc comparisons in females revealed that those exposed to 6mg/kg PCBs had a shorter latency to onset of wild running than the NE controls (p<.05) and NN controls (p<.05).
Figure 2.
Latency to onset of wild running in females (A) and males (B). *p<.05 vs NE controls, #p<.05 vs NN controls, †p<.05 vs 1 mg/kg
In addition, analyses of wild running incidence revealed a main effect of treatment in female rats [χ2 (4,N=36)=16.28, p<.005]. Shown in Figure 2B, females in the 6mg/kg PCB group had a higher incidence of wild running compared to the NE controls (p<.05) and NN controls (p<.05), and females in the 3mg/kg PCB group also had a higher incidence of wild running compared to the NE controls (p<.05) and NN controls (p<.05).
The numbers of males used in these analyses were 9, 8, 7, 6, and 6 for the NE control, NN control, 1, 3 and 6 mg/kg PCB groups respectively. There was a significant main effect of treatment in male rats [χ2 (4,N=36)=11.48, p<.05] in mean latencies to onset of wild running. Shown in Figure 2C, post hoc comparisons in males showed that rats exposed to 6mg/kg PCBs had a significantly shorter latency to onset of wild running than the NN controls (p<.01), and a trends towards a shorter latency compared to NE controls (p=.07).
Analyses of wild running incidence revealed a main effect of treatment in male rats [χ2 (4,N=36)=12.32, p<.05]. Shown in Figure 2D, wild running incidence in males was significantly higher in rats exposed to 6mg/kg PCBs compared to rats from the NN control group (p<.005) and the 1mg/kg PCB group (p<.05).
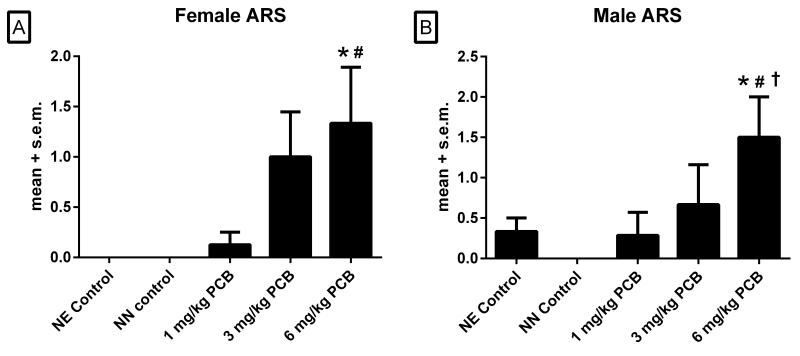
3.3 Audiogenic Response Score (ARS)
The numbers of females used in this analysis were 9, 8, 8, 5, and 6 for the NE control, NN control, 1, 3, and 6 mg/kg PCB groups, respectively. There were significant main effects of treatment in both female [χ2 (4,N=36)=16.24, p<.005] and male rats [χ2 (4,N=36)=12.00, p<.005] in ARS scores. In females (Figure 3A), post hoc comparisons revealed that the 6 mg/kg PCB groups had significantly higher ARS scores than the NE control group (p<.05) and the NN control group (p<.05). There were also trends for differences in females when comparing the 3 mg/kg PCB groups to the NE control group (p=.08) and the NN control group (p=.09) and when comparing the 6 mg/kg group to the 1 mg/kg group (p=.08).
Figure 3.
ARS in females (A) and males (B). *p<.05 vs NE controls, #p<.05 vs NN controls, †p<.05 vs 1 mg/kg
The numbers of males used in this analysis were 9, 8, 7, 6, and 6 for the NE control, NN control, 1, 3 and 6 mg/kg PCB groups respectively. Shown in Figure 3B, males exposed to 6mg/kg PCB had significantly higher ARS scores than the NE controls (p=.05), NN controls (p<.01) and the 1mg/kg PCB group (p=.05).
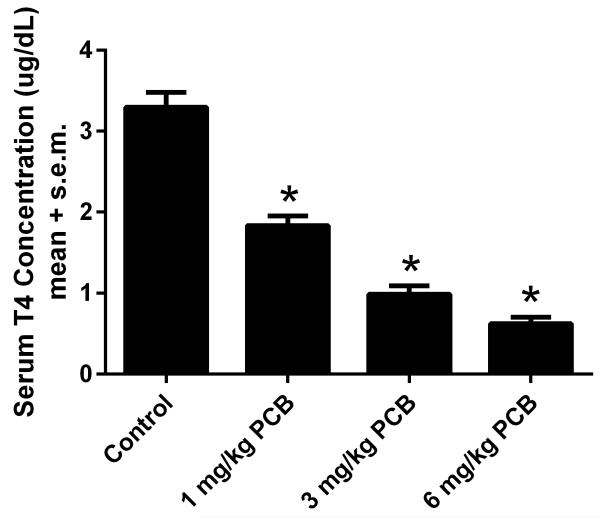
3.4 Thyroid Hormone Concentrations
Figure 4 shows serum total T4 levels (averages include both sexes) measured at weaning (PND 21) in littermates of the rats used in this study. The number of litters was 9, 8, 6 and 6 for control, 1, 3, and 6 mg/kg PCB groups. There was a significant effect of treatment [F(3,25)=45.406, p<0.001]. Tukey’s tests revealed that all treatment groups had significantly lower T4 levels than the control group (p<0.001). There was no main effect of sex or sex by treatment interaction.
Figure 4.
Pup serum T4 concentrations taken at weaning. *p<.001 vs controls
4. DISCUSSION
The current study used a systematic audiogenic seizure testing paradigm to confirm that developmentally PCB-exposed rats are, in fact, more susceptible to audiogenic seizures when exposed to loud noise in adulthood. The study demonstrated that developmentally PCB-exposed rats were more prone to audiogenic seizures, and this was more pronounced in females than males. PCB-exposed males showed a patterns similar to that seen in the females, but comparisons to the NE control group did not reach statistical significance, most likely because more NE control males than expected experienced audiogenic seizures. The higher than expected number of NE control males exhibiting seizures may have been a result of their previous exposure to noise. Thus, we also compared the 6 mg/kg PCB males, less than half of which had previous exposure to noise, to NN control males, revealing a significant increase in the number of PCB-exposed male rats experiencing seizures.
There is a sex difference in susceptibility to audiogenic seizures, with females showing a greater susceptibility to audiogenic seizures. Consistently, our effects were clearer in the females than the males. Specifically, female GEPR-9 rats had a higher frequency of more severe seizures than males (Mishra et al. 1988). Another study showed that female Sprague-Dawley rats that were ovariectimized and given testosterone exhibited decreased audiogenic seizures responses, while castrated males given estrogen exhibited increased audiogenic seizures responses (Werboff and Corcoran 1961).
Furthermore, previous studies have shown that neonatal hypothyroidism ranging from severe to mild can increase susceptibility to audiogenic seizures. Administration of propylthiouracil to rat dams from gestational day 6 to postnatal day 21 resulted in an increased sensitivity to seizures after exposure to the convulsant, pentylenetrazol (Gilbert et al. 2014), and rat pups treated with propylthiouracil from PND 0-19 had higher incidences of audiogenic seizures at 4 months of age (Kato et al. 1996). Rat dams fed low iodine diets throughout gestation and lactation had hypothyroid offspring that also had higher incidences of audiogenic seizures (Van Middlesworth 1977). Also, mild and transient maternal hypothyroxinemia induced by exposure to 2-mercapto-1-methyl-imidazole (MMI) during embryonic (E) days 12-15 led to an increase in the percentage of adolescent offspring displaying wild runs and audiogenic seizures (Ausó et al. 2004). Interestingly, supplementation of T4 from E13-16 to MMI-treated dams fully reversed the effect that MMI had on increasing seizures. Lastly, defective inhibitory synaptic activity in the lateral superior olivary complex and hippocampus is seen after developmental hypothyroidism (Friauf et al. 2008), suggesting that disruption of the thyroid hormone system may lead to increased excitation in the brain. Therefore, prenatal T4 plays a critical role in mediating susceptibility to audiogenic seizures.
Thyroxine hormone levels measured just after the end of the critical period of cochlear development were dramatically reduced in the littermates of the PCB-exposed rats in this study compared to controls. Other studies have also shown that developmental exposure to PCBs causes temporary hypothyroxinemia (lowered T4, without alterations in TSH) (Goldey et al. 1995; Morse et al. 1996; Poon et al. 2011). Therefore, hypothyroxinemia during early development could be leading to permanent changes in brain auditory pathways that make PCB exposed rats more susceptible to audiogenic seizures. In our study, levels of T4 at weaning did not reveal sex differences, suggesting that the difference in susceptibility between males and females to audiogenic seizures is not mediated through T4 or that an additional mechanism other than T4 is attenuating the seizure susceptibility in males or exacerbating it in females.
Another possible mechanism for the increased susceptibility of the PCB-exposed rats to audiogenic seizures is an imbalance between the excitatory and inhibitory neurochemical systems in the brain, as evident by resulting increased sensitivity to epileptogenic treatments. Perinatal exposure to a specific PCB congener (PCB 95) or to Aroclor 1254, but not to the vehicle, produced after-discharges (epileptic waveforms) in the evoked potentials of hippocampal slices when challenged with picrotoxin, a GABAA channel blocker (Kim and Pessah 2011). Additionally, rats developmentally exposed to 1 mg/kg/day PCB 95 had significantly shorter latencies to the first stage of myoclonus and onset of tonic-clonic seizures when exposed to flurothyl, a convulsive drug, and rats exposed to the same dose of PCB 95 also kindled seizures significantly faster when exposed to pentylenetetrazole (Lein et al. 2010). The investigators speculated that the lower seizure threshold in PCB rats was due to PCBs binding to the ryanodine receptor which, in turn, released calcium from intracellular stores, altering neuronal excitability (Wong et al. 1997). Importantly, the PCB mixture (Fox River) used in the current study has high ryanodine receptor activity (Kostyniak et al. 2005).
Many believe that audiogenic seizures are induced by an imbalance in the inhibitory/excitatory GABA and glutamate circuits in the inferior colliculus (Faingold 2002; Ross and Coleman 2000). Although there is no direct evidence of imbalance between inhibitory and excitatory circuits in the inferior colliculus of PCB-exposed rats, PCBs have been shown to inhibit uptake of glutamate and GABA into rat brain synaptosomes in vitro (Mariussen and Fonnum 2001). Additionally, developmental exposure to the PCB congener, Aroclor 1254, increased the pop spike amplitude and peak of the postsynaptic potential, while reducing paired pulse depression, suggesting an increase in the excitability of the dentate gyrus of the hippocampus (Gilbert 2003). Lastly, developmental PCB exposure altered the tonography, plasticity, and balance of neuronal inhibition to excitation in the auditory cortex (Kenet et al. 2007).
Audiogenic seizures are only rarely seen in humans, but are well-documented in mammals in which the auditory system matures postnatally (Ross and Coleman 1999). Genetically susceptible rodents with hearing loss, or rodents primed by sound exposure, sound deprivation, or kanamycin exposure (Pierson and Swann 1988) during postnatal development show altered excitability levels in the auditory system such that they are more susceptible to audiogenic seizures (Ross and Coleman 1999). Although audiogenic seizures do not present clinically in humans (Fisher 1989), many of the same substrates that are involved in audiogenic seizures are also involved in generalized human seizures (Ross and Coleman 2000). The findings of Kim and Pessah (2011) and Lein et al. (2010) suggest that early PCB exposure may also lead to a greater sensitivity to other types of generalized seizures, such as forebrain seizures, that are more relevant to humans.
4.1 Conclusions
Developmental exposure to PCBs during the critical period for cochlear development caused rats to be more susceptible to audiogenic seizures in adulthood. PCB exposed rats also exhibited a shorter latency to onset of seizures and a greater severity of seizures. The effect was statistically more robust in females than males. Groups with increased audiogenic seizure susceptibility had significant decreases in T4 during cochlear development. This is important because hypothyroidism during cochlear development has been linked to an increase in audiogenic seizures, suggesting a potential mechanism for the effect. One study suggests that developmental exposure to PCBs can also result in a lower threshold for forebrain seizures (Lein et al. 2010). Together with our findings, this suggests a generalized reduction in seizure threshold after early PCB exposure. Future studies are needed to fully assess whether this is the case, and, if so, to determine how the biological substrates for these seizures have been altered in the brains of PCB-exposed rats.
Highlights.
Dams were exposed to 0, 1, 3, or 6 mg/kg/day PCBs during gestation and lactation
Offspring were tested for audiogenic seizure susceptibility in adulthood
Exposure to 3 and 6 mg/kg PCBs increased the incidence and severity of seizures
PCB-treated groups had lower levels of thyroxine at weaning compared to controls
Acknowledgements
The authors would like to thank Drs. Larry G. Hansen and Paul J. Kostyniak for their help in formulating the PCB mixture, Dr. Paul A. Eubig and Supida Monaikul for their help with the animals, and Dr. Mary Gilbert for help with classifying the seizures. This work was supported by a grant from the National Institute of Environmental Health Sciences (NIEHS) [R01 ES015687 to SLS].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ausó E, Lavado-Autric R, Cuevas E, Del Rey FE, Morreale De Escobar G, Berbel P. A moderate and transient deficiency of maternal thyroid function at the beginning of fetal neocorticogenesis alters neuronal migration. Endocrinology. 2004;145:4037–4047. doi: 10.1210/en.2004-0274. [DOI] [PubMed] [Google Scholar]
- Coleman JR, Ross KC, Mullaney MM, Cooper WA. Latency alterations of the auditory brainstem response in audiogenic seizure-prone Long-Evans rats. Epilepsy Res. 1999;33:31–38. doi: 10.1016/s0920-1211(98)00075-8. [DOI] [PubMed] [Google Scholar]
- Crinnion W. Polychlorinated biphenyls: persistent pollutants with immunological, neurological, and endocrinological consequences. Altern Med Rev. 2011;16:5–13. [PubMed] [Google Scholar]
- Crofton KM, Ding D, Padich R, Taylor M, Henderson D. Hearing loss following exposure during development to polychlorinated biphenyls: a cochlear site of action. Hear. Res. 2000;144:196–204. doi: 10.1016/s0378-5955(00)00062-9. [DOI] [PubMed] [Google Scholar]
- Eells JB, Clough RW, Browning RA, Jobe PC. Comparative fos immunoreactivity in the brain after forebrain, brainstem, or combined seizures induced by electroshock, pentylenetetrazol, focally induced and audiogenic seizures in rats. Neuroscience. 2004;123:279–292. doi: 10.1016/j.neuroscience.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Faingold CL. Role of GABA abnormalities in the inferior colliculus pathophysiology - audiogenic seizures. Hear. Res. 2002;168:223–237. doi: 10.1016/s0378-5955(02)00373-8. [DOI] [PubMed] [Google Scholar]
- Fisher RS. Animal models of the epilepsies. Brain Res. Brain Res. Rev. 1989;14:245–278. doi: 10.1016/0165-0173(89)90003-9. [DOI] [PubMed] [Google Scholar]
- Friauf E, Wenz M, Oberhofer M, Nothwang HG, Balakrishnan V, Knipper M, Löhrke S. Hypothyroidism impairs chloride homeostasis and onset of inhibitory neurotransmission in developing auditory brainstem and hippocampal neurons. Eur J Neurosci. 2008;28:2371–2380. doi: 10.1111/j.1460-9568.2008.06528.x. [DOI] [PubMed] [Google Scholar]
- Gilbert ME. Perinatal exposure to polychlorinated biphenyls alters excitatory synaptic transmission and short-term plasticity in the hippocampus of the adult rat. Neurotoxicology. 2003;24:851–860. doi: 10.1016/S0161-813X(03)00073-1. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Ramos RL, McCloskey DP, Goodman JH. Subcortical band heterotopia in rat offspring following maternal hypothyroxinaemia: structural and functional characteristics. J Neuroendocrinol. 2014;26:528–541. doi: 10.1111/jne.12169. [DOI] [PubMed] [Google Scholar]
- Goldey ES, Kehn LS, Lau C, Rehnberg GL, Crofton KM. Developmental exposure to polychlorinated biphenyls (Aroclor 1254) reduces circulating thyroid hormone concentrations and causes hearing deficits in rats. Toxicol. Appl. Pharmacol. 1995;135:77–88. doi: 10.1006/taap.1995.1210. [DOI] [PubMed] [Google Scholar]
- Ishida N, Kato N, Kanai H, Watanabe Y, Kuroda Y, McEwen BS. Audiogenic seizure induces c-fos mRNA expression in the inferior colliculus and not in the hippocampus. Psychiatry Clin. Neurosci. 1995;49:S280–282. doi: 10.1111/j.1440-1819.1995.tb02207.x. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Fein GG, Jacobson SW, Schwartz PM, Dowler JK. The transfer of polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs) across the human placenta and into maternal milk. Am J Public Health. 1984;74:378–379. doi: 10.2105/ajph.74.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusko TA, Sisto R, Iosif AM, Moleti A, Wimmerová S, Lancz K, Tihányi J, Sovčíková E, Drobná B, Palkovičová L, Jurečková D, Thevenet-Morrison K, Verner MA, Sonneborn D, Hertz-Picciotto I, Trnovec T. Prenatal and Postnatal Serum PCB Concentrations and Cochlear Function in Children at 45 Months of Age. Environ Health Perspect. 2014;122:1246–1252. doi: 10.1289/ehp.1307473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N, Ishida N, Kanai H, Watanabe Y, Kuroda Y, McEwen BS. Expression of c-fos mRNA after audiogenic seizure in adult rats with neonatal hypothyroidism. Brain Res. Mol. Brain Res. 1996;38:85–90. doi: 10.1016/0169-328x(95)00332-m. [DOI] [PubMed] [Google Scholar]
- Kenet T, Froemke RC, Schreiner CE, Pessah IN, Merzenich MM. Perinatal exposure to a noncoplanar polychlorinated biphenyl alters tonotopy, receptive fields, and plasticity in rat primary auditory cortex. Proc. Natl. Acad. Sci. U. S. A. 2007;104:7646–7651. doi: 10.1073/pnas.0701944104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Pessah IN. Perinatal exposure to environmental polychlorinated biphenyls sensitizes hippocampus to excitotoxicity ex vivo. Neurotoxicology. 2011;32:981–985. doi: 10.1016/j.neuro.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyniak PJ, Hansen LG, Widholm JJ, Fitzpatrick RD, Olson JR, Helferich JL, Kim KH, Sable HJ, Seegal RF, Pessah IN, Schantz SL. Formulation and characterization of an experimental PCB mixture designed to mimic human exposure from contaminated fish. Toxicol. Sci. 2005;88:400–411. doi: 10.1093/toxsci/kfi338. [DOI] [PubMed] [Google Scholar]
- Lasky RE, Widholm JJ, Crofton KM, Schantz SL. Perinatal exposure to Aroclor 1254 impairs distortion product otoacoustic emissions (DPOAEs) in rats. Toxicol. Sci. 2002;68:458–464. doi: 10.1093/toxsci/68.2.458. [DOI] [PubMed] [Google Scholar]
- Lein PJ, Kim KH, Berman RF, Pessah IN. Exposure of the developing brain to polychlorinated biphenyls influences the suceptibility of the adult brain to stress. In: Wang C, Slikker W, editors. Developmental Neurotoxicolgy Research: Principles, Models, Techniques, Strategies and Mechanisms. John Wiley & Sons Inc; Hoboken, NJ: 2010. pp. 211–229. [Google Scholar]
- Mariussen E, Fonnum F. The effect of polychlorinated biphenyls on the high affinity uptake of the neurotransmitters, dopamine, serotonin, glutamate and GABA, into rat brain synaptosomes. Toxicology. 2001;159:11–21. doi: 10.1016/s0300-483x(00)00374-7. [DOI] [PubMed] [Google Scholar]
- Mishra PK, Dailey JW, Reigel CE, Tomsic ML, Jobe PC. Sex-specific distinctions in audiogenic convulsions exhibited by severe seizure genetically epilepsy-prone rats (GEPR-9s) Epilepsy Res. 1988;2:309–316. doi: 10.1016/0920-1211(88)90039-3. [DOI] [PubMed] [Google Scholar]
- Morse DC, Wehler EK, Wesseling W, Koeman JH, Brouwer A. Alterations in rat brain thyroid hormone status following pre- and postnatal exposure to polychlorinated biphenyls (Aroclor 1254) Toxicol. Appl. Pharmacol. 1996;136:269–279. doi: 10.1006/taap.1996.0034. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health . Public Health Service Policy on Humane Care and Use of Laboratory Animals. NIH/Office of Laboratory Animal Welfare; Rockville, MD: 2002. [Google Scholar]
- National Research Council, Institute for Laboratory Animal Research . Guidelines for the Care of Use of Mammals in Neuroscience and Behavioral Research. National Academy Press; Washington, DC: 2003. [Google Scholar]
- Pierson MG, Swann JW. The sensitive period and optimum dosage for induction of audiogenic seizure susceptibility by kanamycin in the Wistar rat. Hear. Res. 1988;32:1–10. doi: 10.1016/0378-5955(88)90142-6. [DOI] [PubMed] [Google Scholar]
- Poon E, Powers BE, McAlonan RM, Ferguson DC, Schantz SL. Effects of developmental exposure to polychlorinated biphenyls and/or polybrominated diphenyl ethers on cochlear function. Toxicol. Sci. 2011;124:161–168. doi: 10.1093/toxsci/kfr214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouyatos B, Gearhart C, Fechter L. Acrylonitrile potentiates hearing loss and cochlear damage induced by moderate noise exposure in rats. Toxicol. Appl. Pharmacol. 2005;204:46–56. doi: 10.1016/j.taap.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Powers BE, Poon E, Sable HJ, Schantz SL. Developmental exposure to PCBs, MeHg, or both: long-term effects on auditory function. Environ. Health Perspect. 2009;117:1101–1107. doi: 10.1289/ehp.0800428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers BE, Widholm JJ, Lasky RE, Schantz SL. Auditory deficits in rats exposed to an environmental PCB mixture during development. Toxicol. Sci. 2006;89:415–422. doi: 10.1093/toxsci/kfj051. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Ross KC, Coleman JR. Developmental and genetic audiogenic seizure models: behavior and biological substrates. Neuroscience biobehavioral reviews. 2000;24:639–653. doi: 10.1016/s0149-7634(00)00029-4. [DOI] [PubMed] [Google Scholar]
- Ross KC, Coleman JR. Audiogenic seizures in the developmentally primed Long-Evans rat. Dev. Psychobiol. 1999;34:303–313. doi: 10.1002/(sici)1098-2302(199905)34:2<303::aid-dev6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Trnovec T, Sovcikova E, Pavlovcinova G, Jakubikova J, Jusko TA, Hustak M, Jureckova D, Palkovicova L, Kocan A, Drobna B, Lancz K, Wimmerova S. Serum PCB concentrations and cochlear function in 12-year-old children. Environ. Sci. Technol. 2010;44:2884–2889. doi: 10.1021/es901918h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Middlesworth L. Audiogenic seizures in rats after severe prenatal and perinatal iodine depletion. Endocrinology. 1977;100:242–245. doi: 10.1210/endo-100-1-242. [DOI] [PubMed] [Google Scholar]
- Werboff J, Corcoran JB. Effects of sex hormone manipulation on audiogenic seizures. Am. J. Physiol. 1961;201:830–832. doi: 10.1152/ajplegacy.1961.201.5.830. [DOI] [PubMed] [Google Scholar]
- Wong PW, Joy RM, Albertson TE, Schantz SL, Pessah IN. Ortho-substituted 2,2′,3,5′,6-pentachlorobiphenyl (PCB 95) alters rat hippocampal ryanodine receptors and neuroplasticity in vitro: evidence for altered hippocampal function. Neurotoxicology. 1997;18:443–456. [PubMed] [Google Scholar]