Abstract
Toxic effects of sulfide come from a poisoning of a number of enzymes, especially cytochrome c oxidase, which catalyzes the terminal step in mitochondrial aerobic respiration. Despite this, some estuarine plants live in sulfide-rich sediments. We hypothesized estuarine and flooding-tolerant species might be more tolerant of sulfide compared to upland species, and this was tested by measures of root cytochrome c oxidase and alcohol dehydrogenase activities in extracts exposed to sulfide. Enzyme activities were measured in 0, 5, 10, 15, and 20 µM sodium sulfide, and compared among 17 species of plants. Activities of alcohol dehydrogenase and cytochrome c oxidase were both reduced by increasing sulfide concentration, but cytochrome c oxidase was more sensitive to sulfide compared to alcohol dehydrogenase. Activities of cytochrome c oxidase were reduced to near zero at 5 to 10 µM sulfide whereas alcohol dehydrogenase activities were only reduced by about 50% at 10 µM sulfide. All species were sensitive to increasing sulfide, but to different degrees. Cytochrome c oxidase in flooding-sensitive species was decreased to near zero activity at 5 µM sulfide, whereas activities in some flooding-tolerant species were still detectable until 15 µM sulfide. Cytochrome c oxidase activities in some estuarine species were low even in the absence of sulfide, perhaps an adaptation to avoid sulfide vulnerability in their native, sulfide-rich habitat. This illustrates the potent metabolic effects of sulfide, and this is the first demonstration of varying sensitivities of cytochrome c oxidase to sulfide across organisms, making these data of novel importance.
Keywords: Alcohol dehydrogenase, Cytochrome c oxidase, Estuarine plants, Flooding-sensitive plants, Flooding-tolerant plants, Respiration, Sulfide
1. INTRODUCTION
Hydrogen sulfide is a potent metabolic toxin (Raven and Scrimgeour, 1997). Sulfide exposure to plants can reduce nutrient uptake (King et al., 1982), growth (DeLaune et al., 1983), and photosynthesis (Pezeshki et al., 1991). Many of the toxic effects of sulfide come from a poisoning of enzymes, especially competitive inhibition of cytochrome c oxidase (mitochondrial Complex IV; Cytochrome a/a3; EC 1.9.3.1) (Bagarinao, 1992), which catalyzes the terminal step in aerobic respiration in eukaryotes. Cytochrome c oxidase (“CytOx”) is also inhibited by cyanide and azide, but on a per molar basis, sulfide is more toxic than either cyanide or azide (Gonzalez-Meler et al., 1992). Nanomolar concentrations of sulfide are enough to inhibit CytOx activity in some animals (reviewed by Bagarinao, 1992). Despite what is known, different tolerances of CytOx to sulfide across species have not been reported. Greater enzymatic tolerance to sulfide toxicity would be a clear advantage for life in a sulfide-rich environment.
Toxic effects of sulfide have long been recognized in organisms (Beauchamp et al., 1984), but specific physiological effects of sulfide have been investigated in few metabolic systems. Sulfide exposure has been linked to straighthead disease (Joshi et al., 1975), among 12 diseases in rice (Allam and Hollis, 1972; Bagarinao, 1992). Sulfide toxicity involves numerous aspects in the organism and its environment (Wang and Chapman, 1999), but much of what is known about sulfide toxicity comes from its inhibition of CytOx. CytOx is embedded in the inner mitochondrial membrane and serves as the terminal oxidase in the mitochondrial electron transport chain, transferring electrons from cytochrome c to oxygen (Siedow and Day, 2000). Complete inhibition of CytOx in plants can occur with as little as 1 to 10 µM sulfide (Raven and Scrimgeour, 1997).
Other toxic effects of sulfide have been recognized, including toxicity to at least 20 other enzymes (Pearson and Havill, 1988; Bagarinao, 1992). One enzyme of interest in sulfide-rich habitats is alcohol dehydrogenase (“ADH”; aldehyde reductase; EC 1.1.1.1), which is active in anaerobic fermentation. ADH catalyzes the regeneration of NAD+ from the anaerobic processing of pyruvate (Huang et al., 2002), specifically from the reduction of acetaldehyde to ethanol in alcoholic fermentation (Mendelssohn et al., 1981). Regeneration of NAD+ is a crucial step during anaerobic conditions, as it keeps glycolysis running (Huang et al., 2002). Sulfide has been shown to inhibit ADH (Koch et al., 1990), which indicates a broader spectrum of toxicity that includes an enzyme crucial for life in anaerobic sediments (Mendelssohn et al., 1981).
Despite the potent toxicity of sulfide, some plants live in sulfide-rich sediments on tidal mudflats with millimolar concentrations of sulfide (King et al., 1982; Bagarinao, 1992), especially estuarine grasses that inhabit low marsh settings like Spartina alterniflora (Lee et al., 1999) and S. anglica (Lee, 2003). In fact, sulfide concentration has been linked to distribution of Phragmites australis and Spartina alterniflora (Chambers et al., 1998) and other species (Ingold and Havill, 1984) in salt marshes. To account for these substantial differences in sulfide tolerance between species, it becomes necessary to understand physiological mechanisms of sulfide tolerance in plants. Physiological mechanisms of plants tolerating sulfide include oxidizing the rhizosphere (McKee et al., 1988; Lee, 2003), excluding sulfide (Dooley et al, 2013), enzymatic and non-enzymatic oxidation of sulfide (Lee et al., 1999), and low respiration enzyme activities (Maricle et al., 2006). Another possible mechanism of tolerating sulfide would be a tolerant form of CytOx that can resist inhibition by sulfide. CytOx is well conserved across eukaryotes and there have been no previous demonstrations of varying sensitivities of CytOx to sulfide across organisms (Bagarinao, 1992). Nonetheless, minor differences in tolerance to sulfide could pose a substantial advantage for life in sulfide-rich habitats.
Because estuarine species are adapted to life in a highly sulfidic environment, we hypothesized estuarine and flooding-tolerant species might be more tolerant of sulfide on a metabolic level compared to flooding-sensitive species. An isozyme of CytOx that is more tolerant of sulfide would provide a significant advantage for life in a sulfide-rich environment, and this was tested by measures of root CytOx and ADH activities in extracts exposed to sulfide. Greater tolerance to sulfide would be evident by high enzymatic activities in samples exposed to sulfide and greater sensitivity to sulfide would be evident by enzyme inhibition at relatively low concentrations of sulfide.
2. METHODS
Cytochrome c oxidase (CytOx) and alcohol dehydrogenase (ADH) activities were measured in roots from 17 species of plants. Most plants were collected from sites of growth near Hays, KS, USA or were obtained through the USDA NRCS Plant Materials Program (Table 1). Phalaris arundinacea L. was collected north of Hays, KS, USA (38° 57′ N, 99° 23′ W). Tamarix ramosissima Ledeb. and Distichlis spicata (L.) Greene were collected from Quivira National Wildlife Refuge near Stafford, KS, USA (38° 13′ N, 98° 29′ W) and Spartina foliosa Trin. was collected in San Francisco Bay, CA, USA (37° 45′ N, 122° 15′ W) (Table 1). Plugs of Solidago sempervirens L. “Monarch,” Spartina patens (Aiton) Muhl. “Avalon,” Spartina cynosuroides (L.) Roth, as well as seeds of Spartina alterniflora Loisel. “Bayshore” and Schizachyrium littorale (Nash) E.P. Bicknell “Dune Crest” were obtained from the Cape May Plant Materials Center of Cape May, NJ, USA. Plugs of Panicum virgatum L. “Blackwell,” and rhizomes of Phragmites australis (Cav.) Trin. ex Steud. “Southwind” and Spartina pectinata Bosc ex Link “Atkins” were obtained from the Manhattan Plant Materials Center of Manhattan, KS, USA. Plants of Juncus effusus L. “Sumter” were obtained from the Jimmy Carter Plant Materials Center in Americus, GA, USA. Phaseolous vulgaris L., Vicia faba L., Pisum sativum L., and Zea mays L. were grown from seed purchased commercially. All plant species were categorized based on their natural habitat, from information drawn from previous work. Plant categories ranged from estuarine species to flooding tolerant species to flooding sensitive species (Table 1).
Table 1.
Plant species studied for tolerance to sulfide. The natural environment and plant source are included with each.
| Species | Common Name | Natural Environment* | From | Germplasm (where known) |
|---|---|---|---|---|
| Spartina alterniflora | smooth cordgrass | Estuarine Species (a, b) | Cape May Plant Materials Center | “Bayshore” |
| Spartina patens | saltmeadow cordgrass | Estuarine Species (a, b) | Cape May Plant Materials Center | “Avalon” |
| Spartina foliosa | California cordgrass | Estuarine Species (a, c) | San Francisco Bay, CA; 37° 45′ N, 122° 15′ W | |
| Distichlis spicata | salt grass | Estuarine Species (d) | Stafford, KS; 38° 13′ N, 98° 29′ W | |
| Phalaris arundinacea | reed canarygrass | Flooding-Tolerant Species (e) | Hays, KS; 38° 57′ N, 99° 23′ W | |
| Juncus effusus | soft rush | Flooding-Tolerant Species (f) | Jimmy Carter Plant Materials Center | “Sumter” |
| Phragmites australis | common reed | Flooding-Tolerant Species (e) | Manhattan Plant Materials Center | “Southwind” |
| Tamarix ramosissima | saltcedar | Flooding-Tolerant Species (g) | Stafford, KS; 38° 13′ N, 98° 29′ W | |
| Spartina cynosuroides | big cordgrass | Flooding-Tolerant Species (a) | Cape May Plant Materials Center | |
| Spartina pectinata | prairie cordgrass | Flooding-Tolerant Species (a, e) | Manhattan Plant Materials Center | “Atkins” |
| Phaseolus vulgaris | common bean | Flooding-Sensitive Species (h) | commercial seeds | |
| Vicia faba | broad bean | Flooding-Sensitive Species (h) | commercial seeds | |
| Pisum sativum | pea | Flooding-Sensitive Species (h) | commercial seeds | |
| Zea mays | maize | Flooding-Sensitive Species (h) | commercial seeds | |
| Panicum virgatum | switchgrass | Flooding-Sensitive Species (i) | Manhattan Plant Materials Center | “Blackwell” |
| Schizachyrium littorale | coastal little bluestem | Flooding-Sensitive Species (i) | Cape May Plant Materials Center | “Dune Crest” |
| Solidago sempervirens | seaside goldenrod | Flooding-Sensitive Species (i) | Cape May Plant Materials Center | “Monarch” |
Sources for determining natural environment:
All plants and seeds were planted in potting soil in 11 cm × 11 cm pots and grown in greenhouse conditions in Hays, KS, USA. Greenhouse conditions were natural lighting with an average PPFD of 1100 µmol m−2 s−1 at midday. Temperatures were near 33°C during afternoons and 21°C at night. Plants grew for one to two months, at which point roots were harvested, rinsed clean, frozen in liquid nitrogen, and stored at −20°C (n = 4 to 10 individuals per species).
Effects of increasing sulfide concentrations were measured on activities of two enzymes involved in respiration. Enzyme activities were measured spectrophotometrically in solutions of 0 to 20 µM Na2S, similar to concentrations tested by Allam and Hollis (1972) and González-Meler et al. (1992), but much lower than concentrations tested by Erskine and Koch (2000).
CytOx assays were performed after Maricle et al. (2006), as modified from Smith (1955). Root samples were ground in a mortar and pestle with liquid nitrogen. CytOx extraction buffer was added at 4 mL g−1, which contained 0.1 M Na-phosphate buffer (pH 7.1) with 0.1% v/v Triton X-100. This mixture was homogenized with a mortar and pestle, filtered through Miracloth (Calbiochem; San Diego, CA, USA), and centrifuged at 1000 g for 20 min at 4°C. The resulting supernatant was assayed for CytOx activity spectrophotometrically at 25°C. Enzyme activity was determined as the rate of cytochrome c oxidation, measured as a decrease in absorbance at 550 nm. Rates of CytOx activity were corrected for background rates of cytochrome c oxidation, then standardized to g fresh root mass (Maricle et al., 2006).
ADH assays were performed after Maricle et al. (2006), as modified from John and Greenway (1976). Root samples were ground in a mortar and pestle with liquid nitrogen. ADH extraction buffer was added at 5 mL g−1, which contained 50 mM HEPES (pH 8.0), 5 mM MgCl2, 2 mM cysteine hydrochloride, and 2% w/v PVP-40. The slurry was ground in a mortar and pestle and centrifuged at 10,000 g at 4°C for 10 min. ADH activity was assayed spectrophotometrically on the supernatant at 25°C. ADH activity was calculated from rates of NADH oxidation, measured as a decrease in absorbance at 340 nm. Background rates of substrate reduction were determined in the presence of 80 µM NADH in 40 mM bicine and 5 mM MgCl2 at a pH of 8.0. Reaction rates were determined following addition of 10 mM acetaldehyde and ADH activities were standardized to g fresh root mass (Maricle et al., 2006).
The sulfide concentration needed to reduce enzyme activity by 50 percent (the inhibition constant, Ki) was calculated from the initial decrease (slope) of the enzyme activity in relation to sulfide concentration. Enzyme activity data were analyzed with analysis of covariance, with species as a fixed effect and Na2S concentration as a covariate. Ki data were analyzed with one-way analysis of variance, with species as a fixed effect. The category of species from their natural environment (Table 1) was also used as a fixed effect. Results are presented for species and summarized for both species and categories of species. Post-hoc comparisons were performed with Fisher’s protected least significant difference. All analyses were performed at α = 0.05 (StatView 5.0, 1998 SAS Institute, Inc., Cary, NC, USA).
3. RESULTS AND DISCUSSION
Many wetland plants experience sulfide in sediments, creating toxic conditions (Havill et al., 1985); sulfide inhibits cytochrome c oxidase (CytOx) and alcohol dehydrogenase (ADH) activity, thereby impeding aerobic and fermentative metabolism, decreasing ATP production and reducing nitrogen uptake and plant growth (Koch et al., 1990; Bradley and Morris, 1990; Raven and Scrimgeour, 1997). Activities of both enzymes were reduced by sulfide in the present study (ANCOVA, P<0.001), but CytOx activities were reduced more than ADH activities, indicating greater sensitivity to sulfide. Furthermore, CytOx from most estuarine and flooding tolerant species was more tolerant of sulfide compared to flooding sensitive species (ANOVA, P<0.001). This is the first demonstration of varying sensitivities of CytOx to sulfide across organisms, perhaps an adaptation to life in highly sulfidic estuaries.
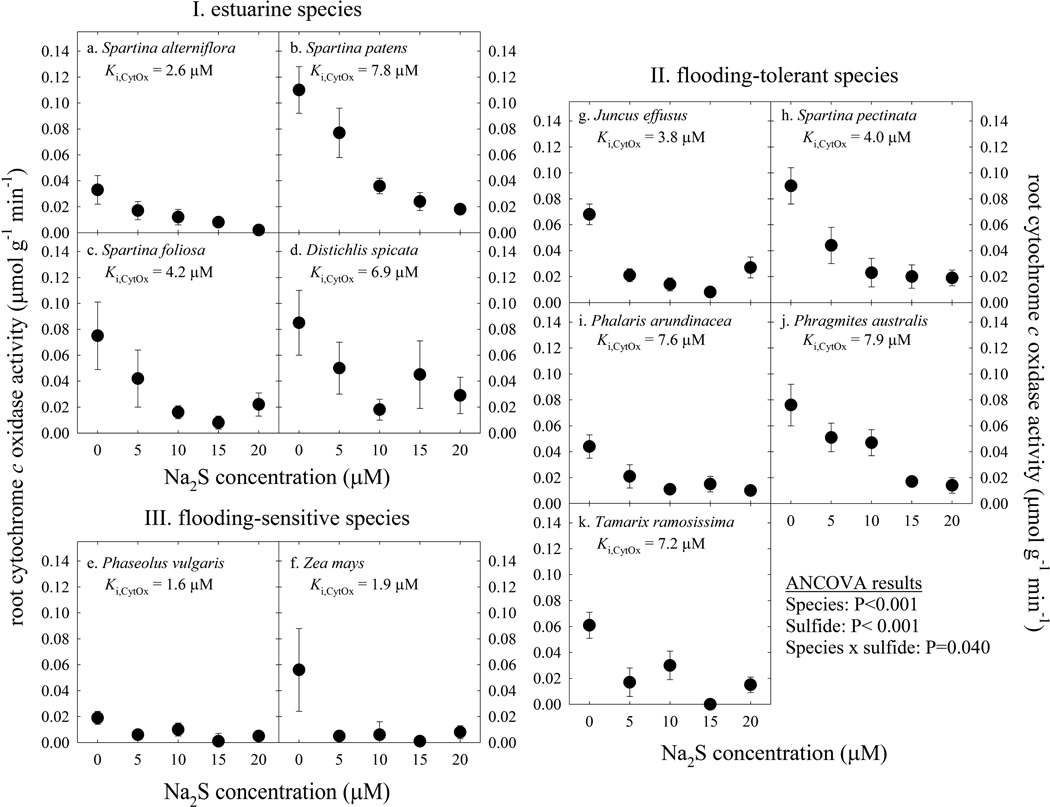
CytOx activities were very sensitive to sulfide, with a significant reduction in activity with increasing sulfide concentration (ANCOVA, P<0.001; Fig. 1). When looking at CytOx, most of the estuarine and flooding-tolerant species were more tolerant than the flooding-sensitive species (ANCOVA, species × sulfide interaction, P=0.040). CytOx activities in flooding-sensitive species like Zea mays were decreased to near zero at 5 µM sulfide. The sulfide concentration that resulted in a 50 percent reduction in CytOx activity (Ki,CytOx) was 1.6 to 1.9 µM in flooding-sensitive species (Fig. 1, Table 2), the lowest among species in this study (ANOVA, P<0.001). In contrast, CytOx activities in the flooding-tolerant species Spartina pectinata, Phalaris arundinacea, and Phragmites australis and all estuarine species were not reduced to zero until 10 or 15 µM sulfide (Fig. 1). Ki,CytOx was as high as 7.9 µM in some estuarine and flooding tolerant species, significantly higher compared to flooding-sensitive species (ANOVA, P<0.001; Table 2). We hypothesize this is an adaptation of the CytOx enzyme to life in high sulfide conditions, and this is the first demonstration of different sensitivities of CytOx to sulfide between species as related to ecological zonation. Furthermore, there was a significant difference between species regarding CytOx activities (ANCOVA, P<0.001). Most of the estuarine and flooding-tolerant species had high CytOx activities, consistent with three species of the estuarine plant Salicornia, as outlined by Pearson and Havill (1988). In that study, high CytOx activities correlated with tolerance to sulfide (Pearson and Havill, 1988). By contrast, Spartina alterniflora, an estuarine species in the present study, was more tolerant of sulfide because it had constitutively low activities of CytOx (Fig. 1), perhaps an adaptation to avoid sulfide toxicity in its native, sulfide-rich habitat. This is the reverse of the strategy as Salicornia as outlined by Pearson and Havill (1988). In the present study, constitutively low CytOx activities appear to correlate with high sulfide tolerance in S. alterniflora, consistent with results of Maricle et al. (2006) and Maricle and Lee (2007).
Figure 1.
The effect of increasing sulfide on activities of cytochrome c oxidase (CytOx) extracted from plant roots. Plant species are grouped by their natural habitat, including (I.) the estuarine species Spartina alterniflora (a.), Spartina patens (b.), Spartina foliosa (c.), and Distichlis spicata (d.), (II.) the flooding-tolerant species Juncus effusus (g.), Spartina pectinata (h.), Phalaris arundinacea (i.), Phragmites australis (j.), and Tamarix ramosissima (k.), and (III.) the flooding-sensitive species Phaseolus vulgaris (e.) and Zea mays (f.). Enzyme activities were measured in 0 to 20 µM Na2S. Points are means of 4 to 10 replicate individuals ± SE. The mean sulfide concentration that resulted in a 50 percent decrease in activity of CytOx (Ki,CytOx) is shown for each species.
Table 2.
The mean ± SE (n) sulfide concentration that resulted in a 50 percent decrease in activity of CytOx (Ki,CytOx) and the sulfide concentration that resulted in a 50 percent decrease in activity of ADH (Ki,ADH). The natural environment is listed with each species (see Table 1).
| Species | Natural Environment | Ki,CytOx (µM) | Ki,ADH (µM) |
|---|---|---|---|
| Spartina alterniflora | Estuarine Species | 2.6 ± 1.6 µM (10)C | 11.6 ± 7.2 µM (4)A |
| Spartina patens | Estuarine Species | 7.8 ± 1.3 µM (10)A | 11.2 ± 2.7 µM (10)A |
| Spartina foliosa | Estuarine Species | 4.2 ± 1.7 µM (9)ABC | |
| Distichlis spicata | Estuarine Species | 6.9 ± 2.6 µM (4)ABC | |
| Phalaris arundinacea | Flooding-Tolerant Species | 7.6 ± 1.6 µM (10)A | 12.0 ± 2.9 µM (10)A |
| Juncus effusus | Flooding-Tolerant Species | 3.8 ± 1.6 µM (10)ABC | |
| Phragmites australis | Flooding-Tolerant Species | 7.9 ± 1.6 µM (10)A | 7.9 ± 3.3 µM (10)A |
| Tamarix ramosissima | Flooding-Tolerant Species | 7.2 ± 1.4 µM (10)AB | |
| Spartina cynosuroides | Flooding-Tolerant Species | 11.1 ± 3.6 µM (10)A | |
| Spartina pectinata | Flooding-Tolerant Species | 4.0 ± 1.4 µM (10)ABC | 11.3 ± 3.6 µM (10)A |
| Phaseolus vulgaris | Flooding-Sensitive Species | 1.6 ± 2.6 µM (4)C | 8.8 ± 4.7 µM (7)A |
| Vicia faba | Flooding-Sensitive Species | 11.7 ± 5.6 µM (5)A | |
| Pisum sativum | Flooding-Sensitive Species | 11.6 ± 7.2 µM (4)A | |
| Zea mays | Flooding-Sensitive Species | 1.9 ± 2.1 µM (6)C | 8.0 ± 3.9 µM (10)A |
| Panicum virgatum | Flooding-Sensitive Species | 12.7 ± 3.6 µM (10)A | |
| Schizachyrium littorale | Flooding-Sensitive Species | 12.7 ± 3.6 µM (10)A | |
| Solidago sempervirens | Flooding-Sensitive Species | 8.2 ± 3.8 µM (10)A | |
Capital letters that accompany means indicate significant differences of P<0.05. Means that share a letter are not significantly different.
CytOx catalyzes the final reaction in the mitochondrial electron transport chain (Siedow and Day, 2000). CytOx is a key enzyme in determining oxygen uptake rates in wetland plants (Maricle et al., 2006; Maricle and Lee, 2007). Furthermore, the primary effect of sulfide is inhibition of CytOx (Raven and Scrimgeour, 1997), resulting in significant challenges to maintain aerobic respiration in the presence of sulfide. Nanomolar concentrations of sulfide reduce CytOx activity by 50% in some animals (Bagarinao, 1992), and complete inhibition of CytOx can occur at 1 to 10 µM sulfide in estuarine plants (Raven and Scrimgeour, 1997). Indeed, sediment sulfide concentration can determine plant distributions in salt marshes (DeLaune et al., 1983; Ingold and Havill, 1984; Chambers et al., 1998). Consequently, vulnerability of CytOx to sulfide is likely to be one of the primary factors determining plant-level sulfide tolerance.
Mechanisms of sulfide tolerance in organisms are numerous, but mostly include ways to avoid sulfide exposure, oxidize sulfide, or exclude it from the body (Bagarinao, 1992). Our results (Fig. 1, Table 2) suggest two more possible mechanisms to tolerate sulfide in the environment: 1.) constitutively low CytOx activities, as seen in the estuarine species S. alterniflora, and 2.) an isozyme of CytOx that is more tolerant of sulfide, as seen by higher Ki values in the estuarine species S. patens and D. spicata and the flooding-tolerant species P. australis, T. ramosissima, and P. arundinacea. CytOx is the primary target of sulfide toxicity (Bagarinao, 1992), so it seems fitting that mechanisms of tolerance involve this enzyme.
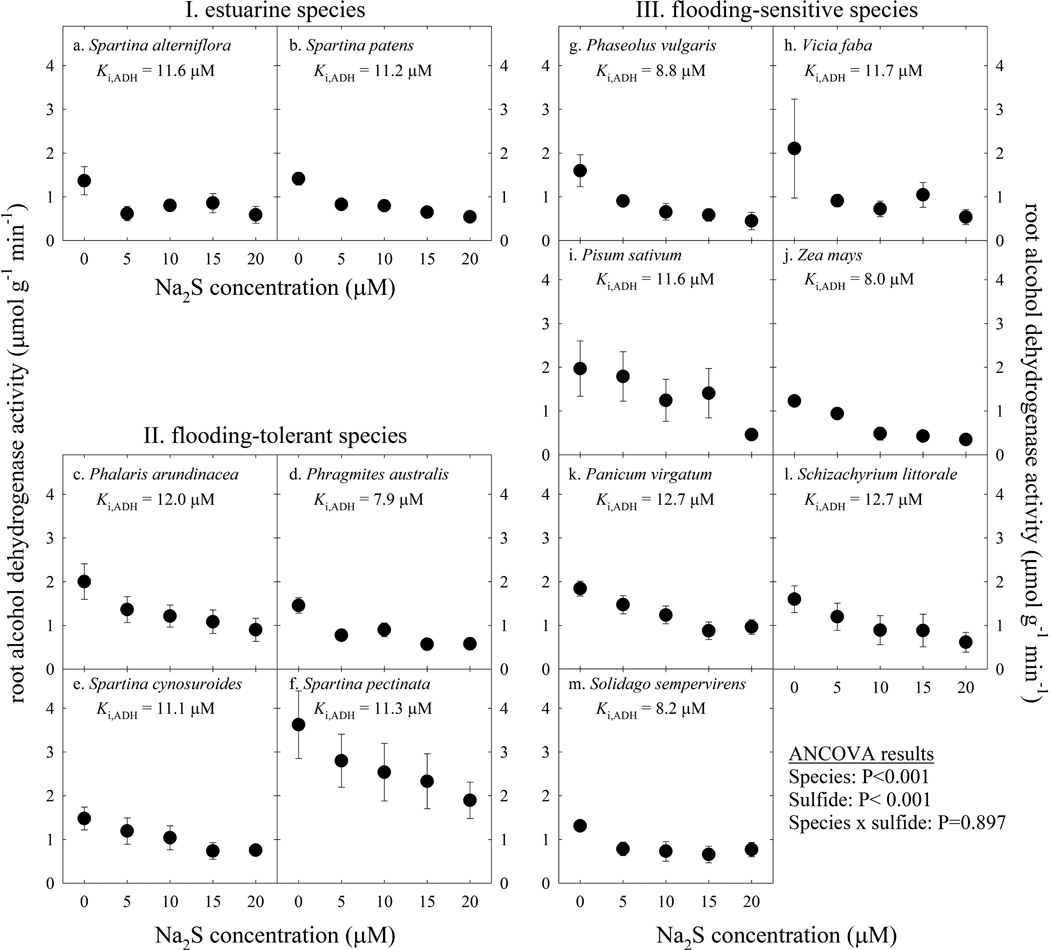
ADH was also sensitive to sulfide exposure, but to a lesser degree than CytOx. 11 to 12 µM sulfide typically reduced ADH activities by about fifty percent (Fig. 2) whereas CytOx activities were reduced to near zero by 5 to 10 µM sulfide (Fig. 1). Although there was a difference in ADH activity between species (ANCOVA, P<0.001; Fig. 2), ADH in all species responded to increasing sulfide to the same degree (ANCOVA, species × sulfide interaction, P=0.897). The sulfide concentration that resulted in a 50 percent reduction of ADH activities (Ki,ADH) ranged from 7.9 to 12.7 µM (Fig. 2, Table 2), with no difference between species (ANOVA, P=0.997). Therefore, there were no differences between species from different environments and apparently no evolutionary trend toward protecting ADH in estuarine species. Results presented by Pearson and Havill (1988) indicate increases in root ADH activity in sulfide treatments, but those treatments involved roots of intact plants. In contrast, ADH activities presented by Koch et al. (1990) indicate inhibition of ADH activity at high sulfide concentrations, also from intact plant roots. Decreasing ADH activity with increasing sulfide indicates a toxic effect of sulfide, consistent with results of the present study. When extracted enzymes were assayed with increasing sulfide concentration in the present study, ADH activity was reduced (ANCOVA, P<0.001). All species were sensitive to sulfide in the same degree regarding poisoning of ADH. Nonetheless, sulfide is toxic to ADH, which means sulfide is not only toxic to CytOx, but other enzymes as well (Bagarinao, 1992).
Figure 2.
The effect of increasing sulfide on activities of alcohol dehydrogenase (ADH) extracted from plant roots. Plant species are grouped by their natural habitat, including (I.) the estuarine species Spartina alterniflora (a.) and Spartina patens (b.), (II.) the flooding-tolerant species Phalaris arundinacea (c.), Phragmites australis (d.), Spartina cynosuroides (e.), and Spartina pectinata (f.), and (III.) the flooding-sensitive species Phaseolus vulgaris (g.), Vicia faba (h.), Pisum sativum (i.), Zea mays (j.), Panicum virgatum (k.), Schizachyrium littorale (l.), and Solidago sempervirens (m.). Enzyme activities were measured in 0 to 20 µM Na2S. Points are means of 4 to 10 replicate individuals ± SE. The mean sulfide concentration that resulted in a 50 percent decrease in activity of ADH (Ki,ADH) is shown for each species.
ADH catalyzes the terminal step in anaerobic fermentation (Maricle et al., 2006) and ADH activity correlates with flooding tolerance. There was a significant difference between categories of species regarding ADH activities (ANCOVA, P<0.001). The estuarine and flooding-tolerant species had ADH activities that were significantly higher than flooding-sensitive species (Fig. 2), consistent with previous studies on flooding tolerance in plants (Mendelssohn et al., 1981; Caudle and Maricle, 2012). The ability to increase ADH activity appears to be an adaptation of estuarine and flooding-tolerant plants to tolerate their natural habitats (Maricle et al., 2006); these habitats sometimes also contain sulfide.
While anaerobic fermentation is necessary for life in anaerobic sediments (Mendelssohn et al., 1981; Pearson and Havill, 1988), vulnerability of ADH to sulfide could lead to a broad-spectrum sensitivity to environmental sulfide. If sulfide toxicity inhibits aerobic and anaerobic respiration, plants will not survive. Higher concentrations of sulfide were needed to poison ADH in intact roots (Pearson and Havill, 1988; Koch et al., 1990) compared to extracts in the present study. This indicates plants are effective at excluding sulfide, a likely mechanism for tolerating sulfide in the environment (Dooley et al, 2013). The role of exclusion in sulfide tolerance has been investigated in some systems (e.g., Koch and Mendelssohn, 1989; Armstrong and Armstrong, 2005; Dooley et al., 2013), but how this might differ between species largely remains an area for future research. However, the greater sensitivity of CytOx to sulfide suggests tolerance for sulfide in the environment might come from protecting it from sulfide exposure (Maricle et al., 2006).
4. CONCLUSIONS
Estuarine plants exhibit a range of mechanisms for tolerating sulfide in the environment. It appears mechanisms of exclusion (Dooley et al., 2013) and external oxidation (McKee et al., 1988; Lee, 2003) are effective at protecting CytOx in estuarine plants. Our results suggest additional mechanisms for tolerating sulfide, which involve more tolerant CytOx and constitutively low activities of CytOx. Furthermore, all aerobic organisms have CytOx, including humans, indicating these results could have broader implications than structuring plant zonation in estuaries. People who work in oil wells and refineries, coal-burning plants, paper and textile mills, tanneries, waste water treatment plants, sewers, landfills, and manure pits are exposed to sulfide (Bagarinao, 1992). Low doses of sulfide can irritate the eyes, nose, and throat and cause difficulty breathing (Agency for Toxic Substances and Disease Registry, 2006). Even brief exposure to 500 ppm hydrogen sulfide can cause loss of consciousness; exposure to >1000 ppm hydrogen sulfide has caused deaths in sewers, animal processing plants, waste dumps, sludge plants, oil and gas well drilling sites, and tanks and cesspools (Agency for Toxic Substances and Disease Registry, 2006). Therefore, sensitivities of key enzymes in response to sulfide could be useful in several contexts, including plant physiology, human health, marine ecology, and evolutionary adaptation to environmental pressures.
Research Highlights.
We compared sulfide toxicity among 17 plant species adapted to different habitats
We investigated effects of sulfide on two enzymes of respiration in plant roots
There are varying sensitivities of cytochrome c oxidase to sulfide across species
Estuarine species have cytochrome c oxidase that is more tolerant of sulfide
Alcohol dehydrogenase is less sensitive to sulfide
ACKNOWLEDGMENTS
We thank K-INBRE, the Kansas Academy of Science, and the Department of Biological Sciences of Fort Hays State University for support of this project. The following USDA-NRCS Plant Materials Centers graciously provided plant materials: Cape May, NJ, Americus, GA, and Manhattan, KS. We thank Keri Caudle for plant maintenance. This project was supported by a Kansas Academy of Science Student Research Grants and grants from the National Center for Research Resources (P20RR016475) and the National Institute of General Medical Sciences (P20GM103418) from the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONTRIBUTIONS
NMM helped conceive the study, collected data, and helped write the paper. BRM helped conceive the study, analyzed data, and helped write the paper.
LITERATURE CITED
- Agency for Toxic Substances and Disease Registry. Public Health Statement: Hydrogen Sulfide. 2006 CAS #: 7783-06-4. [Google Scholar]
- Allam AI, Hollis JP. Sulfide inhibition of oxidases in rice roots. Phytopathology. 1972;62:634–639. [Google Scholar]
- Armstrong J, Armstrong W. Rice: Sulfide-induced barriers to root radial oxygen loss, Fe2+ and water uptake, and lateral root emergence. Ann. Bot. 2005;96:625–638. doi: 10.1093/aob/mci215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres DR, Smith DL, Zaremba K, Klohr S, Strong DR. Spread of exotic cordgrasses and hybrids (Spartina sp.) in the tidal marshes of San Francisco Bay, California, USA. Biol. Inv. 2004;6:221–231. [Google Scholar]
- Bagarinao T. Sulfide as an environmental factor and toxicant: Tolerance and adaptations in aquatic organisms. Aquat. Toxicol. 1992;24:21–62. [Google Scholar]
- Beauchamp RO, Bus JS, Popp JA, Boreiko CJ, Andjelkovich DA. A critical review of the literature on hydrogen sulfide toxicity. CRC Crit. Rev. Toxicol. 1984;13:25–97. doi: 10.3109/10408448409029321. [DOI] [PubMed] [Google Scholar]
- Bertness MD. Zonation of Spartina patens and Spartina alterniflora in a New England salt marsh. Ecology. 1991;72:138–148. [Google Scholar]
- Bradley PM, Morris JT. Influence of oxygen and sulfide concentration on nitrogen uptake kinetics in Spartina alterniflora. Ecology. 1990;71:282–287. [Google Scholar]
- Caudle KL, Maricle BR. Effects of flooding on photosynthesis, chlorophyll fluorescence, and oxygen stress in plants of varying flooding tolerance. Trans. Kansas Acad. Sci. 2012;115:5–18. [Google Scholar]
- Caudle KL, Maricle BR. Physiological relationship between oil tolerance and flooding tolerance in marsh plants. Environ. Exp. Bot. 2014;107:7–14. [Google Scholar]
- Chambers RM, Mozdzer TJ, Ambrose JC. Effects of salinity and sulfide on the distribution of Phragmites australis and Spartina alterniflora in a tidal saltmarsh. Aquat. Bot. 1998;62:161–169. [Google Scholar]
- DeLaune RD, Smith CJ, Patrick WH. Relationship of marsh elevation, redox potential, and sulfide to Spartina alterniflora productivity. Soil Sci. Soc. Am. J. 1983;47:930–935. [Google Scholar]
- Dooley FD, Wyllie-Echeverria S, Roth MB, Ward PD. Tolerance and response of Zostera marina seedlings to hydrogen sulfide. Aquat. Bot. 2013;105:7–10. [Google Scholar]
- Erskine JM, Koch MS. Sulfide effects on Thalassia testudinum carbon balance and adenylate energy charge. Aquat. Bot. 2000;67:275–285. [Google Scholar]
- González-Meler MA, Aranda X, Ribas-Carbó M, Peñuelas J, Azcón-Bieto J. Titration of cytochrome pathway by azide, cyanide and sulfide in leaf slices of Pisum sativum L. In: Lambers H, van der Plas LHW, editors. Molecular, Biochemical and Physiological Aspects of Plant Respiration. the Hague: Academic Publishing; 1992. pp. 603–607. [Google Scholar]
- Hansen DJ, Dayanandan P, Kaufman PB, Brotherson JD. Ecological adaptations of salt marsh grass, Distichlis spicata (Gramineae), and environmental factors affecting its growth and distribution. Amer. J. Bot. 1976;63:635–650. [Google Scholar]
- Havill DC, Ingold A, Pearson J. Sulphide tolerance in coastal halophytes. Vegetatio. 1985;62:279–285. [Google Scholar]
- Huang Y, Picha DH, Kilili AW. Atmospheric oxygen level influences alcohol dehydrogenase and pyruvate decarboxylase activities in sweet potato roots. J. Plant Physiol. 2002;159:129–136. [Google Scholar]
- Ingold A, Havill DC. The influence of sulphide on the distribution of higher plants in salt marshes. J. Ecol. 1984;72:1043–1054. [Google Scholar]
- John CD, Greenway H. Alcoholic fermentation and activity of some enzymes in rice roots under anaerobiosis. Aust. J. Plant Physiol. 1976;3:325–336. [Google Scholar]
- Joshi MM, Ibrahim IKA, Hollis JP. Hydrogen sulfide: Effects on the physiology of rice plants and relation to straighthead disease. Phytopathology. 1975;65:1165–1170. [Google Scholar]
- King GM, Klug MJ, Wiegert RG, Chalmers AG. Relation of soil water movement and sulfide concentration to Spartinan alterniflora production in a Georgia salt marsh. Science. 1982;218:61–63. doi: 10.1126/science.218.4567.61. [DOI] [PubMed] [Google Scholar]
- Koch MS, Mendelssohn IA. Sulphide as a soil phytotoxin: Differential responses in two marsh species. J. Ecol. 1989;77:565–578. [Google Scholar]
- Koch MS, Mendelssohn IA, McKee KL. Mechanism for the hydrogen sulfide-induced growth limitation in wetland macrophytes. Limnol. Oceanogr. 1990;35:399–408. [Google Scholar]
- Lee RW, Kraus DW, Doeller JE. Oxidation of sulfide by Spartina alterniflora roots. Limnol. Oceanogr. 1999;44:1155–1159. [Google Scholar]
- Lee RW. Physiological adaptations of the invasive cordgrass Spartina anglica to reducing sediments: rhizome metabolic gas fluxes and enhanced O2 and H2S transport. Mar. Biol. 2003;143:9–15. [Google Scholar]
- Maricle BR, Crosier JJ, Bussiere BC, Lee RW. Respiratory enzyme activities correlate with anoxia tolerance in salt marsh grasses. J. Exp. Mar. Biol. Ecol. 2006;337:30–37. [Google Scholar]
- Maricle BR, Lee RW. Root respiration and oxygen flux in salt marsh grasses from different elevational zones. Mar. Biol. 2007;151:413–423. [Google Scholar]
- McKee KL, Mendelssohn IA, Hester MW. Reexamination of pore water sulfide concentrations and redox potentials near the aerial roots of Rhizophora mangle and Avicennia germinans. Amer. J. Bot. 1988;75:1352–1359. [Google Scholar]
- Mendelssohn IA, McKee KL, Patrick WH. Oxygen deficiency in Spartina alterniflora roots: Metabolic adaptation to anoxia. Science. 1981;214:439–441. doi: 10.1126/science.214.4519.439. [DOI] [PubMed] [Google Scholar]
- Mobberley DG. Taxonomy and distribution of the genus Spartina. Iowa State Coll. J. Sci. 1956;30:471–574. [Google Scholar]
- Pearson J, Havill DC. The effect of hypoxia and sulphide on culture-grown wetland and non-wetland plants II. Metabolic and physiological changes. J. Exp. Bot. 1988;29:431–439. [Google Scholar]
- Pezeshki SR, DeLaune RD, Pan SZ. Relationship of soil hydrogen sulfide level to net carbon assimilation of Panium hemitomon and Spartina patens. Vegetatio. 1991;95:159–166. [Google Scholar]
- Polacik KA, Maricle BR. Effects of flooding on photosynthesis and root respiration in saltcedar (Tamarix ramosissima), an invasive riparian shrub. Environ. Exp. Bot. 2013;89:19–27. [Google Scholar]
- Raven JA, Scrimgeour CM. The influence of anoxia on plants of saline habitats with special reference to sulphur cycle. Ann. Bot. 1997;79(Suppl. A):79–86. [Google Scholar]
- Siedow JN, Day DA. Respiration and photorespiration. In: Buchanan BB, Gruissem W, Jones RL, editors. Biochemistry and Molecular Biology of Plants. Rockville, Maryland: American Society of Plant Physiologists; 2000. pp. 676–728. [Google Scholar]
- Smith L. Spectrophotometric assay of cytochrome c oxidase. Meth. Biochem. Anal. 1955;2:427–434. doi: 10.1002/9780470110188.ch13. [DOI] [PubMed] [Google Scholar]
- Visser EJW, Bogemann GM. Aerenchyma formation in the wetland plant Juncus effusus is independent of ethylene. New Phytol. 2006;171:305–314. doi: 10.1111/j.1469-8137.2006.01764.x. [DOI] [PubMed] [Google Scholar]
- Wang F, Chapman PM. Biological implications of sulfide in sediment--A review focusing on sediment toxicity. Environ. Toxicol. Chem. 1999;18:2526–2532. [Google Scholar]
- Waring EF, Maricle BR. Photosynthetic variation and carbon isotope discrimination in invasive wetland grasses in response to flooding. Environ. Exp. Bot. 2012;77:77–86. [Google Scholar]