Abstract
Background
Planning for renal replacement therapy, such as referral for arteriovenous fistula placement and transplantation, is often guided by level of estimated glomerular filtration rate (eGFR). The use of risk equations might enable more accurate estimation of time to end-stage renal disease (ESRD), thus improving patient care.
Study Design
Prospective observational study.
Setting & Participants
1,094 participants in the African-American Study of Kidney Disease and Hypertension (AASK) cohort.
Predictor
Age, sex, urine protein-creatinine ratio ≥1 g/g, APOL1 high-risk status, and 3-year antecedent eGFR decline.
Outcome
Cumulative incidence of ESRD from five different starting points: eGFR values of 30 and 15 ml/min/1.73 m2, and a 5%, 10%, and 20% 1-year ESRD risk, estimated by a published, 4-variable kidney failure risk equation.
Results
There were 566 participants who developed an eGFR of 30 ml/min/1.73 m2, 244 who developed eGFR of 15 ml/min/1.73 m2, and 437, 336, and 259 who developed a 5%, 10%, and 20% 1-year ESRD risk, respectively. The 1-year cumulative incidence of ESRD was 4.3% from eGFR 30 ml/min/1.73 m2, 49.0% from eGFR 15 ml/min/1.73 m2, 6.7% from 5% ESRD risk, 15.0% from 10% ESRD risk, and 29% from 20% ESRD risk. From eGFR 30 ml/min/1.73 m2, there were several risk factors that predicted ESRD risk. From eGFR 15 ml/min/1.73 m2, only level of proteinuria did; median time to ESRD was 9 and 19 months in those with higher and lower proteinuria, respectively. Median times were less variable from corresponding ESRD risk thresholds. For example, median time to ESRD from 20% ESRD risk was 22 and 25 months among those with higher and lower proteinuria, respectively.
Limitations
Relatively homogeneous population of African Americans with hypertensive kidney disease.
Conclusions
The results of the present study suggest the potential benefit of incorporating kidney failure risk equations into clinical care, with selection of a specific threshold guided by its intended use.
Keywords: end-stage renal disease (ESRD), estimated glomerular filtration rate (eGFR), proteinuria, kidney failure risk equations, risk, disease trajectory, disease progression, prognosis, clinical decision making, African-American Study of Kidney Disease and Hypertension (AASK), hypertensive kidney disease
Advanced chronic kidney disease (CKD) is associated with mortality and significant morbidity, including decline in kidney function and progression to end-stage renal disease (ESRD).1–7 Estimating time to ESRD is important both for patient counseling and for the timing of interventions. Procedures such as arteriovenous fistula placement and kidney transplantation are considered optimal when implemented before the initiation of dialysis, but there may be unnecessary expense and/or risk in performing these procedures too early in the course of kidney function decline.8–11 However, the time until ESRD is often difficult to estimate, since it varies by population and potentially by age, sex, and the presence of certain comorbidities.12–22
Historically, clinical decision-making in nephrology has hinged on level of estimated glomerular filtration rate (eGFR). Guidelines recommend nephrology referral once a patient reaches an eGFR of 30 ml/min/1.73 m2; transplant programs typically initiate wait-listing for kidney transplantation once eGFR declines to 20 ml/min/1.73 m2.11 Some kidney disease experts have begun to advocate for clinical decision-making based on risk probabilities, methods long used in specialties such as cardiology. Kidney failure risk equations have been developed and externally validated,7, 23, 24 and the 2012 KDIGO (Kidney Disease: Improving Global Outcomes) guidelines incorporate these equations, recommending the initiation of renal replacement therapy planning in persons with >10% 1-year risk of ESRD.11
To investigate whether kidney failure risk equations might be useful in informing the timing of interventions in advanced kidney disease, we determined the cumulative incidence of and time to ESRD among participants in the African American Study of Kidney Disease and Hypertension (AASK) from five different starting points: two eGFR levels (30 and 15 ml/min/1.73 m2) and three 1-year ESRD risk thresholds (5%, 10%, and 20%) estimated from a kidney failure risk equation. We hypothesized that incidence of and time to ESRD would be less variable between subgroups of age, gender, degree of proteinuria, prior eGFR slope, and APOL1 risk status when estimated from an incident kidney failure risk threshold compared to the more traditional approach based on eGFR levels.
METHODS
Study Population
The AASK study was designed as a multicenter, randomized clinical trial to test the efficacy of three antihypertensive medications and two levels of blood pressure control.25 Study participants were African-American individuals aged 18–70 years with an eGFR of 20–65 ml/min/1.73 m2 as estimated by renal clearance of I125 iothalamate. Persons with diabetes, a urine protein-creatinine ratio (PCR) >2.5 g/g, heart failure, severe systemic disease, or malignant or secondary hypertension were excluded. At the completion of the trial, all subjects who were alive and had not yet initiated RRT were invited to continue in the AASK observational cohort study.26 Of the 1,094 original participants, 787 were eligible for the observational cohort study, and 691 agreed. Follow-up range was 3.0–6.4 years during the trial phase and 8.8–12.4 years during the full study.
For the purposes of the present study, study populations were created for each of 5 starting points: eGFR 30 ml/min/1.73 m2, eGFR 15 ml/min/1.73 m2, and 5%, 10%, and 20% 1-year ESRD risk. A participant was included in a given study population at the first study visit (including study visit 1) in which their eGFR or 1-year ESRD risk crossed the specified threshold value. By definition, the study populations are not mutually exclusive, and a given participant could be included in all five study populations at different times during follow-up. The 1-year risk of ESRD was calculated at each study visit using the 4-variable equation published by Tangri and colleagues,24 ie, Model 3, where 1-year risk = 1 − (0.987104504)((−0.55668*eGFR/5) − 0.2201) × (age/10) + (0.246738*(1 − female)) + (0.451013× ln(ACR)) + 3.11246); the one-year risk equation and method for converting urine PCR to urine albumin-creatinine ratio (ACR) was obtained through personal communication with Dr. Tangri. In the full AASK population, the C-statistic for this equation was 0.9832 at one year and 0.8329 at five years.
Laboratory Measurements
As in previous studies, the AASK estimating equation was used to approximate measured GFR: eGFR= 329 × (serum creatinine)−1.096 × (age)−0.294 × (0.736 if female). Serum creatinine was measured twice at baseline, then at follow-up months 3 and 6, and then every 6 months thereafter; all samples were auto-analyzed at the AASK Central Biochemistry Laboratory in the Department of Laboratory Medicine at the Cleveland Clinic. Urine protein and creatinine were measured using the pyrogallol red technique and the modified Jaffe reaction. The PCR was dichotomized as ≤ or >1 g/g, a higher threshold than previous AASK studies given the selection for more advanced CKD. Urine protein was also expressed continuously as a log-transformed ACR, in order to utilize existing risk equations. The PCR was converted to ACR by dividing by 0.0017566 if female and 0.002655 if male.24
Covariate and Outcome Ascertainment
Study visits were conducted at months 3, 6, and every 6 months thereafter. Serum creatinine was measured at each visit, as was weight and blood pressure. Height at study enrollment was used in the calculation of BMI. Hematocrit, urine protein, and urine creatinine were measured approximately yearly. Separately, for each of the five starting points, antecedent 3-year slope was estimated for each participant using linear regression of eGFR on time during all visits in the previous 3 years. Rapid progression was defined as an antecedent 3-year slope <−5 ml/min/year. APOL1 risk status was defined as the presence of two APOL1 risk alleles, corresponding to the G1 (rs73885319 [leading to a serine to glycine substitution at amino acid 342] and rs60910145 [leading to an isoleucine to methionine substitution at amino acid 384) and G2 (rs71785313) variants. Single nucleotide polymorphisms were typed using ABI Taqman, and G1 or G2 homozygote status or G1/G2 compound heterozygous status were determined based on inferred haplotypes using PLINK.27 All but 2 individuals had a posterior probability of 1 given the high linkage disequilibrium between the G1 and G2 alleles. The study outcome was ESRD, defined as the self-reported initiation of dialysis or transplantation. For the assessment of competing events, pre-ESRD death was also considered.
Statistical Analysis
Five different starting points were evaluated: eGFR 30 ml/min/1.73 m2, eGFR 15 ml/min/1.73 m2, and 5%, 10%, and 20% 1-year ESRD risk. For each starting point, “baseline” was defined as the first visit in which a participant crossed the specified eGFR or 1-year ESRD risk threshold. For example, for analyses from eGFR 30 ml/min/1.73 m2, baseline characteristics represent the values at the earliest study visit in which eGFR was <30 ml/min/1.73 m2. Since hematocrit and PCR were not measured at every study visit, missing values at the baseline visit were imputed with the most recent value obtained during a study visit in the previous 12 months. Participants were followed from the qualifying visit date until ESRD, death, or end of study (June 30, 2007), whichever came first. Median times to ESRD or death were derived using Kaplan-Meier survival methods.
Competing risk models were used to separately estimate the cumulative incidence of ESRD and pre-ESRD death, adjusting for baseline (in other words, the first visit after a participant reached the specified threshold) eGFR in analyses of eGFR thresholds and 1-year ESRD risk in analyses of kidney failure risk thresholds. The adjustment for baseline eGFR or 1-year ESRD risk was necessary given that actual eGFR was used (i.e., not a modeled trajectory), and thus “baseline” eGFR or 1-year ESRD risk was quite variable. In order to evaluate whether thresholds completely captured information pertaining to ESRD risk, competing risk models using the methods of Fine and Gray were run adding age, gender, BMI, systolic blood pressure, log-transformed ACR, APOL1 risk status, and 3-year antecedent eGFR slope individually to the eGFR- or 1-year risk-adjusted models as well as all together.28 The interaction between APOL1 risk status and log-transformed ACR was tested, but it was not significant and thus not included in the final model. Confidence intervals for cumulative incidence curves were estimated using a bootstrap method with 1,000 repetitions (user-written Stata program “stcompadj”). Median, 25th percentile, and 75th percentile times were derived from cumulative incidence curves from each starting threshold (eGFR 30 or 15 ml/min/1.73 m2, 1-year ESRD risk of 5%, 10%, or 20%) for the following subgroups: age (< 50, ≥50 years), sex (male, female), APOL1 status (<2, ≥2 risk alleles), proteinuria (<1, ≥1 g/g), and up to 3-year antecedent eGFR decline (<5, ≥5 ml/min/1.73 m2 per year). All cumulative incidence curves and median time to ESRD estimates were adjusted for baseline eGFR or baseline 1-year ESRD risk, as appropriate.
All analyses were performed using Stata/MP 13.1 (StataCorp LP, College Station, TX). A p-value <0.05 was considered statistically significant.
RESULTS
Baseline Characteristics
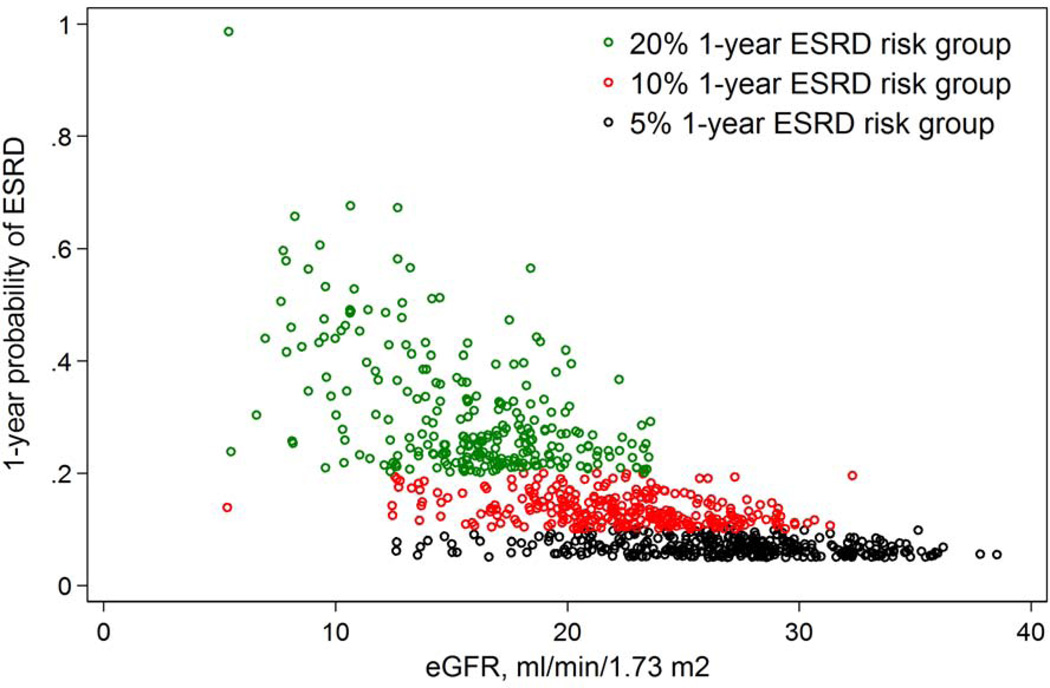
A total of 1094 AASK participants were followed up for a median of 7.8 years. Of the original enrollees, 566 developed eGFR 30 ml/min/1.73 m2 (median baseline eGFR, 26 ml/min/1.73 m2), 244 developed eGFR 15 ml/min/1.73 m2 (median baseline eGFR, 13 ml/min/1.73 m2), 437 developed a 5% 1-year ESRD risk (median eGFR, 26 ml/min/1.73 m2), 336 developed a 10% 1-year ESRD risk (median eGFR, 22 ml/min/1.73 m2), and 259 developed a 20% 1-year ESRD risk (median eGFR, 16 ml/min/1.73 m2) (Table 1). Median eGFR in the 5% ESRD risk category and the 20% ESRD risk category were most similar to those in the eGFR 30 and 15 ml/min/1.73 m2 groups, respectively, although there was a wide range of baseline eGFR in each population defined by 1-year ESRD risk (Figure 1). Compared with those crossing eGFR 30 ml/min/1.73 m2, among those crossing the 5% ESRD risk threshold there was a higher proportion with PCR >1 g/g (48.7% vs. 30.0%) and a fairly similar proportion with ≥2 APOL1 high-risk alleles (31.8% vs. 30.2%) and previous rapid progression (36.2% vs. 35.3%). Compared with those crossing eGFR 15 ml/min/1.73 m2, persons crossing the 20% ESRD risk threshold had higher median baseline eGFR (16 vs. 13 ml/min/1.73 m2), and higher proportion with PCR >1 g/g (77.2% vs. 64.4%) or ≥2 APOL1 high-risk alleles (35.4% vs. 32.7%).
TABLE 1.
Characteristics of AASK participants at 5 starting thresholds
| eGFR 30 ml/min/1.73 m2 |
eGFR 15 ml/min/1.73 m2 |
5% ESRD risk | 10% ESRD risk |
20% ESRD risk | |
|---|---|---|---|---|---|
| No. of participants | 566 | 244 | 437 | 336 | 259 |
| Time from randomization (mo) | 25 [0–67] | 50 [27–88] | 26 [0–65] | 37 [8–73] | 45 [20–80] |
| Age (y) | 56.3 ±11.9 | 55.1 ±11.2 | 53.9 ±11.7 | 53.9 ±12.0 | 53.9 ±11.6 |
| Male sex | 325 (57.4) | 125 (51.2) | 260 (59.5) | 197 (58.6) | 141 (54.4) |
| eGFR (ml/min/1.73 m2) | 26 [24–29] | 13 [11–14] | 26 [22–29] | 22 [18–24] | 16 [13–18] |
| BMI (kg/m2) | 31.1 ±7.6 | 31.8 ±7.4 | 31.8 ±7.9 | 32.0 ±7.9 | 32.3 ±7.9 |
| Systolic blood pressure (mmHg) | 138.4 (23.4) | 137.8 (21.5) | 142.8 (23.2) | 143.5 (22.3) | 140.2 (21.7) |
| Diastolic blood pressure (mmHg) | 84.1 (16.7) | 81.0 (13.5) | 86.7 (16.3) | 86.7 (15.9) | 83.6 (14.7) |
| Hematocrit (g/dL) | 37.0 (5.1) | 33.4 (5.0) | 36.9 (5.3) | 36.2 (5.1) | 34.6 (5.4) |
| Urine ACR (mg/g)*** | 179 [38–541] | 641 [229–1131] | 457 [231–770] | 633 [345–948] | 854 [551–1242] |
| Urine PCR >1 g/g*** | 164 (30.0) | 148 (64.3) | 213 (48.7) | 219 (65.2) | 200 (77.2) |
| APOL1 high-risk status* | 117 (30.2) | 56 (32.4) | 96 (31.8) | 80 (34.3) | 63 (35.4) |
| Antecedent eGFR slope** (ml/min/1.73 m2 per y) | −2.7 (20.8) | −6.0 (7.8) | −3.3 (10.4) | −3.9 (9.1) | −5.6 (10.3) |
| Rapid eGFR progression** | 133 (35.3) | 134 (55.6) | 104 (36.2) | 101 (38.6) | 118 (49.6) |
| Follow-up to ESRD or death (mo) | 44 [25–83] | 9 [4–19] | 35 [18–55] | 23 [12–39] | 13 [6–24] |
| Proportion reaching outcome | |||||
| Administrative censoring | 190 (33.6) | 30 (12.3) | 103 (23.6) | 47 (14.0) | 29 (11.2) |
| Death before ESRD | 80 (14.1) | 12 (4.9) | 46 (10.5) | 28 (8.3) | 12 (4.6) |
| ESRD | 296 (52.3) | 202 (82.8) | 288 (65.9) | 261 (77.7) | 218 (84.2) |
ACR, albumin-creatinine ratio; BMI, body mass index; PCR, protein-creatinine ratio; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease;
Note: Kidney failure risk equation uses age, sex, urine ACR, and eGFR to estimate 1-year risk of ESRD. Values for categorical variables represent number (percentage) and values for continuous variables represent mean ± standard deviation or median [interquartile range] during the first study visit at which the participant had eGFR <30 ml/min/1.73 m2, eGFR <15 ml/min/1.73 m2, or 1-year ESRD risk >5%, >10%, or >20%. ACR was estimated from PCR using a linear conversion factor separately for men and women. Antecedent eGFR slope was calculated from all eGFR values measured in study visits during the preceding 3 years. Rapid eGFR progression was defined as an antecedent eGFR slope of lesser magnitude than −5 ml/min/1.73 m2 per year.
Genotype data available for 388, 173, 302, 233, and 178 participants with eGFR <30 ml/min/1.73 m2, eGFR <15 ml/min/1.73 m2, and 1-y ESRD risks of >5%, >10%, and >20%, respectively.
eGFR slope available for 377, 241, 287, 262, and 238 participants with eGFR <30 ml/min/1.73m2, eGFR <15 ml/min/1.73m2, 1-year ESRD risk >5%, 1-year ESRD risk >10%, and 1-year ESRD risk >20%, respectively. Follow-up to ESRD or death was estimated using Kaplan-Meier methods.
PCR available for 546 and 230 participants with eGFR <30 ml/min/1.73m2 and eGFR <15 ml/min/1.73m2, respectively.
Figure 1.
Cross-sectional distribution of eGFR and 1-year probability of end-stage renal disease at the first visit at which an AASK participant crosses the threshold of 5% 1-year ESRD risk (black circles), 10% 1-year ESRD risk (red circles), and 20% 1-year ESRD risk (green circles)
Outcomes From eGFR and 1-Year ESRD Risk Starting Points
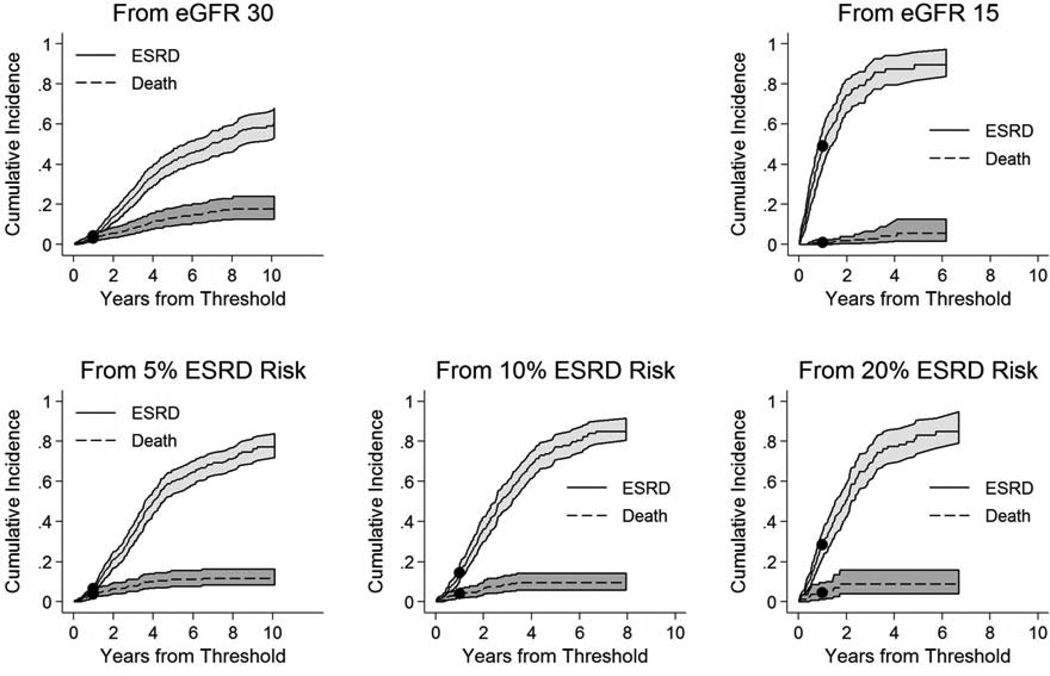
At 1 year, the cumulative incidence of ESRD was 4.3% from eGFR 30 ml/min/1.73 m2, 49.0% from eGFR 15 ml/min/1.73 m2, 6.7% from a 5% ESRD risk threshold, 15.0% from a 10% ESRD risk threshold, and 29% from a 20% ESRD risk threshold (Figure 2). The corresponding cumulative 1-year incidence of pre-ESRD death was 3.0% from eGFR 30 ml/min/1.73 m2, 1.0% from eGFR 15 ml/min/1.73 m2, 3.9% from a 5% ESRD risk threshold, 4.1% from a 10% ESRD risk threshold, and 4.5% from a 20% ESRD risk threshold. At 5 years, the cumulative incidence of ESRD was 41.2%, 89.5%, 60.3%, 77.3%, and 83.1%, and the corresponding 5-year cumulative incidence of pre-ESRD death was 13.4%, 5.7%, 11.3%, 9.7%, and 8.9%, respectively.
Figure 2.
Cumulative incidence of end-stage renal disease and death prior to end-stage renal disease from eGFR 30 ml/min/1.73 m2, eGFR 15 ml/min/1.73 m2, and 5%, 10%, and 20% 1-year risk of end-stage renal disease†
† Curves reflect cumulative incidence of ESRD and pre-ESRD mortality, where each outcome is treating as a competing event for the other. Confidence intervals were calculated by a boot-strap method using 10,000 repetitions. Models were adjusted to eGFR 30 and 15 ml/min/1.73 m2 in the eGFR threshold analyses and 5%, 10%, and 20% 1-year ESRD risk in the kidney failure risk threshold analyses. Solid circles represent incidence at 1 year.
Factors Associated With ESRD Risk From eGFR and 1-Year ESRD Risk Starting Points
From eGFR 30 ml/min/1.73 m2, younger age, higher BMI, higher systolic blood pressure, higher proteinuria, the presence of two APOL1 high-risk alleles, steeper antecedent 3-year eGFR decline, and rapid CKD progression were all significantly associated with higher risk of ESRD in analyses treating pre-ESRD as a competing risk and adjusting for baseline eGFR (Table 2). In multivariable-adjusted analysis of this population, younger age and higher proteinuria remained significantly associated with a greater risk of ESRD. From eGFR 15 ml/min/1.73 m2, only higher proteinuria was consistently associated with ESRD. From 5% 1-year ESRD risk (determined using age, sex, eGFR, and ACR in the 4-variable equation), APOL1 high-risk status was associated with higher ESRD risk, but this was not significant after adjustment for ACR. From 20% 1-year ESRD risk, older age was associated with higher ESRD risk in both crude (adjusted only for baseline ESRD risk) and multivariable-adjusted analysis. No tested variable was significantly associated with ESRD from 10% 1-year ESRD risk in crude or multivariable-adjusted analysis.
TABLE 2.
Crude and multivariable associations with ESRD in the setting of the competing risk of pre-ESRD death, from five starting thresholds
| eGFR 30 ml/min/1.73m2 | eGFR 15 ml/min/1.73m2 | 1-y ESRD risk 5% | 1-y ESRD risk 10% | 1-y ESRD risk 20% | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Crude Associations (adjusted only for eGFR or 1-y probability of ESRD) | ||||||||||
| Covariate | sHR (95% CI) | P | sHR (95% CI) | P | sHR (95% CI) | P | sHR (95% CI) | P | sHR (95% CI) | P |
| Age, per 10-y older | 0.69 (0.63–0.77) | <0.001 | 0.88 (0.77–1.01) | 0.08 | 0.92 (0.83–1.03) | 0.1 | 0.99 (0.88–1.11) | 0.9 | 1.15 (0.99–1.32) | 0.06 |
| Male sex | 1.09 (0.87–1.38) | 0.5 | 1.11 (0.83–1.48) | 0.5 | 1.10 (0.87–1.40) | 0.4 | 1.11 (0.87–1.43) | 0.4 | 1.11 (0.83–1.47) | 0.5 |
| BMI, per 5 kg/m2 greater | 1.11 (1.02–1.20) | 0.01 | 0.98 (0.88–1.08) | 0.6 | 1.00 (0.92–1.09) | 0.9 | 0.96 (0.88–1.04) | 0.3 | 0.93 (0.85–1.02) | 0.1 |
| SBP, per 10-mmHg greater | 1.08 (1.04–1.13) | <0.001 | 1.01 (0.94–1.09) | 0.8 | 1.01 (0.97–1.06) | 0.6 | 1.00 (0.95–1.06) | 0.9 | 0.99 (0.93–1.06) | 0.8 |
| ACR, per 2-fold higher | 1.41 (1.32–1.50) | <0.001 | 1.29 (1.16–1.43) | <0.001 | 1.08 (0.98–1.18) | 0.1 | 0.94 (0.83–1.06) | 0.3 | 0.91 (0.78–1.06) | 0.2 |
| APOL1 high-risk status | 1.92 (1.45–2.55) | <0.001 | 1.10 (0.77–1.58) | 0.6 | 1.47 (1.11–1.95) | 0.007 | 1.28 (0.96–1.70) | 0.09 | 1.05 (0.74–1.47) | 0.8 |
| Antecedent eGFR decline, per 5-ml/min/1.73 m2 per y faster | 1.15 (1.09–1.21) | <0.001 | 1.19 (0.99–1.44) | 0.07 | 1.06 (0.99–1.13) | 0.09 | 1.06 (0.99–1.13) | 0.08 | 1.05 (0.97–1.13) | 0.2 |
| Rapid eGFR progression | 1.67 (1.23–2.28) | 0.001 | 1.26 (0.93–1.70) | 0.1 | 1.13 (0.82–1.56) | 0.5 | 1.20 (0.89–1.60) | 0.2 | 1.15 (0.86–1.54) | 0.3 |
| Multivariable Associations (also adjusted for eGFR or 1-y probability of ESRD) | ||||||||||
| Age, per 10-y older | 0.75 (0.62–0.90) | 0.002 | 0.91 (0.75–1.09) | 0.3 | 0.97 (0.79–1.19) | 0.8 | 1.07 (0.91–1.26) | 0.4 | 1.26 (1.02–1.55) | 0.03 |
| Male sex | 1.49 (0.96–2.31) | 0.08 | 1.07 (0.69–1.67) | 0.8 | 0.96 (0.65–1.41) | 0.8 | 0.92 (0.63–1.34) | 0.7 | 0.65 (0.43–1.00) | 0.05 |
| BMI, per 5-kg/m2 greater | 0.96 (0.85–1.08) | 0.5 | 0.90 (0.80–1.01) | 0.06 | 0.97 (0.86–1.10) | 0.6 | 1.02 (0.92–1.12) | 0.7 | 0.97 (0.88–1.07) | 0.6 |
| SBP, per 10-mm Hg greater | 1.08 (0.96–1.20) | 0.2 | 0.90 (0.81–0.99) | 0.03 | 1.02 (0.92–1.12) | 0.7 | 0.99 (0.91–1.08) | 0.9 | 0.98 (0.90–1.07) | 0.6 |
| ACR, per 2-fold higher | 1.36 (1.20–1.54) | <0.001 | 1.40 (1.22–1.62) | <0.001 | 1.13 (0.99–1.28) | 0.08 | 0.98 (0.82–1.17) | 0.8 | 0.87 (0.69–1.08) | 0.2 |
| APOL1 high-risk status | 1.26 (0.82–1.96) | 0.3 | 0.82 (0.56–1.21) | 0.3 | 1.36 (0.93–2.00) | 0.1 | 1.26 (0.90–1.76) | 0.2 | 1.01 (0.70–1.44) | 0.9 |
| Antecedent eGFR decline. per 5-ml/min/1.73 m2 per y faster | 1.10 (0.97–1.24) | 0.1 | 1.06 (0.97–1.15) | 0.2 | 1.03 (0.95–1.12) | 0.5 | 1.07 (0.93–1.23) | 0.4 | 1.01 (0.94–1.09) | 0.8 |
Note: Minimally-adjusted analyses represents competing risk regression of the risk of ESRD associated with the covariate, adjusting for eGFR in the analyses using eGFR thresholds and 1-y ESRD risk in the analyses of ESRD risk thresholds.28 Multivariable associations reflect competing risk regression of the risk of ESRD associated with all specified covariates as well as eGFR in the analyses using eGFR thresholds and 1-year ESRD risk in the analyses of ESRD risk thresholds. In all regressions, pre-ESRD death is treated as a competing event. Baseline for each analysis is the first study visit at which a participant crosses the specified threshold (eGFR 30 or 15 ml/min/1.73 m2, 1-year ESRD risk of 5%, 10%, or 20%).
ACR, albumin-creatinine ratio; BMI, body mass index; PCR, protein-creatinine ratio; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; sHR indicates sub-distribution hazard ratio ; CI, confidence interval; SBP, systolic blood pressure
Median Time to ESRD by Patient Characteristic
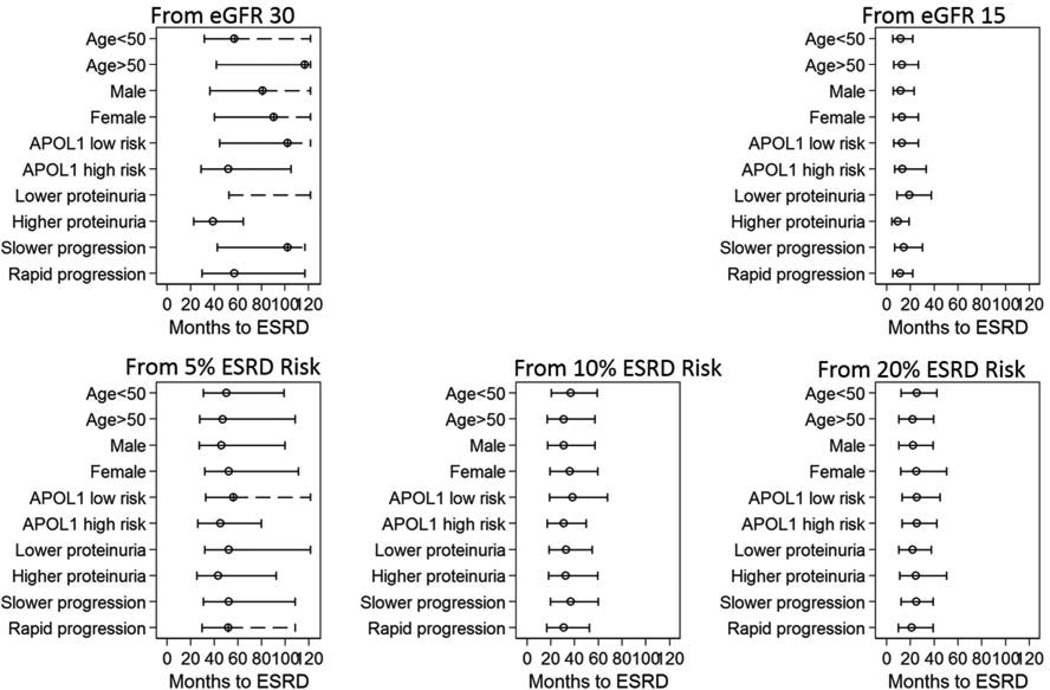
There was large variation in time to ESRD, particularly from the starting point of eGFR 30 ml/min/1.73 m2 (Figure 3). For example, the median time to ESRD from eGFR 30 ml/min/1.73 m2 was 57 months in participants aged 50 years or younger and 116 months in participants older than 50 years. There was less variability from the starting point of 5% 1-year ESRD risk, where median time to ESRD was 50 months in participants aged 50 years or younger and 47 months in participants older than 50 years. Variability in time to ESRD was lower in those with more advanced disease. From eGFR 15 ml/min/1.73 m2, median times to ESRD were 11 vs. 13 months in those aged 50 years or younger vs. older than 50 years, and 19 vs. 9 months in the low proteinuria vs. higher proteinuria group. From 20% 1-year ESRD risk, the corresponding times to ESRD were 22 vs. 26 months and 22 vs. 25 months for age and proteinuria subgroups, respectively.
Figure 3.
Median (25th percentile – 75th percentile) times to end-stage renal disease, by patient characteristic, from five starting points: eGFR 30 ml/min/1.73 m2, eGFR 15 ml/min/1.73 m2, and 5%, 10%, and 20% 1-year risk of end-stage renal disease†
†Times to end-stage renal disease estimated accounting for the competing risk of death and adjusting to eGFR 30 or 15 ml/min/1.73 m2 in analyses from eGFR thresholds and 5%, 10%, and 20% 1-year end-stage renal disease (ESRD) risk in analyses from ESRD risk thresholds. Dashed lines represent an imputed interquartile range and are truncated at the last observed follow-up time.
DISCUSSION
In this prospective study of African Americans with hypertension and CKD, a kidney failure risk equation that incorporates age, sex, proteinuria, and eGFR appeared to be a useful tool in estimating time to ESRD. Using the more traditional approach of estimation of ESRD risk from level of eGFR, there was significant heterogeneity in time to ESRD despite having selected only participants with advanced CKD. For example, from eGFR 30 ml/min/1.73 m2, median times to ESRD varied by nearly 5 years for lower vs. higher levels of proteinuria; from eGFR 15 ml/min/1.73 m2, this variation was attenuated but still statistically and clinically significant at 10 months. There was less variation in times to ESRD from thresholds of 1-year ESRD risk (5%, 10%, 20%), suggesting that kidney failure risk equations may enable more accurate patient counseling and clinical decision-making.
Variation in time to ESRD is smaller in situations in which ESRD risk is higher; hence, direct comparison of eGFR and ESRD risk thresholds is difficult. That said, despite similar baseline eGFR for the 5% ESRD risk and the eGFR 30 ml/min/1.73 m2 groups, the time to ESRD appeared more predictable in the 5% ESRD risk group, with similar median times to ESRD across all subgroups tested. This observation may be driven in part by the kidney failure risk equation’s incorporation of albuminuria, an important risk factor for ESRD as well as for acute kidney injury, patterns of CKD progression, and mortality.2, 29, 30
Incorporation of other risk factors in the kidney failure risk equation might further improve performance. In Tangri’s original kidney failure risk equation study, both a 7-variable (age, sex, eGFR, ACR, systolic blood pressure, diastolic blood pressure, body weight) and an 8-variable (age, sex, eGFR, ACR, serum albumin, serum phosphate, serum bicarbonate, and serum calcium) model had slightly higher discriminatory ability than the 4-variable model used in the present study (C-statistics: 0.92, 0.92, and 0.91 for the 7-, 8-, and 4-variable models, respectively).24 Thus, incorporating additional risk factors (or using the 8-variable equation) might further reduce heterogeneity in times to ESRD, but the magnitude of improvement might be small.
Heterogeneity in times to ESRD may have multiple causes, including heterogeneity in etiology of kidney disease, the competing risk of mortality, non-linear or non-progressing eGFR trajectories, interceding events such as acute kidney injury, and provider variation in the timing of dialysis initiation.18, 30–33 Participants in the AASK study are relatively homogenous, selected to represent a single etiology (hypertensive kidney disease), and they experienced a lower than expected rate of pre-ESRD death.1 Despite these factors, time to ESRD was quite variable from eGFR thresholds. Nonlinear trajectories and periods of prolonged non-progression were common in the AASK cohort; however, these eGFR patterns were far more prevalent in participants with eGFR ≥40 ml/min/1.73 m2 or proteinuria <0.22 g/g, a subgroup with very little representation in the present study.30 Unfortunately, we have no data on acute kidney injury events or the timing of dialysis initiation in the AASK cohort. To the extent that acute kidney injury and dialysis timing are also affected by age, gender, and albuminuria, however, one could expect that the kidney failure risk equations might more completely capture these risks.
Risk equations have long been used in cardiology, albeit not without controversy.34–37 The Framingham study equations for incident cardiovascular disease have been used to determine therapeutic interventions such as aspirin and statin therapy.36 The CHADS2 index has been used to predict stroke risk in atrial fibrillation and drive choice of antithrombotic therapy.38 Kidney failure risk equations should be especially useful in nephrology: referrals for nephrology, vascular access, and transplantation might be better informed by absolute risk of kidney failure rather than eGFR alone. Kidney failure risk equations could be used to optimize patient selection and statistical power for clinical trials in kidney disease. Selection of a specific risk threshold would be guided by its purpose. For example, although we include the 10% 1-year ESRD risk threshold given its prominence in the KDIGO CKD guideline,11 a different risk threshold would be required to target a shorter or longer time to ESRD. In the case of vascular access, a higher threshold might be appropriate, so that a person receives permanent access within 6 months of RRT initiation and the probability of pre-ESRD death is lower. In the case of transplantation referral, a lower threshold might be appropriate, given the length of the kidney transplant waitlist and the importance of early transplantation. To our knowledge, no study has directly compared interventions guided by eGFR threshold to those guided by kidney failure risk.
This study also demonstrates that traditional ESRD risk factors may be less important in more advanced CKD. Several recent studies, including those with participants from AASK, have demonstrated the importance of high risk variants in the APOL1 gene with respect to ESRD and CKD progression.39–41 The present study finds an APOL1-associated ESRD risk from the milder disease thresholds of eGFR 30 ml/min/1.73 m2 and 5% 1-year ESRD risk, but no significant risk associations from eGFR 15 ml/min/1.73 m2 or 10% and 20% ESRD risk. This may be due to the exceedingly high risk of ESRD from the latter starting points or, in the case of the 10% and 20% ESRD risk populations, it may indicate that much of the risk associated with APOL1 is captured by measures of proteinuria. The association of age also differed based on threshold: older age was associated with lower ESRD risk from eGFR 30 and 15 ml/min/1.73 m2 (the latter non-significant) and higher ESRD risk from the 20% ESRD risk threshold. This observation may stem from the fact that the AASK population is an extremely high-risk cohort, with a higher rate of ESRD than pre-ESRD death, which is different than an older, general CKD population like the one in which the Tangri equation was developed.1, 5, 24
Strengths of this study include a large cohort followed up at least semi-annually for a median of 7.8 years. In contrast to a clinical cohort, all serum creatinine, urine protein, and urine creatinine measurements were done per protocol, not for cause, obviating any treatment by indication bias. Evaluated covariates were time-varying, mimicking what would be seen in clinical practice. Decline in kidney function were observed and not modeled, and loss to follow up was small. On the other hand, not all participants were genotyped for APOL1, and ACR was estimated from urine PCR rather than directly measured. The AASK study was originally a clinical trial, and findings may not be generalizable to the general population of African-American patients with CKD attributed to hypertension. Finally, each “starting point” corresponds to a different study population, thereby complicating direct comparisons (as commonly done when analyses are nested within the same population).
The results of the present study suggest the potential benefit of incorporating kidney failure equations into clinical care, and they provide useful data supporting the KDIGO-recommended threshold of 10% 1-year ESRD risk. The robust performance of the kidney failure risk equation in the AASK study—a cohort quite different from the original administrative cohort in which the kidney failure risk equation was developed—is a reassuring confirmation of the kidney failure risk equation itself. The incorporation of kidney failure risk equations may enhance counseling in CKD and optimize clinical decision-making with respect to the timing of interventions.
ACKNOWLEDGEMENTS
The authors thank the staff and participants of the AASK study for their important contributions.
Support: MG is supported by National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant K08DK092287. AC was supported by the NIDDK grant T32DK007732. LL, THG, and LJA were supported by NIDDK grant R01DK090046. The funders of this study had no role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Some data presented in this study were shown at the American Society of Nephrology Kidney Week in San Diego on November 3, 2012.
Financial Disclosure: Dr Kao died before this article was accepted for publication, but Dr Grams affirms, to the best of her knowledge, that Dr Kao had no relevant financial interests. The other authors declare that they have no other relevant financial interests.
Contributions: research idea and study design: MEG, LL, THG; data acquisition: THG, WHLK, MSL, JTW, BCA, LJA; data analysis/interpretation: MEG, LL, THG, AT, YS, BCA, AC; statistical analysis: MEG, YS, AT; supervision or mentorship: THG, LJA, WHLK. WHLK died during the manuscript consideration process; MEG affirms that she contributed to data acquisition and supervision or mentorship and vouches for her coauthorship status; all other authors approved the final author list. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. MEG takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
REFERENCES
- 1.Alves TP, Wang X, Wright JT, Jr, et al. Rate of ESRD exceeds mortality among African Americans with hypertensive nephrosclerosis. Journal of the American Society of Nephrology : JASN. 2010;21:1361–1369. doi: 10.1681/ASN.2009060654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gansevoort RT, Matsushita K, van der Velde M, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80:93–104. doi: 10.1038/ki.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. The New England journal of medicine. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 4.Ishani A, Grandits GA, Grimm RH, et al. Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. Journal of the American Society of Nephrology : JASN. 2006;17:1444–1452. doi: 10.1681/ASN.2005091012. [DOI] [PubMed] [Google Scholar]
- 5.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Archives of Internal Medicine. 2004;164:659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 6.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson ES, Thorp ML, Platt RW, Smith DH. Predicting the risk of dialysis and transplant among patients with CKD: a retrospective cohort study. American Journal of Kidney Diseases : The Official Journal of the National Kidney Foundation. 2008;52:653–660. doi: 10.1053/j.ajkd.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 8.Akkina SK, Connaire JJ, Snyder JJ, Matas AJ, Kasiske BL. Earlier is not necessarily better in preemptive kidney transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8:2071–2076. doi: 10.1111/j.1600-6143.2008.02381.x. [DOI] [PubMed] [Google Scholar]
- 9.Grams ME, Massie AB, Coresh J, Segev DL. Trends in the Timing of Pre-emptive Kidney Transplantation. Journal of the American Society of Nephrology : JASN. 2011 Sep;22(9):1615–1620. doi: 10.1681/ASN.2011010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishani A, Ibrahim HN, Gilbertson D, Collins AJ. The impact of residual renal function on graft and patient survival rates in recipients of preemptive renal transplants. American Journal of Kidney Diseases : The Official Journal of the National Kidney Foundation. 2003;42:1275–1282. doi: 10.1053/j.ajkd.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 11.Kidney Disease: Improving Global O. Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013;3:1–150. [Google Scholar]
- 12.Barbour SJ, Er L, Djurdjev O, Karim M, Levin A. Differences in progression of CKD and mortality amongst Caucasian, Oriental Asian and South Asian CKD patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2010 Nov;25(11):3663–3672. doi: 10.1093/ndt/gfq189. [DOI] [PubMed] [Google Scholar]
- 13.Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Hernandez GT, O'Hare AM. White/black racial differences in risk of end-stage renal disease and death. The American Journal of Medicine. 2009;122:672–678. doi: 10.1016/j.amjmed.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans M, Fryzek JP, Elinder CG, et al. The natural history of chronic renal failure: results from an unselected, population-based, inception cohort in Sweden. American Journal of Kidney Diseases : The Official Journal of the National Kidney Foundation. 2005;46:863–870. doi: 10.1053/j.ajkd.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 15.Hanratty R, Chonchol M, Miriam Dickinson L, et al. Incident chronic kidney disease and the rate of kidney function decline in individuals with hypertension. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2010;25:801–807. doi: 10.1093/ndt/gfp534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iseki K, Iseki C, Ikemiya Y, Fukiyama K. Risk of developing end-stage renal disease in a cohort of mass screening. Kidney international. 1996;49:800–805. doi: 10.1038/ki.1996.111. [DOI] [PubMed] [Google Scholar]
- 17.Jafar TH, Schmid CH, Stark PC, et al. The rate of progression of renal disease may not be slower in women compared with men: a patient-level meta-analysis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2003;18:2047–2053. doi: 10.1093/ndt/gfg317. [DOI] [PubMed] [Google Scholar]
- 18.Nakayama M, Sato T, Sato H, et al. Different clinical outcomes for cardiovascular events and mortality in chronic kidney disease according to underlying renal disease: the Gonryo study. Clinical and experimental nephrology. 2010;14:333–339. doi: 10.1007/s10157-010-0295-y. [DOI] [PubMed] [Google Scholar]
- 19.Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. Journal of the American Society of Nephrology : JASN. 2000;11:319–329. doi: 10.1681/ASN.V112319. [DOI] [PubMed] [Google Scholar]
- 20.O'Hare AM, Choi AI, Bertenthal D, et al. Age affects outcomes in chronic kidney disease. Journal of the American Society of Nephrology : JASN. 2007;18:2758–2765. doi: 10.1681/ASN.2007040422. [DOI] [PubMed] [Google Scholar]
- 21.van der Velde M, Bakker SJ, de Jong PE, Gansevoort RT. Influence of Age and Measure of eGFR on the Association between Renal Function and Cardiovascular Events. Clinical journal of the American Society of Nephrology : CJASN. 2010 Nov;5(11):2053–2059. doi: 10.2215/CJN.08851209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xue JL, Eggers PW, Agodoa LY, Foley RN, Collins AJ. Longitudinal study of racial and ethnic differences in developing end-stage renal disease among aged medicare beneficiaries. Journal of the American Society of Nephrology : JASN. 2007;18:1299–1306. doi: 10.1681/ASN.2006050524. [DOI] [PubMed] [Google Scholar]
- 23.Elley CR, Robinson E, Kenealy T, Bramley D, Drury PL. Derivation and Validation of a New Cardiovascular Risk Score for People With Type 2 Diabetes The New Zealand Diabetes Cohort Study. Diabetes care. 2010;33:1347–1352. doi: 10.2337/dc09-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tangri N, Stevens LA, Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA : the journal of the American Medical Association. 2011;305:1553–1559. doi: 10.1001/jama.2011.451. [DOI] [PubMed] [Google Scholar]
- 25.Sika M, Lewis J, Douglas J, et al. Baseline characteristics of participants in the African American Study of Kidney Disease and Hypertension (AASK) Clinical Trial and Cohort Study. American Journal of Kidney Diseases : The Official Journal of the National Kidney Foundation. 2007;50:78–89. doi: 10.1053/j.ajkd.2007.03.004. 89.e71. [DOI] [PubMed] [Google Scholar]
- 26.Appel LJ, Middleton J, Miller ER, 3rd, et al. The rationale and design of the AASK cohort study. Journal of the American Society of Nephrology : JASN. 2003;14:S166–S172. doi: 10.1097/01.asn.0000070081.15137.c0. [DOI] [PubMed] [Google Scholar]
- 27.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. American Journal of Human Genetics. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 29.Grams ME, Astor BC, Bash LD, Matsushita K, Wang Y, Coresh J. Albuminuria and Estimated Glomerular Filtration Rate Independently Associate with Acute Kidney Injury. Journal of the American Society of Nephrology : JASN. 2010 Oct;21(10):1757–1764. doi: 10.1681/ASN.2010010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li L, Astor BC, Lewis J, et al. Longitudinal progression trajectory of GFR among patients with CKD. American Journal of Kidney Diseases : The Official Journal of the National Kidney Foundation. 2012;59:504–512. doi: 10.1053/j.ajkd.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grams ME, Coresh J, Segev DL, Kucirka LM, Tighiouart H, Sarnak MJ. Vascular Disease, ESRD, and Death: Interpreting Competing Risk Analyses. Clinical journal of the American Society of Nephrology : CJASN. 2012 Oct;7(10):1606–1614. doi: 10.2215/CJN.03460412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Hare AM, Batten A, Burrows NR, et al. Trajectories of kidney function decline in the 2 years before initiation of long-term dialysis. American Journal of Kidney Diseases. 2012;59:513–522. doi: 10.1053/j.ajkd.2011.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosansky SJ, Clark WF, Eggers P, Glassock RJ. Initiation of dialysis at higher GFRs: is the apparent rising tide of early dialysis harmful or helpful? Kidney international. 2009;76:257–261. doi: 10.1038/ki.2009.161. [DOI] [PubMed] [Google Scholar]
- 34.D'Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 35.Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014 Jul 1;63(25 Pt B):2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 37.National Cholesterol Education Program Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 38.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA : the journal of the American Medical Association. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 39.Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. Journal of the American Society of Nephrology : JASN. 2013;24:1484–1491. doi: 10.1681/ASN.2013010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanji Z, Powe CE, Wenger JB, et al. Genetic variation in APOL1 associates with younger age at hemodialysis initiation. Journal of the American Society of Nephrology : JASN. 2011;22:2091–2097. doi: 10.1681/ASN.2010121234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parsa A, Kao WH, Xie D, et al. APOL1 risk variants, race, and progression of chronic kidney disease. The New England journal of medicine. 2013;369:2183–2196. doi: 10.1056/NEJMoa1310345. [DOI] [PMC free article] [PubMed] [Google Scholar]