Abstract
Purpose
To evaluate the association between cystoid macular edema (CME) observed in very preterm infants and developmental outcomes at 18 to 24 months corrected age.
Design
Cohort study.
Participants
Infants born at or less than 1500 g or at or less than 30 weeks postmenstrual age who underwent screening for retinopathy of prematurity (ROP) in an intensive care nursery.
Methods
Bedside handheld spectral-domain optical coherence tomography (SD OCT; Envisu, Bioptigen, Inc, Research Triangle Park, NC) imaging was obtained from preterm infants who were being screened for ROP and graded for presence of CME, central foveal thickness (CFT), inner nuclear layer thickness, and foveal-to-parafoveal thickness ratio. At 18 to 24 months corrected age, the children were assessed with the Bayley Scales of Infant and Toddler Development, Third Edition.
Main Outcome Measures
Scores on the Bayley cognitive, language, and motor subscales.
Results
Among 77 children with SD OCT imaging, 53 were evaluated with the Bayley Scales. Compared with children who did not have CME as infants (n = 22), the mean score for children who had CME (n = 31) was 7.3 points (95% confidence interval [CI], −15.5 to 0.9; P = 0.08) lower on the cognitive subscale, 14.1 points (95% CI, −22.7 to −5.5; P = 0.002) lower for the language subscale, and 11.5 points (95% CI, −21.6 to −1.3; P = 0.03) lower for the motor subscale. Differences were maintained after adjusting for gestational age and birth weight. Severity of CME, as assessed by foveal-to-parafoveal thickness ratio, within the CME group correlated with poorer cognitive (R2 = 0.16, P = 0.03) and motor (R2 = 0.15, P = 0.03) development.
Conclusions
Cystoid macular edema observed on SD OCT in very preterm infants screened for ROP is associated with poorer language and motor skills at 18 to 24 months corrected age. Evaluation of the retina with SD-OCT may serve as an indicator of neurodevelopmental health for very preterm infants in the intensive care nursery.
The emergence of portable, handheld spectral-domain optical coherence tomography (SD OCT) in the intensive care nursery has increased our understanding of eye development and pediatric ophthalmologic diseases by providing high-resolution, noncontact, in vivo cross-sectional imaging of the posterior retina of neonates.1–9 One notable ocular finding recently discovered is the high prevalence (approximately 50%) of cystoid macular edema (CME) in very preterm infants screened for retinopathy of prematurity (ROP).10–12 These foveal cystoid structures appear as hyporeflective spaces in the inner nuclear layer (INL) that commonly persist at the fovea along with the other inner retinal layers in preterm infants.13 Because CME usually is not detected on traditional indirect ophthalmoscopy, little is known about this relatively new finding. The cause remains speculative, with theories ranging from increased levels of vascular endothelial growth factor to mechanical traction. Resolution of CME is reported in some, but not all, cases, making it unclear whether this subclinical finding is a physiologic process during normal retinal development or a marker of underlying pathologic features.11,12,14
Neurosensory retina is central nervous system (CNS) tissue, and therefore is likely to reflect ongoing brain development or maldevelopment. Previous studies of infants with ROP link structural abnormalities of the retina and its vasculature to substantial developmental delays,15 even among those patients with favorable vision.16,17 Although the phenomenon of subclinical CME observed on perinatal SD OCT in very preterm infants has been characterized, no previous investigation has assessed correlations between CME findings and neurodevelopmental outcome.10–12,14 The purpose of this analysis was to determine if very preterm infants with CME observed on SD OCT in the intensive care nursery will have poorer neurodevelopmental outcomes at 18 to 24 months corrected age compared with very preterm infants without CME. Additionally, we aimed to determine if severity of CME, represented by various measures of retinal thickness and deformation, correlates with worse neurodevelopmental outcomes.
Methods
The current analysis is part of a larger prospective study that was approved by the Duke University Institutional Review Board and adhered to the Health Insurance Portability and Accountability Act and the tenets of the Declaration of Helsinki. The original protocol for the study did not include neurodevelopmental testing; however, we later found that the testing was being performed fairly regularly as part of routine care. The neurodevelopmental study is a secondary analysis, and all neurodevelopmental data were captured as described below by clinicians masked to SD OCT imaging data. From January 2009 through April 2014, preterm infants admitted to the Duke University Hospital intensive care nursery who underwent ROP screening and were considered stable enough for research imaging were eligible, with the written informed consent of a parent or legal guardian, for enrollment as part of a larger study to evaluate retinal and optic nerve microanatomic features during ROP screening. Infants screened for ROP included those born at or less than 1500 g or at or less than 30 weeks postmenstrual age (PMA) who were at least 30 weeks PMA and 4 weeks of age at the time of their first eye examination.18 Demographic information, including gestational age (GA), birth weight, gender, race and ethnicity, and ROP status, were recorded at the initial imaging session. All imaging was performed with a portable, noncontact, handheld SD OCT imaging system (either an early research system or the Envisu, Bioptigen, Inc, Research Triangle Park, NC) using a previously described protocol.2 Spectral-domain optical coherence tomography imaging occurred immediately after routine ROP examinations and all subsequent clinically indicated ophthalmic imaging examinations when the infant was determined to be clinically stable for research imaging. Very preterm infants from the larger study were eligible for the current study if they had at least 1 readable SD OCT volume scan with a gradable fovea and Bayley Scales of Infant and Toddler Development evaluation at 18 to 24 months corrected age. The scan must have been captured before 43 weeks PMA and before any retinal therapy (laser photocoagulation or injection of bevacizumab, an anti–vascular endothelial growth factor used off label for ROP treatment).
Spectral-domain optical coherence tomography analysis was performed by 2 trained graders (A.L.R. and D.T.-V) masked to all infant data other than PMA at imaging. The highest quality foveal scan of 1 randomly chosen eye was selected from each available imaging session by criteria including good contrast, ability to see inner retinal layers, and lack of tilt. Only 1 eye per infant was considered for analysis because Maldonado et al11 demonstrated bilaterally of CME in all infants with both eyes imaged as well as a correlation coefficient of 0.989 for central foveal thickness (CFT) between study and fellow eyes. Each foveal scan was graded by 1 of the 2 graders qualitatively for the presence of CME and quantitatively for CFT, parafoveal thickness at 1000 µm distance inferiorly and superiorly from the foveal center for vertical scans or nasally and temporally from the foveal center for horizontal scans, and INL thickness. Cystoid macular edema was defined, as described by Maldonado et al,11 as single or multiple “hyporeflective round or elongated structures separated in most instances by a vertical hyperreflective band.” Retinal thicknesses were measured from Bruch’s membrane to the internal limiting membrane (Fig 1). Foveal-to-parafoveal (FP) thickness ratio was calculated by dividing the CFT by the average parafoveal thickness and served as a marker of foveal bulging from more severe macular edema. All SD OCT measurements were made using the caliper tool with OsiriX software version 4.1.2 (Pixmeo, Bernex, Switzerland). The imaging session with the greatest CFT was included in the current analysis to ensure that the imaging session with the greatest edema captured on SD OCT was considered. Twenty randomly selected SD OCT imaging sessions were regraded by each grader for reproducibility analysis.
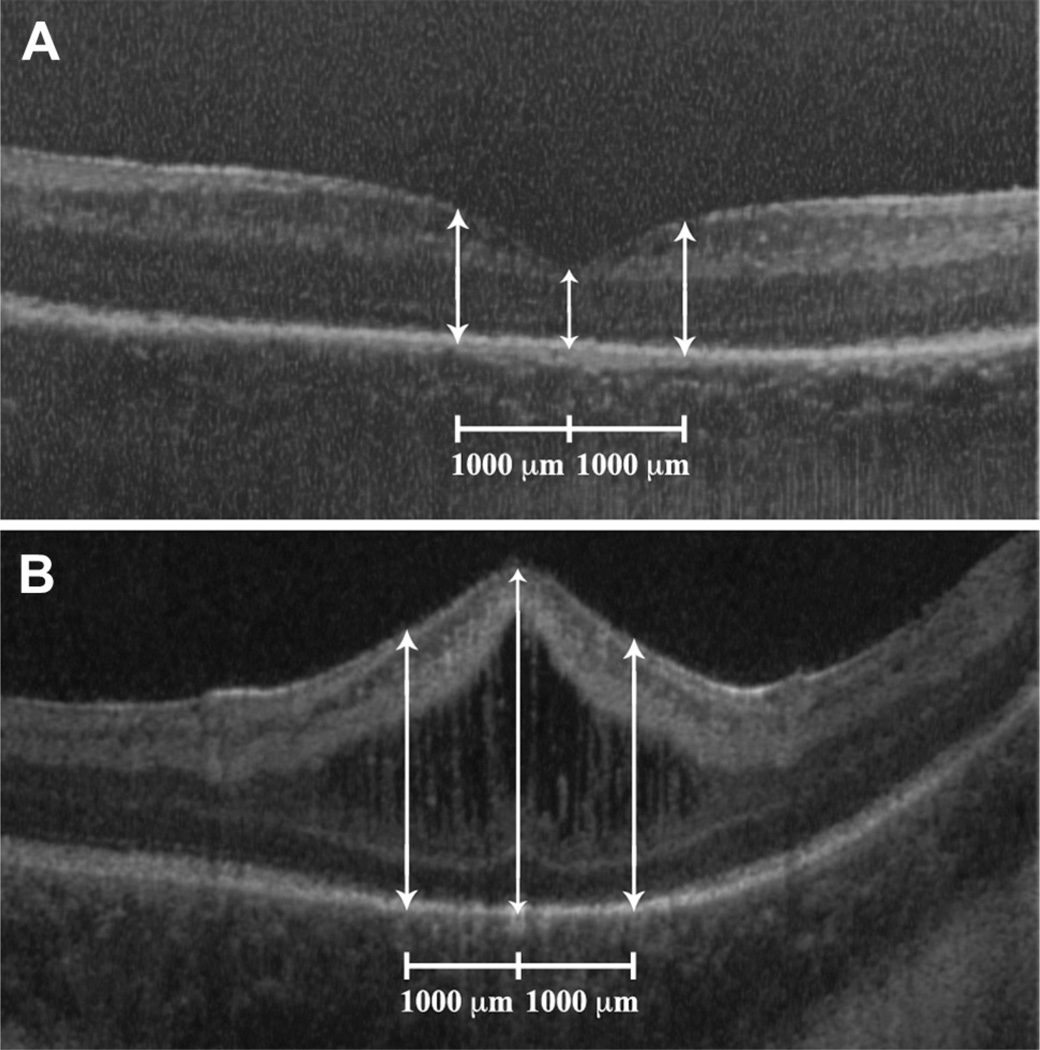
Figure 1.
A, Cross-sectional spectral-domain optical coherence tomography (SD OCT) scan of the retina of a premature infant at 33 weeks postmenstrual age (PMA) and with no retinopathy of prematurity (ROP). White arrows represent central foveal thickness as well as parafoveal thickness measurements obtained at 1000 µm from the foveal center. This infant had no cystoid macular edema (CME) on SD OCT and later had relatively normal cognitive, language, and motor Bayley Scales of Infant and Toddler Development, Third Edition, scores of 100, 83, and 100, respectively, at 18 to 24 months corrected age. B, Cross-sectional SD OCT scan of the retina of a premature infant at 34 week PMA and with stage 2 ROP. White arrows represent central foveal thickness as well as parafoveal thickness measurements obtained at 1000 µm from the foveal center. This infant had severe CME on SD OCT and later had subnormal cognitive, language, and motor Bayley Scales of Infant and Toddler Development, Third Edition, scores of 60, 74, and 49, respectively, at 18 to 24 months corrected age.
The primary measure of neurodevelopment in the current analysis was the Bayley Scales of Infant and Toddler Development, Third Edition (Pearson, San Antonio, TX), administered at 18 to 24 months corrected age. The Bayley Scales were assessed by a licensed clinical psychologist (K.E.G.) certified as a Bayley examiner by the National Institute of Health Neonatal Research Network and masked to SD OCT imaging data and grading. This standardized assessment consists of separate cognitive, language, and motor scales that result in normalized scores (Bayley scores) with a mean score of 100 and a standard deviation of 15.
All analyses were performed using either JMP Pro software version 10.0, SAS software version 9.4 (SAS Institute Inc, Cary, NC), or R software version 3.02. (Vienna, Austria). P values were considered statistically significant if less than 0.05. Intragrader and intergrader reproducibility of presence of CME, CFT, INL thickness, and FP thickness ratio were summarized by percent exact agreement and κ value for categorical measures and by the intra-class correlation coefficient for continuous measures. Chi-square tests were used to compare proportions between 2 groups and 2-sample t tests were used to compare means. Ninety-five percent confidence intervals (CIs) were calculated for the differences in means and the differences in proportions. Plots of locally weighted scatterplot smoothing curves computed using second-degree polynomials, a bandwidth of 0.8, and locally weighted least squares regression were used to summarize the relationship between severity of CME and each Bayley subscale. Mean cognitive, language, and motor Bayley scores were compared among groups based on maximum ROP stage or maximum plus disease status using analysis of variance with a test for trend in means in the ordered categories, and the mean Bayley score for each disease severity was compared with all others by a Student t test of means comparison; future need for retinal therapy was compared with each Bayley score by a 2-sample t test.
Results
Demographics
A total of 128 very preterm infants were imaged from January 2009 through April 2014. Ninety-nine of these infants had at least 1 gradable foveal SD OCT scan obtained before 43 weeks PMA and before retinal therapy, if treated. One infant died after discharge, whereas 21 infants had not reached 18 months corrected age at the time of data analysis. Fifty-three of the remaining 77 very preterm infants were evaluated by the Bayley Scales of Infant and Toddler Development, Third Edition, at 18 to 24 months corrected age and are included in this study. Of the remaining infants who were at least 18 months corrected age but were not included in the current study, 11 were lost to follow-up, 8 were tested on the Bayley Scales outside of 18 to 24 months corrected age, and 5 transferred care to another health care system. The mean ± standard deviation GA of infants assessed with the Bayley Scales was lower than those infants without Bayley Scales scores (25.8±2.3 weeks PMA vs. 27.1±2.6 weeks PMA; P = 0.05). There was no significant difference in birth weight, age at imaging, gender, race, ethnicity, eye imaged, maximum ROP stage, plus disease status, or need for laser (no infants received bevacizumab) between infants later assessed with the Bayley Scales at 18 to 24 months versus those who were not assessed with the Bayley Scales. Additionally, there was no significant difference in presence of CME, CFT, INL thickness, or FP thickness ratio between these 2 groups.
Thirty-one (58.5%) of the 53 very preterm infants with graded SD OCT scans and Bayley scores had CME observed on perinatal retinal SD OCT, whereas 22 (41.5%) of 53 infants did not have CME noted in the analyzed eye. Characteristics of the infants in each group are displayed in Table 1. The differences in mean GA (−0.3 weeks PMA; 95% CI, −1.6 to 0.9) and birth weight (−46 g; 95% CI, −201 to 110) between infants with and without CME did not differ significantly. However, infants with CME had an imaging session with the greatest CFT at an older age than infants without CME (1.8 weeks PMA; 95% CI, 0.1–3.5; P = 0.04). Although there was no significant difference in gender, race, or eye imaged, a lower proportion of children with CME (3.2%) than without CME (22.7%) were Hispanic, but not to a statistically significant degree (P = 0.07). With very few infants in either group demonstrating severe ROP, there was also no significant difference in maximum ROP stage, plus disease status, or need for laser between infants with and without CME.
Table 1.
Baseline Characteristics of Preterm Infants by Cystoid Macular Edema Status
| Parameter | Cystoid Macular Edema (n = 31) | No Cystoid Macular Edema (n = 22) | P Value |
|---|---|---|---|
| Gestational age (wks), mean (SD) | 25.7 (2.3) | 26.0 (2.3) | 0.60 |
| Birth weight (g), mean (SD) | 813 (263) | 859 (299) | 0.56 |
| PMA at imaging (wks), mean (SD) | 36.3 (3.3) | 34.5 (2.6) | 0.04 |
| Gender, n (%) | 0.17 | ||
| Male | 18 (58) | 8 (36) | |
| Race, n (%) | 0.66 | ||
| White | 15 (48) | 10 (45) | |
| Black | 16 (52) | 11 (50) | |
| Multiracial | 0 (0) | 1 (5) | |
| Ethnicity, n (%) | 0.07 | ||
| Non-Hispanic | 30 (97) | 17 (77) | |
| Hispanic | 1 (3) | 5 (23) | |
| Eye, n (%) | 0.78 | ||
| Right | 11 (35) | 9 (41) | |
| Maximum ROP stage, n (%) | 0.72 | ||
| 0 | 4 (13) | 5 (23) | |
| 1 | 4 (13) | 4 (18) | |
| 2 | 17 (55) | 9 (40) | |
| 3 | 6 (19) | 4 (18) | |
| Maximum plus disease, n (%) | 0.54 | ||
| None | 22 (71) | 16 (73) | |
| Pre-plus disease | 2 (6) | 3 (14) | |
| Plus disease | 7 (23) | 3 (14) | |
| Laser, n (%)* | 1.00 | ||
| Yes | 7 (23) | 5 (26) |
PMA = postmenstrual age; ROP = retinopathy of prematurity; SD = standard deviation.
Laser, if administered, occurred after the imaging visit.
Reliability of Grading of Retinal Morphologic Features
There was 100% exact agreement with a κ value of 1 for presence of CME between the 2 graders. Intergrader intraclass correlation for CFT, INL thickness, and FP thickness ratio were 0.99 (95% CI, 0.98–1.00), 0.99 (95% CI, 0.97–1.00), and 0.96 (95% CI, 0.91–0.98), respectively. There was 90% exact agreement with a κ value of 0.79 (95% CI, 0.53–1.00) for presence of CME for 1 grader at 2 different grading sessions. Intragrader intraclass correlation for CFT, INL thickness, and FP thickness ratio were 0.99 (95% CI, 0.98–1.00), 0.99 (95% CI, 0.98–1.00), and 0.98 (95% CI, 0.95–0.99), respectively.
Retinal Morphologic Features and Bayley Scale Scores
All quantitative measures deemed markers of CME were substantially greater in those infants with CME than in those without CME (P<0.001). The mean ± standard deviation CFT and INL thickness in infants with CME versus those without CME were 417±165 µm versus 185±35 µm and 257±149 µm versus 74±26 µm, respectively. Additionally, the mean ± standard deviation FP thickness ratio of infants with CME versus those without CME was 1.05±0.18 versus 0.71±0.13, respectively.
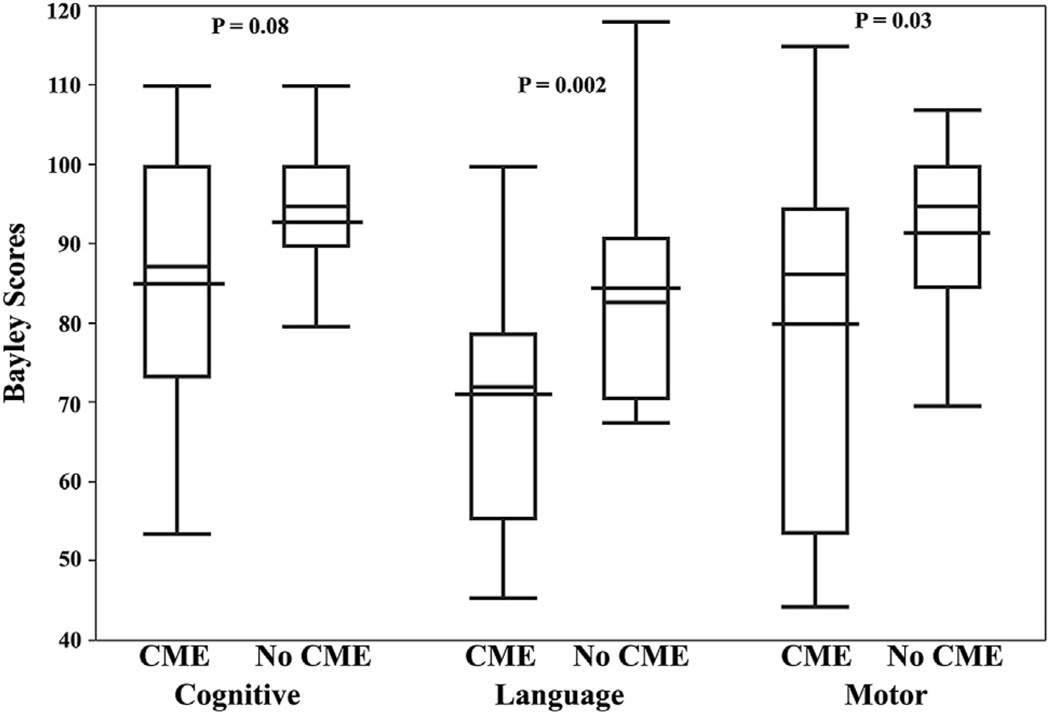
Children with CME had significantly lower language and motor Bayley scores than those without CME (Fig 2). Children with CME also had lower cognitive scores, but not to a statistically significant degree. Compared with children who did not have CME as infants, the mean score for children who had CME was 7.3 points (95% CI, −15.5 to 0.9; P = 0.08) lower on the cognitive subscale, 14.1 points (95% CI, −22.7 to −5.5; P = 0.002) lower for the language subscale, and 11.5 points (95% CI, −21.6 to −1.3; P = 0.03) lower for the motor subscale. Adjusting the differences in Bayley scores between CME groups for GA and birth weight had little impact; mean differences decreased by less than 1 point, whereas P values increased from 0.08 to 0.10 for cognitive scores and 0.03 to 0.04 for motor scores and remained the same at 0.002 for language scores.
Figure 2.
Box-and-whisker plot showing cognitive, language, and motor Bayley Scales of Infant and Toddler Development, Third Edition, scores at 18 to 24 months corrected age for children with cystoid macular edema (CME) compared with those without CME on perinatal spectral-domain optical coherence tomography (SD OCT). The box-and-whisker plots represent the median, quartiles, maximums, and minimums of each Bayley score for each group. The wider black line represents the mean for each group. Language and motor Bayley scores were significantly lower for children with versus without CME on perinatal SD OCT imaging. There was also a trend in lower cognitive Bayley scores for infants with versus without CME on perinatal SD OCT. Relationships were maintained after adjustment for gestational age and birth weight.
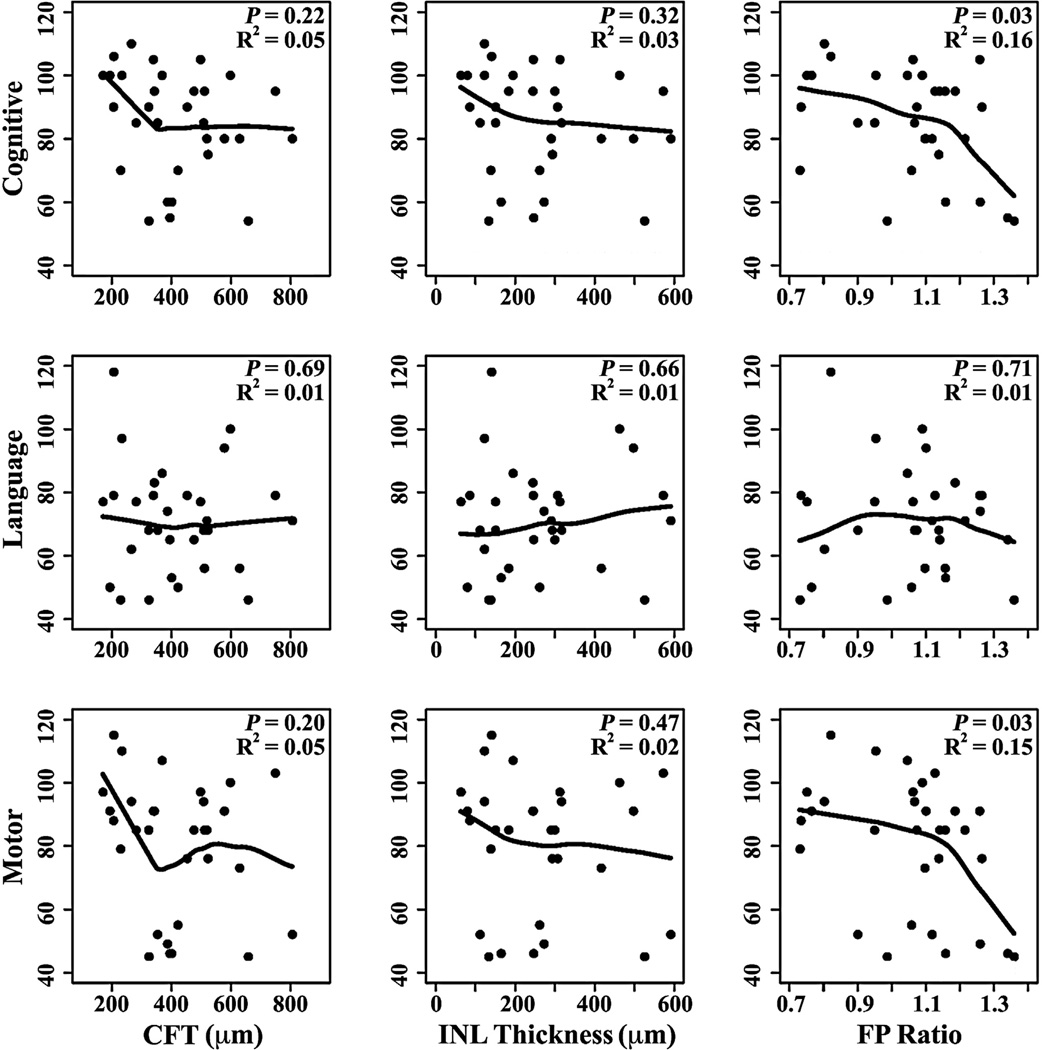
The relationships between severity of CME and Bayley scores were summarized by locally weighted scatterplot smoothing curves within the subgroup of 31 infants with CME (Fig 3). Increasing FP thickness ratio correlated with lower cognitive (R2 = 0.16, P = 0.03) and motor scores (R2 = 0.15, P = 0.03). There was no linear correlation between lower scores and increasing CFT or INL thickness.
Figure 3.
Scatterplots showing correlation between cognitive, language, and motor Bayley Scales of Infant and Toddler Development, Third Edition, scores at 18 to 24 months corrected age and central foveal thickness (CFT), inner nuclear layer (INL) thickness, and foveal-to-parafoveal (FP) thickness ratio measured on spectral-domain optical coherence tomography (SD OCT) in 31 preterm infants with cystoid macular edema (CME). Increased FP thickness ratio on perinatal SD OCT correlated with lower cognitive and motor Bayley scores, suggesting that the severity of CME may relate to the extent of delayed neurodevelopmental outcomes.
Three infants with CME at 34, 38, and 33 weeks PMA on initial SD OCT imaging had resolution of CME on SD OCT after 1, 1, and 2 weeks, respectively; the remaining infants with CME had persistent CME on additional imaging sessions. Infants with CME had CME observed on SD OCT for a mean ± standard deviation of 1.8±1.4 weeks. There was no significant difference in the mean ± standard deviation number of weeks infants were imaged between infants with CME (2.3±1.7 weeks) and without CME (2.1±2.1 weeks; P = 0.73).
Bayley scores also were compared for different measures of ROP severity (Table 2). Cognitive and motor scores were lower with increasing maximum ROP stage (P = 0.04 and P = 0.048, respectively). Motor scores in children with a history of ROP requiring laser were lower than in children who did not require laser, but not to a statistically significant degree (P = 0.06). Finally, there was no significant difference in means for any of the 3 Bayley scores for different categories of maximum plus disease status.
Table 2.
Bayley Scores by Severity of Retinopathy of Prematurity
| Parameter | No. | Cognitive | P Value | Language | P Value | Motor | P Value |
|---|---|---|---|---|---|---|---|
| Maximum ROP stage | 0.04 | 0.10 | 0.048 | ||||
| 0 | 9 | 96 (15) | 84 (17) | 92 (18) | |||
| 1 | 8 | 96 (9) | 84 (20) | 96 (11) | |||
| 2 | 26 | 84 (15) | 74 (12) | 81 (20) | |||
| 3 | 10 | 87 (16) | 75 (22) | 80 (18) | |||
| Maximum plus disease | 0.43 | 0.18 | 0.20 | ||||
| None | 38 | 89 (15) | 79 (15) | 87 (19) | |||
| Pre-plus | 5 | 87 (10) | 77 (17) | 85 (18) | |||
| Plus | 10 | 85 (17) | 71 (21) | 78 (19) | |||
| Laser* | 0.30 | 0.31 | 0.06 | ||||
| No | 41 | 90 (15) | 78 (15) | 88 (18) | |||
| Yes | 12 | 84 (16) | 73 (21) | 76 (21) |
ROP = retinopathy of prematurity.
Data are mean (standard deviation) unless otherwise indicated.
Laser, if administered, occurred after the imaging visit.
Discussion
In this study, we prospectively identified morphologic abnormalities observed in very preterm infants on SD OCT in the intensive care nursery that are associated with future neurodevelopment delays at 18 to 24 months corrected age. Infants with CME on neonatal SD OCT macular scans had statistically significantly lower language and motor Bayley scores as well as lower cognitive scores (P = 0.08) at 18 to 24 months corrected age. Within the macular edema group, severity of macular edema, as assessed by FP thickness ratio, was associated modestly with worse cognitive and motor scores, but not language scores (Fig 3).
Cystoid macular edema in very preterm infants was first reported by Lee et al,10 who compared SD OCT and indirect ophthalmoscopy in 38 infants during routine ROP examinations conducted under standard screening criteria. Thirty-nine percent of the infants had intraretinal cystoid structures observed on SD OCT, whereas none of the traditional ophthalmic examinations masked to imaging data identified this abnormality. They noted that the cystoid structures usually appeared in the INL, rarely involved other retinal layers, and persisted over several imaging sessions. In a population of infants born older and at greater birth weight (mean GA > 30 weeks and birth weight >1240 g), Vinekar et al12 similarly compared indirect ophthalmoscopy and SD OCT in infants with and without ROP. They found CME in 29% of the eyes with stage 2 ROP, but normal retinal morphologic features for age at imaging in all infants with no ROP or stage 1 ROP. They qualitatively characterized the CME based on foveal distortion and whether the cystoid structures were elongated with hyperreflective septae. All eyes with CME demonstrated resolution of edema without treatment when infants were reimaged at 52 weeks PMA.
Maldonado et al11 conducted a thorough analysis of 42 very preterm infants to characterize the CME phenomenon further. They found subclinical CME in 50% of infants, and all infants with bilateral imaging had bilateral CME with highly correlated CFT in both eyes. Central foveal thickness, inner retinal layer thickness, and FP thickness ratio were significantly greater in infants who required laser treatment, had plus disease, or had stage 3 ROP. They also performed an extensive comparison of systemic health factors related to CME and found an association between bronchopulmonary dysplasia and photoreceptor retinal layer thickness. Notably, there was no association between CME measures and Apgar scores, culture-proven sepsis, surgery for necrotizing enterocolitis or patent ductus arteriosus, intraventricular hemorrhage, periventricular leukomalacia, or hydrocephalus.
Dubis et al14 studied 46 preterm infants and found bilateral CME in 56% of infants. Infants with CME had a significantly younger GA. Three infants had resolution of CME observed on repeat imaging. There was no association between maximum ROP stage and presence of CME. In 3 of 8 infants without CME who required laser therapy, CME developed 1 to 2 weeks after retinal laser. Four infants received bevacizumab; 3 infants with CME before treatment did not have resolution of CME after bevacizumab, whereas in 1 infant without CME before treatment, CME developed after injection.
This study demonstrated results consistent with those reported in the literature. This study included 20 very preterm infants included in prior publications by Lee et al10 and Maldonado et al,11 11 of whom had CME on SD OCT. The 58.5% incidence of CME in this study is slightly higher than those previously reported. Vinekar et al12 had a much lower incidence rate at 29%, but the group’s infant population had a greater mean GA and birth weight than in other published data.11,14 The higher incidence in the current study may be explained partially by the possibility that infants without CME did not maintain enrollment in the high-risk clinic because they had lower developmental risk and thus did not undergo Bayley Scales testing at the same rate as infants with CME.19 Three infants with CME in this study showed an absence of CME in subsequent imaging sessions, which is consistent with the paucity of CME resolution during the neonatal period reported in the literature. The relatively lower incidence of CME in the Hispanic infant population has not been noted previously. The lack of association between presence of CME and ROP severity differs from the results of Vinekar et al,12 but is consistent with those of Maldonado et al11 and other invesitgators.14 Although Maldonado et al11 noted a correlation between markers of CME and severity of ROP, the current study, which includes more infant data, did not find this association.
The cause of CME observed in preterm infants remains unknown. One hypothesis, reflected in the relatively high prevalence of CME, is that this may be a common variant of preterm macular development that is only now appreciated with the advent of SD OCT. The associations between quantitative markers of CME and ROP outcomes reported by Maldonado et al11 and the absence of CME in infants with stage 0 or 1 ROP by Vinekar et al12 suggest that VEGF may play a role, perhaps by increasing vascular permeability; these cystoid structures may form from leakage secondary to increased intracapillary hydrostatic pressure. However, reports of CME development after laser and bevacizumab therapy as well as persistence of CME after bevacizumab administration suggest other factors play a role.14 A mechanical theory that suggests traction from the ridge as a cause of these foveal changes seems unlikely because infants in whom stage 3 or greater ROP never developed also have been reported to have CME.12 Although Maldonado et al11 did not find any systemic health factors associated with CME, a case report of an infant with bilateral CME that resolved after liver transplantation resulting from hemochromatosis suggests the cause of CME may be extraophthalmic systemic health factors in some eyes.11,20 Comparisons of retinal layers observed on SD OCT with histopathologic results have been validated in the preterm population; the hyperreflective stranding between the hyporeflective spaces may represent Müller cells whose plasticity protects the retina from damage when there is a fluid overload.21,22 These Müller cells maintain ionic and hydrostatic homeostasis in the retina by upregulating aquaporin and downregulating potassium channels when stressed, leading to water retention.23,24 This study suggests that CME is a pathologic, rather than physiologic, process because it is associated with poorer neurodevelopment outcomes at a later age.
Because the retina and optic nerve are extensions of the CNS, manifestations of neurologic and developmental health may be observed on SD OCT. This study demonstrated a strong association between a retinal phenomenon and future functional outcomes. Tong et al25 demonstrated that optic nerve parameters observed on SD OCT in term equivalent-aged very preterm infants correlated with functional outcomes and brain pathologic features. They found a weak correlation between increased optic nerve cup-to-disc ratio and cognitive Bayley scores at 18 to 24 months corrected age. Infants with periventricular leukomalacia had a larger vertical cup diameter and cup-to-disc ratio, whereas infants with posthemorrhagic hydrocephalus had a shallower optic cup.
Cystoid macular edema in the premature infant occurs during a period of rapid and potentially vulnerable CNS and retinal maturation. Infants examined for ROP are of lower birth weight and GA than the general preterm population, and thus are a particularly susceptible group.26 Some survivors of preterm birth demonstrate cognitive and behavioral deficits at an older age, and the pathologic mechanisms causing these deficits are not well understood.27,28 Recently, diffusion tensor magnetic resonance imaging of the brain has shown microscopic structural changes in infants and adults with a history of premature birth.29–31 Perinatal inflammation may contribute to CNS vulnerability or injury.32–34 Alternately, cerebrovascular immaturity may play a role.27 Cystoid macular edema within the retinal tissue may reflect cellular events that also are occurring in the CNS structures of the brain. Thus, the retina and its microstructure, as CNS tissues, may be sensitive biomarkers for CNS events, whether these events are associated with inflammation or with normal or abnormal development or result from systemic insult.
Retinal cystoid structures have been implicated with neurologic pathologic features in adults. Hyporeflective spaces in the INL have been documented on macular SD OCT of adult patients with optic atrophy secondary to multiple sclerosis and hydrocephalus. These INL microcysts are associated with a younger age as well as thinning of the overlaying nerve fiber layer and ganglion cell layer.35 Patients with multiple sclerosis who demonstrated these cystoid structures on SD OCT had greater disease progression, lower visual acuity, and thinner retinal nerve fiber layer than those without the cystoid spaces.36 Because of lack of fluorescein angiographic leakage in these cases, the cystoid spaces may be the result of ganglion cell loss and Müller cell degeneration or a retrograde cellular degeneration.37 They differ from CME observed in neonates because the cystoid structures in neonates are symmetrically centered on the macula (Fig 1B). Children with a history of preterm birth and ROP have not demonstrated any cystoid structures on SD OCT of the macula at school age.38–40 Thus, although CME in preterm infants seems to be associated negatively with neurologic health, the pronounced location centered on the fovea, the severity of the edema, and the presumed resolution by school age differ notably from the presumed degeneration reported in multiple sclerosis. However, school-aged children with a history of ROP do have thinner retinal nerve fiber layer, and a future analysis of retinal nerve fiber layer thickness in infants and older children with a history of CME could help to elucidate whether CME in preterm infants is mechanistically related to the cystoid structures observed in optic atrophy.41
The Bayley Scales of Infant and Toddler Development, Third Edition, is a well-accepted standardized assessment tool for neurodevelopment in young children.42 The Bayley scores obtained in the current study seem lower than others reported in the literature in similar populations. Pineda et al43 report mean ± standard deviation cognitive, language, and motor Bayley scores at 2 years corrected age for 86 very preterm infants of 86±9, 88±11, and 83±11, respectively. Another study that included 1477 infants born at less than 27 weeks PMA had cognitive and language Bayley scores of 90±15 and 85±17, respectively.44 Discrepancies in Bayley scores between studies may reflect the higher developmental risk of our infants, who were born extremely prematurely, admitted to our intensive care nursery, and then referred to the intensive care nursery developmental follow-up clinic, rather than transferred to a community hospital for preparation and transition to home.
Previous work by Msall et al16 demonstrated that ROP severity is associated with functional disability. They followed up with infants from the Cryotherapy for Retinopathy of Prematurity Study and evaluated functional skill with the Functional Independence Measure for Children at 5.5 years and found that a history of threshold ROP was a greater risk factor for severe disability than all other demographic parameters except an abnormal neurologic assessment. When these children were 8 years of age, a history of threshold ROP was found to be associated with developmental, educational, and social challenges. Even after stratifying the children by vision, those children with better vision still demonstrated substantial rates of functional delays.17 These findings suggest that there are neurologic deficits associated with ROP separate from visual acuity. In addition, Allred et al34 found children with severe ROP were more likely to have scores on the second edition of Bayley Scales that were 2 to 3 standard deviations less than the expected mean score. They believe the preterm infant lacks growth factors, such as insulin-like growth factor 1, normally supplied by the mother in utero that protect against both retinal and brain damage. This study, even with a relatively small sample size, found a correlation between severity of ROP and Bayley scores (Table 2). Increasing ROP stage correlated with worsening mean cognitive and language Bayley scores, and infants who required laser therapy had lower mean motor Bayley scores. Additionally, all indicators of ROP severity demonstrated a general decrease in each mean Bayley score as ROP worsened. This should be evaluated further in a future study with a larger sample size in which Bayley scores are captured across all children according to a standardized protocol.
There are several limitations to this study. The sample size was limited by the number of infants who were assessed with Bayley Scales at 18 to 24 months corrected age. This may introduce bias toward lower Bayley scores because children with less pronounced development delays may be less likely to return for follow-up care at the Duke Special Infant Care Clinic.19 This may be reflected in trend toward lower GA of children who underwent Bayley scores assessment than those who did not undergo formal assessment. However, the associations between CME and Bayley scores did not change when adjusting for demographic parameters, and there was no difference in CME or CME severity between these 2 groups. In addition, the size of the 2 groups may explain why there were weaker coefficients of determination despite strong P values when comparing severity of FP thickness ratio with cognitive and motor scores. Only very preterm infants eligible for ROP screening were included in this study; therefore, the prevalence of CME and its potential relationship with functional outcomes in the preterm population as a whole remains unknown. Because infants underwent research imaging only when clinically stable during a routine ophthalmic examination, there were a variable number of weeks between imaging sessions, leading to all intervals reported as weeks and not number of imaging sessions. Although all SD OCT scans were gradable, image quality varied by infant and imaging session. However, the high intragrader and intergrader reproducibility suggests that the SD OCT grading system is valid. Fluctuations in retinal thicknesses and FP thickness ratio throughout the day or in relation to changes in the infant (position, blood pressure, fluid status, etc.) remain unknown. All imaging sessions were reviewed to determine the largest measures of CME; however, each imaging session still is only a snapshot of CME status at the time of SD OCT capture. The variable relationship between severity of CME and Bayley scores may be clarified with greater statistical power. Although Maldonado et al11 established no association between CME and a battery of systemic health factors, there may be an unidentified confounding health condition that is responsible for lower Bayley scores in infants with CME. Thus, all associations between CME and Bayley scores should be emphasized as correlations because of an unidentified, underlying cause. Although visual acuity was not known and may have affected Bayley scores, Vinekar et al12 demonstrated that CME was transient and resolved in all infants with followup at 52 weeks PMA. In addition, children with known visual impairment do not undergo Bayley Scales assessment and all of the children who completed the test had functional vision. Although it seems unlikely that CME interferes with maturation of the visual system during this brief time, further research is required to understand the role of CME during this dynamic period of vision development.
Spectral-domain optical coherence tomography allows for noninvasive, cross-sectional assessment of retinal health in preterm infants. We found that CME observed on SD OCT in very preterm infants while still in the intensive care nursery correlates with poorer neurodevelopment outcomes. A prospective, multicenter, observational study that includes neonatal SD OCT eye imaging with subsequent visual acuity and magnetic resonance imaging analysis as well as more consistent neurodevelopmental follow-up would clarify the usefulness of monitoring the neurologic and systemic health of neonates with SD OCT of the retina and optic nerve and would help to clarify the role of ROP and vascular endothelial growth factor dysregulation in CME formation and resolution. Other anatomic indicators of neurosensory tissue development and health such as retinal nerve fiber layer thickness and photoreceptor maturity also could be investigated. Thus, additional research is required before incorporating these findings into the clinical practice of predicting functional outcomes based on SD OCT imaging and gaining a better understanding of any linkage of mechanisms of maldevelopment in the eyes and brains of very preterm infants.
Acknowledgment
The authors thank Ramiro S. Maldonado, MD, for assistance in imaging infants.
Supported in part by The Hartwell Foundation; The Andrew Family Charitable Foundation; Research to Prevent Blindness, Inc, New York, New York; the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research, Bethesda, Maryland (grant no.: 1UL1RR024128-01); and the National Eye Institute (NEI), NIH (grant no.: P30 EY001583). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR, NEI, or NIH. The sponsors or funding organizations had no role in the design or conduct of this research.
Abbreviations and Acronyms
- CFT
central foveal thickness
- CI
confidence interval
- CME
cystoid macular edema
- CNS
central nervous system
- FP
foveal-to-parafoveal
- GA
gestational age
- INL
inner nuclear layer
- PMA
postmenstrual age
- ROP
retinopathy of prematurity
- SD OCT
spectral-domain optical coherence tomography
Footnotes
Financial Disclosure(s):
The author(s) have made the following disclosure(s): C.A.T.: Financial support – Bioptigen Inc., Research Triangle Park, NC, and Genentech Inc., San Francisco, CA; Royalties – Alcon Inc., Fort Worth, TX; unlicensed patents pending in OCT imaging and analysis.
References
- 1.Chong GT, Farsiu S, Freedman SF, et al. Abnormal foveal morphology in ocular albinism imaged with spectral-domain optical coherence tomography. Arch Ophthalmol. 2009;127:37–44. doi: 10.1001/archophthalmol.2008.550. [DOI] [PubMed] [Google Scholar]
- 2.Maldonado RS, Izatt JA, Sarin N, et al. Optimizing hand-held spectral domain optical coherence tomography imaging for neonates, infants, and children. Invest Ophthalmol Vis Sci. 2010;51:2678–2685. doi: 10.1167/iovs.09-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott AW, Farsiu S, Enyedi LB, et al. Imaging the infant retina with a hand-held spectral-domain optical coherence tomography device. Am J Ophthalmol. 2009;147:364–373. doi: 10.1016/j.ajo.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Chavala SH, Farsiu S, Maldonado R, et al. Insights into advanced retinopathy of prematurity using handheld spectral domain optical coherence tomography imaging. Ophthalmology. 2009;116:2448–2456. doi: 10.1016/j.ophtha.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothman AL, Folgar FA, Tong AY, Toth CA. Spectral domain optical coherence tomography characterization of pediatric epiretinal membranes. Retina. 2014;34:1323–1334. doi: 10.1097/IAE.0000000000000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day S, Maldonado RS, Toth CA. Preretinal and intraretinal exudates in familial exudative vitreoretinopathy. Retina. 2011;31:190–191. doi: 10.1097/IAE.0b013e3182019c04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maldonado RS, Yuan E, Tran-Viet D, et al. Three-dimensional assessment of vascular and perivascular characteristics in subjects with retinopathy of prematurity. Ophthalmology. 2014;121:1289–1296. doi: 10.1016/j.ophtha.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maldonado RS, Toth CA. Optical coherence tomography in retinopathy of prematurity: looking beyond the vessels. Clin Perinatol. 2013;40:271–296. doi: 10.1016/j.clp.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreno TA, O’Connell RV, Chiu SJ, et al. Choroid development and feasibility of choroidal imaging in the preterm and term infants utilizing SD-OCT. Invest Ophthalmol Vis Sci. 2013;54:4140–4147. doi: 10.1167/iovs.12-11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee AC, Maldonado RS, Sarin N, et al. Macular features from spectral-domain optical coherence tomography as an adjunct to indirect ophthalmoscopy in retinopathy of prematurity. Retina. 2011;31:1470–1482. doi: 10.1097/IAE.0b013e31821dfa6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maldonado RS, O’Connell R, Ascher SB, et al. Spectral-domain optical coherence tomographic assessment of severity of cystoid macular edema in retinopathy of prematurity. Arch Ophthalmol. 2012;130:569–578. doi: 10.1001/archopthalmol.2011.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vinekar A, Avadhani K, Sivakumar M, et al. Understanding clinically undetected macular changes in early retinopathy of prematurity on spectral domain optical coherence tomography. Invest Ophthalmol Visual Sci. 2011;52:5183–5188. doi: 10.1167/iovs.10-7155. [DOI] [PubMed] [Google Scholar]
- 13.Maldonado RS, O’Connell RV, Sarin N, et al. Dynamics of human foveal development after premature birth. Ophthalmology. 2011;118:2315–2325. doi: 10.1016/j.ophtha.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubis AM, Subramaniam CD, Godara P, et al. Subclinical macular findings in infants screened for retinopathy of prematurity with spectral-domain optical coherence tomography. Ophthalmology. 2013;120:1665–1671. doi: 10.1016/j.ophtha.2013.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt B, Davis PG, Asztalos EV, et al. Association between severe retinopathy of prematurity and nonvisual disabilities at age 5 years. JAMA. 2014;311:523–525. doi: 10.1001/jama.2013.282153. [DOI] [PubMed] [Google Scholar]
- 16.Msall ME, Phelps DL, DiGaudio KM, et al. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Severity of neonatal retinopathy of prematurity is predictive of neurodevelopmental functional outcome at age 5.5 years. Pediatrics. 2000;106:998–1005. doi: 10.1542/peds.106.5.998. [DOI] [PubMed] [Google Scholar]
- 17.Msall ME, Phelps DL, Hardy RJ, et al. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Educational and social competencies at 8 years in children with threshold retinopathy of prematurity in the CRYO-ROP multicenter study. Pediatrics. 2004;113:790–799. doi: 10.1542/peds.113.4.790. [DOI] [PubMed] [Google Scholar]
- 18.American Academy of Pediatrics Section on Ophthalmology, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus, American Association of Certified Orthoptists. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013;131:189–195. doi: 10.1542/peds.2012-2996. [DOI] [PubMed] [Google Scholar]
- 19.Castro L, Yolton K, Haberman B, et al. Bias in reported neurodevelopmental outcomes among extremely low birth weight survivors. Pediatrics. 2004;114:404–410. doi: 10.1542/peds.114.2.404. [DOI] [PubMed] [Google Scholar]
- 20.Maldonado RS, Freedman SF, Cotten CM, et al. Reversible retinal edema in an infant with neonatal hemochromatosis and liver failure. J AAPOS. 2011;15:91–93. doi: 10.1016/j.jaapos.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vajzovic L, Hendrickson AE, O’Connell RV, et al. Maturation of the human fovea: correlation of spectral-domain optical coherence tomography findings with histology. Am J Ophthalmol. 2012;154:779–789. doi: 10.1016/j.ajo.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hendrickson A, Possin D, Vajzovic L, Toth CA. Histologic development of the human fovea from midgestation to maturity. Am J Ophthalmol. 2012;154:767–778. doi: 10.1016/j.ajo.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhargava P, Calabresi PA. The expanding spectrum of aetiologies causing retinal microcystic macular change. Brain. 2013;136:3212–3214. doi: 10.1093/brain/awt295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bringmann A, Reichenbach A, Wiedemann P. Pathomechanisms of cystoid macular edema. Ophthalmic Res. 2004;36:241–249. doi: 10.1159/000081203. [DOI] [PubMed] [Google Scholar]
- 25.Tong AY, El-Dairi M, Maldonado RS, et al. Evaluation of optic nerve development in preterm and term infants using handheld spectral-domain optical coherence tomography. Ophthalmology. 2014;121:1818–1826. doi: 10.1016/j.ophtha.2014.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kidokoro H, Anderson PJ, Doyle LW, et al. Brain injury and altered brain growth in preterm infants: predictors and prognosis. Pediatrics. 2014;134:e444–e453. doi: 10.1542/peds.2013-2336. [report online] [DOI] [PubMed] [Google Scholar]
- 27.Back SA, Riddle A, McClure MM. Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke. 2007;38:724–730. doi: 10.1161/01.STR.0000254729.27386.05. [DOI] [PubMed] [Google Scholar]
- 28.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwata S, Nakamura T, Hizume E, et al. Qualitative brain MRI at term and cognitive outcomes at 9 years after very preterm birth. Pediatrics. 2012;129:e1138–e1147. doi: 10.1542/peds.2011-1735. [report online] [DOI] [PubMed] [Google Scholar]
- 30.Allin MP, Kontis D, Walshe M, et al. White matter and cognition in adults who were born preterm. [Accessed September 14, 2014];PloS One. 2011 6:e24525. doi: 10.1371/journal.pone.0024525. [serial online] Available at: http://dx.plos.org/10.1371/journal.pone.0024525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skiold B, Vollmer B, Bohm B, et al. Neonatal magnetic resonance imaging and outcome at age 30 months in extremely preterm infants. J Pediatr. 2012;160:559–566. doi: 10.1016/j.jpeds.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 32.Van Steenwinckel J, Schang AL, Sigaut S, et al. Brain damage of the preterm infant: new insights into the role of inflammation. Biochem Soc Trans. 2014;42:557–563. doi: 10.1042/BST20130284. [DOI] [PubMed] [Google Scholar]
- 33.Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: implications for neurologic and neuropsychiatric disease in children and adults. Ann Neurol. 2012;71:444–457. doi: 10.1002/ana.22620. [DOI] [PubMed] [Google Scholar]
- 34.Allred EN, Capone A, Jr, Fraioli A, et al. Retinopathy of prematurity and brain damage in the very preterm newborn. J AAPOS. 2014;18:241–247. doi: 10.1016/j.jaapos.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abegg M, Dysli M, Wolf S, et al. Microcystic macular edema: retrograde maculopathy caused by optic neuropathy. Ophthalmology. 2014;121:142–149. doi: 10.1016/j.ophtha.2013.08.045. [DOI] [PubMed] [Google Scholar]
- 36.Gelfand JM, Nolan R, Schwartz DM, et al. Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain. 2012;135:1786–1793. doi: 10.1093/brain/aws098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolff B, Basdekidou C, Vasseur V, et al. Retinal inner nuclear layer microcystic changes in optic nerve atrophy: a novel spectral-domain OCT finding. Retina. 2013;33:2133–2138. doi: 10.1097/IAE.0b013e31828e68d0. [DOI] [PubMed] [Google Scholar]
- 38.Park KA, Oh SY. Analysis of spectral-domain optical coherence tomography in preterm children: retinal layer thickness and choroidal thickness profiles. Invest Ophthalmol Vis Science. 2012;53:7201–7207. doi: 10.1167/iovs.12-10599. [DOI] [PubMed] [Google Scholar]
- 39.Ecsedy M, Szamosi A, Karko C, et al. A comparison of macular structure imaged by optical coherence tomography in preterm and full-term children. Invest Ophthalmol Visual Sci. 2007;48:5207–5211. doi: 10.1167/iovs.06-1199. [DOI] [PubMed] [Google Scholar]
- 40.Recchia FM, Recchia CC. Foveal dysplasia evident by optical coherence tomography in patients with a history of retinopathy of prematurity. Retina. 2007;27:1221–1226. doi: 10.1097/IAE.0b013e318068de2e. [DOI] [PubMed] [Google Scholar]
- 41.Akerblom H, Holmstrom G, Eriksson U, Larsson E. Retinal nerve fibre layer thickness in school-aged prematurely-born children compared to children born at term. Br J Ophthalmol. 2012;96:956–960. doi: 10.1136/bjophthalmol-2011-301010. [DOI] [PubMed] [Google Scholar]
- 42.Albers CA, Grieve AJ. Test review: Bayley, N. (2006). Bayley Scales of Infant and Toddler Development—Third Edition. San Antonio, TX: Harcourt Assessment. J Psychoeduc Assess. 2007;25:180–190. [Google Scholar]
- 43.Pineda RG, Neil J, Dierker D, et al. Alterations in brain structure and neurodevelopmental outcome in preterm infants hospitalized in different neonatal intensive care unit environments. J Pediatr. 2014;164:52–60. doi: 10.1016/j.jpeds.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adams-Chapman I, Bann CM, Vaucher YE, Stoll BJ Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) Association between feeding difficulties and language delay in preterm infants using Bayley Scales of Infant Development-Third Edition. J Pediatr. 2013;163:680–685. doi: 10.1016/j.jpeds.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]