Abstract
Kv1.1 subunits of low voltage-activated (Kv) potassium channels are encoded by the Kcna1 gene and crucially determine the synaptic integration window to control the number and temporal precision of action potentials in the auditory brainstem of mammals and birds. Prior electrophysiological studies showed that auditory signaling is compromised in monaural as well as in binaural neurons of the auditory brainstem in Kv1.1 knockout mice (Kcna1−/−). Here we examine the behavioral effects of Kcna1 deletion on sensory tasks dependent on either binaural processing (detecting the movement of a sound source across the azimuth), monaural processing (detecting a gap in noise), as well as binaural summation of the acoustic startle reflex (ASR). Hearing thresholds measured by auditory brainstem responses (ABR) do not differ between genotypes, but our data show a much stronger performance of wild type mice (+/+) in each test during binaural hearing which was lost by temporarily inducing a unilateral hearing loss (through short term blocking of one ear) thus remarkably, leaving no significant difference between binaural and monaural hearing in Kcna1−/− mice. These data suggest that the behavioral effect of Kv1.1 deletion is primarily to impede binaural integration and thus to mimic monaural hearing.
Keywords: Kv1.1, potassium channels, prepulse inhibition, acoustic startle reflex, sound localization, gap-detection
1. INTRODUCTION
The auditory nerve and many auditory brainstem neurons express Kv1 ion channels, comprised of heterogeneous tetramers that include the low-threshold potassium channel subunit Kv1.1, encoded by the Kcna1 gene (Adamson et al., 2002; Grigg et al., 2000; Rosenberger et al., 2003). Kv1.1 containing channels in cell membranes serve to limit the duration and amplitude of perturbations in the resting potential and thus to maintain brief integration windows for coincident inputs (Klug et al., 2006; Mathews et al., 2010; Oertel et al., 2000). The importance of Kv1.1 for maintaining neural function in auditory processing is apparent in in-vitro and in-vivo single-unit electrophysiological studies of Kcna1−/− mice, in which first, the firing pattern of monaural neurons in the medial nucleus of the trapezoid body are no longer synchronous with their acoustic inputs (Gittelman and Tempel, 2006; Kopp-Scheinpflug et al., 2003); second, binaural neurons in the lateral nucleus of the superior olivary complex are less sensitive to interaural level differences (Karcz et al., 2011); and third; coincidence windows in the midbrain inferior colliculus are less precise (Karcz et al., 2012). These data suggest the hypothesis that a major contribution of Kv1.1 to neural processing is to refine the temporal integration of coincident inputs in both monaural and binaural auditory pathways. The functional significance of these electrophysiological results that were obtained in slice preparations or in anesthetized mice is demonstrated by behavioral experiments showing that Kcna1−/− mice are less sensitive to sounds moving from one spatial position to another across the frontal azimuth compared to their wild type littermates (Allen et al., 2012).
A series of different point mutations in the KCNA1 gene causes episodic ataxia type 1 (EA1) in humans, a neurological disorder of autosomal dominant inheritance (Heeroma et al., 2009; Herson et al., 2003; Imbrici et al., 2008; Rajakulendran et al., 2007; Zuberi et al., 1999). Recent studies of families with the EA1 phenotype report the presence of hearing loss and suggests a link between Kv1.1 channel malfunction and hearing impairment (Strupp, 2013; Tomlinson et al., 2013). Given the known importance for Kv1.1 for coincidence detection in the auditory system, it is conceivable that Kv1.1 channels are necessary for encoding the temporal fine structure of acoustic signals which is especially important for binaural hearing. Although Kv1.1 containing channels are strongly expressed in fast firing neurons of the auditory periphery and lower brainstem, temporal processing deficits caused by malfunctioning of these channels are likely to decrease a listener's ability to combine information from the two: for example, inaccuracies in perceptual grouping based on incorrect temporal integration of auditory signals have been described for other forms of ataxia (Rance et al., 2008; Rance et al., 2012) and are likely to apply to all forms of neural integration that rely on the synchrony or temporal precision of the incoming signals.
In the present study we test whether the lack of Kv1.1 channel subunits can account for perceptual deficits similar to those acquired by the loss of binaural hearing. Therefore in our experiments we structured “monaural” and “binaural” processing in two ways, first by comparing performance in a task that is primarily dependent on binaural processing (behavioral sensitivity to changes in sound location) with one primarily dependent on monaural processing (gap detection); and second, by comparing performance of mice with normal binaural hearing to the same mice with monaural hearing, produced by inserting a foam plug into one ear. The degree of attenuation in thresholds across the frequency spectrum was assessed by the ABR audiogram.
2. METHODS
2.1 Animals
The C3HeB/FeJ-Kcna1tm1Tem mice were bred in the University of Rochester vivarium from heterozygotic (+/−) breeding pairs obtained from Jackson Laboratory (Bar Harbor, ME). Animal husbandry and genotyping procedures are described in Allen and Ison (2012). A total of 36 mice began testing: 21 Kcna1+/+ (10 male, 11 female) and 15 Kcna1−/− (6 male, 9 female). Testing began at about 4 weeks of age and ended 7 to 10 days later. Body weight in +/+ mice increased from 15.4 ±0.5 g to 20.4 ±2.1 g and in −/− mice from 12.2 ±0.9g to 13.8 ±0.9 g. The University of Rochester Committee on Animal Resources approved all procedures.
2.2 Apparatus and Procedures
The experimental procedures for assessing sensory processing were based on “Reflex Modification Audiometry” as described by Young and Fechter (1983). The method depends on the common observation that even at near threshold levels, preliminary stimuli that precede the elicitation of the acoustic startle reflex (ASR) at an appropriate brief lead time modify the expression of the motor response. The behavioral apparatus and procedures used here are detailed in Allen and Ison (2012) and in Allen et al. (2008). The experiment was conducted within a large (2.7m long, 1.8m wide, 2.4 m high) sound-attenuating room (Industrial Acoustics; IAC Inc.) with echo-attenuating Sonex acoustical foam lining the walls. The eliciting stimulus (ES) was generated using a Tucker-Davis Technology (TDT, Alachua, FL) RP2.1 Real-time Processor amplified with an Adcom (East Brunswick, NJ) GFA-535 II amplifier and broadcast via a Yamaha JA4281B compression tweeter. This ES stimulus was a 20 ms noise burst (120 dB SPL). The carrier stimulus for spatial sound localization and gap detection was a wide band noise (70 dB SPL) digitally generated using a second RP2 and broadcast from one of two matched TDT-ES1 electrostatic speakers located 50 cm from the mouse's head in the azimuth. The two electrostatic speakers were matched over the frequency range of the white noise, 1-50kHz. Sound levels were measured with a 1/4″ B&K model 4135 microphone.
The test cage was mounted at the center of a semicircle of 50 cm radius, with the carrier speakers placed on the semicircle with angular separations of 120° or 90° for spatial testing, or one speaker centered in front for the gap experiment. For spatial experiments the cage was either oriented so that the mouse faced the mid-line between the two speakers placed left and right (front position) or was rotated 90° and the speakers were thus located front and back directly to the left of the mouse (side position). For gap detection a single speaker placed in the midline position along the semicircle provided the wide band noise carrier for the gaps. The force of the ASR was transduced by an accelerometer and its amplitude was scored as the integrated RMS voltage of this signal for 100 ms after the delivery of the ES. The experimental stimuli were controlled and the responses recorded by a PC using a custom LabView (National Instruments) front-end. The intertrial interval, during which the background noise was continuously present, averaged 20 s and was randomly selected from the range 15 to 25 s. Due to this relatively long intertrial intervals and rests between test days in our experiments we found little systematic habituation of the ASR or of PPI.
2.3 Ear plugging procedure and ABR validation
Earplugs were inserted under ketamine/xylazine anesthesia (120 and 10 mg/kg body weight, respectively) at the time of the ABR measurements. The earplugs consisted of small pieces cut from an “E.A.R. Classic” human foam earplug (Etymotic, Chicago, IL), compressed and inserted into the right external auditory meatus and sealed with Vetbond tissue adhesive. In addition, a polyvinyl siloxane ear impression material (Ready-Press; Microsonic®) was mixed to cover the external auditory meatus and fill the concha of the outer ear. Our experience was that the plug lasted for on average about 7 days with a range of 3 to 12 days; this limited the duration of behavioral testing in some mice. For the ABR measures the left meatus was temporarily filled with a similar earplug but not sealed. The free field ABR methods followed Allen and Ison (2012). In brief, the mice were anesthetized with a mixture of ketamine/xylazine (see above) and tested twice in order to determine the degree of sound attenuation across spectral frequencies provided by the insertion of the earplugs. The mouse was kept warm during the procedure with a circulating-water heating pad. Miniature subdermal needle electrodes (Nicolet) were inserted at the vertex (reference), over the bulla (active), and just above the hind limb (ground). Calibrated tone bursts (5 ms duration, 0.5 ms rise–fall time, phase alternating 90 deg) were synthesized with SigGenRP software on a TDT RP2.1 real-time processor and presented from a lateral speaker aligned with the right ear at a rate of 29/s at frequencies of 3, 6, 12, 24, 32, and 36 kHz. ABR waveforms were recorded using a TDT RA4LI low-impedance digital headstage and RA4PA Medusa preamplifier controlled by a RA16 digital signal processor, and averaged in response to 300 tone bursts (150 x 2 replicates) at each tested frequency/amplitude combination. At each frequency, the amplitude of the signal was attenuated (PA5; TDT) in 5 dB steps from 80 dB SPL until the two average ABR waveforms obtained from 150 repetitions each were no longer distinguishable by eye from the background. The traces were recorded with filter settings of 100 to 3,000 Hz. By convention, threshold was established as the response at 5 dB above this final level.
2.4 Behavioral Procedures
Two behavioral test protocols were used, first to measure sensitivity for a gap in noise (detailed in Allen et al., 2008) and second, to measure sensitivity to a change in sound location as detailed in Allen and Ison 2012). For the spatial experiment a prepulse trial began with a continuous noise from one speaker that lasted for 15 to 25 s, with its offset coincident with noise onset in the second speaker. For the gap experiment the prepulse trials also began with a continuous noise but there was a 10 ms quiet period in the noise before the noise returned to the same speaker. For the control trials the ES was presented from an overhead position at the normal time (15-20 s) after the beginning of the trial, with no prior change in position or no gap. There were two rounds of behavioral testing, the second round changing in detail to reflect the transitory effect of the plug on hearing. The first round of tests included 8 spatial tests comprised of 4 spatial separations from typically 45 to 120 degrees, each of these presented in the frontal field or in the lateral field (for example, starting with a speaker at 45o to the right of center of the mouse and moving from right to left across the midline prior to the ES; or starting at 45o in back of the imaginary line through the auditory meatus and then moving from the behind to ahead of the mouse). These spatial conditions were given in separate sessions which consisted of 21 blocks of 6 trials, two control trials (with no prior switch in the speaker location) and four prepulse trials with the switch preceding the startle stimulus by 7.5, 15, 30, or 60 ms, lasting for approximately 40 minutes. In the second round of tests the spatial protocol was provided in just two tests at 90 degrees and 120 degrees, with a constant lead time of the switch before the ES by a constant 20 ms. These tests required just 20 minutes. In both rounds of experiments the gap experiment preceded each spatial test. For the gap experiment the background noise was always presented from a frontal speaker and the prepulse was a 10 ms gap in the noise beginning either 10 ms or 60 ms before the ES. Each test day usually consisted of two different spatial conditions and two repetitions of the gap experiment. One set of tests preceded the insertion of the ear plug. Following ear plugging procedure each mouse was again tested in both experiments (gap detection and speaker swap). The first binaural series of tests preceded the insertion of the ear plug, and the second set of monaural tests began after a two day recovery from this procedure. The second round of tests was completed within 4-5 days after the ear plugging procedure.
2.5 Data Analysis
The median of the ASR and ACT (measure of “restless activity”) values for each condition was calculated for each mouse on each test after excluding the first block of trials to avoid the effects of the novelty of the ES. ASR amplitudes are given in arbitrary voltage units (aV-units), the RMS of the accelerometer output for 100 ms after the ES. ACT values were provided by the RMS of the accelerometer output over a 100 ms period just before the onset of either the prepulse or the ES on control trials. The prepulse inhibition scores (PPI) were calculated as a ratio of each subject's median response amplitude for the appropriate prestimulus conditions (ASRp) compared with the no-prepulse control baseline (ASRc), using the formula PPI=1–[ASRp/ASRc] and then expressed in percent PPI. A precaution in interpreting the strength of PPI as a measure of subjective intensity or salience of the prepulse is that the control ASR should be sufficiently greater than the level of spontaneous activity to avoid a “ceiling effect” on the PPI level. In the present experiments the maximum possible PPI ceiling was (mean/SD) 0.90 ±0.06 in the +/+ mice and 0.80 ±0.08 in the −/− mice for the binaural condition. For the monaural condition with its lower startle levels, the PPI ceilings were 0.71 ±0.15 in the +/+ mice and 0.68 ±0.12. The obtained levels of PPI never approached these possible maximum limits.
The analysis of the data was accomplished primarily with ANOVA (SPSS), using as within-Subject(repeated) variables the binaural vs. monaural conditions, and variously front vs. side presentations in the spatial experiment (after summing over the 90° and 120° which were not different within a genotype). The between-Subject variable was Kcna1+/+ vs. −/−. The within-Subject p-values provided by the ANOVA were adjusted via the Hunyh-Feldt method for non-homogeneity of between-cell correlations. Effect sizes for the ANOVA were determined by SPSS partial-Eta-squared measures (ηp2), and follow-up t-tests (Pearson or Welch's as appropriate) based on Graphpad Prism with effect size given by R2.
3. RESULTS
The focus of this study was to determine the role of Kv1.1 in monaural vs. binaural auditory processing by combining three experimental strategies: i) Kcna1+/+ and Kcna1−/− mice were temporarily monauralized by plugging one ear; ii) Kcna1+/+ and Kcna1−/− mice had to perform tasks based on monaural and binaural cues and iii) Kcna1+/+ and Kcna1−/− mice were solving the binaural tasks either in the frontal hemifield or they were rotated by 90° and were now solving the tasks in the lateral hemifield.
3.1 Inserting ear plugs resulted in elevated ABR thresholds with no difference between genotypes
Hearing thresholds were assessed by auditory brainstem recordings for Kcna1+/+ and Kcna1−/− mice before and immediately after ear plugging using auditory brainstem responses (ABR; Fig. 1a, b). Binaural mice showed no genotype specific differences in thresholds across all tested frequencies (Fig. 1c). The increase in ABR thresholds produced by the ear plugging procedure accelerated from 4.8 ±8.5dB at 3 kHz; to 11.8 ±9.1dB at 6 kHz, and up to 35.5 ±7.7dB for the higher frequencies (mean/±SD). The hearing sensitivity in small headed mammals like mice for frequencies below 3 kHz is reportedly low (Heffner et al., 2003), so that the less effective earplug at these low frequencies can be disregarded.
Figure 1. ABR thresholds are equally elevated in Kcna1+/+ and Kcna1−/− mice after plugging one ear.
A, B) Averaged ABR waveforms in response to 12kHz/80dB SPL tone stimulation in A) Kcna1+/+ mice and B) Kcna1−/− mice before (black) and after (red) ear plugging procedure. C) Mean (±SEM) ABR thresholds for each tested frequency in Kcna1+/+ (closed symbols, solid lines) and Kcna1−/− mice (open symbols, broken lines) before (black) and after (red) ear plugging procedure. D) Both genotypes showed a 60-70% loss in threshold sensitivity.
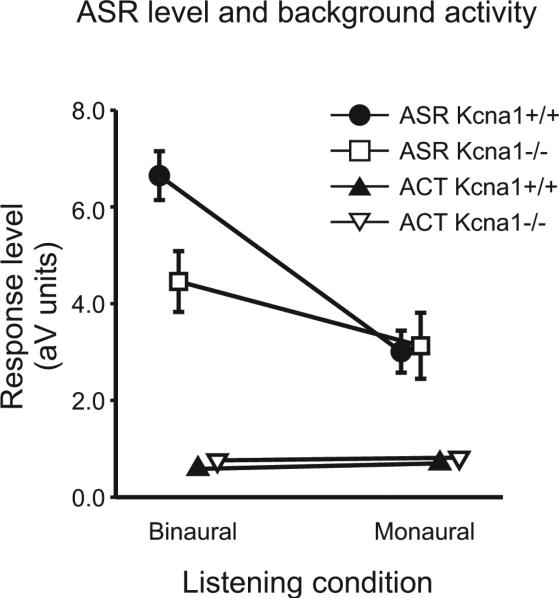
3.2 The Acoustic Startle Reflex was more vigorous for binaural listening in Kcna1+/+ but not Kcna1−/− mice
The acoustic startle reflex (ASR) is a short latency reflex elicited by a sudden, loud sound and mediated via a short sensory-motor pathway integrating neural signals from both sides of the brain. Shown in Figure 2, the stimulus-elicited ASR amplitude was significantly lower in the −/− compared to the +/+ mice when tested in the binaural condition (by 49%; p=0.003), while in the monaural condition both genotypes were nearly identical (just 4% apart). Figure 2 also shows that the ASR amplitudes were significantly larger than the background levels of restless activity (ACT) in each group and hearing condition (all p≤0.001). A significant difference between the ASR and ACT measures verified that the startle eliciting stimulus (ES) had successfully triggered the startle response in every condition and in each group of mice ([.Delta]+/+: 6.46, n=19; p≤0.001; Δ−/−: 3.07, n=12; p≤0.01).
Figure 2. Amplitudes of the acoustic startle reflex (ASR) are decreased in monaural wild types as well as in monaural and binaural Kcna1 mice.
ASR amplitudes (squares and circles) and restless activity (ACT) scores (triangles) for Kcna1+/+ (black symbols; n=19) and Kcna1−/− mice (white symbols; n=12), with normal hearing (left) and with one ear plugged (right). All data are given as Mean (±SEM).
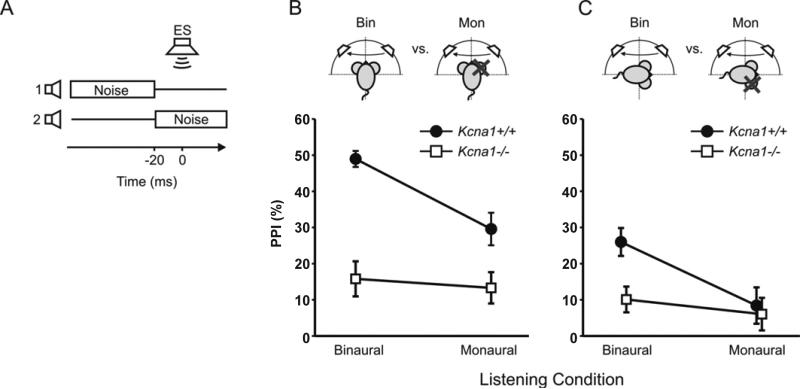
3.3 Binaural hearing assists sound localization in Kcna1+/+ but not in Kcna1 −/− mice
Sound localization along the azimuth in mice is predominantly based on interaural intensity differences for matched acoustic inputs that are received within a small temporal window: and thus, based on binaural hearing. We suggest the hypothesis that Kcna1−/− mice have prolonged temporal integration windows and will therefore have a reduced ability to detect differences in sound location. The swap of a continuous noise from one speaker to another was used as a prepulse, which if detected will inhibit the ASR (= prepulse inhibition or PPI). Since previous studies showed that Kcna1−/− mice can only detect differences in the sound location if these are at least 90° apart (Allen and Ison, 2012), here only 90° and 120° speaker separation were tested. Within each genotype no significant differences were found between 90° and 120° separation and the data for both angles were therefore pooled within genotypes, leaving six groups to be compared: Kcna1+/+ vs. Kcna1−/−, monaural vs. binaural and frontal hemifield vs. lateral hemifield. Figure 3 shows that for binaural listening Kcna1+/+ mice were significantly more responsive than the Kcna1−/− mice to sound source movement along the azimuth in both the frontal and lateral field (p≤0.001). Further, when the sound source moved across the frontal midline monaural Kcna1+/+ mice did better than monaural Kcna1−/− mice (Fig. 3B; p≤0.005), although the Kcna1+/+ mice lost this advantage when the sound moved through the same angular separations but within the lateral field (Fig. 3C). For Kcna1−/− mice, no differences were measured between binaural vs. monaural listening, nor between frontal vs. lateral changes in location.
Figure 3. Kcna1+/+ mice but not Kcna1−/− mice benefit from binaural hearing in sound source discrimination tasks.
A) For the spatial discrimination experiment, stimulus conditions illustrating the change of noise location from speaker 1 to speaker 2 at 20 ms before the startle eliciting stimulus (ES) that is presented at time point 0 s. B) PPI scores for Kcna1+/+ (circles, n=17) and Kcna1−/− mice (squares, n=12) with binaural (left) or monaural listening (right), with the test stimulus to the front of the mouse (cartoon), C) For the spatial discrimination experiment, same condition as in B except for the test stimulus presented to the side of the mouse (cartoon), PPI scores for Kcna1+/+ (circles, n=17) and Kcna1−/− mice (squares, n=12) with binaural (left) or monaural listening (right). All data are given as Mean (±SEM).
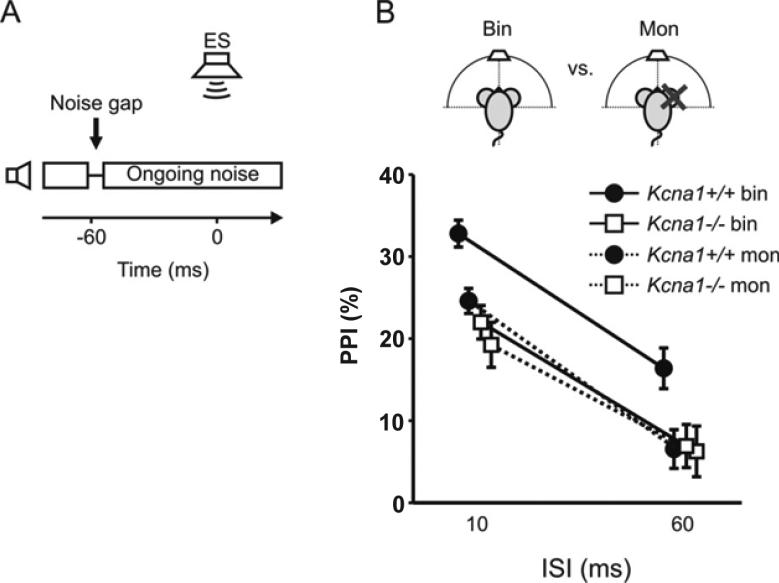
3.3 Gap detection in Kcna1+/+ benefits from binaural hearing
The ability to detect brief intervals of silence within a carrier signal, such as noise, is an important listening skill and a widely used measure of temporal processing proficiency. Mice of both genotypes exhibited strong prepulse inhibition (PPI) of the startle response for gaps starting 10 ms before the ES stimulus under binaural conditions, while less PPI was observed for gaps starting 60 ms before the ES (Fig. 4). Binaural listening is not essential for gap-detection in human listeners and is in fact thought of as a monaural process because high acuity requires that the noise offset at the start of the gap and the noise onset at its end must be received in the same ear (Boehnke et al., 2005). However, we found that Kcna1+/+ mice did show a benefit from binaural stimulus input, while no such benefit was observed in Kcna1−/− mice (p≤0.001). This suggests that even for tasks that do not depend on binaural difference cues in order to be detected, Kcna1+/+ mice seem to benefit from binaural integration.
Figure 4. Kcna1+/+ mice but not Kcna1−/− mice benefit from binaural hearing in gap detection tasks.
A) Stimulus condition showing the offset and onset of otherwise ongoing noise presented by one speaker to produce a 10 ms gap starting 60 ms before the ES at time point 0 s. B) PPI scores for the Kcna1+/+ (circles, n=19) and the Kcna1−/− mice (squares, n=12) with binaural (solid lines) and monaural listening (dashed lines, cartoon) for ISIs between noise offset and ES of 10 ms (left) and 60 ms (right). All data are given as Mean (±SEM).
4. DISCUSSION
Consistent with our initial hypotheses that a major benefit of Kv1.1 for neural processing is to strengthen the integration of coincident inputs, our data showed that Kcna1+/+ mice have a maximal advantage compared to the Kcna1−/− mice in the binaural sound localization experiment and less so for monaural listening, that is, for gap detection. An alternative hypothesis is that because the monaural tests always followed the binaural tests, it is at least conceivable that the reduction in ASR amplitudes and in the strength of PPI was a result of habituation produced by the series of initial binaural tests. However, contrary to this second hypothesis, the first round of testing demonstrated that following the insertion of the ear plug there was a precipitous decrement in both the ASR and in PPI, and when the plug fell out there was an immediate recovery in both of these behavioral endpoints.
4.1 The effect of binaural stimulation on the ASR
The hypothesis of a binaural advantage in Kcna1+/+ mice was strongly supported in the startle amplitude data shown in Figure 2: when presented with binaural input, the Kcna1+/+ mice had a more vigorous startle response than the Kcna1−/− mice, but with monaural input ASR amplitudes declined in both groups and there was no discernible difference between Kcna1+/+ and Kcna1−/− mice. Binaural summation of the ASR has been previously observed in human listeners (Bradley et al., 1991; Grillon et al., 1995), but the neural site of binaural integration was not certain. In rodents it is most likely that the responsible target cells that receive binaural information are on the motor side rather than the sensory side of the reflex pathways. As described in the rat by Lopez et al. (Lopez et al., 1999), the afferent component of the primary ASR reflex arc lies in the cochlear root nucleus which is projecting bilaterally to the motor neurons in the contralateral and ipsilateral caudal pontine reticular nucleus. From there, the caudal pontine reticular nucleus neurons form synapses onto the motor neurons in the facial motor nucleus (pinna reflex) and onto the spinal cord (whole body startle) with the whole reflex arc taking less than 10ms in total. Since there is no fast binaural integration between the cochlear nuclei, which could pass information about binaural summation onto the pontine reticular formation, it is more likely that the ascending projection of the cochlear root neurons converge onto neurons in the pontine reticular formation. Single unit recordings from pontine reticular nucleus neurons show higher firing rates when stimulated binaurally proving them to be the neural site for binaural summation (Gomez-Nieto et al., 2014). As Kv1.1 is expressed in the reticular formation of rodents (Verma-Kurvari et al., 1997), we suggest that the loss of binaural summation of the ASR in the Kcna1−/− mice results from failures in binaural summation in these sensory-motor reflex pathways because the absence of Kv1.1 containing channels will impede integration of coincident or near coincident inputs.
4.2 Binaural hearing was beneficial for sound localization in Kcna1+/+ but not in Kcna1−/− mice
The data in Figure 2 show a significant benefit of binaural hearing on sound localization in the Kcna1+/+ mice. Accordingly, the lack of binaural hearing benefits in Kcna1−/− mice suggests possible hearing deficits in EA1 patients especially in advanced functions like identifying sources of complex sounds or understanding speech in the presence of competing noises as observed in patients carrying a different potassium channel mutation (Middlebrooks et al., 2013).
Interestingly, there was also a small but significant advantage of the Kcna1+/+ mice in monaural hearing but only when the stimuli cross the midline on the frontal azimuth. This better performance of monaural Kcna1+/+ mice in the frontal position is consistent with the observed contribution of monaural spectral stimuli to sound localization in mice, and the conclusion that these cues are most apparent in the frontal azimuth (Lauer et al., 2011). Our data suggest that the monaural Kcna1+/+ mouse, akin to the monaural human (Slattery et al., 1994), can use the monaural spectral cues detected at both ears independently to gain a modest benefit for sound localization. The fact that Kcna1−/− mice cannot use monaural spectral cues confirms a contribution of Kv1.1 to monaural coincidence detection in addition to the more apparent binaural coincidence detection.
4.3 Tasks that do not depend on binaural difference cues also benefit from binaural integration in Kcna1+/+ but not in Kcna1−/− mice
The gap detection task was intended as a test for monaural processing of acoustic signals and in fact, a previous study of gap detection in Kcna1−/− mice used a more lateral speaker configuration that would mostly concur with our monaural listening condition and had revealed no threshold differences between Kcna1+/+ and −/− mice (Allen et al., 2008). In our current study the placement of the speaker in the front of the animal provided gap-in-noise cues to both ears in the binaural condition, which appear to be integrated to improve performance. A similar finding in human listeners has been reported by Roberts and Lister (2004), showing that binaural presentation of the noise carriers for the gap provided significantly lower gap thresholds than monaural presentation. Recent work suggested neurons in the superior paraolivary nucleus (SPN) in the auditory brainstem are reliable detectors of gaps in noise (Kadner et al., 2008; Kadner et al., 2006; Kopp-Scheinpflug et al., 2011; Kulesza et al., 2003; Yassin et al., 2014). The broad frequency tuning in wild type SPN neurons (Dehmel et al., 2002) enables across frequency integration to the benefit of better temporal resolution. The correct integration of convergent information of both SPNs within the inferior colliculus (Felix et al., 2014) might be the reason for the better performance of Kcna1+/+ in the binaural gap detection task.
The observation that the contribution of Kv1.1 was not limited to sound source localization tasks based on interaural signal comparisons but was also apparent in the monaural tasks suggests that the role of Kv1.1 in binaural hearing is to ensure the integration of near coincident inputs that may have their origin in either monaural or interaural signal detection. This finding is corroborated by unilaterally deaf individuals where the lack of proper binaural integration not only causes sound localization deficits, but also impairments in temporal processing and speech comprehension (Wie et al., 2010). Since Kv1.1 is expressed in the spiral ganglion neurons (Liu et al., 2014) it is conceivable that both monaural and binaural hearing would be influenced by the lack of Kcna1. The fact that monaural processing is less dependent on Kv1.1 than binaural processing could have at least three reasons: First, the features of Kv1 channels in spiral ganglion cells of the auditory nerve are dominated by Kv1.2 and Kv1.4 rather than Kv1.1 (Wang et al., 2013), so that the output of the auditory nerve is not significantly affected by the Kcna1 deletion. This is corroborated by the similarity of P1 amplitudes and ABR thresholds between Kcna1−/− mice and their wild type littermates (Allen and Ison, 2012). Second, once neurons in monaural nuclei such as the MNTB are compromised by the Kcna1 deletion (Brew et al., 2003; Gittelman and Tempel, 2006; Kopp-Scheinpflug et al., 2003), the effect on cellular signal processing will be summative when passed on to the next (binaural) neurons of the LSO and will consequently result in more severe deficits. Third, Kv1.1 channels are not only expressed at neural cell bodies but also in the juxtaparanodal regions of myelinated axons (Poliak et al., 2003; Wang et al., 1993). Therefore the deletion of Kcna1 might lead to axonal signal conduction deficits which will have stronger affects with increasing axon length, e.g. connecting one side of the brain with the other as found in the binaural signal processing pathway in the brainstem. Implications for KV1.1 function in those juxtaparanodal locations is given by mutations in the human KCNA1 which cause several neuropathies including Episodic Ataxia Type 1 (EA1) and neuromyotonia (Browne et al., 1994; Lassche et al., 2014; Tomlinson et al., 2013; Zuberi et al., 1999). Our results support the findings of recent studies suggesting the role of Kv1.1 in hearing impairment (Strupp, 2013; Tomlinson et al., 2013) and extend these to demonstrate its importance to auditory processing.
5. CONCLUSIONS
Our results support the hypothesis that Kv1.1 containing channels are needed to ensure accurate binaural integration in the auditory brainstem. Genetic deletion of the Kcna1 gene reduced the performances in sound localization and gap detection tasks to the level of monaural listening conditions. Therefore when testing for auditory functions in humans with mutations in the potassium channel genes, special attention should be paid to deficits that are known to occur under challenging listening conditions, such as perceiving temporal cues in noisy environments.
HIGHLIGHTS.
We measured binaural and monaural auditory processing in Kcna1+/+ and −/− mice.
Binaural Kcna1+/+ mice had the highest acoustic startle reflexes.
Binaural Kcna1+/+ mice had better sound source discrimination and gap detection.
Monaural performances of Kcna1+/+ mice were very similar to Kcna1−/− mice.
We conclude that the many sensory benefits of binaural hearing depend on Kcna1.
Acknowledgments
This research was supported by USPHS NIH-NIA P01 Grant AG09524, NIH-NIDCD P30 Grant DC005409, and by the Schmitt Foundation for Integrative Brain Research, and DAAD-D/07/40536 (AK), and the DFG SFB870/2 A-10 (CKS). The authors thank Dr. Robert Frisina and Xiaoxia Zhu for genotyping, and John Housel for colony management. This project was part of the PhD dissertation of AK, and we thank Professor Rudolf Rübsamen for his continuing encouragement and support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions: AK performed experiments and analyzed data, PDA designed the behavioral apparatus and developed the software, JW designed the ABR apparatus and developed the software and the earplugging procedure, JRI analyzed and interpreted the data, CKS conceived the project and posed the experimental questions, all authors contributed to writing the paper.
REFERENCES
- Adamson CL, Reid MA, Mo ZL, Bowne-English J, Davis RL. Firing features and potassium channel content of murine spiral ganglion neurons vary with cochlear location. The Journal of comparative neurology. 2002;447:331–50. doi: 10.1002/cne.10244. [DOI] [PubMed] [Google Scholar]
- Allen PD, Ison JR. Kcna1 gene deletion lowers the behavioral sensitivity of mice to small changes in sound location and increases asynchronous brainstem auditory evoked potentials but does not affect hearing thresholds. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:2538–43. doi: 10.1523/JNEUROSCI.1958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen PD, Schmuck N, Ison JR, Walton JP. Kv1.1 channel subunits are not necessary for high temporal acuity in behavioral and electrophysiological gap detection. Hearing research. 2008;246:52–8. doi: 10.1016/j.heares.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehnke SE, Phillips DP. The relation between auditory temporal interval processing and sequential stream segregation examined with stimulus laterality differences. Perception & psychophysics. 2005;67:1088–101. doi: 10.3758/bf03193634. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Startle and emotion: lateral acoustic probes and the bilateral blink. Psychophysiology. 1991;28:285–95. doi: 10.1111/j.1469-8986.1991.tb02196.x. [DOI] [PubMed] [Google Scholar]
- Brew HM, Hallows JL, Tempel BL. Hyperexcitability and reduced low threshold potassium currents in auditory neurons of mice lacking the channel subunit Kv1.1. The Journal of physiology. 2003;548:1–20. doi: 10.1113/jphysiol.2002.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne DL, Gancher ST, Nutt JG, Brunt ER, Smith EA, Kramer P, Litt M. Episodic ataxia/myokymia syndrome is associated with point mutations in the human potassium channel gene, KCNA1. Nature genetics. 1994;8:136–40. doi: 10.1038/ng1094-136. [DOI] [PubMed] [Google Scholar]
- Dehmel S, Kopp-Scheinpflug C, Dorrscheidt GJ, Rubsamen R. Electrophysiological characterization of the superior paraolivary nucleus in the Mongolian gerbil. Hearing research. 2002;172:18–36. doi: 10.1016/s0378-5955(02)00353-2. [DOI] [PubMed] [Google Scholar]
- Felix RA, 2nd, Magnusson AK, Berrebi AS. The superior paraolivary nucleus shapes temporal response properties of neurons in the inferior colliculus. Brain structure & function. 2014 doi: 10.1007/s00429-014-0815-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittelman JX, Tempel BL. Kv1.1-containing channels are critical for temporal precision during spike initiation. Journal of neurophysiology. 2006;96:1203–14. doi: 10.1152/jn.00092.2005. [DOI] [PubMed] [Google Scholar]
- Gomez-Nieto R, Horta-Junior Jde A, Castellano O, Millian-Morell L, Rubio ME, Lopez DE. Origin and function of short-latency inputs to the neural substrates underlying the acoustic startle reflex. Frontiers in neuroscience. 2014;8:216. doi: 10.3389/fnins.2014.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg JJ, Brew HM, Tempel BL. Differential expression of voltage-gated potassium channel genes in auditory nuclei of the mouse brainstem. Hearing research. 2000;140:77–90. doi: 10.1016/s0378-5955(99)00187-2. [DOI] [PubMed] [Google Scholar]
- Grillon C, Davis M. Acoustic startle and anticipatory anxiety in humans: effects of monaural right and left ear stimulation. Psychophysiology. 1995;32:155–61. doi: 10.1111/j.1469-8986.1995.tb03307.x. [DOI] [PubMed] [Google Scholar]
- Heeroma JH, Henneberger C, Rajakulendran S, Hanna MG, Schorge S, Kullmann DM. Episodic ataxia type 1 mutations differentially affect neuronal excitability and transmitter release. Disease models & mechanisms. 2009;2:612–9. doi: 10.1242/dmm.003582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner HE, Heffner RS. Audition. In: Davis S, editor. Handbook of Research Methods in Experimental Psychology. Blackwell; 2003. pp. 413–440. [Google Scholar]
- Herson PS, Virk M, Rustay NR, Bond CT, Crabbe JC, Adelman JP, Maylie J. A mouse model of episodic ataxia type-1. Nature neuroscience. 2003;6:378–83. doi: 10.1038/nn1025. [DOI] [PubMed] [Google Scholar]
- Imbrici P, Gualandi F, D'Adamo MC, Masieri MT, Cudia P, De Grandis D, Mannucci R, Nicoletti I, Tucker SJ, Ferlini A, Pessia M. A novel KCNA1 mutation identified in an Italian family affected by episodic ataxia type 1. Neuroscience. 2008;157:577–87. doi: 10.1016/j.neuroscience.2008.09.022. [DOI] [PubMed] [Google Scholar]
- Kadner A, Berrebi AS. Encoding of temporal features of auditory stimuli in the medial nucleus of the trapezoid body and superior paraolivary nucleus of the rat. Neuroscience. 2008;151:868–87. doi: 10.1016/j.neuroscience.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner A, Kulesza RJ, Jr., Berrebi AS. Neurons in the medial nucleus of the trapezoid body and superior paraolivary nucleus of the rat may play a role in sound duration coding. Journal of neurophysiology. 2006;95:1499–508. doi: 10.1152/jn.00902.2005. [DOI] [PubMed] [Google Scholar]
- Karcz A, Rubsamen R, Kopp-Scheinpflug C. Low-threshold potassium currents stabilize IID-sensitivity in the inferior colliculus. Frontiers in neural circuits. 2012;6:60. doi: 10.3389/fncir.2012.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcz A, Hennig MH, Robbins CA, Tempel BL, Rubsamen R, Kopp-Scheinpflug C. Low-voltage activated Kv1.1 subunits are crucial for the processing of sound source location in the lateral superior olive in mice. The Journal of physiology. 2011;589:1143–57. doi: 10.1113/jphysiol.2010.203331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug A, Trussell LO. Activation and deactivation of voltage-dependent K+ channels during synaptically driven action potentials in the MNTB. Journal of neurophysiology. 2006;96:1547–55. doi: 10.1152/jn.01381.2005. [DOI] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Fuchs K, Lippe WR, Tempel BL, Rubsamen R. Decreased temporal precision of auditory signaling in Kcna1-null mice: an electrophysiological study in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:9199–207. doi: 10.1523/JNEUROSCI.23-27-09199.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Tozer AJ, Robinson SW, Tempel BL, Hennig MH, Forsythe ID. The sound of silence: ionic mechanisms encoding sound termination. Neuron. 2011;71:911–25. doi: 10.1016/j.neuron.2011.06.028. [DOI] [PubMed] [Google Scholar]
- Kulesza RJ, Jr., Spirou GA, Berrebi AS. Physiological response properties of neurons in the superior paraolivary nucleus of the rat. Journal of neurophysiology. 2003;89:2299–312. doi: 10.1152/jn.00547.2002. [DOI] [PubMed] [Google Scholar]
- Lassche S, Lainez S, Bloem BR, van de Warrenburg BP, Hofmeijer J, Lemmink HH, Hoenderop JG, Bindels RJ, Drost G. A novel KCNA1 mutation causing episodic ataxia type I. Muscle & nerve. 2014;50:289–91. doi: 10.1002/mus.24242. [DOI] [PubMed] [Google Scholar]
- Lauer AM, Slee SJ, May BJ. Acoustic basis of directional acuity in laboratory mice. Journal of the Association for Research in Otolaryngology : JARO. 2011;12:633–45. doi: 10.1007/s10162-011-0279-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Lee E, Davis RL. Heterogeneous intrinsic excitability of murine spiral ganglion neurons is determined by Kv1 and HCN channels. Neuroscience. 2014;257:96–110. doi: 10.1016/j.neuroscience.2013.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez DE, Saldana E, Nodal FR, Merchan MA, Warr WB. Projections of cochlear root neurons, sentinels of the rat auditory pathway. The Journal of comparative neurology. 1999;415:160–74. [PubMed] [Google Scholar]
- Mathews PJ, Jercog PE, Rinzel J, Scott LL, Golding NL. Control of submillisecond synaptic timing in binaural coincidence detectors by K(v)1 channels. Nature neuroscience. 2010;13:601–9. doi: 10.1038/nn.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrooks JC, Nick HS, Subramony SH, Advincula J, Rosales RL, Lee LV, Ashizawa T, Waters MF. Mutation in the kv3.3 voltage-gated potassium channel causing spinocerebellar ataxia 13 disrupts sound-localization mechanisms. PloS one. 2013;8:e76749. doi: 10.1371/journal.pone.0076749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel D, Bal R, Gardner SM, Smith PH, Joris PX. Detection of synchrony in the activity of auditory nerve fibers by octopus cells of the mammalian cochlear nucleus. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11773–9. doi: 10.1073/pnas.97.22.11773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliak S, Salomon D, Elhanany H, Sabanay H, Kiernan B, Pevny L, Stewart CL, Xu X, Chiu SY, Shrager P, Furley AJ, Peles E. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. The Journal of cell biology. 2003;162:1149–60. doi: 10.1083/jcb.200305018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakulendran S, Schorge S, Kullmann DM, Hanna MG. Episodic ataxia type 1: a neuronal potassium channelopathy. Neurotherapeutics : the Journal of the American Society for Experimental NeuroTherapeutics. 2007;4:258–66. doi: 10.1016/j.nurt.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Rance G, Fava R, Baldock H, Chong A, Barker E, Corben L, Delatycki MB. Speech perception ability in individuals with Friedreich ataxia. Brain : a journal of neurology. 2008;131:2002–12. doi: 10.1093/brain/awn104. [DOI] [PubMed] [Google Scholar]
- Rance G, Ryan MM, Carew P, Corben LA, Yiu E, Tan J, Delatycki MB. Binaural speech processing in individuals with auditory neuropathy. Neuroscience. 2012;226:227–35. doi: 10.1016/j.neuroscience.2012.08.054. [DOI] [PubMed] [Google Scholar]
- Roberts RA, Lister JJ. Effects of age and hearing loss on gap detection and the precedence effect: broadband stimuli. Journal of Speech Language & Hearing Research. 2004;47:965–78. doi: 10.1044/1092-4388(2004/071). [DOI] [PubMed] [Google Scholar]
- Rosenberger MH, Fremouw T, Casseday JH, Covey E. Expression of the Kv1.1 ion channel subunit in the auditory brainstem of the big brown bat, Eptesicus fuscus. The Journal of comparative neurology. 2003;462:101–20. doi: 10.1002/cne.10713. [DOI] [PubMed] [Google Scholar]
- Slattery WH, 3rd, Middlebrooks JC. Monaural sound localization: acute versus chronic unilateral impairment. Hearing research. 1994;75:38–46. doi: 10.1016/0378-5955(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Strupp M. Ion channel disorders: still a fascinating topic--news on episodic ataxia type 1. Journal of neurology, neurosurgery, and psychiatry. 2013;84:1063–4. doi: 10.1136/jnnp-2012-304857. [DOI] [PubMed] [Google Scholar]
- Tomlinson SE, Rajakulendran S, Tan SV, Graves TD, Bamiou DE, Labrum RW, Burke D, Sue CM, Giunti P, Schorge S, Kullmann DM, Hanna MG. Clinical, genetic, neurophysiological and functional study of new mutations in episodic ataxia type 1. Journal of neurology, neurosurgery, and psychiatry. 2013 doi: 10.1136/jnnp-2012-304131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma-Kurvari S, Border B, Joho RH. Regional and cellular expression patterns of four K+ channel mRNAs in the adult rat brain. Brain research. Molecular brain research. 1997;46:54–62. doi: 10.1016/s0169-328x(96)00271-9. [DOI] [PubMed] [Google Scholar]
- Wang H, Kunkel DD, Martin TM, Schwartzkroin PA, Tempel BL. Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature. 1993;365:75–9. doi: 10.1038/365075a0. [DOI] [PubMed] [Google Scholar]
- Wang W, Kim HJ, Lv P, Tempel B, Yamoah EN. Association of the Kv1 family of K+ channels and their functional blueprint in the properties of auditory neurons as revealed by genetic and functional analyses. Journal of neurophysiology. 2013;110:1751–64. doi: 10.1152/jn.00290.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wie OB, Pripp AH, Tvete O. Unilateral deafness in adults: effects on communication and social interaction. The Annals of otology, rhinology, and laryngology. 2010;119:772–81. [PubMed] [Google Scholar]
- Yassin L, Radtke-Schuller S, Asraf H, Grothe B, Hershfinkel M, Forsythe ID, Kopp-Scheinpflug C. Nitric oxide signalling modulates synaptic inhibition in the superior paraolivary nucleus (SPN) via cGMP-dependent suppression of KCC2. Frontiers in neural circuits. 2014 doi: 10.3389/fncir.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JS, Fechter LD. Reflex inhibition procedures for animal audiometry: a technique for assessing ototoxicity. The Journal of the Acoustical Society of America. 1983;73:1686–93. doi: 10.1121/1.389391. [DOI] [PubMed] [Google Scholar]
- Zuberi SM, Eunson LH, Spauschus A, De Silva R, Tolmie J, Wood NW, McWilliam RC, Stephenson JB, Kullmann DM, Hanna MG. A novel mutation in the human voltage-gated potassium channel gene (Kv1.1) associates with episodic ataxia type 1 and sometimes with partial epilepsy. Brain : a journal of neurology. 1999;122(Pt 5):817–25. doi: 10.1093/brain/122.5.817. [DOI] [PubMed] [Google Scholar]