Abstract
Background
Attacks of neuropathic pain, usually abdominal, are characteristic of the acute porphyrias and are accompanied by overproduction of heme-precursor molecules, specifically delta-aminolevulinic acid and porphobilinogen. The basis for the acute symptoms in these diseases has been speculative.
Methods
We review genetic acute porphyria, hereditary tyrosinemia, and an acquired condition, lead poisoning. All perturb heme synthesis and present with a very similar pain syndrome.
Results
While each of these conditions has characteristic urine biochemistry, all exhibit excess delta-aminolevulinic acid. Moreover, in all, treatment with hemin reduces delta-aminolevulinic acid and relieves symptoms. In contrast, use of recombinant porphobilinogen deaminase to knock down porphobilinogen in acute porphyria was ineffective.
Conclusion
There is now convincing evidence that delta-aminolevulinic acid is the cause of pain in the acute porphyrias. The efficacy of hemin infusion is due mainly, if not entirely, to its inhibition of hepatic delta-aminolevulinic acid synthase-1, the enzyme that catalyzes delta-aminolevulinic acid formation. Delta-aminolevulinic acid synthase-1 is a rational target for additional therapies to control symptoms in acute porphyria.
Keywords: Acute porphyria, abdominal pain, delta-aminolevulinic acid, lead poisoning, Ayurveda
INTRODUCTION
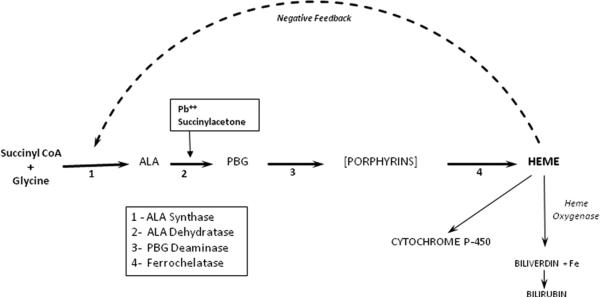
The acute porphyrias comprise four diseases*, each representing a genetic defect in the pathway of heme synthesis that potentially limits production of heme (Figure 1). Expression is most important in hepatocytes, where a critically low level of intracellular heme triggers overproduction of the precursor molecules, delta-aminolevulinic acid and porphobilinogen. The symptoms of acute porphyria include abdominal pain, nausea, and tachycardia.1 In an attack, delta-aminolevulinic acid and porphobilinogen are invariably elevated in plasma and urine.1 Specific treatment is intravenous hemin (Panhematin® or Normosang®, Recordati Rare Diseases). 1, 2
Figure 1.
Schematic pathway of heme synthesis and utilization, showing the enzymes mediating formation of delta-aminolevulinic acid, porphobilinogen and the initial porphyrin (uroporphyrinogen), respectively. Also shown is the regulatory feedback loop involving the end-product of the pathway, heme, and delta-aminolevulinic acid synthase activity. Inhibition of delta-aminolevulinic acid dehydratase by lead (Pb++) is indicated.
Hereditary tyrosinemia and lead poisoning also affect the heme synthetic pathway (Figure 1). In tyrosinemia, the genetic defect in tyrosine metabolism results in hepatocellular accumulation of succinylacetone, which potently inhibits ALA dehydratase.3 In lead poisoning, the metal directly inhibits the same enzyme by binding to sulfhydryl groups. In either condition, the pathway is activated as in acute porphyria, but the result is overproduction of ALA only; porphobilinogen remains normal, reflecting the impaired conversion of delta-aminolevulinic acid to porphobilinogen (Fig. 1). In a case of tyrosinemia, neurological crisis was treated with hemin infusion to good effect.4 The present paper includes a case of lead poisoning in which the symptoms similarly responded to hemin.
Case
A previously healthy woman, age 32, sought evaluation for fluctuating abdominal pain and nausea that had been present since age 26. Extensive testing had been unrevealing except for anemia (Hgb 8.6) and elevated urine coproporphyrins (2070 mcg/g creatinine; reference range 23-130). She was referred to our tertiary facility for presumed acute porphyria. Urine delta-aminolevulinic acid and porphobilinogen had been requested but were still pending. Without the latter data, the porphyria diagnosis was provisional only. However, in light of her worsening symptoms, she was started on daily intravenous hemin as for acute porphyria.1,2 With the third infusion, she reported marked improvement in pain and nausea. After two additional infusions, her symptoms were nearly resolved, and she was discharged to outpatient followup. When reports from pre-treatment testing arrived, they were notable for urine delta-aminolevulinic acid 29.4 mg/g creatinine (normal <7.5) and porphobilinogen 2 mg/g creatinine (normal <2). The blood lead was 83 mcg/dL (mean level in U.S. adults, <1). A repeat blood lead 2 weeks later was 91. RBC Zn-protoporphyrin was 285 (normal <40 mcg/dL). Urine delta-aminolevulinic acid and porphobilinogen became undetectable after the second hemin infusion.
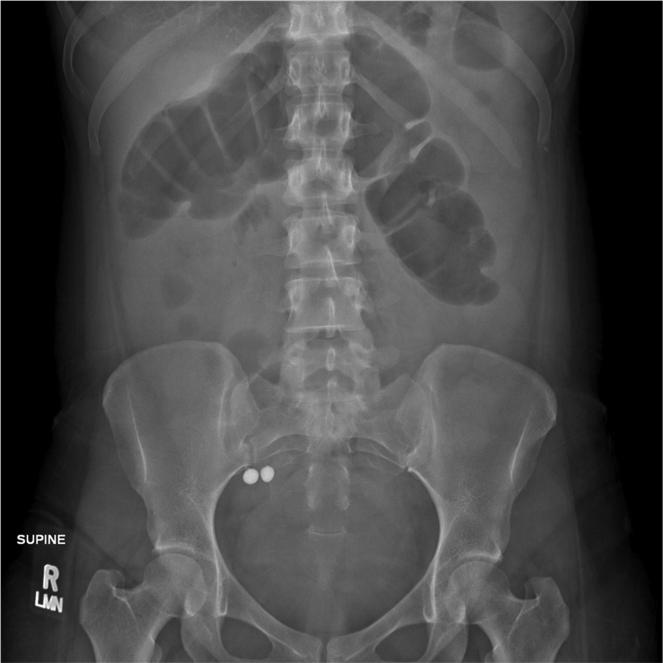
The patient was Asian Indian, born and raised in the U.S. On further questioning, she described using around 40 traditional (Ayurvedic) medications over several years for her abdominal symptoms. She provided samples of those taken during the 6 months prior to her hospitalization. Analysis by the California Department of Public Health's Food and Drug Laboratory Branch showed that all had detectable lead, four with distinctly high levels; some had mercury as well (Table 1). In retrospect, abdominal imaging at the referring hospital had shown two radio-opaque objects in the proximal colon of ca. 8 mm diameter (Figure 2), which matched the sixth item in Table 1 (Vatchintamani Rasa, Brihat). Chelation therapy was begun according to current guidelines for lead intoxication. 5 As the blood lead dropped from 91 to less than 10, her symptoms resolved completely, and the hemoglobin rose from 8.6 to 14.5.
Table 1.
Recent Ayurvedic preparations taken by the patient; Analysis for lead and mercury.
| NAME | APPEARANCE | LEAD (limit 0.25 ug/g) | MERCURY (limit 0.10 ug/g) |
|---|---|---|---|
| (proprietary) | Purple-grey powder | 37 ug/g | 25,000 ug/g |
| (proprietary) | Tan powder | 0.88 | <0.1 |
| (proprietary) | Brown powder | 1.2 | <0.1 |
| (custom tablet) | Black pill | 1.5 | 37 |
| Vatchintamani Ras (Baidyanath) | Pink pill | 37 | 270,000 |
| Vatchintamani Rasa (Brihat) | Brown pill | 36 | 80,000 |
| Muktashukti Bhasma (Baidyanath) | White powder | 48 | 230 |
| (proprietary) | Tan powder | 0.88 | <0.1 |
Figure 2.
Plain film of the abdomen showing two radio-opaque objects, ca. 8 mm in diameter, in the proximal colon.
SOURCES OF LEAD POISONING
Adult lead poisoning in the United States historically is from occupational exposure but in recent years has been linked also to traditional Indian (Ayurvedic) medications.6 The latter are available worldwide via the internet. A 2008 report describes purchase on line of an assortment in the U.S., of which 20% contained potentially toxic amounts of lead.7 Ayurvedic medications may be formulated with mercury and arsenic as well as lead, and three of the products tested did have a large amount of mercury (Table 1). The only metal detected in the patient's blood, however, was lead.This is consistent with Ayurveda practice, which stipulates the use of inorganic mercury only, a form that is not absorbed by the intestine. The case underscores the importance of a detailed history of supplement use (and occupation) in patients with abdominal pain of uncertain cause.
THE DIFFERENTIAL DIAGNOSIS OF DISORDERS OF HEME SYNTHESIS
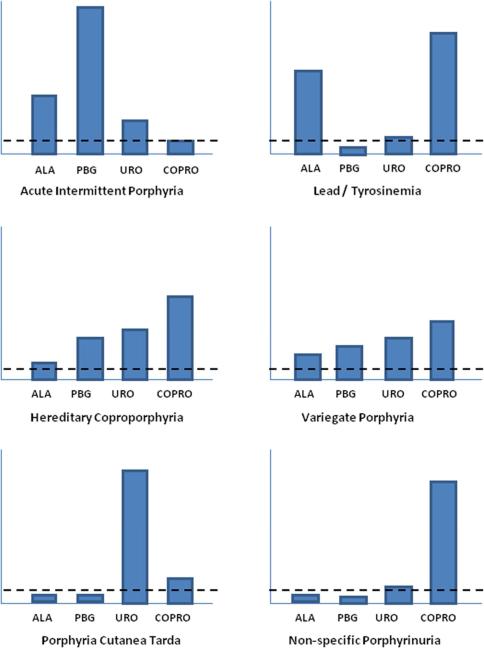
The profile of heme precursors in blood, urine and stool is unique for each type of porphyria and is the key to diagnosis (Figure 3). When the presentation suggests acute porphyria but urine studies show elevated delta-aminolevulinic acid and coproporphyrin only (with normal porphobilinogen), a blood lead should be ordered. Another important clinical point is that coproporphyrin elevation in isolation (with normal delta-aminolevulinic acid and porphobilinogen) is non-specific and not associated with any of the conditions under discussion.
Figure 3.
Profile of heme precursors in urine for individual types of acute porphyria, lead poisoning and non-specific porphyrinuria. The bars are not to scale. They represent the general pattern of heme precursor excretion in individual disturbances of the pathway. The increases are shown relative to the upper limit of normal (horizontal dashed line).
PATHOGENESIS OF THE ABDOMINAL SYMPTOMS IN ACUTE PORPHYRIA
The cause of neuropathic symptoms in acute porphyria has not been defined. Hypotheses have ranged from neural heme deficiency to direct toxic effects of delta-aminolevulinic acid and/or porphobilinogen (for a detailed review, see reference 8). Both metabolites are invariably and strikingly elevated in attacks. However, the absolute concentrations vary among individuals, and threshold values for symptoms have not been defined. People with chronically elevated delta-aminolevulinic acid (and porphobilinogen) do appear to be predisposed to acute attacks, by comparison with those who have normal (or nearly normal) values.9 The variation among individuals implies a role for genetic and/or environmental modifiers, which is an area of active investigation.
A direct pathogenic effect of porphobilinogen was examined in 2005 when recombinant human porphobilinogen deaminase (Porphozyme®) was given to people with frequent attacks and markedly elevated delta-aminolevulinic acid and porphobilinogen. A rapid, selective decrease in plasma porphobilinogen10 was seen, but symptoms were unchanged. Around this time, the first liver transplant for acute porphyria was performed in a very ill patient with recurrent, debilitating attacks. In this and subsequent cases, both delta-aminolevulinic acid and porphobilinogen normalized within days, and symptoms resolved. .11
The Porphozyme data and the transplant results, taken together, strongly point to delta-aminolevulinic acid as the cause of symptoms in porphyria. The experience with hemin infusions in tyrosinemia and lead poisoning further support the hypothesis.
Hemin inhibits the initial enzyme of the pathway, hepatic delta-aminolevulinic acid synthase-1, through a feedback loop (Fig. 1), thereby reducing production of delta-aminolevulinic acid, porphobilinogen and subsequent pathway intermediates. Its efficacy in acute porphyria has been documented in numerous individual patients and small series.1,2 It also relieves symptoms when only delta-aminolevulinic acid is present in excess, as in tyrosinemia and the present case of lead poisoning. It is important to note that hemin was used inadvertently in our patient: It is not a recommended treatment for lead poisoning.
IMPLICATIONS FOR ACUTE PORPHYRIA TREATMENT
Intravenous hemin is the only specific therapy for acute attacks of porphyria and can be life-saving but is less than ideal. Its effect is short-lived. Patients with frequent spontaneous attacks may require infusions at intervals of 1-4 weeks. The free solution is mildly caustic, causing painful inflammation of small peripheral veins. Thus it is often given through a central line, which has its own complications. Finally, with breakdown of hemin to bile pigment, iron is released and may accumulate over time to a level that requires chelation therapy. The stage is set for new approaches to reducing delta-aminolevulinic acid, including down-regulation of the enzyme catalyzing its formation (delta-aminolevulinic acid synthase-1) or correction of the inherited deficiency in porphobilinogen deaminase that leads to its overproduction.
Clinical Significance.
Acute porphyria, tyrosinemia and lead poisoning all present similarly, with a clinical picture that is dominated by abdominal pain, nausea and neuropathy. All three exhibit striking elevation of delta-aminolevulinic acid in urine or blood.
Pain resolves with treatment that shuts down overproduction of this heme pathway intermediate, confirming its pathogenic role.
Future therapy for the acute porphyrias should target overproduction of delta-aminolevulinic acid.
ACKNOWLEDGMENT
Timur Durrani MD, MPH and Robert Kosnick MD provided treatment and follow-up for lead poisoning.
Funding Sources: Support is from an NIH grant (U54 DK083909) to the Porphyrias Consortium of the Rare Disease Clinical Research Network. Dr. Lai receives support from a Career Development Award of the American College of Gastroenterology, the UCSF Liver Center (P30 DK026743) and the American Porphyria Foundation. The opinions expressed are those of the authors and not of NIH or the U.S. Department of Health and Human Services.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no competing interests with respect to the subject matter of this paper. All had access to the data and participated in writing the manuscript.
The principal acute porphyrias include acute intermittent porphyria (AIP), hereditary coproporphyria (HCP) and variegate porphyria (VP). A fourth type, delta-aminolevulinic acid dehydratase deficiency, is a very rare recessive form and not considered here.
REFERENCES
- 1.Anderson KE, Bloomer JR, Bonkovsky HL, et al. Recommendations for the diagnosis and treatment of the acute porphyrias. Ann Intern Med. 2005;142:439–50. doi: 10.7326/0003-4819-142-6-200503150-00010. [DOI] [PubMed] [Google Scholar]
- 2.Bissell DM. Treatment of acute hepatic porphyria with hematin. J Hepatol. 1988;6(1):1–7. doi: 10.1016/s0168-8278(88)80456-2. [DOI] [PubMed] [Google Scholar]
- 3.Sassa S, Kappas A. Hereditary tyrosinemia and the heme biosynthetic pathway. Profound inhibition of delta-aminolevulinic acid dehydratase activity by succinylacetone. J Clin Invest. 1983;71:625–34. doi: 10.1172/JCI110809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rank JM, Pascual-Leone A, Payne W, et al. Hematin therapy for the neurologic crisis of tyrosinemia. J Pediatr. 1991;118:136–9. doi: 10.1016/s0022-3476(05)81867-0. [DOI] [PubMed] [Google Scholar]
- 5.Kosnett MJ, Wedeen RP, Rothenberg SJ, et al. Recommendations for medical management of adult lead exposure. Environ Health Perspect. 2007;115:463–71. doi: 10.1289/ehp.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lead Poisoning in Pregnant Women Who Used Ayurvedic Medications from India — New York City, 2011–2012. Morbid Mortal Weekly Rep (MMWR) 61(33):641–646. [PubMed] [Google Scholar]
- 7.Saper RB, Phillips RS, Sehgal A, et al. Lead, mercury, and arsenic in US- and Indian-manufactured Ayurvedic medicines sold via the Internet. JAMA. 2008;300(8):915–23. doi: 10.1001/jama.300.8.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bissell DM. The porphyrias. In: Rosenberg RN, Pascual JM, editors. Rosenberg's Molecular and Genetic Basis of Neurological and Psychiatric Disease. 5th Edition. Academic Press; 2014. (in press) [Google Scholar]
- 9.von und zu Fraunberg M, Pischik E, Udd L, Kauppinen R. Clinical and biochemical characteristics and genotype-phenotype correlation in 143 Finnish and Russian patients with acute intermittent porphyria. Medicine (Baltimore) Jan. 2005;84(1):35–47. doi: 10.1097/01.md.0000152455.38510.af. [DOI] [PubMed] [Google Scholar]
- 10.Sardh E, Rejkjaer L, Andersson DE, et al. Safety, pharmacokinetics and pharmocodynamics of recombinant human porphobilinogen deaminase in healthy subjects and asymptomatic carriers of the acute intermittent porphyria gene who have increased porphyrin precursor excretion. Clin Pharmacokinet. 2007;46(4):335–349. doi: 10.2165/00003088-200746040-00006. [DOI] [PubMed] [Google Scholar]
- 11.Singal AK, Parker C, Bowden C, Thapar M, Liu L, McGuire BM. Liver transplantation in the management of porphyria. Hepatology. 2014;60(3):1082–89. doi: 10.1002/hep.27086. [DOI] [PMC free article] [PubMed] [Google Scholar]