Abstract
Despite the emergence of the PD-1:PD-1 ligand (PD-L) regulatory axis as a promising target for treating multiple human diseases, remarkably little is known about how this pathway regulates responses to extracellular bacterial infections. We found that PD-1−/− mice, as well as wild type mice treated with a PD-1 blocking antibody, exhibited significantly increased survival against lethal Streptococcus pneumoniae infection following either priming with low-dose pneumococcal respiratory infection or S. pneumoniae-capsular polysaccharide immunization. Enhanced survival in mice with disrupted PD-1:PD-L interactions was explained by significantly increased proliferation, isotype switching, and IgG production by pneumococcal capsule-specific B cells. Both PD-1 ligands, B7-H1 and B7-DC, contributed to PD-1-mediated suppression of protective capsule-specific IgG. Importantly, PD-1 was induced on capsule-specific B cells and suppressed IgG production and protection against pneumococcal infection in a B cell-intrinsic manner. These results provide the first demonstration of a physiologic role for B cell-intrinsic PD-1 expression in vivo. In summary, our study reveals that B cell-expressed PD-1 plays a central role in regulating protection against S. pneumoniae, and thereby represents a promising target for bolstering immunity to encapsulated bacteria.
Introduction
Streptococcus pneumoniae is a leading source of morbidity and mortality globally, causing life-threatening diseases including pneumonia, meningitis, and sepsis (1). Antibodies (Ab) directed against the capsular polysaccharide of the pneumococcus play a major role in promoting clearance (2, 3). The Pneumovax 23 (PPV23) vaccine consisting of 23 native pneumococcal polysaccharides (PPS) from the most common disease-causing serotypes elicits rapid, persistent PPS-specific Ab production but induces sub-optimal levels of IgG in humans, even when PPS are conjugated to a carrier protein (4). Antibodies of the IgG isotype confer superior protection over IgM and IgA isotypes against pneumococcal infection in mouse studies (5, 6) and thus, eliciting increased PPS-specific IgG levels is a major goal of pneumococcal vaccination in humans (7).
PD-1 is a B7/CD28 superfamily receptor expressed on activated lymphoid and myeloid cells (8, 9). Upon engagement of its ligands (PD-L), B7-H1 (PD-L1) and B7-DC (PD-L2), PD-1 negatively regulates critical signaling events. Recent interest in exploiting the PD-1:PD-L regulatory axis for treatment of chronic viral infections, cancer, and autoimmunity is supported by numerous mouse, non-human primate and human studies (8–11). Nonetheless, remarkably little is known about how this immunoregulatory pathway influences the immune response to bacterial infections. Studies with two distinct intracellular bacteria yielded divergent results, with PD-1 suppressing protective responses to Listeria monocytogenes via dendritic cell regulation (12) but promoting survival in response to Mycobacterium tuberculosis infection via suppression of excessive inflammation (13, 14). To date, the sole investigation of PD-1 effects on acute extracellular bacterial infection employed a cecal ligation puncture model, wherein PD-1 expression on macrophages was found to promote macrophage dysfunction and lethality due to sepsis (15). The potential for PD-1 to regulate immune responses against common respiratory infections caused by extracellular bacteria has not been explored.
In this study, we examined the role of PD-1 and its ligands in the host response to S. pneumoniae. Susceptibility to acute respiratory and systemic S. pneumoniae infections was normal in naïve mice lacking PD-1. However, a primary subclinical respiratory infection in PD-1−/− mice, but not wild type mice, elicited significant protection against subsequent lethal systemic pneumococcal challenge, suggesting a role for PD-1 in regulating the protective adaptive immune response to S. pneumoniae. Consistent with this, PD-1 was found to suppress protective anti-capsular IgG levels produced in response to a respiratory pneumococcal infection and native PPS immunization. Immunized PD-1−/− mice, as well as wild type mice treated with a PD-1 blocking Ab at the time of immunization, therefore had a significant survival advantage during S. pneumoniae infection. Our results support a crucial role for B cell-intrinsic PD-1 expression in suppressing protective humoral immune responses to S. pneumoniae via inhibiting clonal expansion and IgG production by capsule-specific B cells, thereby providing the first evidence for B cell-expressed PD-1 in regulating immunity to infectious disease.
Materials and Methods
Mice
C57BL/6 and μMT mice were obtained from Jackson Laboratories. PD-1−/− (16), B7-DC−/− (17) and B7-H1−/− (18) mice were on a C57BL/6 background. Permission to use PD-1−/− mice was kindly obtained from Tasuku Honjo (Kyoto University, Kyoto, Japan). B6.129P2-PtrpcaIghtm1Mnz/J (VHB1-8hi transgenic) mice were from Jackson Laboratories. Mice were housed under specific pathogen free conditions, except during infection experiments. Mice were used at 2–4 months of age and were age-matched for experiments. All studies and procedures were approved by the Wake Forest Animal Care and Use Committee.
Infections, Immunizations, and in vivo mAb blockade
Mice were infected with serotype 3 WU2 strain S. pneumoniae and monitored every 12 hrs for signs of distress as previously described (19, 20). Strain WU2 was obtained in 2002 from Dr. David Briles (University of Alabama-Birmingham) with stocks prepared as originally described (19). In serum transfer experiments, μMT mice challenged with 200 CFU WU2 i.p. received 10 μL of pooled serum (i.p.) from either wild type or PD-1−/− mice harvested 14d post i.n. infection with 106 CFU WU2. Lung (1 mL PBS homogenate) and blood CFU were determined by plating serial dilutions on 5% TSA-II sheep red blood agar plates (BBL) coated with 4 μg/mL gentamicin and incubated overnight at 37°C.
Mice were immunized i.p. or s.c. with diluted, purified serotype 3 pneumococcal polysaccharide (PPS3) (ATCC; Merck) or vaccine-grade Pneumovax23 (PPV23; Merck, Whitehouse Station, NJ) containing either 0.1 μg (referred to as “0.1 μg dose”) or 1 μg (referred to as “1 μg dose”) of each of 23 serotypes of PPS or Prevnar-13 (Pfizer, formerly Wyeth Pharmaceuticals, New York, NY) containing ~0.1 μg of each of 13 serotypes of PPS, as previously described (20). TNP65-Ficoll (Biosearch) was administered i.p. (25 μg). PD-1 mAb blockade was performed by administering RMP1-14 or rat IgG2a (eBioscience) i.p. on d1 (200 μg), d3 (100 μg), and d5 (100 μg) post immunization as previously described (21). For ligand blockade experiments, 200 μg B7-H1 (10F.9G2; BioLegend and BioXcell), B7-DC (TY25; BioLegend and BioXcell), or rat IgG control mAbs (BioXcell or eBioscience) was given i.p. on d0, 2, and 4 post immunization.
ELISAs and ELISPOTs
ELISAs were as previously described (20, 21). Serum samples were diluted in TBS containing 1% BSA (TBS-BSA) and incubated with 10 μg/mL cell wall polysaccharide (CWPS, Statens Serum Institut, Denmark) to adsorb non-capsular polysaccharide Abs. PPS3 (ATCC, Manassas, VA) and PPV23-specific Ab levels were determined by adding diluted serum samples to Maxisorp plates coated with 5 μg/mL native PPS3 or PPV23 in PBS and blocked with TBS-BSA. AP-conjugated polyclonal goat anti-mouse IgM, IgG, IgG3, and IgA Abs (Southern Biotechnology Associates) and pNPP (Sigma) were used to detect PPS-specific Ab. For lung Ig levels, lungs were perfused by slowly injecting 5 mL of PBS into the right ventricle of the heart prior to harvest and homogenization in 1 mL PBS. ELISAs were performed using serum dilutions shown to yield OD405nm readings that fall within a linear range. ELISA values are reported as relative absorbance units (AU; OD405nm reading for serum samples minus OD405nm reading from wells with serum omitted). Endpoint titer PPS3-specific ELISAs were performed using 3-fold serial serum dilutions, with titers reported as the reciprocal dilution yielding OD values that were 2.5-fold over background (serum-omitted) OD405nm values. ELISPOTs were performed as previously described (20).
Flow cytometry
Single cell suspensions (2 × 107/mL) were washed with PBS containing 2% newborn calf serum and then pre-blocked in PBS containing 10 μg/mL CWPS and 0.5 μg/mL Fc block (eBioscience), followed by staining with 25 μg/mL biotinylated PPS3 (PPS3bio) at room temperature as previously described (20). Cells were then washed with PBS containing 2% newborn calf serum and stained with streptavidin-FITC, and a combination of the following mAbs conjugated to different fluorochromes: CD5 (53-7.3), B220 (RA3-6B2), CD86 (GL-1), PD-1 (J43), CD11b (M1/70) (Biolegend); CD19 (1D3, eBioscience); CD138 (281-2, BD Biosciences); and IgG3 (Southern Biotechnology Associates). For Ki-67 staining, extracellular marker and Ag staining was first performed, followed by intracellular staining (Fix/Perm kit in conjunction with SolA15; eBioscience). Cells were fixed and analyzed using a FACSCantoII cytometer (Becton Dickinson) with FSC-A/FSC-H doublet exclusion. Dump channels for CD11bhi myeloid cells and dump channels for autofluorescence signals (minus fluorochrome) were used to eliminate non-specific signals. Streptavidin-FITC-only staining by B cells served as a negative gating control. Dual Ag staining experiments using PPS3bio plus streptavidin-FITC and Alexa488-labeled TNP-Ficoll (a neutral sugar) showed PPS3+ B cells selectively bound PPS3 and not TNP-Ficoll. Two million events were collected for Ag-specific B cell analysis. Positive and negative cell populations were determined using non-reactive isotype-matched Ab staining of PPS-binding cells from immune mice. Data was analyzed using FlowJo analysis software (Treestar).
BrdU staining and flow cytometry
Bromodeoxyuridine (BrdU, Sigma, 0.8 mg/mL) was delivered to mice in drinking water as previously described (22). Single cell suspensions (2 × 107/mL) were fixed with 0.5% paraformaldehyde in PBS (pH 7.4), permeabilized with 3 N HCl containing 0.5% Tween 20, and neutralized with 0.1 M disodium tetraborate. The cells were pre-blocked with unlabeled mIgG1 (20 μg/mL) and intracellular staining performed with mouse anti-BrdU-PE (Bu20A, 4 μg/mL). Cells were washed and blocked in PBS containing 10 μg/mL CWPS and 0.5 μg/mL Fc block, and then stained with 20 μg/mL PPS3bio. Cells were washed and stained with streptavidin-FITC and CD19 mAb and analyzed as described above.
Adoptive transfer experiments
Splenic B cells were purified using magnetic beads (Dynal) against Thy1.2, DX5, Gr1, F4/80, and 33D1 as previously described (21). Purified B cells (4 × 107/mouse) were injected i.p. into μMT mice. Two days later, recipient mice were immunized i.p. with PPV23 containing ~0.1 μg of each PPS. Sera were diluted 1:100 for IgM and 1:10 for IgG PPV23-specific detection. Total serum IgM and IgG levels were determined using mouse Ig standards to generate standard curves with linear regression analysis applied to determine Ab concentrations. Peritoneal B-1b cells were isolated from VHB1-8 Tg mice as previously described (21).
Statistical analysis
Data are shown as means ± SEM with differences assessed using Student’s t test. Differences in overall survival or survival curves were assessed using the Fisher’s Exact Test or Log Rank test, respectively.
Results
PD-1−/− mice exhibit increased survival following secondary, but not primary, S. pneumoniae infection
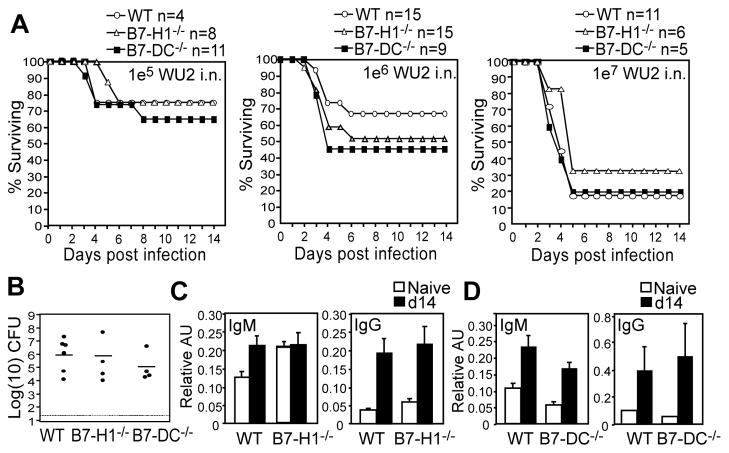
To examine the role of PD-1 during acute S. pneumoniae respiratory infection, we infected PD-1−/− and wild type mice intranasally (i.n.) or intraperitoneally (i.p.) with WU2, a highly virulent invasive serotype 3 strain. PD-1−/− and wild type mice displayed similar susceptibility to S. pneumoniae, regardless of dose or route (105, 106, or 107 CFU i.n., and 101 and 102 CFU i.p.; Fig. 1A, C). Consistent with this, lung bacterial burdens were comparable between PD-1−/− and wild type mice 3 days post high dose (107 CFU) i.n. challenge (Fig. 1B) and 106 CFU i.n. challenge (data not shown). Thus, naïve PD-1−/− and wild type mice display similar susceptibility to primary S. pneumoniae respiratory and systemic infections.
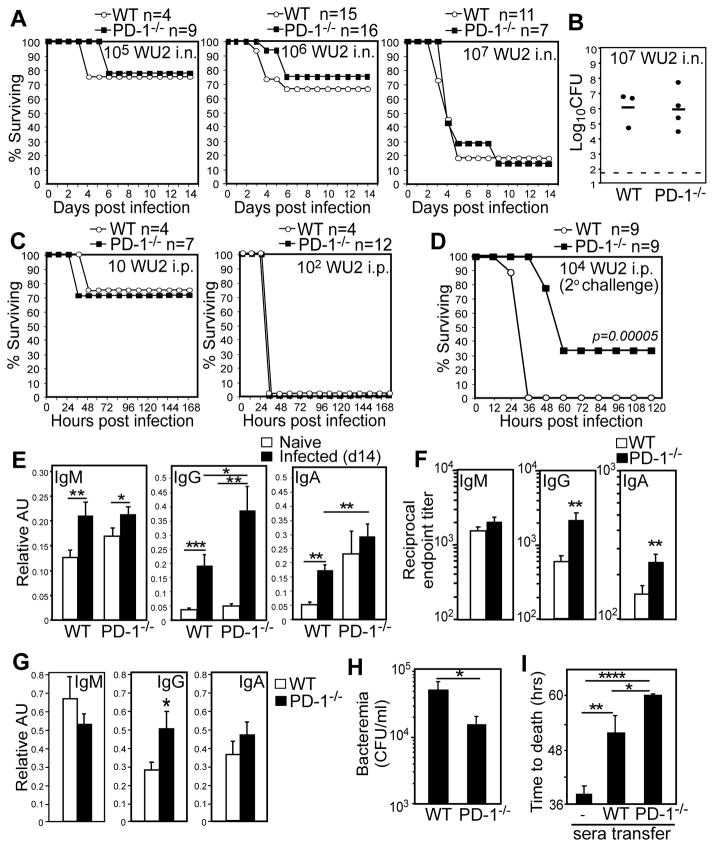
Figure 1. Naïve PD-1−/− mice generate increased capsule-specific IgG following S. pneumoniae respiratory infection and are protected against secondary lethal systemic infection.
Wild type (WT) or PD-1−/− mice were infected i.n. (A–B) or i.p. (C) with WU2 and monitored for signs of morbidity requiring euthanasia. A) Kaplan-Meier survival curves for naïve mice infected i.n. with 105, 106, or 107 CFU WU2. B) Lung bacterial burdens in WT and PD-1−/− mice 3 days post i.n. infection with 107 CFU WU2. C) Kaplan-Meier survival curves for naïve mice infected i.p. with 10 or 102 CFU WU2. D) Survival following secondary systemic pneumococcal infection. Three weeks following primary i.n. infection (106 CFU WU2), survivors were infected with 104 CFU WU2 i.p., with differences in survival curves assessed by Log-rank analysis (p=0.00005). The experiment was performed 3 times using 103 to 104 CFU WU2 (n=19 wild type mice and n=14 PD-1−/− mice), with differences in survival curves (Log-rank analysis, p= 0.002) similar to those shown in (D). E–F) Mean (±SEM) PPS3-specific serum IgM, IgG, and IgA levels (AU values in E and reciprocal endpoint titers in F) 14 days post i.n. infection with 106 CFU WU2 (n≥10 mice/group). Results representative of those obtained in 3 separate experiments. (G) Mean (±SEM) PPS3-specific IgM, IgG, and IgA levels in perfused lung homogenates 14 days post i.n. infection with 106 CFU WU2 (n≥11 mice/group). (H–I) Pooled sera from PD-1−/− (n=10) and WT (n=11) mice 14d post i.n. WU2 infection was administered to μMT mice i.p. concurrently with 200 CFU WU2 i.p. (H) Mean blood bacteria CFU/mL (±SEM) in recipient μMT mice 48 hr post infection. (I) Mean time to death in naïve and recipient μMT mice following i.p. infection (n=6–10 mice/group). Results representative of 2 independent experiments. In E–H, asterisks (*) indicate significant differences in values (p≤0.05, *; p≤0.01, **; p≤0.001, ***; p≤0.0001, ****; unpaired Student’s t test).
A complicating fatal infection that follows pneumococcal pneumonia is bacteremia. We therefore tested whether PD-1 influences the outcome of a secondary systemic infection following a primary respiratory infection. Survivors of the 106 WU2 i.n. challenge were infected with a lethal systemic dose (104 CFU WU2 i.p.; >100 times the LD50 (23)) three weeks following the primary respiratory infection. As shown in Fig. 1D, all wild type mice succumbed to secondary (systemic) infection within 36 hours post challenge. Remarkably, PD-1−/− mice exhibited significantly delayed morbidity (48–60 hrs) and significantly reduced mortality relative to wild type mice (p=0.00005). In contrast, all naïve PD-1−/− mice succumb to a primary systemic infection 36 hours post-challenge (Fig. 1C), similar to naive wild type mice and wild type mice previously challenged with a sub-lethal respiratory infection. Thus, PD-1 deficiency did not affect the outcome of primary pneumococcal infection, but provided a significant survival advantage during secondary infection.
PD-1 deficiency significantly increases anti-capsular IgG levels produced in response to S. pneumoniae infection
Protection against acute systemic pneumococcal infection is largely conferred by pneumococcal-specific Abs, with IgG providing the highest level of protection in mouse studies (5). To assess whether adaptive humoral responses to primary infection were altered by PD-1 deficiency, we assessed Ab levels specific PPS3, the major component of the thick WU2 pneumococcal capsule, 14 days post i.n. infection with 106 CFU. PPS3-specific serum IgM, IgG, and IgA levels were increased in wild type and PD-1−/− mice following infection (Fig. 1E). However, PD-1−/− mice produced significantly higher PPS3-specific serum IgG levels (3.4-fold higher IgG titers; p=0.01) relative to wild type mice in response to infection (Fig. 1E–F). Analysis of perfused lung homogenate Ig levels derived from serum transudation or local synthesis revealed similar findings, with PD-1−/− mice exhibiting significantly higher PPS3-specific lung IgG, but not IgM or IgA, levels compared to wild type mice (Fig. 1G, p=0.02).
To examine whether increased capsule-specific IgG in PD-1−/− mice following sublethal respiratory infection could explain increased protection against subsequent systemic infection (Fig. 1D), the protective capacity of serum harvested from PD-1−/− and wild type mice 14d post infection was assessed in μMT mice challenged with a lethal systemic infection. μMT mice that had received sera from PD-1−/− mice had significantly lower bacteremia at 48 hrs (Fig. 1H; p=0.04) and delayed time to death relative to wild type serum recipient mice (Fig. 1I, p=0.026). Thus, PD-1 deficiency significantly increased the level of protective capsule-specific IgG elicited in response to S. pneumoniae respiratory infection.
PD-1 deficiency and PD-1 mAb blockade significantly increase IgG responses to native PPS, but not protein-conjugated PPS
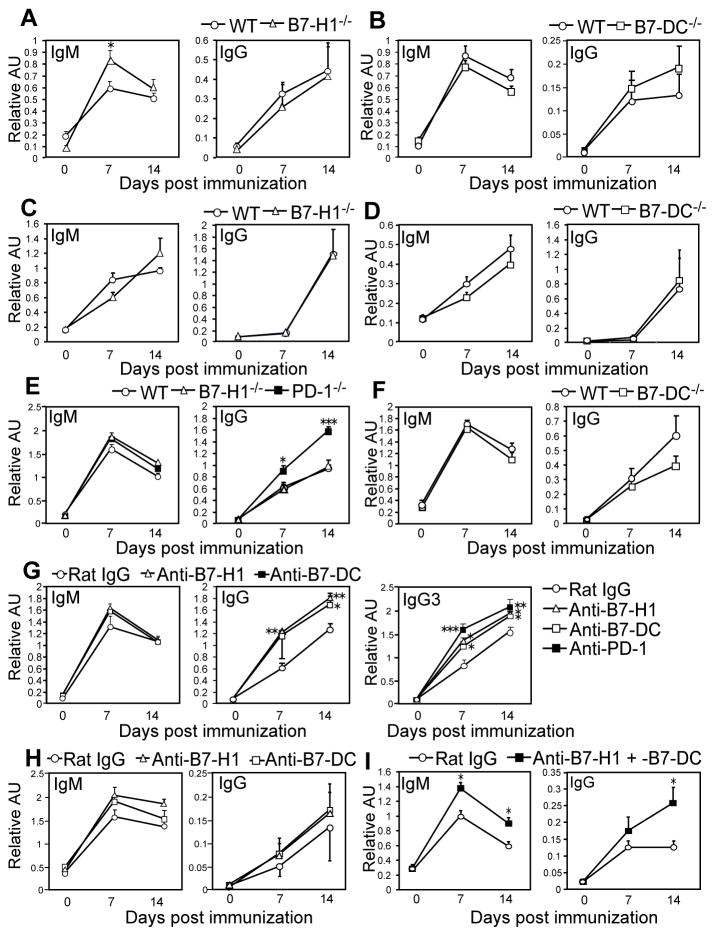
PD-1−/− mice also generated significantly increased PPS3-specific IgG responses following i.p. immunization with purified PPS3 or the PPV23 vaccine (Fig. 2A–B). Subcutaneous immunization, one of the approved routes of administration for the PPV23 vaccine in humans, elicited 9-fold higher PPS3-specific IgG titers in PD-1−/− mice relative to wild type mice (Fig. 2C; p=0.008). PD-1−/− mice generated significantly higher PPV23-specific IgG levels following both 0.1 μg and 1 μg PPS- equivalent PPV23 doses, with the mean d14 titer increased 2.6-fold (Fig. 2D, p=0.03). Notably, PPS doses outside this range are often tolerizing (24). Differences in IgG were largely due to significantly increased PPS-specific IgG3 levels in PD-1−/− mice (Fig. 2A–B), as we did not detect significant increases in PPS3-specific IgG1, IgG2b, and IgG2c levels in immune wild type or PD-1−/− mice relative to day 0 values (data not shown). IgM responses were normal to moderately increased in PD-1−/− mice. Thus, PD-1-deficiency yields significantly higher PPS3-specific IgG responses to both purified PPS3 and PPS3 associated with live S. pneumoniae.
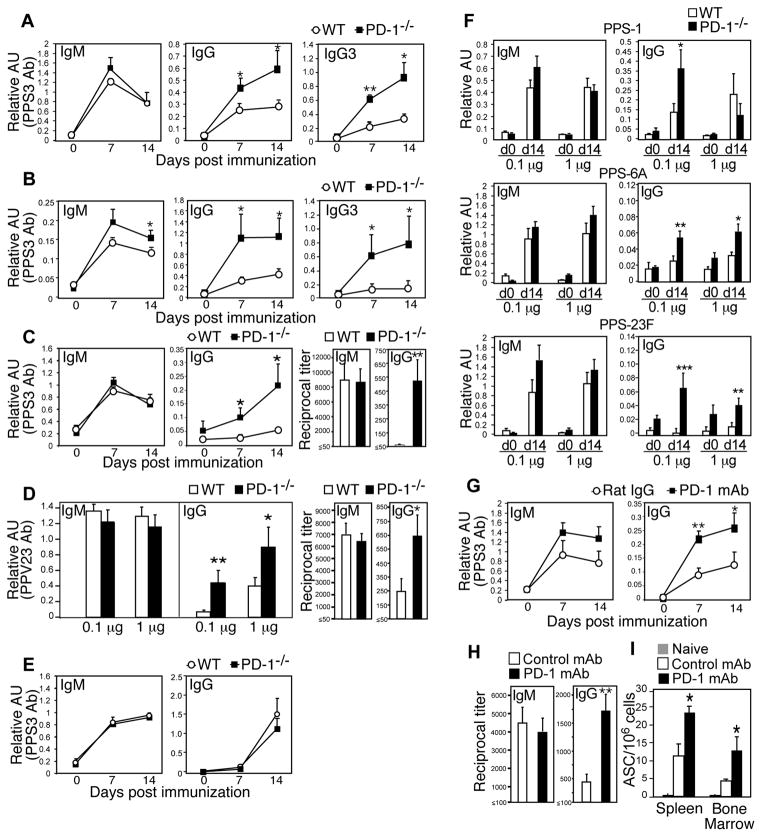
Figure 2. PD-1 suppresses IgG production against the native, but not protein-conjugated, PPS vaccine.
A–C) PPS3-specific serum IgM, IgG, and IgG3 levels in WT and PD-1−/− mice post immunization with: 0.1 μg purified PPS3 (A), an equivalent dose of 1 μg PPS3 contained in the PPV23 vaccine given i.p. (B) or s.c (C). D) PPV23-specific serum IgM (d7) and IgG (d14) levels after immunization with PPV23 containing 0.1 μg or 1 μg of each PPS. In C and D, d14 endpoint dilution titers are also shown. E) PPS3-specific serum IgM and IgG levels in WT and PD-1−/− mice following immunization with an equivalent dose of 0.1 μg PPS3 (CRM197-conjugated) contained within Prevnar-13. F) PPS-1, -6A, and -23F-specific serum IgM (d7) and IgG (d14) levels in WT and PD-1−/− mice following immunization with PPV23 containing 0.1 μg or 1 μg each PPS. G–I) PPS3-specific serum IgM and IgG levels on d0-14 (G), d14 endpoint dilution titers (H), and spleen and bone marrow PPS3-specific Ab-secreting cell frequencies (I; d40) in WT mice that received either PD-1 blocking mAb (RMP1-14) or control rat IgG mAb on d1, 3, and 5 post immunization with purified PPS3. In panels G and I, mice were immunized with 0.1 μg purified PPS3 and in panel H, with 1 μg purified PPS3. (A–I) Mean (±SEM) values are shown (n ≥ 4 mice/group). Asterisks (*) indicate significant differences in values (p≤0.05, *; p ≤ 0.01, **; p ≤ 0.001, ***; unpaired Student’s t test) between PD-1−/− and WT mice (A–F) or between WT mice receiving PD-1 versus control mAb (G–I).
We next assessed Ab responses against structurally unrelated PPS. As shown in Fig. 2F, PD-1−/− and wild type mice generated similar IgM responses against PPS1, -6A, and -23F. Wild type mice produced increased IgG over d0 values in response to the zwitterionic PPS1, which behaves as both a TI-2 and TD Ag (25), but did not produce appreciable IgG responses against PPS-6A and -23F relative to pre-immune levels. In comparison, PD-1−/− mice generated significantly higher IgG levels in response to each of these PPSs, with the exception of the high (1 μg) PPS1 dose (Fig. 2F). The lack of increased PPS1-specific IgG levels in PD-1−/− mice could be due to the opposing role PD-1 plays in promoting TD Ab responses (26). We therefore examined the extent to which PD-1 regulates Ab responses to PPS3 conjugated to the CRM197 protein (Prevnar-13 vaccine). As shown in Fig. 2E, PPS3-specific IgM responses to Prevnar-13 were similar, while IgG responses were slightly reduced in PD-1−/− mice relative to wild type mice. Thus, PD-1 plays a significant role in suppressing IgG responses to multiple clinically relevant native PPS. However, conversion of native PPS to a TD Ag abolishes PD-1-mediated suppression of PPS-specific IgG responses.
Because PD-1−/− mice may have pre-existing phenotypic alterations that could contribute to increased PPS-specific IgG responses, we assessed the effects of blocking PD-1 from interacting with its ligands in normal mice. PD-1 mAb blockade between d1 and d5 post-immunization significantly increased PPS3-specific IgG responses in wild type mice (Fig. 2G–H). This effect was observed with both 0.1 μg (Fig. 2G) and 1 μg (Fig. 2H) PPS3 doses (the optimal dose range for inducing PPS3-specific Ab responses). PD-1 blockade increased mean IgG titers over control mice by ~3.5-fold (Fig. 2H; p=0.008). PPS3-specific ASCs were significantly increased in both spleen (p=0.02) and bone marrow (p=0.03) of PPS3-immune mice that had received PD-1 mAb blockade (Fig. 2I). Thus, PD-1 inhibits PPS-specific Ab responses by suppressing the generation and/or maintenance of splenic and bone marrow ASC.
PD-1 suppresses PPS3-specific B cell proliferation, isotype switching, and plasmablast differentiation
We did not find alterations in the numbers or frequencies of total B cells or B-1b cells in the spleens or peritoneal cavities of PD-1−/− mice compared to wild type mice, although peritoneal B-2 cells were elevated (Fig. S1A). Moreover, PD-1−/− B cells proliferated normally in response to BCR stimulation in vitro (Fig. S1B). We therefore examined the effects PD-1-deficiency had on PPS3-specific B cell responses using flow cytometry, as we have previously described (20). PPS3-specific B cell frequencies and numbers were similar between naïve wild type and PD-1−/− mice (Fig. 3A–B). Thus, increased Ab levels in PD-1−/− mice were not explained by increased PPS3-specific B cell precursor frequencies. Three days post immunization, a small (1.5-fold) increase in PPS3-specific B cell frequencies occurred in wild type mice (Fig. 3A) as we have previously reported (20). However, PPS3-specific B cell frequencies and numbers were increased ~2 to 2.5-fold in PD-1−/− mice (Fig. 3A–B; p=0.038 and p=0.017, respectively). PPS3-specific Ki-67+ B cell frequencies and numbers in PD-1−/− mice were also significantly higher (~2-fold) than in wild type mice at this time point (Fig. 3C; p=0.02 and p=0.02, respectively). In contrast, we did not detect differences in the frequencies of nonAg-specific B cells that were Ki-67+ (wild type, 6.7 ± 0.5% vs. PD-1−/−, 6.4 ± 0.1%). Consistent with these findings, the frequencies and number of PPS3-specific BrdU+ B cells following a 5-day pulse beginning at the time of PPS3 immunization were significantly higher (>2-fold) in PD-1−/− mice than wild type mice (Fig. 3D; p=0.02 and p=0.01, respectively). These differences were selective for Ag-specific cells, as non-Ag-specific BrdU+ B cell frequencies were similar between wild type and PD-1−/− mice (wild type: 18.4 ± 2.3 % vs. PD-1−/−: 20.2 ± 2.4%). Thus, in the absence of PD-1, PPS3-specific B cell clonal expansion was increased following immunization.
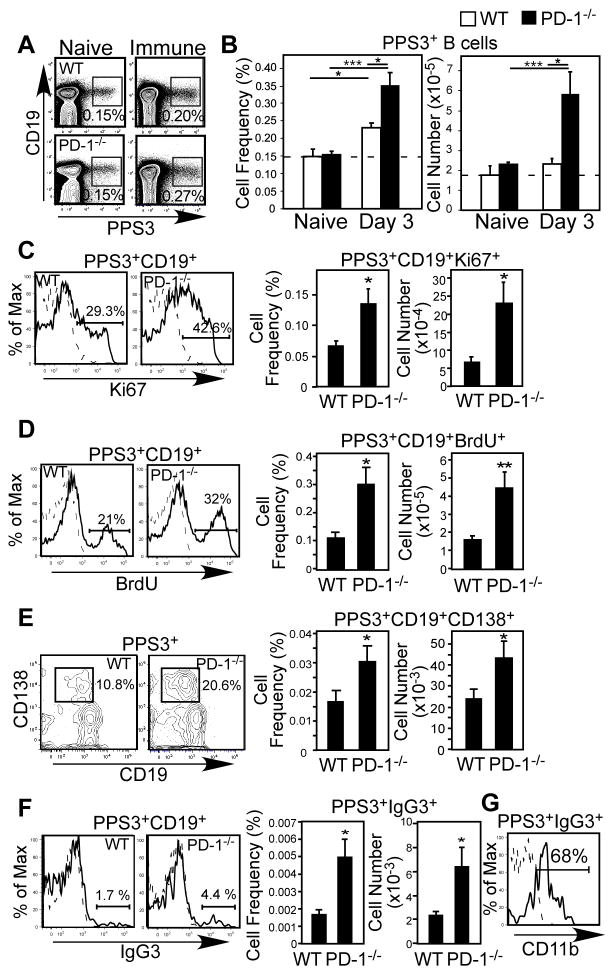
Figure 3. PD-1 is induced on activated PPS3-specific B cells and PD-1−/− mice have significantly increased PPS3-specific B cell proliferation, IgG switching, and ASC generation.
(A–G) Flow cytometric analysis and enumeration of PPS3-binding (Ag-specific) spleen cells from naïve and immune PD-1−/− and WT mice immunized with 0.1 μg purified PPS3. (A) Representative flow cytometric analysis of PPS3-specific splenic CD19+ B cells in naïve (left plots) and immune (d3; right plots) mice. Frequencies of gated cells are indicated. (B) Mean (±SEM) frequencies and cell numbers of splenic PPS3-specific splenic CD19+ B cells in naïve and d3 immune mice. (C) Representative intracellular Ki-67 expression by PPS3-specific splenic CD19+ B cells in d3 immune mice (solid line, left panel). Mean frequencies and numbers (±SEM) of PPS3-specific Ki-67+ cells splenic B cells are graphed. D) BrdU incorporation in PPS3-specific splenic CD19+ B cells 5 days post-immunization following a 5-day BrdU pulse (solid line, left panel). Mean frequencies and numbers (±SEM) of PPS3-specific BrdU+ splenic B cells are indicated. E) Representative PPS3-specific splenic CD19+CD138+ B cell staining in d5 immune mice (solid line, left panel). Mean frequencies and numbers (±SEM) of PPS3-specific CD138+ cells splenic B cells are graphed. F) Representative IgG3 staining of PPS3-specific splenic CD19+ B cells in immune mice (solid line, left panel). Graphs indicate total mean (±SEM) frequencies and cell numbers of PPS3-specific splenic IgG3+CD19+ B cells. (G) CD11b expression by PPS3-specific splenic IgG3+CD19+ B cells in immune PD-1−/− mice (solid line). In C, D, F, and G, the dashed histogram indicates mAb isotype control staining (intracellular staining in C and D or extracellular staining in F and G) for PPS3-specific B cells from immune mice. Asterisks (*) in B–F indicate significant differences between WT and PD-1−/− values (p ≤0.05, *; p ≤ 0.01, **; p ≤ 0.001, ***; n ≥ 3 mice/group). Student’s t test was used in B–E. In F, Welch’s t-test was used due to unequal variance between groups as determined by F-test.
PD-1−/− mice exhibited significantly increased frequencies and numbers of CD138+CD19+PPS3+ splenic plasmablasts 5 days post immunization relative to wild type mice (Fig. 3E; p=0.048 and p=0.035, respectively). Moreover, the frequency and number of IgG3+ class-switched PPS3-specific B cells was significantly higher (~2.5-fold) in PD-1−/− mice compared to wild type mice at this time point (Fig. 3F; p=0.048). Notably, a major fraction of PPS3-specific IgG3+ B cells in PD-1−/− mice expressed CD11b (Fig. 3G), suggesting that PD-1-deficiency may affect IgG3 switching in the B-1b cell population known to participate in PPS3-specific Ab responses (20, 23). Thus, in the absence of PD-1, PPS3-specific B cell division and the frequency of class-switched IgG3+ cells were increased.
B cell-specific PD-1 expression suppresses PPS-specific Ab responses
To assess the potential mechanism by which PD-1 suppresses PPS-specific B cell expansion and IgG production, we first examined whether PD-1 was expressed by PPS3-specific B cells. Naïve PPS3-specific B cells lacked PD-1 expression. However, a fraction (~25%) of PPS3-specific B cells expressed PD-1 following immunization (Fig. 4A). Frequencies of PD-1+ PPS3-non-binding cells were not altered by immunization (not shown). As expected, B cells from immune PD-1−/− mice remained negative. A fraction of PPS3-specific CD11b+ (B-1) B cells expressed PD-1 (Fig. 4B), although not all PD-1-expressing B cells were CD11b+. Activated (FSChiCD86+) PPS3-specific B cells exhibited a ~3-fold increase in PD-1 expression (MFI) levels relative to non-activated (FSCloCD86−) PPS3-specific B cells in both immune (p=0.02) and naïve mice (p=0.02; Fig. 4C). Approximately 50% of activated PPS3-specific B cells expressed PD-1 at levels over background staining (isotype control and PD-1−/− activated B cell staining; Fig. 4D) whereas PD-1 expression by non-activated PPS3-specific B cells was similar between immune and naïve mice (Fig. 4D–E). Consistent with this, PD-1+PPS3+ B cells exhibited increased size (FSC) and CD86 expression (Fig. 4F), whereas PD-1neg PPS3+ B cells from immune mice closely resembled PPS3+ B cells from naïve mice. These data support that PD-1 expression is induced on activated PPS3-specific B cells in immune mice.
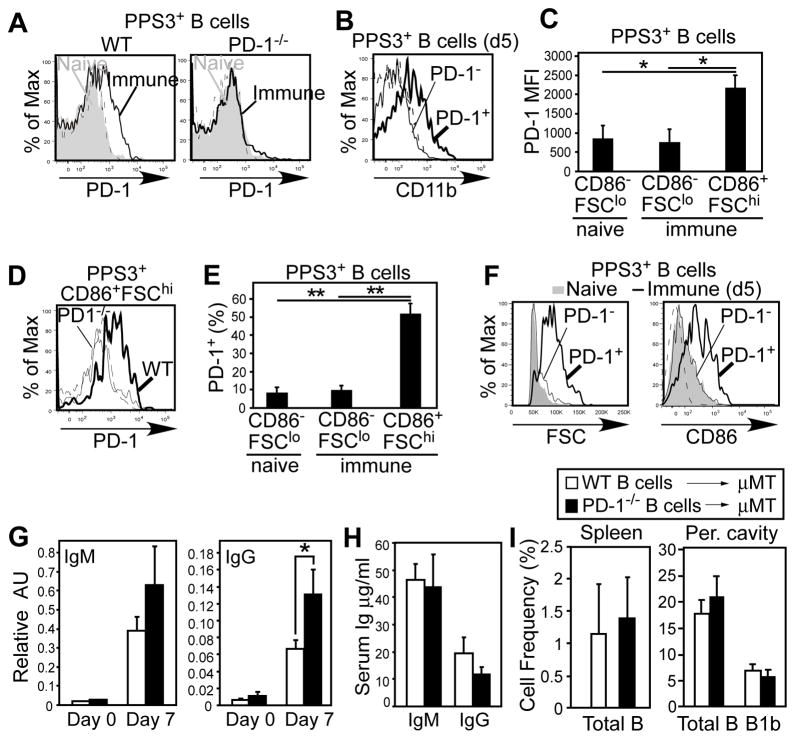
Figure 4. B-cell specific PD-1 expression suppresses PPS3-specific IgG responses.
A) PD-1 expression on naïve (gray shading) and PPS3-immune (thick solid line) PPS3-specific CD19+ splenic B cells from WT and PD-1−/− mice. The dashed histogram indicates mAb isotype control staining for PPS3-specific B cells from immune mice. B) CD11b expression on PPS3-specific CD19+ (PD-1+ and PD-1−) B cells from WT mice 5 days post immunization (0.1 μg PPS3). C) PD-1 expression levels (mean MFI (±SEM)) on PPS3-specific resting (FSCloCD86−) and activated (FSChiCD86+) splenic B cells in immune (d5) and naïve mice (n=3 mice/group). D) PD-1 staining on PPS3-specific CD19+FSChiCD86+ splenic B cells from WT and PD-1−/− mice 5d post immunization. The dashed histogram indicates mAb isotype control staining of PPS3-specific CD19+FSChiCD86+ splenic B cells from WT mice. E) Mean frequency (±SEM) of PPS3-specific resting (FSCloCD86−) and activated (FSChiCD86+) splenic B cells in immune (d5) and naïve mice expressing PD-1 (n=3 mice/group). F) FSC and CD86 expression by naïve PPS3-specific CD19+ B cells (gray shading), and PD-1neg (thin line) and PD-1+ (thick line) PPS3-specific CD19+ B cells in immune (d5) mice. The dashed histogram indicates mAb isotype control staining. G) Mean (±SEM) PPV23-specific serum IgM and IgG levels in μMT mice that were reconstituted with 4 × 107 splenic B cells (i.p. transfer) from WT or PD-1−/− mice and immunized with PPV23 containing 0.1 μg each PPS two days post adoptive transfer (n=6 mice/group; pooled results from 2 experiments). Pre-immune (d2 post transfer) and d7 immune (d9 post transfer) values are shown. H) Total serum IgM and IgG concentrations in μMT mice 9 days post reconstitution. I) Mean (±SEM) spleen B cell and peritoneal cavity total B cell and B-1b cell frequencies in μMT mice 2 weeks post reconstitution. Asterisks (*) indicate significant differences between values (p ≤0.05, *; p ≤ 0.01, **; unpaired Student’s t test, n ≥ 3 mice/group).
Selective up-regulation of PD-1 on Ag-specific B cells suggests a potential mechanism whereby B cell-intrinsic PD-1 expression could regulate PPS-specific B cell responses. To assess this, 4 × 107 wild type or PD-1−/− spleen B cells were adoptively transferred into B cell deficient (μMT) mice, since high numbers (1 × 107) of splenic B cells reconstitute B-1b cells and PPS-specific Ab responses (23). Recipients were immunized with PPV23 two days post transfer. Mice reconstituted with PD-1−/− B cells produced significantly higher PPV23-specific IgG levels relative to recipients of wild type B cells (Fig. 4G). PPV23-specific IgM responses were not significantly different (Fig. 4G). Notably, total (non-specific) reconstituted serum IgM and IgG levels at this time point were not significantly different between recipient groups (Fig. 4H), demonstrating that there was a selective increase in PPV23-specific IgG responses in PD-1−/− B cell-recipient mice. Finally, splenic B cell and peritoneal total B cell and B-1b cell recovery were similar between recipient groups (Fig. 4I), indicating that differential B cell reconstitution was unlikely to have caused the increased PPS-specific IgG responses observed in PD-1−/− B cell recipient mice. Thus, B cell-expressed PD-1 plays a key role in suppressing PPS-specific IgG responses.
Unfortunately, detection of wild type PPS-specific B cells in reconstituted mice is not a feasible strategy to assess the effects of B cell-intrinsic PD-1 expression on Ag-specific B cell proliferation during in vivo responses. To assess this, we adoptively transferred CFSE-labeled allotype-marked (CD45.1+) peritoneal B-1b cells from B1-8high IgH knock-in mice into the peritoneal cavities of CD45.2+ PD-1−/− mice. The recipient mice were immunized with NP40-Ficoll and treated with either a PD-1 blocking mAb (RMP1-14) or an isotype control mAb. Nearly all NP-specific (λ+CD45.1+) CD19+ B cells isolated from the peritoneal cavities of both groups of mice had undergone extensive division by 3 days post-immunization and expressed CD11b and PD-1 (Fig. S2A). In contrast, non-specific (λ −) CD45.1+ B cells were non-dividing, CD11b+/−, and lacked PD-1 expression. Interestingly, NP-specific peritoneal B-1b cells in mice treated with the PD-1 blocking mAb showed significantly increased (~2.5-fold) frequencies of IgG3+ cells (Fig. S2B) and these IgG3+ cells had lost significantly more CFSE than those in mice receiving control mAb (Fig. S2C). Although differences in IgG3+ frequencies were not observed in transgenic B cells recovered from spleens, significantly increased division was observed in spleens from PD-1−/− mice treated with the PD-1 mAb relative to control mAb (Fig. S2D). Thus, B cell-intrinsic PD-1 expression suppresses NP-specific B cell proliferation and the generation and/or proliferation of IgG+ class-switched B-1b cells during in vivo responses to NP-Ficoll.
PD-1 suppresses IgG responses to PPS and other TI-2 Ags through combined interactions with B7-H1 and B7-DC
B7-H1 and B7-DC have been shown to have overlapping or distinct functions, depending on the model system under study (9). We assessed the role of PD-1 ligands, B7-H1 and B7-DC, in PD-1-mediated regulation of protective Ab responses to S. pneumoniae. As observed with PD-1−/− mice (Fig. 1A–B), B7-H1−/− and B7-DC−/− mice exhibited similar susceptibilities and lung bacteria burdens following virulent S. pneumoniae respiratory infection compared to wild type mice (Fig. 5A–B). However, in contrast to PD-1−/− mice which showed significantly increased PPS3-specific IgG relative to wild type mice following pneumococcal respiratory infection (Fig. 1E–G), B7-H1−/− and B7-DC−/− mice produced IgG levels that were similar to wild type mice (Fig. 5C–D). In addition, PPS3-specific IgG levels following PPV23 immunization were not significantly different between B7-H1−/− or B7-DC−/− mice and wild type mice (Fig. 6A–B). B7-H1−/− and B7-DC−/− mice also produced normal Ab responses to the PPS3-conjugate vaccine (Fig. 6C–D). Thus, in contrast to results with PD-1−/− mice, B7-H1−/− and B7-DC−/− mice do not exhibit altered PPS-specific humoral responses to S. pneumoniae infection or PPS immunization.
Figure 5. Naïve B7-H1−/− and B7-DC−/− mice exhibit normal survival kinetics and similar PPS3-specific Ig levels following S. pneumoniae respiratory infection.
C57BL/6 WT, B7-H1−/−, and B7-DC−/− mice were challenged i.n. with 105, 106, or 107 CFU WU2 and monitored for signs of morbidity requiring euthanasia. A) Kaplan-Meier survival curves are shown. No significant differences were found based on Log-rank analysis. B) Lung bacterial burdens 3 days post challenge with 107 CFU WU2. C–D) PPS3-specific serum IgM, IgG, and IgA levels in (C) B7-H1−/− and (D) B7-DC−/− mice 14 days post infection with 106 CFU WU2 (n ≥ 5 mice/group).
Figure 6. B7-H1 and B7-DC are both required for suppression of PPS-specific IgG responses.
A–B) PPS3-specific serum IgM and IgG levels in B7-H1−/− (A) and B7-DC−/− (B) mice 0, 7, and 14 days post PPV23 immunization (~0.1 μg PPS3) relative to WT mice. C–D) PPS3-specific serum IgM and IgG levels in B7-H1−/− (C) and B7-DC−/− (D) mice following immunization with Prevnar-13 (~0.1 μg PPS3). E–F) TNP-specific serum IgM and IgG levels in WT, PD-1−/−, B7-H1−/−, and B7-DC−/− mice following TNP-Ficoll immunization. G) TNP-specific serum IgM, IgG, and IgG3 levels in WT mice administered blocking mAbs against B7-H1, B7-DC, PD-1 or rat IgG control mAbs following TNP-Ficoll immunization. H–I) PPS3-specific IgM and IgG levels in WT mice administered single blocking mAbs against B7-H1 or B7-DC (H) or blocking mAbs against both B7-H1 and B7-DC (I) following PPV23 immunization. A–I) Mean values (± SEM; n ≥4 mice/group) are shown. Asterisks (*) indicate significant differences in values between knockout mice and WT mice (A and E) or WT mice receiving control mAbs and functional PD-1/PDL blocking mAbs (G and I) (p ≤ 0.05, *; p ≤0.01, **; p ≤0.001, ***; unpaired Student’s t test).
PD-1−/− mice also generated significantly higher IgG responses to the synthetic TI-2 Ag, TNP-Ficoll (Fig. 6E), as reported for responses to DNP-Ficoll (16). PD-1−/− mice produced significantly higher levels of TNP-specific IgG1, IgG2b, IgG2c, IgG3, and IgA (data not shown). In contrast, TNP-specific IgM and IgG responses to TNP-Ficoll were unaltered by B7-H1 or B7-DC deficiency (Fig. 6E–F), with the exception of IgG2c, which was selectively increased in B7-H1−/− mice (data not shown). Levels of TNP-specific IgG3, which is the predominant IgG isotype produced in response to this and other TI-2 Ags, were also normal in B7-H1−/− or B7-DC−/− mice. Thus, individual B7-H1 or B7-DC deficiency had little effect on total IgM or IgG responses to PPS (Fig. 6A–F (PPS3); PPS-1, -6A, and -23F; data not shown) or a synthetic TI-2 Ag, TNP-Ficoll.
Given the possibility that B7-H1−/− and/or B7-DC−/− mice have other defects that negatively affect TI-2 Ab responses, we examined the effects of B7-H1 and B7-DC mAb blockade in wild type mice. B7-H1 and B7-DC blockade each significantly increased IgG, but not IgM responses, to TNP-Ficoll relative to rat IgG control-treated mice (Fig. 6G). Consistent with this, B7-H1 and B7-DC mAb blockade each significantly increased TNP-specific IgG3 (Fig. 6G). However, PD-1 blockade produced the greatest increase in TNP-specific IgG3 levels. IgG2b, IgG2c, and IgG1 (but not IgA) TNP-specific levels were also increased in mice receiving B7-H1 and B7-DC mAb blockade (data not shown). Thus, B7-H1 and B7-DC mAb blockade in wild type mice, in contrast to B7-H1 and B7-DC deficiency, significantly increased IgG responses to TNP-Ficoll. However, PD-1 mAb blockade produced greater increases than single blockade alone, suggesting potential overlapping functions for B7-H1 and B7-DC in carrying out PD-1-mediated suppression of IgG responses.
Single B7-H1 or B7-DC mAb blockade moderately increased PPS3-specific IgG responses relative control mice (Fig. 6H), although B7-H1, but not B7-DC, blockade significantly increased PPS3-specific IgM levels, similar to what was observed in B7-H1−/− mice (Fig. 6A). To test the combined roles of B7-H1 and B7-DC in PD-1-mediated suppression of PPS Ab responses, we co-administered B7-H1 and B7-DC blocking mAbs to wild type mice following PPV23 immunization. As shown in Fig. 6I, dual blockade significantly increased PPS3-specific IgM and IgG levels (p=0.036) comparable to what was observed with PD-1 mAb blockade and PD-1 deficiency (Fig. 2). Collectively, these results demonstrate B7-H1 and B7-DC have overlapping functions in carrying out PD-1-mediated suppression of PPS Ab responses.
PD-1 deficiency and mAb blockade in wild type mice suppresses protective humoral immune responses to S. pneumoniae
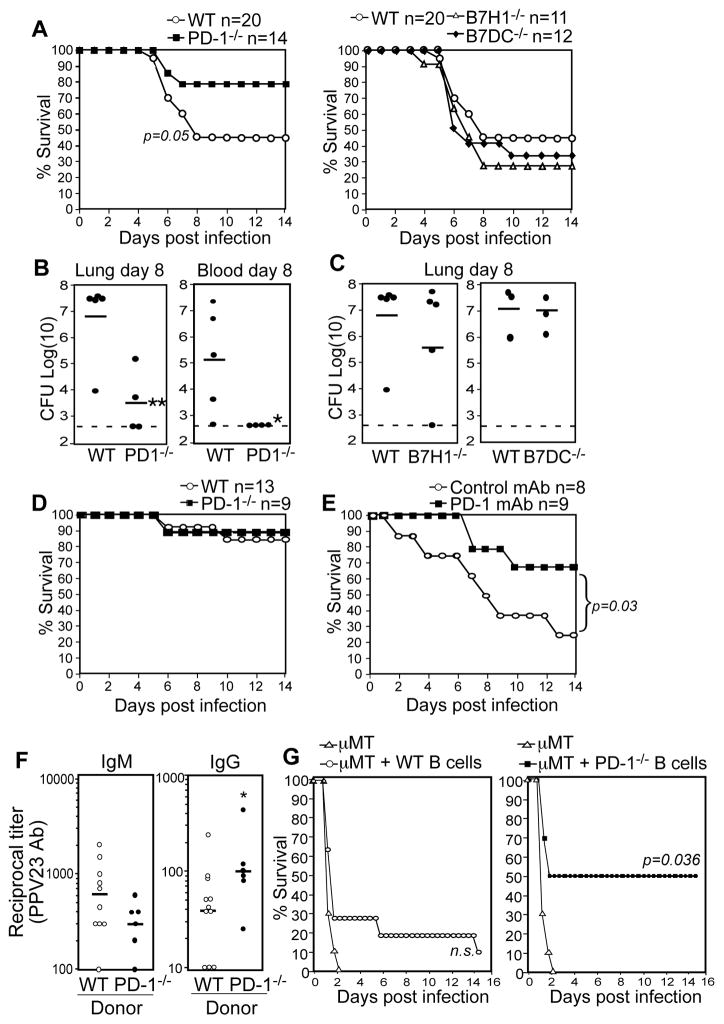
Based on studies in mice, IgG Abs raised against the S. pneumoniae capsule and cell wall constituents provide superior protection relative to IgM Abs (27). We therefore examined the physiological relevance of PD-1-mediated suppression of capsule-specific IgG responses by challenging immune wild type and PD-1−/− mice with a lethal i.n. dose (1 × 107 CFU) of WU2 28 days following immunization. PPV23-immunized wild type mice were offered moderate protection (~40% survival) whereas immune PD-1−/− mice had significantly increased survival (~80%; Fig. 7A). Consistent with these results, PD-1−/− mice had significantly lower bacterial counts in the lung and blood (bacteremia) (Fig. 7B, p=0.01 and p=0.04, respectively). In contrast, survival and bacterial counts in immune B7-H1−/− and B7-DC−/− mice were similar to wild type mice (Fig. 7A, C). Thus, despite the similar sensitivities of naïve PD-1−/−, B7-H1−/−, B7-DC−/−, and wild type mice to this infection (Figs. 1A–C, 5A–B), immunization provided a selective advantage to PD-1−/− mice, likely owing to increased PPS3-specific IgG in these mice. No differences in survival were observed between wild type and PD-1−/− mice that had been immunized with Prevnar-13 (Fig. 7D), which elicits normal PPS Ab responses in these mice (Fig. 2E). Finally, wild type mice receiving transient PD-1 mAb blockade following PPV23 immunization demonstrated significantly increased survival relative to control mAb-treated mice following respiratory challenge (Fig. 7E, p=0.03). Thus, PD-1-mediated suppression of PPS-specific humoral immunity impairs vaccine-mediated protection against lethal pneumococcal respiratory infections.
Figure 7. PPS immunization in the context of PD-1 deficiency or blockade significantly increases survival against lethal respiratory S. pneumoniae infection and is dependent on B cell-intrinsic PD-1 expression.
A–C) Purified PPS3-immunized WT, PD-1−/−, B7-H1−/−, and B7-DC−/− mice were challenged with a lethal i.n. dose (1 × 107 CFU) of WU2 28 days following immunization. A–B) Survival analysis shows a significant difference between PD-1−/− and WT mice (p=0.05; Fisher’s Exact test). B–C) Total lung (B and C) and blood (per mL; B only) CFU 8 days post challenge. Asterisks (*) indicate significant differences in CFU between WT and PD-1−/− mice (p ≤0.05, *; p ≤ 0.01, **; Student’s t test). D) Survival in Prevnar-13-immunized WT and PD-1−/− mice challenged i.n. with 107 CFU WU2 28 days following immunization. E) Survival in WT mice given PD-1 blocking or control mAbs at the time of purified PPS3 immunization (as described in Fig. 6 legend) and challenged i.n. with 1 × 107 CFU WU2 28 days following immunization (n=8–9 mice/group). Survival curves were significantly different as determined by Log-rank analysis (p=0.03). F–G) Reconstitution of μMT mice with B cells from PD-1−/− mice yields significantly increased PPS-specific IgG responses and significantly increases protection against infection. Control μMT mice (n=10), and μMT mice reconstituted with 2 × 107 wild type (n=11) or PD-1−/− (n=6) spleen B cells, were immunized on d1 as in Figure 4. F) PPV23-specific IgM and IgG reciprocal endpoint dilution titers were determined for d7 sera and were defined as the dilution yielding an OD405nm value 3-fold higher than values for non-reconstituted μMT mice. (*p=0.04, unpaired Student’s t test). G) Mice were infected i.p. with 200 CFU WU2 on d8, with differences in overall survival assessed by Fisher’s Exact test.
B cell-intrinsic PD-1 expression regulates protection against S. pneumoniae infection
To assess whether PD-1 expression on B cells directly regulates protection against S. pneumoniae infection, we transferred wild type or PD-1−/− B cells into B cell-deficient mice. We then immunized recipients and challenged them with a lethal S. pneumoniae infection. As observed in earlier experiments (Fig. 4G), μMT mice reconstituted with PD-1−/− B cells produced significantly higher IgG (~2.5-fold higher titers on d7), but not IgM levels in response to PPV23 (Fig. 7F; p=0.04). Importantly, PPV23-immunized μMT mice reconstituted with PD-1−/− B cells were offered significant protection against systemic S. pneumoniae infection (50% survival) relative to non-reconstituted mice (0% survival; Fig. 7G). In contrast, overall survival in μMT mice reconstituted with wild type B cells did not significantly differ from non-reconstituted μMT mice (9% versus 0%; Fig. 7G). Thus, B cells from PD-1−/−, but not wild type, mice afforded B cell-deficient mice with significant protection against systemic pneumococcal infection.
Discussion
Herein, we show that the PD-1:PD-L regulatory axis suppresses protection against respiratory and invasive S. pneumoniae infections by regulating the protective adaptive humoral immune response to one of the most potent virulence factors of S. pneumoniae – the capsular polysaccharide (28). For the first time, our study provides definitive evidence that PD-1 expression on B cells has profound physiological consequences for the regulation of immunity to infection. Specifically, our data demonstrates that PD-1 suppresses B cell clonal expansion and IgG production in response to native and bacterial-associated PPS, but not protein-conjugated PPS, revealing a unique role for the PD-1:PD-L axis in selectively suppressing TI responses to pathogens. Our data further indicate that B7-H1 and B7-DC both contribute to PD-1-mediated suppression. Finally, our results showing that PD-1 blockade following PPS immunization significantly increases protection against lethal S. pneumoniae infection has important implications for future strategies aimed at enhancing protection against life-threatening encapsulated bacterial infections.
Interestingly, PD-1 or PD-L deficiency had little effect on lung bacterial burdens or survival following an acute respiratory or systemic infection with a highly invasive S. pneumoniae serotype 3 strain (20, 29) - a serotype associated with one of the highest fatality rates in humans (30, 31). In contrast to our results, naive PD-1−/− mice are more resistant to peritoneal polymicrobial sepsis (15) and Listeria monocytogenes challenge (12) due to the role PD-1 plays in suppressing innate cells, yet are more susceptible to Mycobacteria tuberculosis respiratory infection due its role in controlling excessive inflammatory responses which may be largely promoted by CD4+ T cells (13, 14). The lack of effect PD-1 and PD-L deficiency have on primary pneumococcal respiratory challenge in our study may reflect dual roles for the PD-1:PD-L pathway in suppressing innate cell clearance mechanisms while limiting inflammation or alternatively, a limited role for the PD-1:PD-L pathway in regulating innate responses to a rapidly progressing lethal pneumococcal infection.
Nonetheless, PD-1 suppressed the generation of a protective humoral response during acute respiratory pneumococcal infection. Although Ab responses were generated too slowly to impact the outcome of primary infection with highly virulent pneumococci, this suppression was physiologically relevant since 1) sera from S. pneumoniae-infected PD-1−/− mice was superior in reducing bacteremia and delaying time to death in S. pneumoniae-challenged μMT mice compared to sera from wild type mice and 2) PD-1−/− mice that survived a low lethal dose respiratory infection were offered significant protection against subsequent lethal systemic pneumococcal infection, in contrast to wild type mice. These findings are likely explained by the significantly increased levels of capsule-specific serum IgG in PD-1−/− mice, which enables optimal clearance of heavily-encapsulated bacteria by promoting FcγR-mediated uptake, complement activation, and phagocyte activation and survival (2, 3). Indeed, multiple studies in mice have demonstrated the importance of IgG in eliciting protection against pneumococcus (2, 3, 5, 6, 19, 32). Importantly, PD-1 deficiency and transient PD-1 mAb blockade in wild type mice following immunization both yielded significantly increased PPS-specific IgG levels and protection against lethal S. pneumoniae challenge. Adoptive transfer experiments revealed that these effects were dependent on B cell-expressed PD-1. Thus, the PD-1:PD-L pathway plays a central role in suppressing highly protective IgG responses against the pneumococcal capsule, and may similarly regulate immunity to other polysaccharide-bearing pathogens.
To our knowledge, our study provides the first definitive evidence of a physiological role for PD-1 expression on B cells in vivo. Other studies have shown PD-1-mediated effects on B cell responses in vivo (21, 26, 33, 34). However, to date only T cell-intrinsic PD-1 signaling has been shown to regulate B cell responses (26, 34). PPS3 induced PD-1 expression on PPS3-specific B cells, some of which belonged to the B-1b cell subset, similar to that observed for hapten-specific B-1b cells from mice and non-human primates following immunization with NP- or TNP-Ficoll (35). Indeed, PD-1 may be a global suppressor of T cell independent responses, as PD-1 also suppresses Ab responses to haptenated Ficoll (16, 21), a T cell independent antigen that differs from PPS3 in several key respects (in contrast to PPS3 and many other types of PPS, it lacks co-associated bacterial contaminants that influence humoral responses (36), lacks charge, lacks tolerogenic potential, can be adjuvanted by TLR agonists (37), and localizes to different accessory cells (38, 39)).
Importantly, our results indicate that PD-1 functions to significantly limit PPS3-specific B cell proliferation and the generation of IgG+ cells. Since proliferation and isotype switching are tightly linked processes (40, 41), it is not surprising that PD-1 had the most significant effects on PPS-specific IgG production. Notably, there was a trend for increased PPS3-specific IgA (albeit not significant) in PD-1−/− mice, and PD-1 deficiency and mAb blockade significantly increased IgA levels in response to TNP-Ficoll. Therefore, we propose PD-1 primarily regulates isotype switching by suppressing the ability of Ag-specific B cells to divide, although it remains possible that PD-1 also influences the production of (or responsiveness to) switch factors. We did not detect splenomegaly or increased splenic or peritoneal total B cell or B-1b cell numbers in PD-1−/− mice, nor did we observe altered BCR-induced in vitro proliferation in splenic B cells from PD-1−/− mice as originally reported by Nishimura et al. (16). These discrepancies could be due to differences in the composition of gut flora between colonies, as Fagarasan and colleagues have elegantly demonstrated that altered flora can alter B cell function in PD-1−/− mice and that antibiotic therapy can normalize their activation phenotype (34, 42). Consistent with the above results, PD-1−/− and wild type spleen B cells reconstituted similar numbers of B-1b cells in B cell-deficient mice. Thus, our findings are not explained by altered B-1b cell numbers or B cell hyperactivity in PD-1−/− mice. This is further supported by the similar effects PD-1 blocking antibodies had on Ag-specific B-1b cell proliferation, IgG3 switching, and IgG production by wild type and VHB1-8 Tg B cells. A large fraction of IgG3-switched cells in PD-1−/− mice were CD11b+, consistent with the key role B-1b cells play in the IgG3 response to PPS3 (20, 23). This finding and the results of our VHB1-8 Tg B-1b cell transfer experiments support the role PD-1 plays in suppressing the ability of B-1b cells to divide and undergo isotype switching. However, it is important to note that our data do not discount a role for PD-1 in regulating the ability of other B cell subsets to participate in PPS-specific Ab responses. Indeed, PD-1, along with additional activation markers (FSChiCD86+), were expressed by both CD11b+ and CD11b− PPS3-specific B cells, and in vitro division of both conventional B cells and B-1b cells can be suppressed by PD-1 engagement (16, 21, 43). The selective sensitivies distinct B cell subsets may have to PD-1-mediated regulation remain to be established. Moreover, whether PD-1 signaling on B cells influences PPS-specific Ab diversity, Ab affinity, or additional B cell functions that are independent of proliferation remains to be determined.
Interestingly, use of a PPS-protein conjugate abolished the effect PD-1 deficiency had on PPS-specific Ab responses and survival during pneumococcal infection. PD-1 has been shown to promote TD antibody responses through interactions between PD-1+ TFH cells and B7-DC+ germinal center B cells (26). Moreover, CD40 ligation overcomes PD-1-mediated suppression of BCR-induced proliferation in vitro (21). Thus, it is conceivable that T cell help via CD40L overcomes B cell-intrinsic, PD-1-mediated suppression during these responses. Normal Ab responses to the PPS-conjugate vaccine in PD-1−/− mice may reflect a balance between PD-1 suppression of the TI-2 Ab response through B cell-intrinsic expression and support of the TD response through TFH cell-intrinsic expression. Collectively, these studies highlight the complex nature of the PD-1:PD-L pathway in regulating humoral immunity.
In contrast to many studies which support a dominant role for PD-1:B7-H1 interactions in mediating T cell suppression, our study shows that both B7-H1 and B7-DC are important for suppression of B cell responses to S. pneumoniae. Unlike single ligand blockade or genetic deficiency, which had no effect (PPS) or modestly increased IgG responses (TNP-Ficoll), simultaneous B7-H1 and B7-DC blockade in wild type mice yielded significant increases in PPS-specific IgG responses comparable to those observed with PD-1 mAb blockade and in PD-1−/− mice. The interaction between PD-1-expressing Ag-specific B cells and B7-H1- and/or B7-DC-expressing cells ultimately controls the extent of Ag-specific B cell proliferation and switching. While B7-H1 expression is ubiquitous, B7-DC expression is largely limited to dendritic cells, macrophages, B-1a cells, and activated and memory B cells (8–11, 44, 45). An intriguing possibility is that homotypic B cell-B cell interactions among Ag-activated B cells stimulate PD-1:PD-L interactions and thereby suppress PPS-specific B cell proliferation. Identification of the PD-L-expressing cells controlling TI-2 Ag-specific B cell activity may offer further insight into PD-1-mediated regulation of B cell function as well as additional signals regulating TI-2 Ab responses.
In conclusion, our results demonstrate B cell-intrinsic PD-1 expression suppresses the protective humoral immune response to the capsule of S. pneumoniae. The selective suppression of TI-2 Ab responses by PD-1 interactions with B7-H1 and B7-DC points to a novel role for PD-1 in regulating Ag-specific B cell responses to carbohydrate Ags. As cell surface self-Ag bearing repetitive epitopes may initiate TI Ab responses, the PD-1:PD-L pathway may represent a key regulatory checkpoint for limiting the initiation of autoimmunity by innate-like B cells, such as B-1b and marginal zone B cells, known to produce Abs in the absence of cognate T cell interactions. Identifying strategies to transiently overcome this regulatory checkpoint to augment protective B cell responses to polysaccharide-bearing pathogens may be key to improving vaccine efficacy and therapeutic interventions for infectious diseases.
Supplementary Material
Acknowledgments
This work was supported by NIH R21AI095800, NIH R56AI08654, and ACS grant RSG-12-170-01-LIB. Shared resources support was provided by a National Cancer Institute Cancer Center Support Grant (CCSG) P30CA012197. JTM was supported by NIH Training grant AI007401.
The authors thank Katiana Garagozlo for assistance with experiments.
References
- 1.Kadioglu A, Weiser JN, Paton JC, Andrew PW. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol. 2008;6:288–301. doi: 10.1038/nrmicro1871. [DOI] [PubMed] [Google Scholar]
- 2.Tian H, Weber S, Thorkildson P, Kozel TR, Pirofski LA. Efficacy of opsonic and nonopsonic serotype 3 pneumococcal capsular polysaccharide-specific monoclonal antibodies against intranasal challenge with Streptococcus pneumoniae in mice. Infect Immun. 2009;77:1502–1513. doi: 10.1128/IAI.01075-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber S, Tian H, van Rooijen N, Pirofski LA. A serotype 3 pneumococcal capsular polysaccharide-specific monoclonal antibody requires Fcgamma receptor III and macrophages to mediate protection against pneumococcal pneumonia in mice. Infect Immun. 2012;80:1314–1322. doi: 10.1128/IAI.06081-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez-Fernandez A, Faro J, Fernandez C. Immune responses to polysaccharides: lessons from humans and mice. Vaccine. 2008;26:292–300. doi: 10.1016/j.vaccine.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 5.Briles DE, Claflin JL, Schroer K, Forman C. Mouse IgG3 antibodies are highly protective against infection with Streptococcus pneumoniae. Nature. 1981;294:88–90. doi: 10.1038/294088a0. [DOI] [PubMed] [Google Scholar]
- 6.McLay J, Leonard E, Petersen S, Shapiro D, Greenspan NS, Schreiber JR. Gamma-3 gene-disrupted mice selectively deficient in the dominant IgG subclass made to bacterial polysaccharides. II. Increased susceptibility to fatal pneumococcal sepsis due to absence of anti-polysaccharide IgG3 is corrected by induction of anti-polysaccharide IgG1. J Immunol. 2002;168:3437–3443. doi: 10.4049/jimmunol.168.7.3437. [DOI] [PubMed] [Google Scholar]
- 7.Romero-Steiner S, Frasch CE, Carlone G, Fleck RA, Goldblatt D, Nahm MH. Use of opsonophagocytosis for serological evaluation of pneumococcal vaccines. Clin Vaccine Immunol. 2006;13:165–169. doi: 10.1128/CVI.13.2.165-169.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013;14:1212–1218. doi: 10.1038/ni.2762. [DOI] [PubMed] [Google Scholar]
- 9.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Ann Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang S, Chen L. Immunobiology of cancer therapies targeting CD137 and B7-H1/PD-1 cosignal pathways. Curr Top Microbiol Immunol. 2011;344:245–267. doi: 10.1007/82_2010_81. [DOI] [PubMed] [Google Scholar]
- 11.Brown KE, Freeman GJ, Wherry EJ, Sharpe AH. Role of PD-1 in regulating acute infections. Curr Opin Immunol. 2010;22:397–401. doi: 10.1016/j.coi.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao S, Wang S, Zhu Y, Luo L, Zhu G, Flies S, Xu H, Ruff W, Broadwater M, Choi IH, Tamada K, Chen L. PD-1 on dendritic cells impedes innate immunity against bacterial infection. Blood. 2009;113:5811–5818. doi: 10.1182/blood-2009-02-203141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lazar-Molnar E, Chen B, Sweeney KA, Wang EJ, Liu W, Lin J, Porcelli SA, Almo SC, Nathenson SG, Jacobs WR., Jr Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proc Natl Acad Sci. 2010;107:13402–13407. doi: 10.1073/pnas.1007394107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barber DL, Mayer-Barber KD, Feng CG, Sharpe AH, Sher A. CD4 T cells promote rather than control tuberculosis in the absence of PD-1-mediated inhibition. J Immunol. 2011;186:1598–1607. doi: 10.4049/jimmunol.1003304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung CS, Ayala A. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci. 2009;106:6303–6308. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishimura H, Minato N, Nakano T, Honjo T. Immunological studies on PD-1 deficient mice: implication of PD-1 as a negative regulator for B cell responses. Int Immunol. 1998;10:1563–1572. doi: 10.1093/intimm/10.10.1563. [DOI] [PubMed] [Google Scholar]
- 17.Shin T, Yoshimura K, Crafton EB, Tsuchiya H, Housseau F, Koseki H, Schulick RD, Chen L, Pardoll DM. In vivo costimulatory role of B7-DC in tuning T helper cell 1 and cytotoxic T lymphocyte responses. J Exp Med. 2005;201:1531–1541. doi: 10.1084/jem.20050072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity. 2004;20:327–336. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 19.Haas KM, Hasegawa M, Steeber DA, Poe JC, Zabel MD, Bock CB, Karp DR, Briles DE, Weis JH, Tedder TF. Complement receptors CD21/35 link innate and protective immunity during Streptococcus pneumoniae infection by regulating IgG3 antibody responses. Immunity. 2002;17:713–723. doi: 10.1016/s1074-7613(02)00483-1. [DOI] [PubMed] [Google Scholar]
- 20.Haas KM, Blevins MW, High KP, Pang B, Swords WE, Yammani RD. Aging Promotes B-1b Cell Responses to Native, but Not Protein-Conjugated, Pneumococcal Polysaccharides: Implications for Vaccine Protection in Older Adults. J Infect Dis. 2014;209:87–97. doi: 10.1093/infdis/jit442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haas KM. Programmed cell death 1 suppresses B-1b cell expansion and long-lived IgG production in response to T cell-independent type 2 antigens. J Immunol. 2011;187:5183–5195. doi: 10.4049/jimmunol.1101990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haas KM, Sen S, Sanford IG, Miller AS, Poe JC, Tedder TF. CD22 ligand binding regulates normal and malignant B lymphocyte survival in vivo. J Immunol. 2006;177:3063–3073. doi: 10.4049/jimmunol.177.5.3063. [DOI] [PubMed] [Google Scholar]
- 23.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Yin JZ, Bell MK, Thorbecke GJ. Effect of various adjuvants on the antibody response of mice to pneumococcal polysaccharides. J Biol Response Mod. 1989;8:190–205. [PubMed] [Google Scholar]
- 25.Kalka-Moll WM, Tzianabos AO, Bryant PW, Niemeyer M, Ploegh HL, Kasper DL. Zwitterionic polysaccharides stimulate T cells by MHC class II-dependent interactions. J Immunol. 2002;169:6149–6153. doi: 10.4049/jimmunol.169.11.6149. [DOI] [PubMed] [Google Scholar]
- 26.Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010;11:535–542. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Briles DE, Nahm M, Schroer K, Davie J, Baker P, Kearney J, Barletta R. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J Exp Med. 1981;153:694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moxon ER, Kroll JS. The role of bacterial polysaccharide capsules as virulence factors. Curr Top Microbiol Immunol. 1990;150:65–85. doi: 10.1007/978-3-642-74694-9_4. [DOI] [PubMed] [Google Scholar]
- 29.Briles DE, Crain MJ, Gray BM, Forman C, Yother J. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun. 1992;60:111–116. doi: 10.1128/iai.60.1.111-116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garau J, Calbo E. Capsular types and predicting patient outcomes in pneumococcal bacteremia. Clin Infect Dis. 2007;45:52–54. doi: 10.1086/518576. [DOI] [PubMed] [Google Scholar]
- 31.Weinberger DM, Harboe ZB, Sanders EA, Ndiritu M, Klugman KP, Ruckinger S, Dagan R, Adegbola R, Cutts F, Johnson HL, O’Brien KL, Scott JA, Lipsitch M. Association of serotype with risk of death due to pneumococcal pneumonia: a meta-analysis. Clin Infect Dis. 2010;51:692–699. doi: 10.1086/655828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haas KM, Poe JC, Tedder TF. CD21/35 promotes protective immunity to Streptococcus pneumoniae through a complement-independent but CD19-dependent pathway that regulates PD-1 expression. J Immunol. 2009;183:3661–3671. doi: 10.4049/jimmunol.0901218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Titanji K, Velu V, Chennareddi L, Vijay-Kumar M, Gewirtz AT, Freeman GJ, Amara RR. Acute depletion of activated memory B cells involves the PD-1 pathway in rapidly progressing SIV-infected macaques. J Clin Invest. 2010;120:3878–3890. doi: 10.1172/JCI43271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawamoto S, Tran TH, Maruya M, Suzuki K, Doi Y, Tsutsui Y, Kato LM, Fagarasan S. The inhibitory receptor PD-1 regulates IgA selection and bacterial composition in the gut. Science. 2012;336:485–489. doi: 10.1126/science.1217718. [DOI] [PubMed] [Google Scholar]
- 35.Yammani RD, Haas KM. Primate B-1 cells generate antigen-specific B cell responses to T cell-independent type 2 antigens. J Immunol. 2013;190:3100–3108. doi: 10.4049/jimmunol.1203058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sen G, Khan AQ, Chen Q, Snapper CM. In vivo humoral immune responses to isolated pneumococcal polysaccharides are dependent on the presence of associated TLR ligands. J Immunol. 2005;175:3084–3091. doi: 10.4049/jimmunol.175.5.3084. [DOI] [PubMed] [Google Scholar]
- 37.Kovarik J, Bozzotti P, Tougne C, Davis HL, Lambert PH, Krieg AM, Siegrist CA. Adjuvant effects of CpG oligodeoxynucleotides on responses against T-independent type 2 antigens. Immunology. 2001;102:67–76. doi: 10.1046/j.1365-2567.2001.01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Humphrey JH. Tolerogenic or immunogenic activity of hapten-conjugated polysaccharides correlated with cellular localization. Eur J Immunol. 1981;11:212–220. doi: 10.1002/eji.1830110310. [DOI] [PubMed] [Google Scholar]
- 39.Harms G, Hardonk MJ, Timens W. In vitro complement-dependent binding and in vivo kinetics of pneumococcal polysaccharide TI-2 antigens in the rat spleen marginal zone and follicle. Infect Immun. 1996;64:4220–4225. doi: 10.1128/iai.64.10.4220-4225.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rush JS, Liu M, Odegard VH, Unniraman S, Schatz DG. Expression of activation-induced cytidine deaminase is regulated by cell division, providing a mechanistic basis for division-linked class switch recombination. Proc Natl Acad Sci. 2005;102:13242–13247. doi: 10.1073/pnas.0502779102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deenick EK, Hasbold J, Hodgkin PD. Switching to IgG3, IgG2b, and IgA is division linked and independent, revealing a stochastic framework for describing differentiation. J Immunol. 1999;163:4707–4714. [PubMed] [Google Scholar]
- 42.Maruya M, Kawamoto S, Kato LM, Fagarasan S. Impaired selection of IgA and intestinal dysbiosis associated with PD-1-deficiency. Gut Microbes. 2013;4:165–171. doi: 10.4161/gmic.23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhong X, Bai C, Gao W, Strom TB, Rothstein TL. Suppression of expression and function of negative immune regulator PD-1 by certain pattern recognition and cytokine receptor signals associated with immune system danger. Int Immunol. 2004;16:1181–1188. doi: 10.1093/intimm/dxh121. [DOI] [PubMed] [Google Scholar]
- 44.Tomayko MM, Steinel NC, Anderson SM, Shlomchik MJ. Cutting edge: Hierarchy of maturity of murine memory B cell subsets. J Immunol. 2010;185:7146–7150. doi: 10.4049/jimmunol.1002163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhong X, Tumang JR, Gao W, Bai C, Rothstein TL. PD-L2 expression extends beyond dendritic cells/macrophages to B1 cells enriched for V(H)11/V(H)12 and phosphatidylcholine binding. Eur J Immunol. 2007;37:2405–2410. doi: 10.1002/eji.200737461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.