Abstract
Transforming growth factor-β1 (TGF-β) is involved in multiple cellular processes through Src activation. In the canonical pathway, Src activation is initiated by pTyr530 dephosphorylation followed by a conformational change allowing Tyr419 auto-phosphorylation. A non-canonical pathway in which oxidation of cysteine allows bypassing of pTyr530 dephosphorylation has been reported. Here, we examined how TGF-β activates Src in H358 cells, a small cell lung carcinoma cell line. TGF-β increased Src Tyr419 phosphorylation, but surprisingly, Tyr530 phosphorylation was increased rather than decreased. Vanadate, a protein tyrosine phosphatase inhibitor, stimulated Src activation itself, but rather than inhibiting Src activation by TGF-β, activation by vanadate was additive with TGF-β showing that pTyr530 dephosphorylation was not required. Thus, the involvement of the non-canonical oxidative activation was suspected. TGF-β increased extracellular H2O2 transiently while GSH-ester and catalase abrogated Src activation by TGF-β. Apocynin, a NADPH oxidase inhibitor, inhibited TGF-β-stimulated H2O2 production. Furthermore, mutation of cysteines to alanine, 248C/A, 277C/A, or 501C/A abrogated, while 490C/A significantly reduced, TGF-β-mediated Src activation. Taken together, the results indicate that TGF-β-mediated Src activation operates largely through a redox dependent mechanism, resulting from enhanced H2O2 production through an NADPH oxidase and that cysteines 248, 277, 490, and 501 are critical for this activation.
Keywords: Src, TGF-β, hydrogen peroxide, oxidative modification, redox signaling
Introduction
Src is a ubiquitously expressed non-receptor tyrosine kinase belonging to the Src family of protein tyrosine kinases (SFKs). By coupling signals from cell surface receptors and various intracellular signaling pathways (1), Src is involved in various fundamental cellular processes, including proliferation, differentiation, and transformation (2). Src also plays a critical role in epithelial-mesenchymal transition (EMT) (3-7), a process implicated in both wound healing and cancer metastasis (8-11).
In the canonical Src activation pathway, under basal conditions in vivo, a Src molecule remains inactive as a result of the intramolecular interactions between its SH3 domain and linker region, and its SH2 domain and the phosphorylated Tyr530 (pTyr530) in the C-terminal negative regulatory region (12,13). Upon stimulation, pTyr530 is dephosphorylated thus dissociating the inhibitory intracellular interactions and allowing Src to autophosphorylate at Tyr419 and restoring full activity (14-17). Thus, in the canonical pathway phosphorylation/dephosphorylation of Tyr530 plays a critical role in Src activation/deactivation. Several kinases and protein tyrosine phosphatases (PTPs) are involved in Src regulation (18), including C-terminal Src kinase (Csk) and Csk homologous kinase (Chk), that phosphorylate Src at Tyr530, and PTP1B, SHP1, PTPα, or PTPγ that reportedly catalyze Tyr530 dephosphorylation (18).
It has been well established that reactive electrophiles, such as H2O2, can participate in signal transduction by acting as second messengers through modification of the activity of signaling molecules. This so called redox signaling mechanism underlies the regulation of signaling pathways induced by many stimuli, including various physiological and/or pathological stimuli. Signaling molecules containing redox sensitive moieties such as cysteine, which readily reacts with electrophiles, have been found to be potential targets of regulation through redox mechanisms. PTPs have been recognized as major switches in redox signaling (19-25). PTPs all have a catalytic site structure in which a reactive cysteine moiety (26,27) is potentially susceptible to oxidative modification by H2O2. The oxidization is however, likely mediated by an enzyme, as the non-enzymatic rate of reaction with H2O2 is too slow to account for the inactivation (28). Redox modification inhibits the activity of PTPs and leads to increased phosphorylation of their corresponding substrate signaling molecules, resulting in altered signaling pathways.
Accumulating evidence suggests that Src can also be activated through a redox dependent mechanism. Purified Src is activated in vitro through either oxidation or alkylation of two cysteine (cys) residues on the protein (29). In vivo, Src is activated by various reactive oxidants such as cigarette smoke (30), acrolein (30), peroxynitrite, and hydrogen peroxide (H2O2) (31-34). In addition, Src activation by many other stimuli including some growth factors seems to involve redox mechanisms (35-38).
There are nine cysteine residues in human Src that are highly conserved among Src family members and species (39), some of which have been implicated in Src activity regulation by oxidative stimuli. Senga first reported that mutation of Cys487 in v-Src (corresponding to Cys 490 in human Src) suppressed its activity while mutation of others changed Src stability (39). In an in vitro assay, Cys277 seemed critical for the homodimerization and activation of Src (40). Giannoni et al. found that Cys245 and Cys487 in v-Src (corresponding to Cys248 and Cys490 in human respectively) were involved in Src activation by oxidants, and dephosphorylation of Tyr530 was important for the early (10min) activation of Src, while a redox mechanism was involved in the late phase of Src activation (45min) in response to extracellular matrix (35).
Transforming growth factor-β1 (TGF-β) is a potent multifunctional growth factor involved in the regulation of cellular proliferation, differentiation and survival, and plays a predominant role in the EMT process (41). TGF-β initiates signaling through binding to its type II receptor, which recruits and phosphorylates type I TGF-β receptor. The type I receptor, a serine/threonine protein kinase, phosphorylates and activates diverse downstream signaling pathways, including ERK, JNK, p38MAPK, PI3K/AKT, and transcription factors such as SMAD2/3 (42).
Many effects of TGF-β are mediated through Src-mediated signaling pathways (43-45). How TGF-β activates Src is not completely clear. Some studies demonstrated that TGF-β could induce the production of oxidants that contributed to TGF-β-mediated effects (43,46), however, little is known about the role of oxidants in the activation of Src by TGF-β. Here, we report that a redox dependent mechanism, most likely involving cysteines of both Src and the PTPs that regulate its activity, is involved in Src activation by TGF-β in a non-canonical redox activation of Src.
Materials and Methods
Chemicals and reagents
Unless otherwise noted, all chemicals were from Sigma (St. Louis, MO). Antibodies to Src and phosphorylated Src were from Cell Signaling Technology, Inc. (Danvers, MA). M-PER Mammalian Protein Extraction Reagent was from Thermal Fisher Scientific Inc. (Thermal Fisher, Rockford, IL). Amplex Red reagent was from Life Technologies (Grand Island, NY). All chemicals used were at least analytical grade.
Cell culture and treatment
A human non-small cell lung carcinoma cell line (H358) was used. H358 cells were cultured in RPMI-1640 medium with 10% fatal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin, in a humidified incubator containing 5% CO2 at 37°C. Cells were treated when about 85% confluent. In catalase experiment, catalase (final concentration is 30 U/ml) was added to the culture medium immediately before TGF-β exposure.
Western Analysis
Briefly, cell lysate was extracted with M-PER and 30 μg protein was electrophoresed on a 4-20% Tris-glycine acrylamide gel (Invitrogen, Carlsbad, CA) and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore Corporation, Bedford, MA). Membranes were blocked with 5% fat-free milk and then incubated overnight at 4°C with primary antibody in 5% BSA dissolved in Tris-buffered saline (TBS). After being washed with 1XTBS containing 0.05% Tween 20 (TTBS), membranes were incubated with secondary antibody at room temperature for 2 h. After TTBS washing, membranes were treated with an enhanced chemiluminescence reagent mixture (Thermal Fisher Scientific, Rockford, IL) for 5 min and then imaged and analyzed using the Biospectrum imaging system (UVP, Upland, CA)
Immunoprecipitation
Cells were washed with cold phosphate-buffered saline (PBS) and collected in 1 ml lysis buffer containing 50 mM HEPES (pH 7.4), 150 mM NaCl, 1% Triton X-100, 0.5% NP40, 50 mM β-glycerophosphate, 1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 1 mM EGTA, 1 mM PMSF, 10% glycerol, 100 mM sodium fluoride, 10 μM DETAPAC, 10 ng/ml leupeptin, and 10 ng/ml aprotinin. 500 μg of whole cell lysate was used for immunoprecipitation with ANTI-FLAG M2 Affinity Gel (Sigma-Aldrich) overnight at 4°C. The complexes were washed three times with TBS, re-suspended in 100 μl of TBS containing 3 μg of 3XFLAG peptide, and incubated at 4°C for 30 min with shaking. After centrifuge, the supernatant was collected and used for Western Blots.
Construction of FLAG-Tagged Src plasmids, site-directed mutagenesis, and transfection
pcDNA3 c-Src (47) (Addgene plasmid 42202) was digested with Hind III and Xba I to get 1.6kb cDNA of human c-Src. The 1.6kb c-Src cDNA was then inserted into p3XFLAG-CMV-8 Expression vector (Sigma). The plasmid was selected with ampicillin and confirmed with DNA sequencing.
Site directed mutagenesis was then performed as described before (48) using a kit (GeneArt Site Directed Mutagenesis System, Invitrogen). The primer sequences were as following (only forward primer was shown): Src248C/A, CCTCACCACCGTGTTCCCCACGTCCAAG; Src277C/A, GCTGGGCCAGGGCTTCTTTGGCGAGGTG; Src490C/A, CTGCCCGCCGGAGTTTCCCGAGTCCCTG; Src501C/A, CCTCATGTGCCAGTTCTGGCGGAAGGAG. The mutations were confirmed with DNA sequencing.
Cells were transfected with plasmids with Lipofectamine 2000 when 90% confluence. Briefly DNA and Lipofectamine were mixed at 1:3.5 ratio and incubated at RT for 15 min before being added to cells. The medium was replaced the next day and 48 h after transfection, cells were treated and collected for experiments.
H2O2 measurement
H2O2 was measured in the extracellular media using Amplex Red reagent (10-acetyl-3,7-dihydroxyphenoxazine). Before treated with TGF-β cell medium was replaced with Krebs–Ringer phosphate glucose (KRPG) (145 mM NaCl, 5.7 mM sodium phosphate, 4.86 mM KCl, 0.54 mM CaCl2, 1.22 mM MgSO4, 5.5 mM glucose, pH 7.35). 120 μl of KRPG was collected at different time points after adding of TGF-β. To measure H2O2, 50 μl of sample was mixed with same volume of reaction buffer containing Amplex red and horseradish peroxidase. The fluorescence signal was detected kinetically with excitation wavelength at 529 nm and emission wavelength at 590 nm. The H2O2 concentration was then calculated based on standard curve.
Statistical analysis
Data were expressed as mean ± standard error. Wilcoxon rank-Sign test was used for statistical analysis of Western densitometry data and t-test was used for analysis of H2O2 data. Statistical significance was accepted when p < 0.05.
Results
TGF-β activated Src in redox-dependent manner
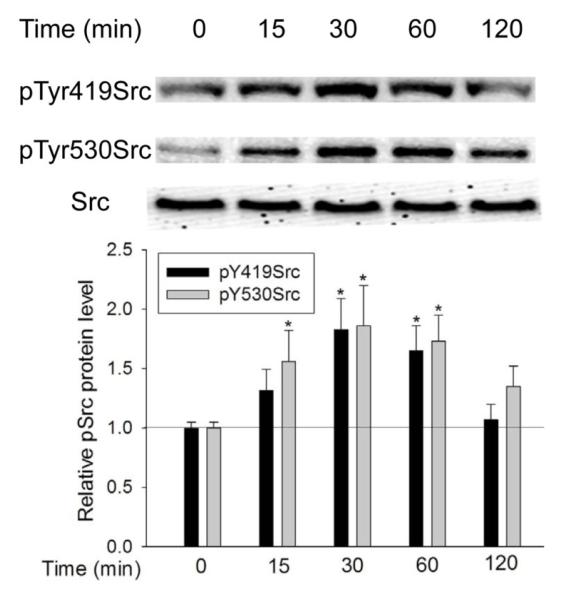
In the canonical pathway, Src activation depends on dephosphorylation of pTyr530 and subsequent phosphorylation at Tyr419. To determine whether Src activation by TGF-β follows this mechanism, we first examined the phosphorylation status of Src at Tyr419 and Tyr530 at different times after TGF-β exposure. As shown in Figure 1, after addition of TGF-β (5 ng/ml), phosphorylation of Src at Tyr419 (pTyr419Src) began to increase at 15 min and reached its highest level at 30 min. Src activation was sustained until it declined to control level after 2 hours of TGF-β exposure. The phosphorylation of Src at Tyr530 (pTyr530Src) followed a similar pattern; it increased at 15 min, reached its highest level at about 30 min, and decreased to control level after 2 hour.
Figure 1.
TGF-β increased Src phosphorylation at both Tyr419 and Tyr530. Cells were exposed to 5 ng/ml TGF-β for the indicated times and pTyr419 and pTyr530 Src were determined by Western Blotting. *, P<0.05, **, P<0.01, compared with control, N=3.
Effect of vanadate on Src activation by TGF-β
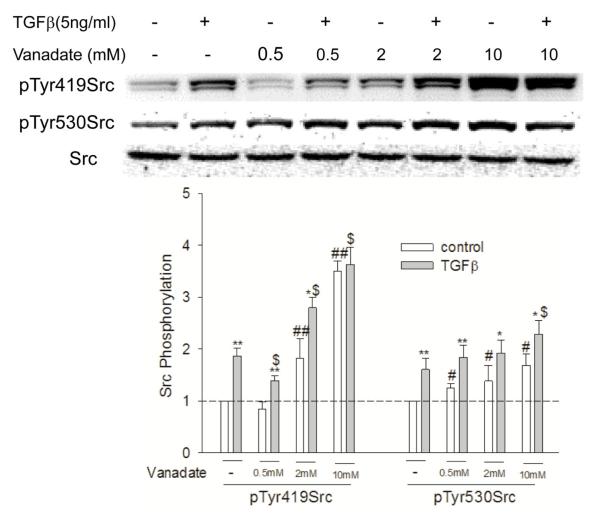
This increase in both pTyr419Src and pTyr530Src phosphorylation upon TGF-β stimulation demonstrated that dephosphorylation of pTyr530 was not essential for TGF-β-mediated Src activation. To further examine this deviation from the canonical pathway, cells were pretreated with sodium orthovanadate (vanadate) for 30 min before being exposed to TGF-β. Vanadate is a general PTP inhibitor and would be expected to block the enzymatic dephosphorylation of Src at Tyr530. Interestingly, vanadate increased both basal and TGF-β-mediated pTyr530 Src (Figure 2). This suggests that both the tyrosine kinase that catalyzes phosphorylation of Tyr530 and the PTP that dephosphorylates Tyr530 are endogenously active.
Figure 2.
Vanadate reveals non-canonical basal and TGF-β-stimulated Src activation. Cells were pretreated with different concentrations of vanadate for 1h before being exposed to TGF-β, the cells were then collected and Src phosphorylation at Tyr419 and Tyr530 was determined by Western Blotting. *, P<0.05, **, p<0.01, compared with vehicle control; #, p<0.05, ##, P<0.01, compared with control of no TGF-β and no vanadate exposure; $, P<0.05 compared with TGF-β treatment, N=3.
The effect of vanadate on the TGFβ-stimulated phosphorylation of Tyr419Src had a more complex dose-dependency. At 0.5 mM, vanadate appeared to inhibit both endogenous Src activation and Src activation by TGFβ. At 2 mM vanadate alone activated Src and appeared additive with TGFβ in activating Src. At 10 mM vanadate alone increased the level of pTyr419Src further and abrogated further stimulation of Src by TGF-β. As Src activation by vanadate was additive with TGF-β, pTyr530 dephosphorylation was clearly not required for Src activation by TGF-β. The results are possibly due to differential sensitivity to vanadate inhibition of PTPs involved in the dephosphorylation of pTyr530 and pTyr419. It appears that Tyr530 dephosphorylation is more sensitive to vanadate, resulting in greater pTyr530 at lower vanadate concentrations, and leading to more Src in the inhibitory form of pTyr530 and less pTyr419. At higher vanadate concentrations, both the dephosphorylation of pTyr530 and pTyr419 were inhibited, and this resulted in increased pTyr530 and pTyr419.
Induction of extracellular H2O2 by TGF-β
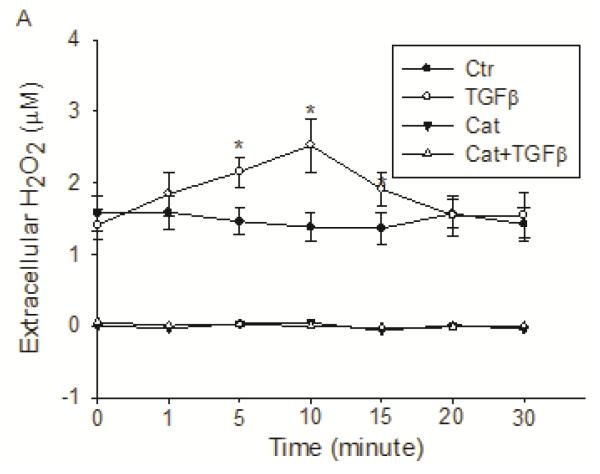
Previous studies have shown that a redox mechanism is implicated in many pathophysiological functions of TGF-β (43,46,49). As Src is a critical mediator of TGF-β signaling and its activity is regulated by oxidants. Thus, we hypothesized that Src activation by TGF-β could be redox-dependent. To test this, we first determined the production of H2O2 by TGF-β using Amplex Red. Upon TGF-β exposure (5 ng/ml), extracellular H2O2 was significantly increased at as early as 1 min, reached its highest level at 10 min, and was back to basal level in 20 min (Figure 3). Catalase (30 U/ml), which catalyzes the dismutation of H2O2 to O2 and H2O, completely removed the basal and TGF-β-produced H2O2 in the extracellular environment. H2O2 was produced continuously by H358 cells (Figure 3). Media incubated without cells did not produce any H2O2.
Figure 3.
TGF-β induced increase in extracellular H2O2. H358 cells were exposed to 5 ng/ml TGF-β for the times indicated and the extracellular H2O2 level was measured with Amplex Red reagent. *, P<0.01 compared with control, N=3.
Redox dependent Src activation by TGF-β
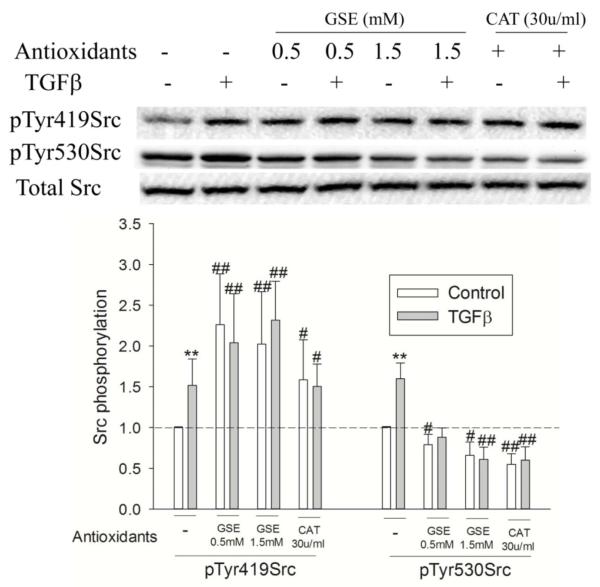
To further study the redox-dependence of Src activation by TGF-β, we next examined the effect of antioxidants. GSH-ester, a precursor of intracellular GSH, or catalase, which removed extracellular H2O2 as shown above, blocked Src activation (pTyr419Src) by TGF-β (Figure 4), indicating that TGF-β-mediated Src activation is accomplished through a redox dependent mechanism. Nonetheless, pretreatment with GSH-ester or catalase increased basal Src activation as shown by a significant elevation of basal pTyr419Src (Figure 4). This occurred concurrently with a decrease in pTyr530Src, suggesting that the basal level of Src activation was through redox dependent dephosphorylation of pTyr530. One possible explanation for this phenomenon could be the relief by GSH-ester or catalase of the inhibition of a PTP, as such enzymes are well known targets for inhibition by H2O2 (26,27).
Figure 4.
Effect of antioxidants on TGF-β-mediated Src activation. Cells were exposed to 5 ng/ml TGF-β for the indicated times and pTyr419 and pTyr530 Src were determined by Western Blotting. *, P<0.05, **, P<0.01 compared with control; #, P<0.05, ##, P<0.01, compared with control of no TGF-β, N=3.
Involvement of NOX in TGF-β-mediated H2O2 production and Src activation
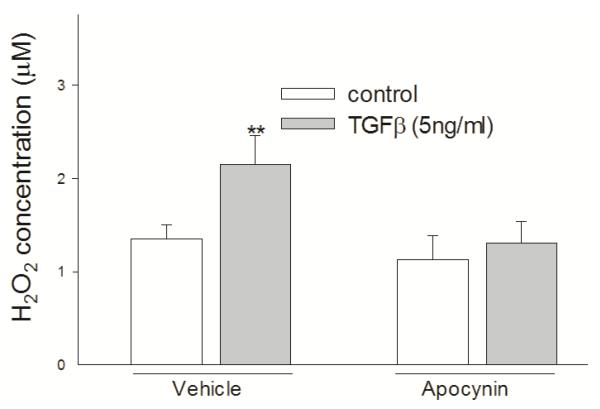
To determine the source of H2O2, cells were pretreated with apocynin, an inhibitor of NADPH oxidases (NOX), before being treated with TGF-β. Pretreatment with 300 μM apocynin for 30 min abrogated the increase of H2O2 caused by TGF-β, suggesting that TGF-β produced H2O2 through activating NOX (Figure 5).
Figure 5.
TGF-β generated H2O2 through NOX. Apocynin abrogated TGF-β-caused increase of extracellular H2O2 level. H358 cells were pretreated with/without 300 μM apocynin for 30 min and then treated with 5 ng/ml TGF-β for 10 min. H2O2 was measured using Amplex Red method. **, P<0.01 compared with control, N=3.
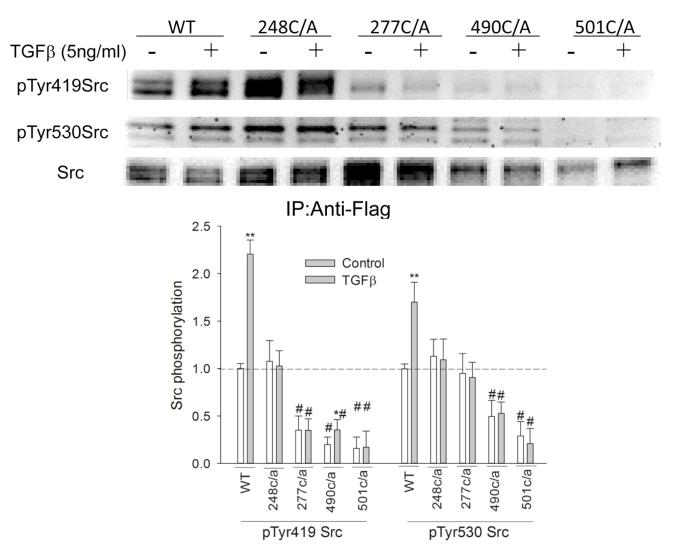
Mutation of cysteine residues decreased Src activation by TGF-β
Cysteine residues in Src are the most likely targets of oxidative modification resulting in Src regulation. To identify the cysteine residue(s) in human Src involved in Src activation by TGF-β, cysteine residues at 248, 277, 490, or 501 were replaced individually with alanine, using site-directed mutation. These cysteine residues were selected because of their conservation among Src kinase family members across species (39), or their reported sensitivity to redox modification (35,40,50). As shown in Figure 6, the phosphorylation of Src at Tyr419 and Tyr530 were affected in different ways by the cysteine/alanine mutation. The 248C/A had no effect on the basal level of pTyr419 or pTyr530 of Src. The 277C/A mutation did not affect the basal pTyr530Src, but decreased the basal level of pTyr419 Src by 50%. The 490C/A mutation decreased the basal level of pTyr530 by 67% and that of pTyr419 by 50%, while the 501C/A mutation decreased the basal level of pTyr530 and pTyr419 both by about 80%. TGF-β-mediated Src activation was abrogated by C/A mutation at residues 248, 277, or 501, while it was decreased significantly in 490C/A Src (52% vs. 120% induction in 490C/A and wild type FLAG-Src respectively).
Figure 6.
Mutation of cysteine residues in Src decreased Src activation by TGF-β . H358 cells were transfected with FLAG-tagged Src plasmids with/without cysteine/alanine mutations for 24 h and then exposed to 5 ng/ml TGF-β for 1h. FLAG-Src was immunoprecipitated with Anti-FLAG agarose beads and then the phosphorylation of Src was determined by Western blotting. *, P<0.05, **, P<0.01 compared with control, #, P<0.01, compared with control of no TGF-β, N=3.
Discussion
Src activation is responsible for many TGF-β-mediated effects (43-45). The mechanism underlying Src activation by TGF-β however, has been unclear. This study demonstrates that Src activation by TGF-β is largely through a redox-dependent pathway. In addition, we verified that some cysteine residues in Src are critical for Src regulation and mutation of cysteine at 277, 490 and 501 to alanine decreases the basal Src activity while mutation of cysteine residues at 248, 277, and 501 abrogates TGF-β-mediated Src activation.
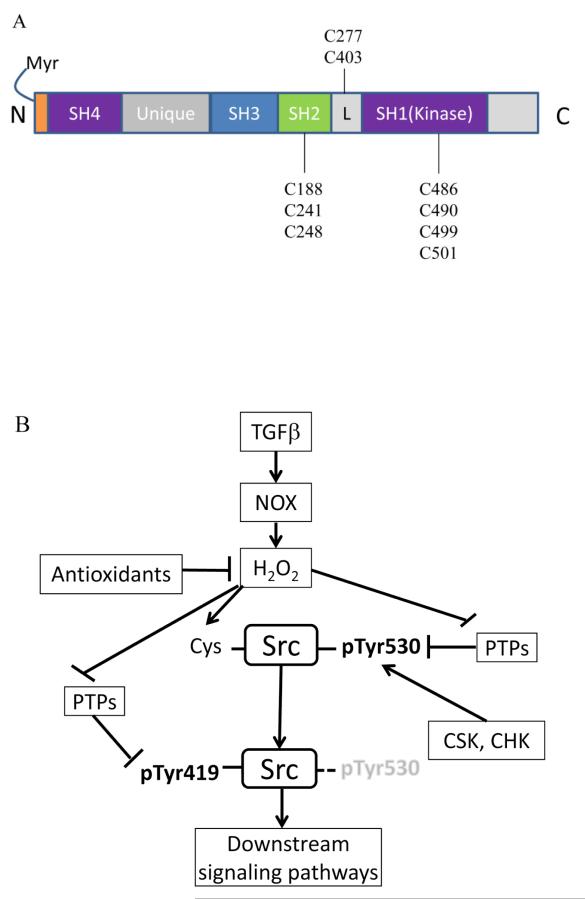
In the canonical pathway, Src activation is initiated by dephosphorylation of pTyr530 followed by a conformational change and subsequent dissociation of intracellular interactions and autophosphorylation of Src at Tyr419 (Figure 7B). Our data (Figure 1) suggest that a non-canonical mechanism, which does not require the dephosphorylation of pTyr530, was involved in TGF-β-mediated Src activation. Inhibition of PTPs (by vanadate at 0.5 and 2 mM concentration), which are responsible for the dephosphorylation of pTyr530, increased pTyr530 Src, but only inhibited TGF-β-mediated Src activation by~40%. In other words, ~60% of Src activation was independent of the dephosphorylation of pTyr530. This evidence further supports that both canonical and non-canonical pathways are involved in TGF-β-mediated Src activation.
Figure 7.
Redox regulation of Src by TGF-β. (A) Location of cysteine residues in Src protein; (B) possible targets of redox regulation in Src activation pathway by TGF-β.
The finding that antioxidants (GSH-ester and catalase) inhibited TGF-β-mediated Src activation indicated that Src activation by TGF-β operated, at least partially, through a redox-dependent mechanism. The results demonstrated a more complex relationship between oxidative Src activation and phosphorylation/dephosphorylation of Src tyrosines. Somewhat surprisingly, antioxidants alone decrease the phosphorylation of Src at Tyr530 while increasing pTyr419. Indeed, this suggests that in H358 cells, there is the potential for endogenous activation of Src through the canonical pathway that is inhibited by the endogenous H2O2. Removing H2O2 then reveals this endogenous Src activation. Most likely, the PTPs that dephosphorylate pTyr530 are inhibited by the endogenous H2O2 so that preventing its inactivation allows dephosphorylation of pTyr530 and subsequent autophosphorylation of Tyr419; i.e., the canonical pathway. In contrast, Src activation by TGF-β was, at least partially, independent of the dephosphorylation of pTyr530. Consistent with our finding, Akhand also found that nitric oxide-mediated Src activation was independent of the phosphorylation status of Tyr530 (51,52). This was further supported by a study from Pu et al. that Hg2+ activated Src in a redox dependent way with the mutation of Tyr530 or inhibition of its phosphorylation (51,52). The PTPs involved in the dephosphorylation of pTyr530, such as PTP1B (26,27) and SHP-1 (53) are redox sensitive. At resting condition, under which H2O2 is constantly produced, a small portion of the PTPs are inhibited. The addition of antioxidants would remove this basal H2O2 and thus reduce the inhibition on PTPs, resulting in increased dephosphorylation of pTyr530 and a subsequent increase in Src activity (the canonical pathway of Src activation).
Except for PTPs, kinases that phosphorylate Tyr530, including CSK and CHK, can also be regulated through redox-dependent mechanisms. Mills et al. reported that CSK activity could be regulated through the oxidation state of the disulfide bond in the SH2 domain, implying that CSK activity can be affected by redox changes in cells (54). H2O2 production may facilitate disulfide bond formation and increase CSK activity, leading to increased pTyr530. Furthermore, CSK activity can also be negatively regulated by Src through its phosphorylation of the CSK binding protein (CBP), which recruits CSK to the membrane to which Src translocates (55). Therefore the phosphorylation status of Tyr530 is regulated by a complex network with the net activity determined by the overall effect of these pathways. In contrast to current findings, Parasassi reported that supplementation with antioxidants such as N-acetylcysteine and dithiothreitol could decrease Src activity by up to 30% in a manner independent of the phosphorylation status of Src530 (56). This inconsistency may be related to differences in kinases and PTPs involved in the regulation of the phosphorylation of either Tyr530 or Tyr419 in the cell types used. Nonetheless, our data suggest that the basal Src activity is regulated through redox-dependent dephosphorylation of pTyr530, while the TGF-β-mediated Src activation operates mainly through a redox-dependent mechanism.
Previous studies have found that TGF-β generates H2O2 through several sources including mitochondria (57), NOX1, NOX2 (58), and NOX4 (59). Here, we measured H2O2 in the extracellular medium where H2O2 is potentially produced by a membrane NADPH oxidase or through the plasma membrane following cytosolic production. The appearance of induced extracellular H2O2 in the current study was observed significantly earlier than in previous reports of TGF-β-induced intracellular production of H2O2, which occurred 30 min after TGF-β exposure and was purportedly due to mitochondria (57) or after 8 h by the intracellular NOX4 (59). Here, TGF-β appears to generate H2O2 through a NOX, as the NOX inhibitor apocynin blocked elevation of H2O2 and Src activation caused by TGF-β (Figure 5). The inhibitory effect of apocynin on NOX depends upon the formation of diapocynin, the dimer form, through a process that is cell-type dependent (60). As it is unclear whether diapocynin is formed in H358 cells, we cannot be certain of the mechanism of the inhibition of apocynin of TGF-β-induced H2O2 production. While H2O2 from NOX4, the only NOX located in the cytosol, may affect Src activity later and modulate later events, the early production of H2O2 and activation of Src observed here implies involvement of a plasma membrane associated NOX rather than NOX4. The evidence supporting this assertion is that the inhibition of TGF-β-induced Src activation by extracellular catalase would not affect intracellular production of H2O2 because the large extracellular/intracellular volume would dilute H2O2 coming from an intracellular source even in the absence of catalase. Instead, the likely source is a membrane oxidase that increases H2O2 production extracellularly, either directly or through superoxide production and subsequent dismutation to H2O2 and O2.
A possible source of TGF-β produced extracellular H2O2 is NOX1 or NOX2. Rac and p47 phox, components of active NOX1 and 2 complex, are potential downstream target of TGF-β signaling (58,61). Another potential source of H2O2 is DUOX1, a predominant membrane NADPH oxidase in bronchial epithelial cells (62). DUOX1 can be activated through association with p47phox as a result of lipid raft clustering caused by stimulators including TNFα (63). Indeed, TGF-β was found to cause lipid raft location of TGF-β receptors and regulate signal transduction (64-66). Which member of the NOX family is involved has not been determined, but is beyond the scope of the present study.
The redox dependence of TGF-β-mediated Src activation is further supported by the finding that activation was abrogated by mutation of cysteine residues in Src. Mutation of Cys248, Cys277, or Cys501 to alanine completely inhibited, while that of Cys490 partially inhibited TGF-β-mediated Src activation; these findings suggest that cysteines 248, 277. 501, and 490 might be the targets of oxidative modification by H2O2 produced by TGF-β. Our results are consistent with those of Giannoni, et al., who found that Cys245 and Cys498 in v-Src (equal to Cys248 and Cys501 respectively in human Src) were critical for Src activation in response to ECM (35). Mutation of 490C/A and 501C/A decreased the basal level of pTyr419 and pTyr530 significantly, indicating that mutation of these two cysteines to alanines might cause conformational change of Src that result in inaccessibility of these tyrosine sites to kinases. Senga, et al. also reported that mutation of Cys498 of v-Src could suppress Src transforming activity (39). However, it remains to be further clarified how these C/A mutations affect Src regulation.
Among the 9 cysteine residues in human Src kinase, Cys 188, 241, and 248 are in the SH2 domain; Cys 277 and 403 are in the linker region; and Cys486, 490, 499 and 501 are in the kinase domain (Figure 7A). The current study and others (35), support the concept that Cys248, 277, 490, and 501 play critical roles in the regulation of Src activity. Indeed, mutation of cysteine residues in the C-terminus (such as Cys490 and 501) makes Src insensitive to redox regulation (29). On the other hand, there are also reports that some cysteine residues in Src might be involved in the suppression of Src activity. For instance, Cys277 was found to be responsible for oxidant-mediated Src homodimerization and inactivation in an in vitro assay (40), and mutation of Cys403 and Cys486 increased Src activity (50). Our data support that cysteine residue 248, 277, 490 and 501 of human Src are essential for Src activation by TGF-β and are potential targets of oxidative modification. Direct modification of cysteine residues in Src has been observed during activation by oxidative stimuli such as NO, herbimycin, and other agents (31,51,67). Interestingly Byeon, et al. demonstrated that a hydroquinone could bind to cysteine residues and cause Src activation (50). This evidence allows for the possibility that some cysteine residues in Src might be direct targets of oxidative modification. It still remains largely unknown, however, how cysteine modification actually causes Src activation. Some studies suggest there are intramolecular disulfide bonds that inhibit Src activity, and that cysteine modification could disrupt these disulfide bonds and activate Src (50). Other studies suggest that disulfide formation activates Src (14). Our current studies show that cysteine mutation to alanine, which prevents formation of disulfide bonds, is more consistent with the latter study.
Conclusion
In summary our data demonstrate three aspects of Src activation by TGF-β in H358 cells. H2O2 generation inhibits Src activation, apparently through inhibition of the PTPs that dephosphorylate pTyr530. In surprising contrast, TGF-β causes Src activation primarily through a redox-dependent, non-canonical pathway that is independent of the dephosphorylation of pTyr530. Finally, mutation of conserved cysteines inhibits TGF-β induced Src activation possibly by preventing disulfide formation.
Highlights.
TGF-β activates Src in H358 cells largely through a non-canonical redox mechanism.
TGF-β induces a transient increase in extracellular H2O2 with a peak at 10 min.
Cysteine mutations in Src abrogate Src activation by TGF-β.
Acknowledgement
This research was supported by NIH grants R21-ES020942 and R01-ES003598.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guarino M. Src signaling in cancer invasion. J Cell Physiol. 2010;223:14–26. doi: 10.1002/jcp.22011. [DOI] [PubMed] [Google Scholar]
- 2.Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23:7906–7909. doi: 10.1038/sj.onc.1208160. [DOI] [PubMed] [Google Scholar]
- 3.Cicchini C, Laudadio I, Citarella F, Corazzari M, Steindler C, Conigliaro A, Fantoni A, Amicone L, Tripodi M. TGFbeta-induced EMT requires focal adhesion kinase (FAK) signaling. Exp Cell Res. 2008;314:143–152. doi: 10.1016/j.yexcr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Xu KP, Yin J, Yu FS. SRC-family tyrosine kinases in wound- and ligand-induced epidermal growth factor receptor activation in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2006;47:2832–2839. doi: 10.1167/iovs.05-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ulianich L, Garbi C, Treglia AS, Punzi D, Miele C, Raciti GA, Beguinot F, Consiglio E, Di Jeso B. ER stress is associated with dedifferentiation and an epithelial-to-mesenchymal transition-like phenotype in PC Cl3 thyroid cells. J Cell Sci. 2008;121:477–486. doi: 10.1242/jcs.017202. [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Orozco R, Navarro-Tito N, Soto-Guzman A, Castro-Sanchez L, Perez Salazar E. Arachidonic acid promotes epithelial-to-mesenchymal-like transition in mammary epithelial cells MCF10A. Eur J Cell Biol. 2010;89:476–488. doi: 10.1016/j.ejcb.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Yang SZ, Zhang LD, Zhang Y, Xiong Y, Zhang YJ, Li HL, Li XW, Dong JH. HBx protein induces EMT through c-Src activation in SMMC-7721 hepatoma cell line. Biochem Biophys Res Commun. 2009;382:555–560. doi: 10.1016/j.bbrc.2009.03.079. [DOI] [PubMed] [Google Scholar]
- 8.Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 9.Gharaee-Kermani M, Hu B, Phan SH, Gyetko MR. Recent advances in molecular targets and treatment of idiopathic pulmonary fibrosis: focus on TGFbeta signaling and the myofibroblast. Curr Med Chem. 2009;16:1400–1417. doi: 10.2174/092986709787846497. [DOI] [PubMed] [Google Scholar]
- 10.Yauch RL, Januario T, Eberhard DA, Cavet G, Zhu W, Fu L, Pham TQ, Soriano R, Stinson J, Seshagiri S, Modrusan Z, Lin CY, O’Neill V, Amler LC. Epithelial versus mesenchymal phenotype determines in vitro sensitivity and predicts clinical activity of erlotinib in lung cancer patients. Clin Cancer Res. 2005;11:8686–8698. doi: 10.1158/1078-0432.CCR-05-1492. [DOI] [PubMed] [Google Scholar]
- 11.Soltermann A, Tischler V, Arbogast S, Braun J, Probst-Hensch N, Weder W, Moch H, Kristiansen G. Prognostic significance of epithelial-mesenchymal and mesenchymalepithelial transition protein expression in non-small cell lung cancer. Clin Cancer Res. 2008;14:7430–7437. doi: 10.1158/1078-0432.CCR-08-0935. [DOI] [PubMed] [Google Scholar]
- 12.Okada M, Nakagawa H. A protein tyrosine kinase involved in regulation of pp60c-src function. J Biol Chem. 1989;264:20886–20893. [PubMed] [Google Scholar]
- 13.Su J, Muranjan M, Sap J. Receptor protein tyrosine phosphatase alpha activates Src-family kinases and controls integrin-mediated responses in fibroblasts. Curr Biol. 1999;9:505–511. doi: 10.1016/s0960-9822(99)80234-6. [DOI] [PubMed] [Google Scholar]
- 14.Giannoni E, Taddei ML, Chiarugi P. Src redox regulation: again in the front line. Free Radic Biol Med. 2010;49:516–527. doi: 10.1016/j.freeradbiomed.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 15.Wang D, Esselman WJ, Cole PA. Substrate conformational restriction and CD45-catalyzed dephosphorylation of tail tyrosine-phosphorylated Src protein. J Biol Chem. 2002;277:40428–40433. doi: 10.1074/jbc.M206467200. [DOI] [PubMed] [Google Scholar]
- 16.Xu W, Doshi A, Lei M, Eck MJ, Harrison SC. Crystal structures of c-Src reveal features of its autoinhibitory mechanism. Mol Cell. 1999;3:629–638. doi: 10.1016/s1097-2765(00)80356-1. [DOI] [PubMed] [Google Scholar]
- 17.Wang SE, Wu FY, Shin I, Qu S, Arteaga CL. Transforming growth factorβ (TGF-β)-Smad target gene protein tyrosine phosphatase receptor type kappa is required for TGF-β function. Mol Cell Biol. 2005;25:4703–4715. doi: 10.1128/MCB.25.11.4703-4715.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sen B, Johnson FM. Regulation of SRC family kinases in human cancers. J Signal Transduct. 2011;2011:865819. doi: 10.1155/2011/865819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrett W, DeGnore JP, Konig S, Fales HM, Keng YF, Zhang ZY, Yim MB, Chock PB. Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry. 1999;38:6699–6705. doi: 10.1021/bi990240v. [DOI] [PubMed] [Google Scholar]
- 20.Chiarugi P, Fiaschi T, Taddei ML, Talini D, Giannoni E, Raugei G, Ramponi G. Two vicinal cysteines confer a peculiar redox regulation to low molecular weight protein tyrosine phosphatase in response to platelet-derived growth factor receptor stimulation. J Biol Chem. 2001;276:33478–33487. doi: 10.1074/jbc.M102302200. [DOI] [PubMed] [Google Scholar]
- 21.Denu J, Tanner KG. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry. 1998;37:5633–5642. doi: 10.1021/bi973035t. [DOI] [PubMed] [Google Scholar]
- 22.Krejsa CM, Nadler SG, Esselstyn JM, Kavanagh TJ, Ledbetter JA, Schieven GL. Role of oxidative stress in the action of vanadium phosphotyrosine phosphatase inhibitors. Redox independent activation of NF-kB. J Biol Chem. 1997;272:11541–11549. doi: 10.1074/jbc.272.17.11541. [DOI] [PubMed] [Google Scholar]
- 23.Lee S, Kwon KS, Kim SR, Rhee SG. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem. 1998;273:15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- 24.Li S, Whorton AR. Regulation of protein tyrosine phosphatase 1B in intact cells by Snitrosothiols. Arch Biochem Biophys. 2003;410:269–279. doi: 10.1016/s0003-9861(02)00696-3. [DOI] [PubMed] [Google Scholar]
- 25.Raugei G, Ramponi G, Chiarugi P. Low molecular weight protein tyrosine phosphatases: small, but smart. Cell and Molecular Life Sciences. 2002;59:941–949. doi: 10.1007/s00018-002-8481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barford D. Protein phosphatases. Curr Opin Struct Biol. 1995;5:728–734. doi: 10.1016/0959-440x(95)80004-2. [DOI] [PubMed] [Google Scholar]
- 27.Neel B, Tonks NK. Protein tyrosine phosphatases in signal transduction. Curr Opin Cell Biol. 1997;2:193–204. doi: 10.1016/s0955-0674(97)80063-4. [DOI] [PubMed] [Google Scholar]
- 28.Forman HJ, Maiorino M, Ursini F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49:835–842. doi: 10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oo ML, Senga T, Thant AA, Amin AR, Huang P, Mon NN, Hamaguchi M. Cysteine residues in the C-terminal lobe of Src: their role in the suppression of the Src kinase. Oncogene. 2003;22:1411–1417. doi: 10.1038/sj.onc.1206286. [DOI] [PubMed] [Google Scholar]
- 30.Zhang H, Liu H, Borok Z, Davies KJ, Ursini F, Forman HJ. Cigarette smoke extract stimulates epithelial-mesenchymal transition through Src activation. Free Radic Biol Med. 2012;52:1437–1442. doi: 10.1016/j.freeradbiomed.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krasnowska EK, Pittaluga E, Brunati AM, Brunelli R, Costa G, De Spirito M, Serafino A, Ursini F, Parasassi T. N-acetyl-l-cysteine fosters inactivation and transfer to endolysosomes of c-Src. Free Radic Biol Med. 2008;45:1566–1572. doi: 10.1016/j.freeradbiomed.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 32.Mallozzi C, Di Stasi AM, Minetti M. Activation of src tyrosine kinases by peroxynitrite. FEBS Lett. 1999;456:201–206. doi: 10.1016/s0014-5793(99)00945-x. [DOI] [PubMed] [Google Scholar]
- 33.Zhuang S, Schnellmann RG. H2O2-induced transactivation of EGF receptor requires Src and mediates ERK1/2, but not Akt, activation in renal cells. Am J Physiol Renal Physiol. 2004;286:F858–865. doi: 10.1152/ajprenal.00282.2003. [DOI] [PubMed] [Google Scholar]
- 34.Mehdi MZ, Pandey NR, Pandey SK, Srivastava AK. H2O2-induced phosphorylation of ERK1/2 and PKB requires tyrosine kinase activity of insulin receptor and c-Src. Antioxid Redox Signal. 2005;7:1014–1020. doi: 10.1089/ars.2005.7.1014. [DOI] [PubMed] [Google Scholar]
- 35.Giannoni E, Buricchi F, Raugei G, Ramponi G, Chiarugi P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol Cell Biol. 2005;25:6391–6403. doi: 10.1128/MCB.25.15.6391-6403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meng D, Shi X, Jiang BH, Fang J. Insulin-like growth factor-I (IGF-I) induces epidermal growth factor receptor transactivation and cell proliferation through reactive oxygen species. Free Radic Biol Med. 2007;42:1651–1660. doi: 10.1016/j.freeradbiomed.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Peng F, Gao B, Ingram AJ, Krepinsky JC. Mechanical strain-induced RhoA activation requires NADPH oxidase-mediated ROS generation in caveolae. Antioxid Redox Signal. 2010;13:959–973. doi: 10.1089/ars.2009.2908. [DOI] [PubMed] [Google Scholar]
- 38.Yan SR, Berton G. Regulation of Src family tyrosine kinase activities in adherent human neutrophils. Evidence that reactive oxygen intermediates produced by adherent neutrophils increase the activity of the p58c-fgr and p53/56lyn tyrosine kinases. J Biol Chem. 1996;271:23464–23471. doi: 10.1074/jbc.271.38.23464. [DOI] [PubMed] [Google Scholar]
- 39.Senga T, Miyazaki K, Machida K, Iwata H, Matsuda S, Nakashima I, Hamaguchi M. Clustered cysteine residues in the kinase domain of v-Src: critical role for protein stability, cell transformation and sensitivity to herbimycin A. Oncogene. 2000;19:273–279. doi: 10.1038/sj.onc.1203296. [DOI] [PubMed] [Google Scholar]
- 40.Kemble DJ, Sun G. Direct and specific inactivation of protein tyrosine kinases in the Src and FGFR families by reversible cysteine oxidation. Proc Natl Acad Sci U S A. 2009;106:5070–5075. doi: 10.1073/pnas.0806117106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katsuno Y, Lamouille S, Derynck R. TGF-beta signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol. 2013;25:76–84. doi: 10.1097/CCO.0b013e32835b6371. [DOI] [PubMed] [Google Scholar]
- 42.Kamato D, Burch ML, Piva TJ, Rezaei HB, Rostam MA, Xu S, Zheng W, Little PJ, Osman N. Transforming growth factor-beta signalling: role and consequences of Smad linker region phosphorylation. Cell Signal. 2013;25:2017–2024. doi: 10.1016/j.cellsig.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Sato M, Kawai-Kowase K, Sato H, Oyama Y, Kanai H, Ohyama Y, Suga T, Maeno T, Aoki Y, Tamura J, Sakamoto H, Nagai R, Kurabayashi M. c-Src and hydrogen peroxide mediate transforming growth factor-beta1-induced smooth muscle cell-gene expression in 10T1/2 cells. Arterioscler Thromb Vasc Biol. 2005;25:341–347. doi: 10.1161/01.ATV.0000152608.29351.8f. [DOI] [PubMed] [Google Scholar]
- 44.Bartscht T, Lehnert H, Gieseler F, Ungefroren H. The Src family kinase inhibitors PP2 and PP1 effectively block TGF-beta1-induced cell migration and invasion in both established and primary carcinoma cells. Cancer Chemother Pharmacol. 2012;70:221–230. doi: 10.1007/s00280-012-1904-0. [DOI] [PubMed] [Google Scholar]
- 45.Ungefroren H, Sebens S, Groth S, Gieseler F, Fandrich F. Differential roles of Src in transforming growth factor-ss regulation of growth arrest, epithelial-to-mesenchymal transition and cell migration in pancreatic ductal adenocarcinoma cells. Int J Oncol. 2011;38:797–805. doi: 10.3892/ijo.2011.897. [DOI] [PubMed] [Google Scholar]
- 46.Samarakoon R, Overstreet JM, Higgins PJ. TGF-beta signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities. Cell Signal. 2013;25:264–268. doi: 10.1016/j.cellsig.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, Caron MG, Lefkowitz RJ. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 48.Zhang H, Liu H, Dickinson DA, Liu RM, Postlethwait EM, Laperche Y, Forman HJ. gamma-Glutamyl transpeptidase is induced by 4-hydroxynonenal via EpRE/Nrf2 signaling in rat epithelial type II cells. Free Radic Biol Med. 2006;40:1281–1292. doi: 10.1016/j.freeradbiomed.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiao YQ, Freire-de-Lima CG, Janssen WJ, Morimoto K, Lyu D, Bratton DL, Henson PM. Oxidants selectively reverse TGF-beta suppression of proinflammatory mediator production. J Immunol. 2006;176:1209–1217. doi: 10.4049/jimmunol.176.2.1209. [DOI] [PubMed] [Google Scholar]
- 50.Byeon SE, Yu T, Yang Y, Lee YG, Kim JH, Oh J, Jeong HY, Hong S, Yoo BC, Cho WJ, Cho JY. Hydroquinone regulates hemeoxygenase-1 expression via modulation of Src kinase activity through thiolation of cysteine residues. Free Radic Biol Med. 2013;57:105–118. doi: 10.1016/j.freeradbiomed.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 51.Akhand AA, Pu M, Senga T, Kato M, Suzuki H, Miyata T, Hamaguchi M, Nakashima I. Nitric oxide controls src kinase activity through a sulfhydryl group modification-mediated Tyr-527-independent and Tyr-416-linked mechanism. J Biol Chem. 1999;274:25821–25826. doi: 10.1074/jbc.274.36.25821. [DOI] [PubMed] [Google Scholar]
- 52.Pu M, Akhand AA, Kato M, Hamaguchi M, Koike T, Iwata H, Sabe H, Suzuki H, Nakashima I. Evidence of a novel redox-linked activation mechanism for the Src kinase which is independent of tyrosine 527-mediated regulation. Oncogene. 1996;13:2615–2622. [PubMed] [Google Scholar]
- 53.Tanner JJ, Parsons ZD, Cummings AH, Zhou H, Gates KS. Redox regulation of protein tyrosine phosphatases: structural and chemical aspects. Antioxid Redox Signal. 2011;15:77–97. doi: 10.1089/ars.2010.3611. [DOI] [PubMed] [Google Scholar]
- 54.Mills JE, Whitford PC, Shaffer J, Onuchic JN, Adams JA, Jennings PA. A novel disulfide bond in the SH2 Domain of the C-terminal Src kinase controls catalytic activity. J Mol Biol. 2007;365:1460–1468. doi: 10.1016/j.jmb.2006.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okada M. Regulation of the SRC family kinases by Csk. Int J Biol Sci. 2012;8:1385–1397. doi: 10.7150/ijbs.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parasassi T, Brunelli R, Costa G, De Spirito M, Krasnowska E, Lundeberg T, Pittaluga E, Ursini F. Thiol redox transitions in cell signaling: a lesson from N-acetylcysteine. ScientificWorldJournal. 2010;10:1192–1202. doi: 10.1100/tsw.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jain M, Rivera S, Monclus EA, Synenki L, Zirk A, Eisenbart J, Feghali-Bostwick C, Mutlu GM, Budinger GR, Chandel NS. Mitochondrial reactive oxygen species regulate transforming growth factor-beta signaling. J Biol Chem. 2013;288:770–777. doi: 10.1074/jbc.M112.431973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murillo MM, Carmona-Cuenca I, Del Castillo G, Ortiz C, Roncero C, Sanchez A, Fernandez M, Fabregat I. Activation of NADPH oxidase by transforming growth factor-beta in hepatocytes mediates up-regulation of epidermal growth factor receptor ligands through a nuclear factor-kappaB-dependent mechanism. Biochem J. 2007;405:251–259. doi: 10.1042/BJ20061846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 61.Nauphar D, Faradz SMH, Micha D, Sistermans E, Pals G. Profiling the Serine– Threonine Kinase Phosphorylation of TGF-β1 Stimulated Fibroblast Using Peptide Microarray. International Journal of Science and Advanced Technology. 2013;3:9. [Google Scholar]
- 62.Geiszt M, Witta J, Baffi J, Lekstrom K, Leto TL. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 2003;17:1502–1504. doi: 10.1096/fj.02-1104fje. [DOI] [PubMed] [Google Scholar]
- 63.Wang L, Zhen H, Yao W, Bian F, Zhou F, Mao X, Yao P, Jin S. Lipid raftdependent activation of dual oxidase 1/H2O2/NF-kappaB pathway in bronchial epithelial cells. Am J Physiol Cell Physiol. 2011;301:C171–180. doi: 10.1152/ajpcell.00363.2010. [DOI] [PubMed] [Google Scholar]
- 64.Schwartz EA, Reaven E, Topper JN, Tsao PS. Transforming growth factor-beta receptors localize to caveolae and regulate endothelial nitric oxide synthase in normal human endothelial cells. Biochem J. 2005;390:199–206. doi: 10.1042/BJ20041182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zuo W, Chen YG. Specific activation of mitogen-activated protein kinase by transforming growth factor-beta receptors in lipid rafts is required for epithelial cell plasticity. Mol Biol Cell. 2009;20:1020–1029. doi: 10.1091/mbc.E08-09-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Midgley AC, Rogers M, Hallett MB, Clayton A, Bowen T, Phillips AO, Steadman R. Transforming growth factor-beta1 (TGF-beta1)-stimulated fibroblast to myofibroblast differentiation is mediated by hyaluronan (HA)-facilitated epidermal growth factor receptor (EGFR) and CD44 co-localization in lipid rafts. J Biol Chem. 2013;288:14824–14838. doi: 10.1074/jbc.M113.451336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fukazawa H, Mizuno S, Uehara Y. Effects of herbimycin A and various SH-reagents on p60v-src kinase activity in vitro. Biochem Biophys Res Commun. 1990;173:276–282. doi: 10.1016/s0006-291x(05)81053-8. [DOI] [PubMed] [Google Scholar]