Summary
Background
Glioblastoma multiforme (GBM) is the most common and aggressive primary brain tumor with a dismal prognosis. Despite intensive study on tumor biology, the underlying mechanisms of the unlimited proliferation and progressive local invasion are still poorly understood, and no effective treatment has been developed for GBM patients.
Aims
We determine the role of TRPM7 channels in the growth, migration, and infiltration of malignant glioma cells.
Methods
Using a combination of RT‐PCR, Western blot, and patch‐clamp techniques, we demonstrated the expression of functional TRPM7 channels of A172 cells, a human glioma cell line, as well as in human glioma tissues. Furthermore, we evaluated the role of TRPM7 in growth, migration, and infiltration of A172 cells with MTT and transwell migration and invasion assays.
Results
We showed the expression of functional TRPM7 channels in both A172 cells and human glioma tissues. Suppression of TRPM7 expression with TRPM7‐siRNA dramatically reduced the proliferation, migration, and invasion of A172 cells. Pharmacological inhibition of TRPM7 channel with 2‐aminoethoxydiphenyl borate (2‐APB) showed a similar effect as TRPM7‐siRNA.
Conclusion
We demonstrate that human glioma cells express functional TRPM7 channel and that activation of this channel plays an important role in the proliferation, migration, and invasion of malignant glioma cells. TRPM7 channel may represent a novel and promising target for therapeutic intervention of malignant glioma.
Keywords: Glioma, Invasion, Migration, Proliferation, TRPM7
Introduction
Gliomas are the most common primary brain tumors of adults, with an estimated 2.5% of all cancer deaths in the United States. The most common and aggressive form of glioma is the glioblastoma multiforme (GBM). Despite decades of research on tumor biology and treatment, GBMs continue to have a poor prognosis, with a median survival of around 1 year after surgery followed by adjuvant therapy. Therefore, it remains a high priority for researchers and clinicians to discover new targets and therapeutic strategies to increase the survival rate and improve the clinical outcomes of GBMs 1. Lines of evidence have suggested that ion channels, the specialized membrane proteins that conduct ion fluxes, are involved in the development of many diseases including cancers 2.
TRP, a superfamily of cation‐permeable channels, are widely expressed in mammalian tissues. TRPM (Melestatin) subfamily, including TRPM1‐TRPM8, have profound influence on various physiological and pathological processes 3, 4, 5, 6, 7. TRPM7 has been demonstrated to be implicated in important physiological processes such as cellular magnesium homeostasis 8, neurotransmitter release 9, and in pathological conditions such as cerebral ischemia 7, 10 and atrial fibrillation 11. In addition, we demonstrated in our previous studies that TRPM7 channels play an important role in the growth and proliferation of human head and neck tumor cells 12. Later on, others have demonstrated that TRPM7 channels also play an important role in breast tumor, bladder and prostate cancer proliferation 13, 14, 15, as well as breast cancer metastasis and pancreatic cancer cell migration 14, 16, 17, 18, 19. However, whether TRPM7 channels are expressed in human glioma cells and whether they play a role in glioma proliferation, migration, and invasion remain unclear.
In this study, we determined the expression of functional TRPM7 channel in A172 cells, a human glioblastoma cell line, as well as in human glioma tissues. We present, for the first time, that TRPM7 channels regulate the proliferation of human glioma cells. More importantly, TRPM7 is a critical determinant of migration and invasion of glioma cells. Together, our findings establish TRPM7 as a novel target to interdict proliferation, migration, and invasion of human malignant glioma.
Materials and Methods
Cell Culture
A human glioblastoma cell line, A172, was obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM, Sigma, St. Louis, MO, USA) plus 10% fetal bovine serum, 50 units/mL penicillin, and 50 μg/mL streptomycin at 37°C with 5% CO2. 2 × 105 cells were plated in 35‐mm dishes for 2–3 days for electrophysiological recording, Western blotting, and reverse transcription–polymerase chain reaction (RT‐PCR) assays.
Isolation and Culture of Adult Glioma Cells from Human Glioma Samples
The protocol for obtaining and using human brain tissues was approved by IRB committee of Legacy Clinical Research Center. Brain tissue samples were obtained with consent from three patients undergoing craniotomies to remove brain tumors (glioblastoma multiforme). Brain tissues were digested enzymatically and isolated as described previously 20. Briefly, the tissues were cut into small pieces, then digested with papain at 3.5 mg/mL and further mechanically dissociated into a single‐cell suspension as described 20. Cells were plated in 35‐mm‐diameter dish coated with poly‐L‐ornithine at a density of 1 × 106 cells per dish and cultured in Neurobasal A medium (Life Technologies, Grand Island, NY, USA) supplemented with 2% of B27, 5 ng/mL FGF2 (Life Technologies), and 0.5 mM glutamine at 37°C with 5% CO2. In general, cells were grown for 2–3 days before experimental use.
Electrophysiology
Whole‐cell patch‐clamp recordings were performed as described previously 21. Data were acquired using an AXOPATCH 200B amplifier with pCLAMP 8.1 software and were filtered at 2 kHz and digitized at 5 kHz using Digidata 1322A (Axon Instruments, Foster City, CA, USA). Unless otherwise specified, cells were voltage‐clamped at −60 mV. Patch electrodes were constructed from thin‐walled borosilicate glass (1.5 mm diameter; WPI, Sarasota, FL, USA) on a two‐stage puller (PP83, Narishige, Tokyo, Japan). Pipettes had a resistance of 2–4 MΩ when filled with the intracellular solution. Membrane capacitance was recorded for each cell as a measure of cell size. For rapid changes of extracellular solutions, a multibarrel perfusion system (SF‐77, Warner Instruments, Hamden, CT, USA) was used. All experiments were performed at room temperature.
Western Blotting
Cells were lysed with lysis buffer containing 20 mM Tris‐HCl, pH 8.0, 140 mM NaCl, 1% Triton X‐100, 10% glycerol, 1 mM EGTA, 1.5 mM MgCl2, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, and 5 μg/mL each of leupeptin, pepstatin A, and aprotinin. After centrifugation at 12,000 × g for 30 min (4°C), the supernatant was collected for protein determination using Bradford reagent (Bio‐Rad Laboratories, Hercules, CA, USA). The samples were resolved by 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and separated proteins were transferred to polyvinylidene difluoride membranes (Bio‐Rad Laboratories) and identified by immunoblotting. Primary antibodies were diluted according to manufacturer's instruction, while secondary antibodies including horseradish peroxidase (HRP)‐conjugated anti‐rabbit and anti‐mouse antibodies were obtained from cell signaling. Blots were developed by an enhanced luminescence kit (Amersham, Piscataway, NJ, USA).
RT‐PCR
Total RNAs were isolated from cells using RNeasy Mini kit (Qiagen, Germantown, MD, USA). cDNA were synthesized from isolated RNA using Oligo(dT)15 primer and SuperScript II cDNA Synthesis kit (Invitrogen, Grand Island, NY, USA). The forward and reverse primers were designed according to the human TRPM7 gene sequence (GenBank accession number NM017672) and the human glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) gene sequence (GenBank accession number M33197), which was used as the internal control. TRPM7 primers were 5′‐CCATACCATATTCTCCAAGGTTCC‐3, and 5′‐CATTCCTCTTCAGATCTGGAAGTT‐3′ and amplify a 399‐bp fragment; GAPDH primers were 5′‐ATGCTGGTGCTGAGTATGTCGTG‐3′ and 5′‐TTACTCCTTGGAGGCCATGTAGG‐3′ and amplify a 743‐bp fragment. Negative control for PCR was performed by replacing cDNA with ultra‐pure water. RT‐PCR amplification was performed using a thermal cycler (MJ Mini, Bio‐Rad laboratories). PCR was conducted in a three‐step 29‐cycle reaction of 94°C for 30 second, 57°C for 15 second, and 72°C for 30 second using Advantage cDNA polymerase mix (Clontech, Mountain View, CA, USA). PCR products were separated by 1.5% agarose gel, visualized by staining with ethidium bromide.
RNA Interference
TRPM7 knockdown experiments were performed as described previously 22, 23. Briefly, special siRNA‐targeting nucleotides 406–426 of human TRPM7 (NM_017672) were synthesized from Invitrogen. Cells were treated with 30 nM siRNA using transfection reagent LipofectamineTM RNAiMAX (Invitrogen) according to the manufacturer's instructions. Nontargeting siRNA (Invitrogen) was used as a negative control.
MTT Assay
Cell growth and viability was determined by 3‐[4,5‐dimethylthiazol‐2‐yl]‐2,5‐diphenyltetrazolium bromide (MTT) incorporation. Cells were seeded in 96‐well culture plates and treated with or without siRNA or 2‐APB for 48 h. MTT assay was performed thereafter. Briefly, aseptically add MTT SOLUTION in an amount equal to 10% of the culture volume, then return cultures to incubator and incubate for 3–4 h at 37°C. After incubation period, remove the culture fluid. Add MTT SOLVENT in an amount equal to the original culture volume. Plates should be read within 1 h after adding MTT SOLVENT. Spectrophotometrically measure absorbance at a wavelength of 570 nm. Subtract background absorbance measured at 690 nm.
Migration and Invasion Assay
Transwell inserts with 8‐μm pores in 12‐well plates (Corning, USA) were used for the migration and invasion assays as described previously 24. In the migration assay, control and treated cells at 5 × 104/well were seeded into the upper chambers containing DMEM with 1% FBS. The upper chambers were soaked in the bottom chamber filled with 0.6 mL DMEM with 10% FBS. 24 h after culture, cells on the upper side of the upper chambers were removed with a cotton swap. The migrating cells on the lower side of the upper chambers were fixed with 4% paraformaldehyde, stained with 0.5% crystal violet in methanol, photographed, and counted. The experiments were repeated at least three times. For invasion assay, the upper chamber filters were precoated with 50 μL of Matrigel (1.25 mg/mL). Control and treated cells at 5 × 105/well were seeded into the upper chambers containing DMEM with 1% FBS. The bottom chambers were filled with DMEM with 10% FBS. 24 h after culture, cells on the upper side of the upper chamber filters were removed. The invasion cells on the lower side of the filters were fixed, stained, photographed, and counted as described above. The experiments were repeated at least three times.
To determine the role of TRPM7 in migration and invasion, A172 cells were treated with 30 nM of TRPM7‐siRNA or 100 μM of 2‐APB as well as corresponding control or vehicle for 48 h followed by serum‐starvation for another 24 h. Thereafter, the migration and invasion assays were conducted as described above.
Solutions and Chemicals
Standard extracellular solution contained (in mM): 140 NaCl, 5.4 KCl, 2 CaCl2, 1 MgCl2, 20 HEPES, 10 glucose (pH 7.4, 320–335 mOsm). For Ca2+ external solutions, CaCl2 was removed, and the osmolarity was adjusted with sucrose. Patch electrodes contained (in mM): 140 Cs‐methanesulphonate, 35 CsOH, 10 HEPES, 1 CaCl2, 11 EGTA, 2 TEA, (pH 7.25 adjusted with CsOH, 290–300 mOsm). 2‐Aminoethoxydiphenyl borate (2‐APB) was purchased from Calbiochem (San Diego, CA, USA). Gadolinium chloride was purchased from Sigma. Monoclonal antibody TRPM7 was purchased from Abcam (Cambridge, MA, USA; cat:ab85016).
Statistical Analysis
Data are expressed as mean ± SE Student's t‐test and ANOVA were performed for data analysis, and significant difference was defined as P < 0.05.
Results
TRPM7‐Like Currents in A172 Glioma Cells
We asked whether A172 human glioma cells express TRPM7 channels. A nondesensitized inward current was induced when lowering extracellular Ca2+ from 2 mM to various lower concentrations as indicated in A172 glioma cells (Figure 1A). The amplitude of Ca2+‐sensing inward currents correlated with decreases of extracellular Ca2+ concentration (Figure 1A). A detail dose–response analysis showed that the inward currents were inhibited by extracellular calcium with a half‐maximal inhibitory concentration (IC50) of 0.18 ± 0.05 mM (Figure 1A, n = 5). The current–voltage (I–V) relationship of the TRPM7‐like currents was then generated by plotting the peak amplitude of the currents against the holding potentials (Figure 1B). TRPM7 currents show outward rectification in whole‐cell recordings with a reversal potential close to 0 mV, and this rectification is largely diminished and became approximately linear when the extracellular divalent cations were removed 10, 25. As shown in Figure 1B, the Ca2+‐sensing currents in A172 cells has a near linear I–V relationship with a reversal potential at ~0 mV (n = 6). Together, these electrophysiological studies provide strong evidence that A172 human glioma cells express functional TRPM7‐like channels.
Figure 1.
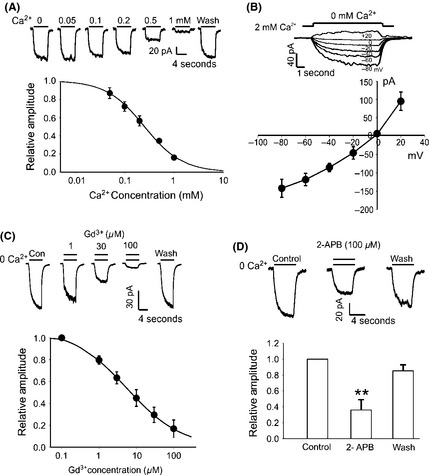
Activation of TRPM7‐like currents in A172 glioma cells. (A) Representative current traces and summary data showing concentration‐dependent inhibition of the inward current in A172 glioma cells by external Ca2+. Dose–response curve was fitted by Hill equation with an average IC50 of 0.18 ± 0.05 mM (n = 5). (B) Representative traces showing the Ca2+‐sensing currents activated at different membrane potentials. Currents were activated by reduction of [Ca2+]o from 2 to 0 mM over a range of holding potentials from −80 to +20 mV. Summary data showing the current–voltage relationship (I–V curve) of the Ca2+‐sensing current (n = 6). (C) Representative traces of the Ca2+‐sensing currents at different concentrations of Gd3+. Holding potential was −60 mV. Summary data showing that the inhibition of Ca2+‐sensing current by Gd3+ is dose dependent with an average IC50 value of 5.53 ± 0.06 μM (n = 5). (D) Representative traces and summary bar graph showing the Ca2+‐sensing current in the absence and presence of 100 μM 2‐APB. The current amplitude was inhibited by ~60% by 100 μM 2‐APB (n = 7, **P < 0.01).
We further determined whether the Ca2+‐sensing currents in A172 glioma cells are mediated by TRPM7 channels. First, we examined the effect of GdCl3 (Gd3+), a nonspecific blocker for TRPM7 channel 26, 27, 28 on the Ca2+‐sensing currents in A172 cells. As shown in Figure 1C, the currents were significantly inhibited by Gd3+ and the inhibition was dose dependent with an IC50 of 5.53 ± 0.06 μM (n = 5, Figure 1C). This finding suggests that Ca2+‐sensing currents in A172 glioma cells might be mediated by TRPM7 channels. To provide additional evidence, we examined the effect of 2‐APB, another nonspecific blocker for TRPM7 channel 29, 30, 31, on the Ca2+‐sensing inward currents. In the presence of 100 μM 2‐APB, the currents in A172 cells were inhibited by ~60% (Figure 1D, n = 7, **P < 0.01). This finding further confirms that the Ca2+‐sensing currents in A172 cells are likely mediated by TRPM7 channels.
TRPM7‐Like Currents in Cells Dissociated from Human Glioma Tissues
Similar to A172 cells, a rapid drop of [Ca2+]o from 2 mM to 0 mM evoked slow nondesensitizing currents that rapidly recovered upon restoration of [Ca2+]o in cells dissociated from human glioma tissue (Figure 2A). Extracellular Ca2+ dose dependently inhibits the inward currents with an IC50 of 0.21 ± 0.03 mM (n = 4) (Figure 2B). Similarly to the currents in A172 cells, the Ca2+‐sensing currents in cells dissociated from human glioma tissues were sensitive to Gd3+ and 2‐APB (Figure 2C and E). Addition of 100 μM Gd3+ inhibited the currents by 66. 7 ± 3.3% (n = 8, **P < 0.01) (Figure 2D), while addition of 100 μM 2‐APB inhibited the currents by 54.5 ± 12.8% (n = 4, *P < 0.05, Figure 2F).
Figure 2.
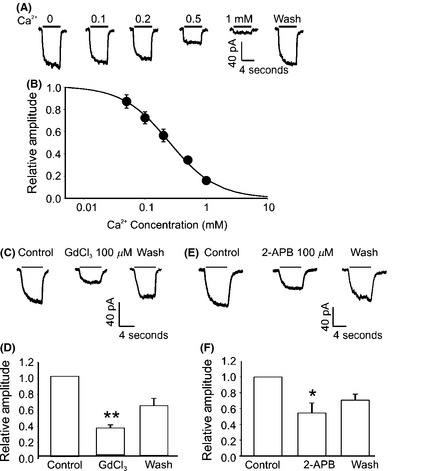
Electrophysiological and pharmacological characterization of the TRPM7‐like currents in human glioma cells from human glioma tissue. (A and B) Representative current traces and summary data showing concentration‐dependent inhibition of TRPM7‐like currents in human glioma cells by external Ca2+. Dose–response curve was fit by Hill equation with an average IC50 of 0.21 ± 0.03 mM (n = 4). (C and D) Representative traces and summary bar graph showing the inhibition of Gd3+ on Ca2+‐sensitive currents recorded from dissociated human glioma cells. Holding potential was −60 mV. The current amplitude was −56.5 ± 7.8 pA in the absence of Gd3+, whereas it became −16.8 ± 1.5 pA after bath perfusion with 100 μM Gd3+ (n = 8, **P < 0.01). (E and F) Representative traces and summary data showing an inhibition of the Ca2+‐sensitive current in human glioma cells by 2‐APB. Addition of 100 μM 2‐APB reduced the amplitude of the inward current from −69.7 ± 2.2 pA to −22.0 ± 5.3 pA (n = 4, *P < 0.05).
Detection of TRPM7 Protein and mRNA Expression in A172 Cells and Human Glioma Tissues
To further confirm the existence of TRPM7 channels in human glioma cells, we next examined TRPM7 protein expression in human glioma tissues. HEK‐293 cells with inducible expression of TRPM7 were used as positive control. HEK‐293 cells were treated with 1 μg/mL tetracycline for 2 days for the induction of TRPM7 expression. TRPM7 protein levels were examined by immunoblotting using mouse monoclonal antibody against TRPM7. A single band of ~220kD, which is identical to that of TRPM7 over expressed in HEK293 cells induced by tetracycline (Figure 3A, B), was detected in human glioma tissues by Western blotting (Figure 3A, C), suggesting that TRPM7 protein is expressed in human glioma. In addition, TRPM7 mRNA levels were analyzed by reverse transcription–polymerase chain reaction (RT‐PCR). As shown in Figure 3B, TRPM7 mRNA (~ 399 bp) was detected in both human glioma tissues (Figure 3B, b & d) and in A172 cells (Figure 3B, f). TRPM7 mRNA levels correlate well with TRPM7 protein expressions.
Figure 3.
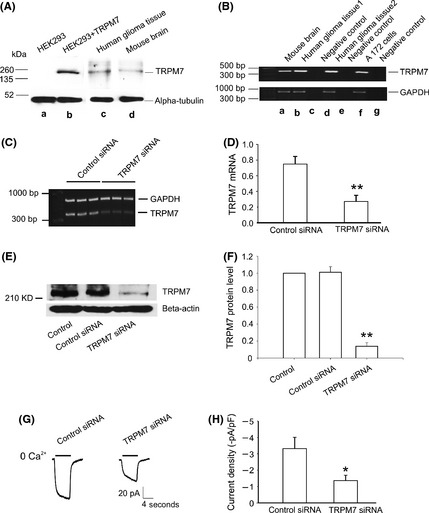
TRPM7 expression in A172 cells and human glioma tissues. (A) Detection of TRPM7 protein in human glioma tissue by Western blotting (A, c). HEK293 cells with inducible expression of TRPM7 by tetracycline were served as a positive control (A, b), and alpha tubulin was used as an internal control. (B) RT‐PCR shows the detection of TRPM7 mRNA in mouse brain tissue (B, a), human glioma tissue (B, b&d), and A172 cells (B, f). GAPDH was used as an internal control. (C and D) Representative RT‐PCR and summary data show the reduction of TRPM7 mRNA in A172 cells transfected with TRPM7‐siRNA (n = 3, **P < 0.01). (E and F) Western blotting shows the decreased TRPM7 protein level in A172 cells transfected with TRPM7‐siRNA (n = 3, **P < 0.01). (G) Representative traces showing the reduction of Ca2+‐sensing currents in TRPM7‐siRNA‐transfected A172 cells. Currents were induced by a drop of [Ca2+]o from 2 mM to 0 mM at −60 mV. Electrophysiological recordings were performed 48 h after TRPM7‐siRNA transfection. (H) Summary data showing the reduction of the Ca2+‐sensing current density in A172 cells by TRPM7‐siRNA (n = 10–13, *P < 0.05).
TRPM7 Silencing Decreases TRPM7 Expression and TRPM7‐Like Currents in A172 Cells
Small interference RNA (siRNA) was employed to provide additional evidence that TRPM7 channels are indeed involved in mediating the Ca2+‐sensing currents in human glioma cells. RNA interference, is a process of posttranscriptional gene silencing that inhibits the expression of native genes with high specificity 32, 33. As shown in Figure 3C and D, after siRNA treatment for 48 h, a significant reduction in TRPM7 mRNA level was detected in cells treated with TRPM7‐siRNA when compared with cells transfected with control‐siRNA (n = 3, **P < 0.01). Similarly, there is a significant reduction of TRPM7 protein level in cells treated with TRPM7‐siRNA compared to cells treated with control‐siRNA (Figure 3E and F, n = 3, **P < 0.01). As expected, TRPM7 currents were largely decreased in A172 cells after TRPM7‐siRNA treatment for 48 h, compared with that of the control‐siRNA‐treated cells (Figure 3G). The current density is −3.5 ± 0.7 pA/pF in control cells, while it is reduced to −1.1 ± 0.3 pA/pF in cells treated with TRPM7‐siRNA (Figure 3H, n = 10–13 cells, *P < 0.05).
2‐APB and Silencing TRPM7 Inhibits the Proliferation of A172 Glioma Cells
We next determined whether TRPM7 influences the growth and proliferation of A172 cells. First, we tested the effect of 2‐APB on the growth/proliferation of A172 cells. The number of A172 cells was significantly reduced after treatment with 2‐APB for 48 h (Figure 4A). MTT assay shows addition of 50 and 100 μM 2‐APB inhibited the growth of A172 cells by 40.33 ± 4.92 and 81.43 ± 5.23%, respectively (Figure 4B, n = 4, **P < 0.01). To further confirm that TRPM7 channels are involved in the growth and proliferation of glioma cells, TRPM7‐siRNA was employed to selectively suppress the expression of TRPM7 channels. As shown in Figure 4C, silencing TRPM7 with 30 nM TRPM7‐siRNA for 48 h significantly inhibited A172 cells growth/proliferation. The cell growth was decreased to 20.93 ± 0.83% by TRPM7‐siRNA (Figure 4D, **P < 0.01, n = 4). Taken together, our data suggest that activation of TRPM7 channels plays an important role in the growth and proliferation of human glioma cells.
Figure 4.
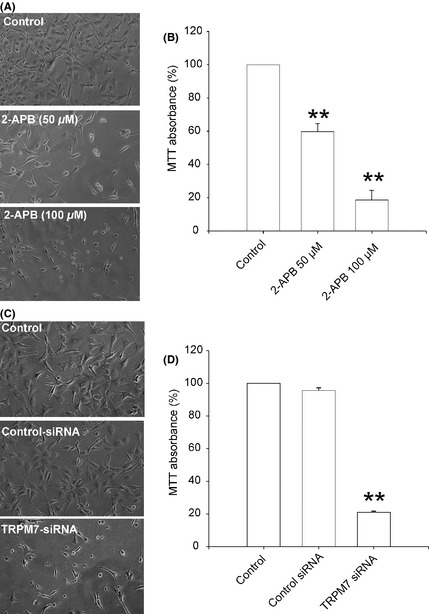
TRPM7 is critical for A172 cell growth and proliferation. (A and B) Concentration‐dependent inhibition of the growth and proliferation of A172 cells by 2‐APB, measured by MTT assay (**P < 0.01, n = 4). (C and D) Inhibition of the proliferation of A172 cells by TRPM7‐siRNA, evaluated by MTT assay (**P < 0.01, n = 4). Data were collected from four independent experiments.
2‐APB and Silencing TRPM7 Inhibits the Migration of A172 Glioma Cells
To examine the potential role of TRPM7 in migration of glioma cells, we inhibited the activation and expression of TRPM7 in A172 cells using 2‐APB and TRPM7‐siRNA, respectively. A172 cells were treated with 100 μM 2‐APB or 30 nM TRPM7‐siRNA for 48 h, followed by serum‐starved for another 24 h. After plating in the transwell chambers for 24 h, some cells migrated from the upper surface of the transwell chambers to the lower surface. As shown in Figure 5A–B, treatment with 2‐APB (100 μM) effectively reduced the number of migrated cells to 35.09% ± 8.49 of control (0.1% DMSO) (**P < 0.01, n = 4). Consistently, treatment with TRPM7‐siRNA greatly decreased the number of migrated cells to 16.31% ± 2.03 of the control‐siRNA‐treated cells (Figure 5C–D, **P < 0.01, n = 3). These data clearly demonstrate that TRPM7 is critical in regulating the migration of human glioma cells.
Figure 5.
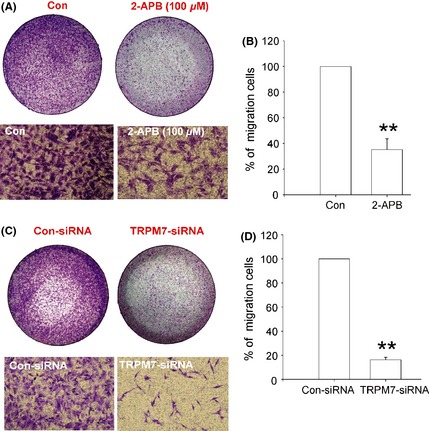
Effect of 2‐APB and TRPM7 silencing on A172 cell migration. (A) Representative images showing the migration of A172 cells in the absence or presence of 100 μM 2‐APB. The upper images showing the whole wells. (B) Summary data showing the percentage of migrated cells (**P < 0.01, n = 4). (C) Representative images showing the migration of A172 cells in the presence of control siRNA or TRPM7‐siRNA. The upper images showing the whole wells. (D) Summary data showing the percentage of migrated cells (**P < 0.01, n = 3). Data were collected from 3 to 4 independent experiments.
2‐APB and Knockdown of TRPM7 Inhibits the Invasion of A172 Cells
We next examined of the role of TRPM7 channels in the invasion of glioma cells. Transwell matrigel invasion assay was used for the invasion study as described previously 24, 34. As shown in Figure 6A, 2‐APB significantly suppresses the invasion of A172 cells. The number of invaded cells was effectively reduced to 19.30% ± 4.22 of the control (0.1% DMSO) by 100 μM 2‐APB (Figure 6B, **P < 0.01, n = 4). TRPM7 knockdown produces a similar result, which decreases the invaded cell number to 13.21% ± 5.11 of the control‐siRNA‐treated cells (Figure 6C–D, **P < 0.01, n = 4). These results further support a critical role of TRPM7 channel in the invasiveness of human glioma cells.
Figure 6.
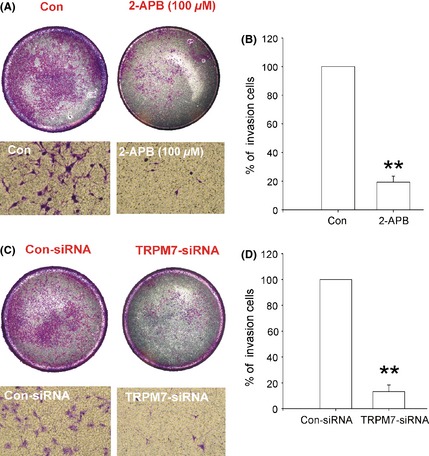
Effect of 2‐APB and TRPM7 silencing on A172 cell invasion. (A) Representative images of the cell invasion. The upper images showing the whole wells. (B) Summary data showing the percentage of invaded cells (**P < 0.01, n = 4). (C) Representative imaging showing the invasion of A172 cells. The upper images showing the whole wells. (D) Summary data showing the percentage of invaded cells (**P < 0.01, n = 4). Data were collected from four independent experiments.
Discussion
In this study, we provided evidence that functional TRPM7 channels are present in human glioma cells including A172 human glioma cell line as well as in dissociated primary glioma cells from human glioma tissues. TRPM7 current has several characteristics including (a) potentiation by decreasing extracellular divalent cations including Ca2+ and Mg2+ 26, 35; (b) reverses at ~0 mV and has a strong outward rectification in the presence of divalent cations and the outward rectification is largely abolished in the absence of divalent cations 35; (c) inhibited by 2‐APB and Gd3+, two nonspecific inhibitors of TRPM7 channels 26; (d) reduction of intracellular Mg2+ potentiates TRPM7 current 35. All these properties are observed in TRPM7‐like currents recorded in A172 glioma cells and glioma cells from human glioma tissues. Additionally, TRPM7‐like current is decreased when TRPM7 is knockdown by siRNA, further confirming that the low calcium induced currents in A172 cells and human glioma tissues are carried largely by TRPM7 channels.
In the central nervous system, reactive nitrogen species under ischemic conditions activate TRPM7 current and produce neuronal death via calcium overload 26 and zinc entry 36. Knockdown of TRPM7 protects hypoxia or cerebral ischemia induced neuronal injury in vitro and in vivo 10, 26. Interestingly, knockdown of TRPM7 has no observable toxicity on cortical neurons or significant influence on series of behavioral tests including learning and memory 10, implying that suppression of TRPM7 would be tolerable. In this regard, TRPM7 may function as a promising therapeutic target for neurological disorders. In the present study, we demonstrated that TRPM7 knockdown significantly inhibits the proliferation of A172 cells as well as primary glioma cells from human glioma tissues. 2‐APB, the nonspecific TRPM7 inhibitor has a similar effect, which further confirms the role of TRPM7 in the proliferation of human glioma cells. Although inhibition of TRPM7 is tolerable and causes no significant side effects in the CNS 10, the side effect may occur in the peripheral system. For example, loss of TRPM7 function was found to induce growth arrest in DT‐40 B‐lymphocytes and osteoblastic cells 37, 38, because the coordination between cellular energy metabolism and Ca2+ and Mg2+ homeostasis was disrupted. In addition, under physiological conditions, TRPM7 is closely associated with cellular growth and development. Global deletion of TRPM7 in mice disrupts embryonic development and thymopoiesis 39. However, it is less likely that TRPM7 disruption causes severe problems in adults. Nevertheless, tumor‐targeted drug delivery may help to avoid the potential side effects.
Malignant gliomas are one of the leading causes of death from central nervous system cancers. They are characterized by unlimited proliferation and progressive local invasion 40, 41. Invasion is a paramount problem that prevents the cure of malignant brain tumors. However, the underlying mechanisms resulting in local invasion of malignant gliomas remain largely unknown, which accounts for the major obstruction in finding effective therapeutic strategies 42. In addition to a critical role of TRPM7 in the proliferation of head and neck cancer cell, TRPM7 is also required for breast tumor cell metastatis and pancreatic cancer cell migration 18, 19. In the present study, we demonstrated that downregulation of TRPM7 expression by TRPM7‐siRNA or suppressing the activity of TRPM7 channel by pharmacological agent impairs the migration and invasion of A172 glioma cells. These data imply that TRPM7 may represent a potential therapeutic target for combating the highly aggressive and refractory malignant glioma.
Although the detailed mechanism of TRPM7 contributing to oncogenesis is largely unknown at present, several potential mechanisms have been proposed. For instance, the downstream activation of Akt/ERK and calpain pathways are important for the proliferation and migration of prostate cancer 14. In addition, annexin‐1 and myosin || heavy chain as the substrates of TRPM7 kinase have been shown be related to cell adhesion and migration 43, 44. A recent study showed that TRPM7 regulates migration and invasion of metastatic breast cancer cells via MAPK pathway 17. In addition, it has been shown that TRPM7 knockdown by siRNA transfection significantly reduces Ca2+ influx and retards cell proliferation by delaying G1/S cell cycle progression 45. Some of the above signaling pathways may be common mechanisms for proliferation, migration, and invasion shared by glioma. Matrix metalloproteinases (MMPs) are a family of zinc‐dependent endopeptidases that are capable of degrading components of the basement membrane and extracellular matrix. Matrix metalloproteinases (MMPs) have been regarded as major critical molecules assisting tumor cells to invade and migrate 46. As TRPM7 is highly permeable to zinc, we speculate that the decreased TRPM7‐mediated zinc entry may cause a decreased function of MMPs, which may account for the decreased migration and invasion ability by TRPM7 knockdown or inhibition. TRPM7 is a strong and independent prognostic marker for breast cancer progression and metastasis, which was found enriched in high‐grade primary tumors. Moreover, Overexpression of TRPM7 is significantly associated with poor prognosis in patients with ovarian cancer 47. However, whether there is any correlation between TRPM7 expression and glioma grade remains to be elucidated.
In conclusion, this study reveals the presence of functional TRPM7 channel in human glioma cell line and human glioma tissue. More importantly, we provide evidence that TRPM7 is required for the proliferation, migration, and invasion of these cells. In summary, this study suggests that TRPM7 may represent a promising therapeutic target for the intervention of malignant gliomas. The future study would be aimed to (1) identify the downstream signaling pathways of TRPM7 that mediate the progression of glioma; (2) identify a highly selective TRPM7 inhibitor with high permeability across the blood–brain barrier or selectively concentrated in the brain for future therapeutic use; (3) investigate the correlation between TRPM7 expression and glioma grades; (4) conduct in vivo study to determine TRPM7 as a novel target for glioma therapy.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgment
This work is supported by R01NS066027, NIMHD S21MD000101, U54NS08932, and AHA 0840132.
The first three authors contributed equally to this work.
References
- 1. Weil RJ. Glioblastoma multiforme–treating a deadly tumor with both strands of RNA. PLoS Med 2006;3:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lehmann‐Horn F, Jurkat‐Rott K. Voltage‐gated ion channels and hereditary disease. Physiol Rev 1999;79:1317–1372. [DOI] [PubMed] [Google Scholar]
- 3. Clapham DE. TRP channels as cellular sensors. Nature 2003;426:517–524. [DOI] [PubMed] [Google Scholar]
- 4. Fleig A, Penner R. The TRPM ion channel subfamily: Molecular, biophysical and functional features. Trends Pharmacol Sci 2004;25:633–639. [DOI] [PubMed] [Google Scholar]
- 5. Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol 2006;68:619–647. [DOI] [PubMed] [Google Scholar]
- 6. Minke B. TRP channels and Ca2 + signaling. Cell Calcium 2006;40:261–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leng T, Shi Y, Xiong ZG, Sun D. Proton‐sensitive cation channels and ion exchangers in ischemic brain injury: New therapeutic targets for stroke? Prog Neurobiol 2014;115:189–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmitz C, Perraud AL, Johnson CO, et al. Regulation of vertebrate cellular Mg2 + homeostasis by TRPM7. Cell 2003;114:191–200. [DOI] [PubMed] [Google Scholar]
- 9. Krapivinsky G, Mochida S, Krapivinsky L, Cibulsky SM, Clapham DE. The TRPM7 ion channel functions in cholinergic synaptic vesicles and affects transmitter release. Neuron 2006;52:485–496. [DOI] [PubMed] [Google Scholar]
- 10. Sun HS, Jackson MF, Martin LJ, et al. Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nat Neurosci 2009;12:1300–1307. [DOI] [PubMed] [Google Scholar]
- 11. Du J, Xie J, Zhang Z, et al. TRPM7‐mediated Ca2 + signals confer fibrogenesis in human atrial fibrillation. Circ Res 2010;106:992–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiang J, Li MH, Inoue K, Chu XP, Seeds J, Xiong ZG. Transient receptor potential melastatin 7‐like current in human head and neck carcinoma cells: Role in cell proliferation. Cancer Res 2007;67:10929–10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guilbert A, Gautier M, Dhennin‐Duthille I, Haren N, Sevestre H, Ouadid‐Ahidouch H. Evidence that TRPM7 is required for breast cancer cell proliferation. Am J Physiol Cell Physiol 2009;297:C493–C502. [DOI] [PubMed] [Google Scholar]
- 14. Sun Y, Sukumaran P, Varma A, Derry S, Sahmoun AE, Singh BB. Cholesterol‐induced activation of TRPM7 regulates cell proliferation, migration, and viability of human prostate cells. Biochim Biophys Acta 2014;1843:1839–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mizuno H, Suzuki Y, Watanabe M, et al. Potential role of transient receptor potential (TRP) channels in bladder cancer cells. J Physiol Sci 2014;64:305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guilbert A, Gautier M, Dhennin‐Duthille I, et al. Transient receptor potential melastatin 7 is involved in oestrogen receptor‐negative metastatic breast cancer cells migration through its kinase domain. Eur J Cancer 2013;49:3694–3707. [DOI] [PubMed] [Google Scholar]
- 17. Meng X, Cai C, Wu J, et al. TRPM7 mediates breast cancer cell migration and invasion through the MAPK pathway. Cancer Lett 2013;333:96–102. [DOI] [PubMed] [Google Scholar]
- 18. Middelbeek J, Kuipers AJ, Henneman L, et al. TRPM7 is required for breast tumor cell metastasis. Cancer Res 2012;72:4250–4261. [DOI] [PubMed] [Google Scholar]
- 19. Rybarczyk P, Gautier M, Hague F, et al. Transient receptor potential melastatin‐related 7 channel is overexpressed in human pancreatic ductal adenocarcinomas and regulates human pancreatic cancer cell migration. Int J Cancer 2012;131:E851–E861. [DOI] [PubMed] [Google Scholar]
- 20. Brewer GJ, Espinosa J, McIlhaney MP, et al. Culture and regeneration of human neurons after brain surgery. J Neurosci Methods 2001;107:15–23. [DOI] [PubMed] [Google Scholar]
- 21. Li M, Inoue K, Branigan D, et al. Acid‐sensing ion channels in acidosis‐induced injury of human brain neurons. J Cereb Blood Flow Metab 2010;30:1247–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sun H, Leng T, Zeng Z, Gao X, Inoue K, Xiong ZG. Role of TRPM7 channels in hyperglycemia‐mediated injury of vascular endothelial cells. PLoS ONE 2013;8:e79540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Inoue K, Xiong ZG. Silencing TRPM7 promotes growth/proliferation and nitric oxide production of vascular endothelial cells via the ERK pathway. Cardiovasc Res 2009;83:547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shu M, Zheng X, Wu S, et al. Targeting oncogenic miR‐335 inhibits growth and invasion of malignant astrocytoma cells. Mol Cancer 2011;19:59–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Runnels LW, Yue L, Clapham DE. TRP‐PLIK, a bifunctional protein with kinase and ion channel activities. Science 2001;291:1043–1047. [DOI] [PubMed] [Google Scholar]
- 26. Aarts M, Iihara K, Wei WL, et al. A key role for TRPM7 channels in anoxic neuronal death. Cell 2003;115:863–877. [DOI] [PubMed] [Google Scholar]
- 27. Liu X, Groschner K, Ambudkar IS. Distinct Ca(2+)‐permeable cation currents are activated by internal Ca(2 + )‐store depletion in RBL‐2H3 cells and human salivary gland cells, HSG and HSY. J Membr Biol 2004;200:93–104. [DOI] [PubMed] [Google Scholar]
- 28. Monteilh‐Zoller MK, Hermosura MC, Nadler MJ, Scharenberg AM, Penner R, Fleig A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J Gen Physiol 2003;121:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Flemming PK, Dedman AM, Xu SZ, et al. Sensing of lysophospholipids by TRPC5 calcium channel. J Biol Chem 2006;281:4977–4982. [DOI] [PubMed] [Google Scholar]
- 30. Ma HT, Patterson RL, van Rossum DB, Birnbaumer L, Mikoshiba K, Gill DL. Requirement of the inositol trisphosphate receptor for activation of store‐operated Ca2 + channels. Science 2000;287:1647–1651. [DOI] [PubMed] [Google Scholar]
- 31. Schindl R, Kahr H, Graz I, Groschner K, Romanin C. Store depletion‐activated CaT1 currents in rat basophilic leukemia mast cells are inhibited by 2‐aminoethoxydiphenyl borate. Evidence for a regulatory component that controls activation of both CaT1 and CRAC (Ca(2 + ) release‐activated Ca(2 + ) channel) channels. J Biol Chem 2002;277:26950–26958. [DOI] [PubMed] [Google Scholar]
- 32. Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21‐nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001;411:494–498. [DOI] [PubMed] [Google Scholar]
- 33. Cullen BR. RNA interference: Antiviral defense and genetic tool. Nat Immunol 2002;3:597–599. [DOI] [PubMed] [Google Scholar]
- 34. Repesh LA. A new in vitro assay for quantitating tumor cell invasion. Invasion Metastasis 1989;9:192–208. [PubMed] [Google Scholar]
- 35. Nadler MJ, Hermosura MC, Inabe K, et al. LTRPC7 is a Mg.ATP‐regulated divalent cation channel required for cell viability. Nature 2001;411:590–595. [DOI] [PubMed] [Google Scholar]
- 36. Inoue K, Branigan D, Xiong ZG. Zinc‐induced neurotoxicity mediated by transient receptor potential melastatin 7 channels. J Biol Chem 2010;285:7430–7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schmitz C, Dorovkov MV, Zhao X, Davenport BJ, Ryazanov AG, Perraud AL. The channel kinases TRPM6 and TRPM7 are functionally nonredundant. J Biol Chem 2005;280:37763–37771. [DOI] [PubMed] [Google Scholar]
- 38. Abed E, Moreau R. Importance of melastatin‐like transient receptor potential 7 and magnesium in the stimulation of osteoblast proliferation and migration by platelet‐derived growth factor. Am J Physiol Cell Physiol 2009;297:C360–C368. [DOI] [PubMed] [Google Scholar]
- 39. Jin J, Desai BN, Navarro B, Donovan A, Andrews NC, Clapham DE. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2 + homeostasis. Science 2008;322:756–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. DeAngelis LM. Brain tumors. N Engl J Med 2001;344:114–123. [DOI] [PubMed] [Google Scholar]
- 41. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–996. [DOI] [PubMed] [Google Scholar]
- 42. Lefranc F, Brotchi J, Kiss R. Possible future issues in the treatment of glioblastomas: Special emphasis on cell migration and the resistance of migrating glioblastoma cells to apoptosis. J Clin Oncol 2005;23:2411–2422. [DOI] [PubMed] [Google Scholar]
- 43. Clark K, Langeslag M, van Leeuwen B, et al. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J 2006;25:290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Callera GE, He Y, Yogi A, et al. Regulation of the novel Mg2 + transporter transient receptor potential melastatin 7 (TRPM7) cation channel by bradykinin in vascular smooth muscle cells. J Hypertens 2009;27:155–166. [DOI] [PubMed] [Google Scholar]
- 45. Hanano T, Hara Y, Shi J, et al. Involvement of TRPM7 in cell growth as a spontaneously activated Ca2 + entry pathway in human retinoblastoma cells. J Pharmacol Sci 2004;95:403–419. [DOI] [PubMed] [Google Scholar]
- 46. Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev 2006;25:9–34. [DOI] [PubMed] [Google Scholar]
- 47. Wang J, Xiao L, Luo CH, et al. Overexpression of TRPM7 is associated with poor prognosis in human ovarian carcinoma. Asian Pac J Cancer Prev 2014;15:3955–3958. [DOI] [PubMed] [Google Scholar]


