Abstract
Background
Survivors of childhood cancer treated with CNS-directed therapy may be at-risk for poor healthcare utilization due to neurocognitive deficits. This study examined associations between neurocognitive function and adherence to routine and risk-based medical evaluations in adult survivors exposed to CNS-directed therapy.
Methods
Neurocognitive function and healthcare utilization were assessed in 1304 adult survivors of childhood cancer enrolled in the St. Jude Lifetime Cohort Study. Adherence to recommended care was defined as meeting guidelines published by the Children's Oncology Group. Multivariable models were used to evaluate associations between neurocognitive function and health screenings. Established predictors of healthcare utilization were included as covariates. Odds ratios (OR) or prevalence ratios (PR) and 95% confidence intervals (CIs) were calculated for variables maintained in the final models.
Results
Adherence to recommended medical care was higher for routine (general physician care: 57.6%; dental care: 49.1%) as opposed to specialized care (survivor-focused care: 21.9%; echocardiogram: 19.9%). Higher intelligence was predictive of general physician care (OR=1.74, 95% CI=1.41 - 2.15) and survivor-focused care (OR=1.44, 95% CI=1.13 – 1.83) compared to no care, while better executive function skills were associated with reduced dental care (PR = 0.94, 95% CI = 0.91-0.98). Echocardiogram monitoring was not associated with neurocognition. Possible late-effects of cancer treatment (pain, reduced cardiorespiratory fitness) were associated with an increased likelihood of receiving specialized medical care.
Conclusion
Survivors with reduced global cognition are at risk for poor healthcare utilization. Education practices regarding recommended healthcare should be personalized to ensure comprehension by survivors with neurocognitive impairment.
Keywords: childhood cancer, long-term survivors, healthcare utilization, neurocognition, late-effects
Introduction
Therapeutic advances have led to a growing population of childhood cancer survivors, with recent estimates indicating that one in every 750 adults in the United States is a long-term survivor1. This population is at-risk for treatment related morbidities; recent findings from the St. Jude Lifetime Cohort Study (SJLIFE) demonstrated that 95.5% of childhood cancer survivors will have at least one chronic health condition by 45 years of age2. Engagement in routine medical care and screening based on treatment exposure is critical for the early detection and management of chronic health conditions; however, adherence to recommended care is variable3-6.
In the general population, adherence to recommended health screenings is approximately 60 to 70%7. Utilization rates differ between children and adults, with adults receiving less preventative care. Healthcare utilization among childhood cancer survivors varies according to service type. While adherence to routine medical care ranges from 72 to 89%, the proportion of survivors adhering to risk-based screening recommendations is significantly lower (echocardiogram: 29.4%; mammogram: 41.1%; colonoscopy: 14.3%)3, 4.
In the general population, barriers to optimal healthcare utilization include lack of insurance, limited access to providers, and demographic characteristics such as gender, age, income, and education level8, 9. In non-cancer populations, neurocognitive impairment is a predictor of less frequent physician and dental care10-12. As nearly half of adult survivors of childhood cancer exposed to central nervous system (CNS) treatment have identified neurocognitive impairment2, these survivors may be at significant risk for poor adherence to recommended healthcare.
A recent report from the Childhood Cancer Survivor Study (CCSS) linked patient-reported cognitive symptoms to recommended healthcare evaluations as outlined by the Children's Oncology Group (COG) Long-Term Follow-Up Guidelines3, 13. Notably, patient self-reported ratings of cognitive abilities are often discrepant with direct performance-based neurocognitive measures due to factors such as response shift and emotional distress14, 15. Further, cognitive impairments may impede patients from accurately assessing functional and skill-based limitations16.
Thus, the aims of the current study were to determine rates of healthcare utilization in adult survivors of childhood cancer treated with CNS directed-therapy and to examine the association between performance-based neurocognitive function and recommended medical care. We hypothesized that survivors with neurocognitive impairment would demonstrate poorer healthcare utilization compared to survivors without impairment. Understanding the impact of cognitive function on healthcare practices is a critical first step towards the development of interventions aimed to promote engagement in routine medical care and risk-based screenings.
Methods
Participants were recruited from the SJLIFE cohort, which includes survivors of childhood cancer treated at St. Jude Children's Research Hospital (SJCRH) who are at least 18 years of age and 10 years post-diagnosis2. All survivors treated at SJCRH for a pediatric malignancy are eligible for SJLIFE, once current age and time since diagnosis criteria have been met. Approximately 75% of eligible survivors complete on-campus comprehensive health evaluations (please see Hudson et al for more detailed descriptions of the SJLIFE study design2,17). A recent report compared participants eligible for SJLIFE to those enrolled on the cohort study across demographic, disease and neighborhood-level socioeconomic variables. Results indicated that differences between participants and nonparticipants were not substantial limiting potential bias secondary to nonparticipation18.
To be eligible for the current study, survivors were enrolled in SJLIFE from October 1, 2007 through April 30, 2012, and were required to be at-risk for neurocognitive impairment based on the COG Long-Term Follow-Up Guidelines13. Survivors had to complete standardized neurocognitive testing and comprehensive questionnaires regarding emotional function, health behaviors and demographic characteristics. As such, findings from the current study may generalize only to cancer survivors treated with similar neurotoxic therapies. Exclusion criteria included a recent visit to SJCRH during which recommended healthcare evaluations were provided. All participants gave written informed consent and approval was obtained from the institutional review board.
Primary Outcomes, Specific Study Samples, and Measurement
Physician Care
At the time of analysis, 2473 survivors were enrolled in SJLIFE. Of these participants, 1143 survivors met study eligibility and were included in the physician care outcome analysis. Participants were excluded if they had not been treated with CNS-directed therapy (n = 943), did not complete the neurocognitive evaluation (n = 47), were unable to complete questionnaires independently (n = 163) or had received medical care at SJCRH within the two years prior to their initial SJLIFE clinical assessment (n = 177). Although it is recommended that survivors receive an evaluation by a physician annually, the current study defined adherence as receiving physician care at least once within the past two years. Survivors indicated if their healthcare visits related to their original cancer diagnosis and responses were categorized as 1) no physician care, 2) general physician care, or 3) general survivor-focused care3.
Dental Care
As dental care is not a routine service provided by SJCRH to long-term survivors, participants were not excluded from analyses relating to dental care, even if they had received recent medical care at SJCRH. A total of 1304 participants were included in the dental care outcome analysis. Although it is recommended that survivors receive a dental exam and cleaning every 6 months, for the current study adherence to dental care was defined as report of at least one dental visit in the past year.
Echocardiogram
To be included in the echocardiogram outcome, survivors had to be at-risk for cardiac toxicity based on childhood cancer treatment exposure with anthracycline chemotherapy and/or chest-directed radiotherapy. Recommended frequency of echocardiogram was based on the COG Long-Term Follow-Up Guidelines, which considers age at treatment, as well as chest radiation and anthracycline dose13. A total of 673 survivors were included in the echocardiogram outcome analyses. Survivors were excluded if they were not exposed to anthracycline chemotherapy or chest-directed radiotherapy (n = 750), had received the recommended echocardiogram at SJCRH (n = 264), did not receive CNS-directed therapy (n = 654), did not complete a neurocognitive evaluation (n = 27) or did not complete the questionnaires independently (n = 105). Adherence to this risk-based screening evaluation was defined as reporting an echocardiogram at the recommended interval based on individual treatment characteristics.
Predictors and Covariates
Neurocognitive Predictors
Neurocognitive function was assessed in five domains, each represented by a single variable: overall reasoning ability (Full Scale Intelligence-Wechsler Abbreviated Scale of Intelligence)19, attention (Variability-Conners' Continuous Performance Task-Second Edition)20, processing speed (Processing Speed Index-Wechsler Adult Intelligence Scale-Third Edition)21, memory (Verbal Learning-California Verbal Learning Test-Second Edition)22, and executive function (Cognitive Flexibility-Trail Making Test Part B)23. Performance scores were transformed into age-adjusted standard scores (mean = 0; SD = 1) based on population norms.
Emotional and Physical Characteristics
Emotional function was assessed through survivor self-report of antidepressant and anxiolytic use and the Brief Symptom Inventory-18 (BSI-18)24. For the BSI-18 gender-specific scores were calculated for the depression, anxiety and somatization sub-scales using a community-based normative sample. Survivors were considered to have excess symptoms of depression if they reported taking antidepressants and/or their T-score on the BSI-18 depression subscale was ≥63. Similarly, excess symptoms of anxiety were considered to be present if survivors reported taking an anxiolytic and/or their T-score on the BSI-18 anxiety subscale was ≥63. Elevated levels of somatization were evaluated by the BSI-18 (T-score ≥63 was considered clinically significant). Survivors also rated their level of bodily pain during the past four weeks. Responses were dichotomized into: 1) no or mild pain (none, very mild, mild) and 2) pain (moderate, severe, very severe).
Physical function was evaluated with the six-minute walk test 25. Distance walked in 6-minutes was compared to healthy controls and impairment was defined as performance <10th percentile. Body mass index (BMI) was calculated according to Center for Disease Control (CDC) guidelines and dichotomized into overweight/obese (BMI ≥ 25) and normal/low weight (BMI ≤24.9)26.
Demographic Characteristics
Demographic characteristics associated with healthcare utilization practices, as outlined above, were selected as covariates3, 5, 27-29. Variables were categorized based on sample distribution: current age at evaluation (<20 years, 20-29 years, 30-39 years, ≥40 years), time since diagnosis (10-19 years, ≥ 20 years), gender (male, female), race (Caucasian, other), education (≤some college, ≥college), marital status (single, married or living as married, divorced or separated), living arrangement (dependent, independent), household income (≤$39,999, ≥$40,000), health insurance (yes, no), and dental insurance (yes, no).
Statistical Analysis
Descriptive statistics were calculated for medical care outcomes, neurocognitive predictors and covariates. Neurocognitive impairment was defined as a z-score ≤ -1.3 standard deviations below the mean. Since the physician care outcome was categorized into three levels, a multinomial generalized logistic regression model was employed to evaluate the relationship between neurocognitive function and physician care. The dental care and echocardiogram outcomes were dichotomized into two levels (those receiving the recommended dental care vs. no dental care and those getting the recommended echocardiogram vs. no echocardiogram) and the relative risk of receiving dental care/an echocardiogram were modeled using log-binomial regression models since the prevalence of the outcomes are about or higher than 20%30. A two stage approach was adopted for selecting the best models. In the first stage all predictors that were significant at level p< 0.20 were selected. Associations with the selected predictors were subsequently assessed using the Akaike Information Criterion (AIC), which promotes model parsimony by applying a penalty function for models with more covariates. Therefore, the model with the lowest AIC was considered optimal31. Among the variables that were not significant at 0.05 level at this stage, the least significant predictor was removed and the new model was considered acceptable if the increase in AIC value was <10 units32. This procedure was continued until the final model, with all insignificant factors (change in AIC value of <10) removed was obtained. Odd ratios (OR) or prevalence ratios (PR) and 95% confidence intervals (CIs) were calculated for variables maintained in the final models. All analyses were performed in SAS version 9.3 (Cary, N.C.).
Results
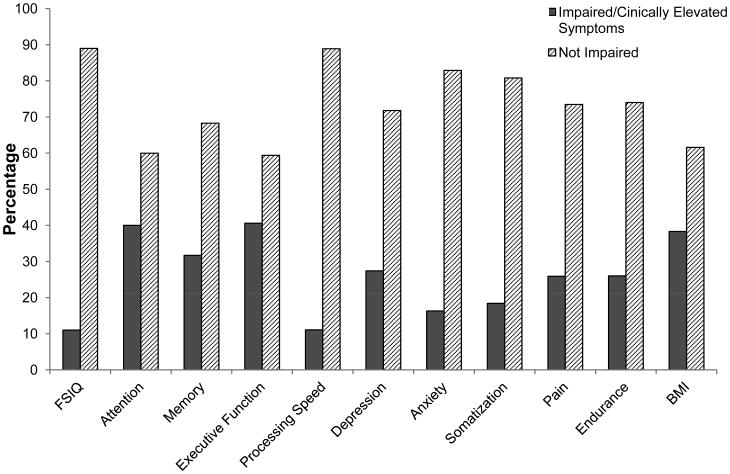
Demographic and treatment characteristics are presented in Table 1. Survivors were on average 31.6 years of age at the time of their neurocognitive assessment and 24.1 years (range: 10.5 – 47.8 years) from their primary cancer diagnosis. The majority had a childhood cancer diagnosis of leukemia (66.9%) and 52% of survivors had received cranial radiation. Information on primary neurocognitive predictors, as well as emotional and physical covariates is included in Figure 1. Prevalence of impairment across cognitive domains ranged from 11.0% to 40.6% with higher prevalence demonstrated on executive function, attention and memory tasks. Pain was reported by 25% of survivors, while 38% were considered overweight or obese based on BMI.
Table 1. Demographic and Treatment Characteristics of Survivors Exposed to CNS-Directed Therapy (N= 1304).
| Mean | SD | |
|---|---|---|
| Age at Evaluation (years) | 31.6 | 7.9 |
| Age at Diagnosis (years) | 7.5 | 5.0 |
|
|
||
| N | %* | |
|
|
||
| Age at Evaluation (years) | ||
| 18-30 | 637 | 48.8 |
| 31-40 | 472 | 36.2 |
| >40 | 195 | 15.0 |
| Age at Diagnosis (years) | ||
| 0-4 | 486 | 37.3 |
| 5-9 | 368 | 28.2 |
| 10-14 | 306 | 23.5 |
| ≥15 | 144 | 11.0 |
| Time since Diagnosis (years) | ||
| 10-19 | 439 | 33.7 |
| ≥20 | 865 | 66.3 |
| Gender | ||
| Male | 654 | 50.2 |
| Female | 650 | 49.8 |
| Ethnicity | ||
| Caucasian | 1145 | 87.8 |
| Other | 159 | 12.2 |
| Education | ||
| ≤Some college | 790 | 62.5 |
| ≥College | 474 | 37.5 |
| Marital Status | ||
| Single, never married | 488 | 38.6 |
| Married, living as married | 601 | 47.6 |
| Divorced, no longer living as married | 175 | 13.8 |
| Living Arrangement** | ||
| Dependent | 390 | 29.9 |
| Independent | 914 | 70.1 |
| Household Income | ||
| <$40,000 | 520 | 47.2 |
| ≥$40,000 | 582 | 52.8 |
| Health Insurance | ||
| Yes | 996 | 76.7 |
| No | 302 | 23.3 |
| Dental Insurance | ||
| Yes | 696 | 55.8 |
| No | 552 | 44.2 |
| Diagnosis+ | ||
| Leukemia | 872 | 66.9 |
| Lymphoma | 129 | 9.9 |
| CNS Tumor | 142 | 10.9 |
| Embryonal | 26 | 2.0 |
| Bone and Soft Tissue Sarcoma | 111 | 8.5 |
| Other | 24 | 1.8 |
| Radiation+ | ||
| Chest | 212 | 16.3 |
| Cranial (CRT) | 674 | 51.7 |
| Chemotherapy+ | ||
| Anthracyclines | 825 | 63.3 |
| Alkylating Agents | 860 | 66.0 |
| Antimetabolites | 990 | 75.9 |
| Anti-Tumor Agents | 67 | 5.1 |
| Corticosteriods | 952 | 73.0 |
| Platinum | 122 | 9.4 |
| CNS Directed Therapy+ | ||
| HD/IV/IT/IO MTX‡ | 1036 | 79.5 |
| HD Ara-C‡‡ | 105 | 8.1 |
| HD/IV/IT/IO MTX and HD Cytarabine | 1057 | 81.1 |
| HD/IV/IT/IO MTX and CRT | 1196 | 91.2 |
| HD Ara-C and CRT | 725 | 55.6 |
| HD/IV/IT/IO MTX, HD Ara-C and CRT | 1199 | 92.0 |
Percentages based on participants with available data
independent living (living alone, with a spouse/roommate or as a caretaker); dependent living (living with parents/family)
variable not included in multivariable models
high dose/intravenous/intrathecal/intra-ommaya methotrexate
high dose cytarabine
Figure 1. Neurocognitive, Emotional and Physical Characteristics.
The percentage of participants with impaired/clinically elevated symptoms versus not impaired across neurocognitive, emotional and physical variables. The expected rate of impairment for cognitive variables is the 10th percentile. Symptoms of depression and anxiety are considered clinically elevated if T-scores ≥63 on BSI-18 and/or report of antidepressant or anxiolytic medication, respectively. T score ≥63 is considered clinically elevated for somatization. Percentages are based on participants with available data.
Adherence to recommended healthcare was variable across medical outcomes (see Table 2). Approximately 16% of survivors reported receiving no physician care and 49% reported receiving recommended dental care. Of the 673 survivors exposed to cardiotoxic therapy and recommended to receive echocardiogram screening, only 20% reported having been screened.
Table 2. Healthcare Utilization.
| Recommended N* | N | % | |
|---|---|---|---|
| Physician Care | 1143 | ||
| No Care | 189 | 16.5 | |
| General Care | 658 | 57.6 | |
| Survivor-Focused Care | 250 | 21.9 | |
| Missing | 46 | 4.0 | |
| Dental Care | 1304 | ||
| No | 664 | 50.9 | |
| Yes | 640 | 49.1 | |
| Echocardiogram | 673 | ||
| No | 391 | 58.1 | |
| Yes | 134 | 19.9 | |
| Missing | 148 | 22.0 |
includes survivors eligible for analysis based on inclusion/exclusion criteria
Physician Care
The final multivariable model identified cognitive, emotional and socio-demographic characteristics as predictors of physician-based care (see Table 3). Higher overall reasoning skills were associated with more physician care (general care OR = 1.74, 95% CI = 1.41 – 2.15; survivor-focused care OR = 1.44, 95% CI =1.13 – 1.83) compared to no care. Specifically, for every one standard deviation increase in Full Scale IQ, survivors were 74% more likely to report general physician care and 44% more likely to report having received survivor-focused care. Other cognitive skills were not retained in the final physician care model. Report of symptoms of depression and/or use of an antidepressant (general care OR = 1.66, 95% CI = 1.01 – 2.72; survivor-focused care OR = 2.19, 95% CI = 1.27 – 3.75) and symptoms of pain (OR = 2.73, 95% CI = 1.58 – 4.74) were associated with increased likelihood of having received physician care. Although the overall effect of years from diagnosis was predictive of physician care, the individual levels were non-significant. Survivors 10-19 years post-diagnosis were more likely to receive survivor-focused care or general care compared to survivors who were more than 20 years from diagnosis (data not shown).
Table 3. Multivariable Model Predicting Physician Care Among Survivors Exposed to CNS-Directed Therapy.
| Physician Care | OR* | 95%CI | p-value | |
|---|---|---|---|---|
| Overall Reasoning (continuous z-score) | <.0001 | |||
| No Care | 1.0 | |||
| General | 1.74 | 1.41 – 2.15 | ||
| Survivor | 1.44 | 1.13 – 1.83 | ||
| Years since Diagnosis (10-19 vs. ≥20 years) | 0.005 | |||
| No Care | 1.0 | |||
| General | 0.68 | 0.44 – 1.03 | ||
| Survivor | 1.18 | 0.73 – 1.91 | ||
| Gender (female vs. male) | 0.036 | |||
| No Care | 1.0 | |||
| General | 1.53 | 1.03 – 2.26 | ||
| Survivor | 1.80 | 1.14 – 2.83 | ||
| Body Pain (≥moderate vs. ≤mild) | <0.001 | |||
| No Care | 1.0 | |||
| General | 1.53 | 0.93 – 2.53 | ||
| Survivor | 2.73 | 1.58 – 4.74 | ||
| Symptoms of Depression (yes vs. no) | 0.017 | |||
| No Care | 1.0 | |||
| General | 1.66 | 1.01 – 2.72 | ||
| Survivor | 2.19 | 1.27 – 3.75 | ||
| Marital Status (single vs. married) | 0.01 | |||
| No Care | 1.0 | |||
| General | 0.63 | 0.43 – 0.94 | ||
| Survivor | 0.95 | 0.60 – 1.51 | ||
| Insurance (yes vs. no) | <.0001 | |||
| No Care | 1.0 | |||
| General | 3.21 | 2.16 – 4.77 | ||
| Survivor | 6.05 | 3.58 – 10.23 |
OR = Odds Ratio
Dental Care
Significant predictors of adherence to routine dental care included neurocognitive, physical and demographic variables (see Table 4). Dental insurance was associated with an increased likelihood of reporting dental care compared to no care (PR = 1.82, 95% CI = 1.58-2.10). In contrast, better executive function and poorer physical endurance, as evaluated by the six-minute walk test, were associated with reduced dental care.
Table 4. Multivariable Model Predicting Dental Care Among Survivors Exposed to CNS-Directed Therapy.
| PR* | 95% CI | p-value | ||
|---|---|---|---|---|
| Overall Reasoning (continuous z-score) | ||||
| No Care | 1.0 | |||
| Dental | 1.04 | 0.97 – 1.12 | 0.29 | |
| Executive Function (continuous z-score) | ||||
| No Care | 1.0 | |||
| Dental | 0.94 | 0.91- 0.98 | 0.001 | |
| Attention (continuous z-score) | ||||
| No Care | 1.0 | |||
| Dental | 0.98 | 0.93 – 1.03 | 0.46 | |
| Processing Speed (continuous z-score) | ||||
| No Care | 1.0 | |||
| Dental | 1.06 | 0.99 – 1.13 | 0.11 | |
| Body Pain (moderate, severe, very severe vs. none, mild, very mild) | ||||
| No Care | 1.0 | |||
| Dental | 0.88 | 0.75 – 1.02 | 0.10 | |
| Symptoms of Somatization (yes vs. no) | ||||
| No Care | 1.0 | |||
| Dental | 0.93 | 0.77 – 1.12 | 0.45 | |
| Ethnicity (Other vs. Caucasian) | ||||
| No Care | 1.0 | |||
| Dental | 0.82 | 0.66 – 1.02 | 0.07 | |
| Marital Status (single, divorced vs. married, living as married) | ||||
| No Care | 1.0 | |||
| Dental | 0.95 | 0.86 – 1.06 | 0.37 | |
| Dental Insurance (yes vs. no) | ||||
| No Care | 1.0 | |||
| Dental | 1.82 | 1.58 – 2.10 | <.0001 | |
| Physical Endurance (impaired vs. not impaired) | ||||
| No Care | 1.0 | |||
| Dental | 0.86 | 0.74 – 0.99 | 0.036 |
PR = prevalence ratio
Echocardiogram
Only insurance (PR = 1.83, 95% CI = 1.12 – 3.00) and poor physical endurance (PR = 1.42, 95% CI = 1.04 – 1.95) were significantly associated with report of echocardiogram. Cognitive and emotional variables were not significantly associated with cardiac risk-based screening and were dropped from the final model.
Discussion
This study identifies a novel predictor of participation in routine medical care, overall reasoning abilities, which were associated with receiving general and survivor-focused physician care. Importantly, for every one standard deviation increase in general intellectual function, survivors were 74% more likely to report routine healthcare, a finding that is statistically and clinically meaningful.
Research has largely focused on the relationship between specific cognitive skills and healthcare utilization, as opposed to general intellectual function3, 33. The identification of specific cognitive deficits enables the implementation of targeted interventions (i.e. receiving a handout that outlines recommended medical care) to increase healthcare utilization. However, the predictive value of a global index of functioning also has several benefits. First, overall reasoning skills can be evaluated using screening tools that take approximately 15 minutes to administer. Such measurement instruments have large normative samples, good psychometric properties and are reliable proxies for more comprehensive measures of intellectual functioning19. Additionally, a global index of functioning is typically included in cognitive assessment batteries, whereas inclusion of instruments to measure cognitive domains (attention, memory, executive function) is more variable. Evaluating specific neurocognitive domains presents challenges as there are many sub-domains, each linked with a different real-life skill. Further, there are many tools available to measure these skills, including both direct-assessment and self-report measures, with limited agreement among researchers and clinicians as to which instrument is ideal; thus, it can be challenging to generalize findings across studies. For instance, in CCSS self-reported difficulties with organization were associated with less dental care, while perceived impairments with task efficiency predicted increased physician care3. It is unclear how these findings relate to the findings of the current study. Importantly, awareness of an individual's global level of function can guide education efforts (i.e. word selection, amount of details provided) regarding treatment related risks and the importance of obtaining recommended healthcare, over and beyond knowledge of specific skill deficits.
Body pain was also associated with report of survivor-focused physician care compared to no care, which is not surprising as pain reported as a late-effect of treatment may stimulate survivors to pursue more specialized care. This finding provides an avenue for intervention of risk-based care prior to the onset of pain symptoms. Specifically, education regarding the potential expression of chronic health conditions and pain secondary to cancer treatment may promote engagement in targeted preventative care34.
Symptoms of depression were associated with increased physician care, while anxiety and somatization were not predictive of healthcare utilization. Studies in the general population have identified both depression and anxiety as predictive of healthcare practices29, 35. In a study of older adults, an increase in depressive symptoms was associated with an increase in missed medical appointments. Notably this study did not take into consideration current use of antidepressant medication29. Previous research has found that survivors who report the greatest number of health concerns and cancer-related worries, as well as poor health status, were less likely to receive risk-based screenings36. In contrast, adult patients diagnosed with generalized anxiety disorder were found to have more medical encounters than a matched comparison group without generalized anxiety disorder35. It would be interesting to see if anxiety specific to health-related concerns or a clinical diagnosis of an anxiety disorder would be predictive of healthcare practices in our current sample.
Higher executive function was predictive of reduced dental care. Executive function skills are associated with higher educational attainment and professional employment37, 38, as well as adherence to medication regimens39. Therefore, it is initially counterintuitive that survivors with better executive function would have worse attendance at dental visits. However, these survivors may be functioning at higher professional positions and have more complex schedules which make it more challenging to attend dental appointments regularly40, the threshold for attending health-related appointments may be lower.
Cognitive status and emotional health were not predictive of receiving an echocardiogram. It is possible that these relationships were not identified due to the small number of survivors who obtained the recommended cardiac evaluation. In contrast, individuals with poor performance on the 6-minute walk test, a proxy for general cardiorespiratory fitness, were more likely to receive an echocardiogram. Therefore, the echocardiogram may have been obtained in response to cancer-related late effects that were already impacting daily function (i.e. reduced physical fitness), rather than as a preventative screening tool. This paradigm is similar to that observed with pain symptoms and again suggests the need for early and effective education regarding treatment-related health risks.
Other predictors of healthcare utilization identified in the current study were generally commensurate with previous research3, 4. Insurance was the strongest predictor of our three medical outcomes; individuals who reported having health insurance were 3-times more likely to receive general physician care and 1.8-times more likely to have an echocardiogram. Survivors with dental insurance were 1.8-times more likely to receive regular dental care. Notably, insurance was associated with a 6-fold increased likelihood of receiving survivor-focused physician care compared to no care suggesting that more comprehensive and targeted physical evaluations are closely related to insurance status. These findings highlight the importance of health and dental insurance. Multidisciplinary teams should confirm that uninsured individuals have the knowledge and resources to apply for insurance coverage. Assistance should be provided to survivors who are unable, potentially secondary to cognitive impairment, to navigate the application system independently. Additionally, information regarding low cost clinics should be provided. For purposes of the current study public v. private insurance type was not modeled due to the small number of survivors who reported having Medicaid (6%) compared to private insurance (70%). Descriptive analyses indicated survivor-focused care was reported at a higher frequency by survivors with Medicaid (43.2%) compared to survivors with private insurance (24.2%). As survivor-focused care is associated with risk-based screening evaluations, it is beneficial to identify factors that promote this type of evaluation4.
The current study has several limitations. First, it is unknown if survivors had knowledge of their treatment-related risks and the associated recommended screening evaluations. Although it is current practice during active treatment and in after therapy care to discuss late effects and long-term follow-up, many of the SJLIFE patients included in the current study received treatment decades before survivorship guidelines and risk factors were well established. Therefore, it is important to determine rates of healthcare utilization in more contemporary cohorts. Further, medical care was collected through survivor self-report. Since the study population was comprised of individuals at-risk for neurocognitive impairment, and a greater proportion of survivors demonstrated memory impairment than expected, it is possible that attendance at medical visits was misreported. However, given that the average global intellectual functioning index for this cohort is within normal limits frequent false reports of medical care outcomes are less likely. Lastly, survivors who did not complete study questionnaires independently, potentially secondary to severe cognitive impairment, were excluded from analyses. Although this limits study generalizability, it is expected that survivors with significant cognitive dysfunction receive support from caregivers and are not independently responsible for scheduling and attending medical appointments.
In summary, the current findings indicate that rates of healthcare utilization among childhood cancer survivors are variable, with poorer adherence demonstrated for more specialized care. Predictors of healthcare include cognitive, emotional and demographic characteristics. Insurance was identified as the strongest predictor of medical care; however, even after adjusting for insurance, better overall reasoning skills were associated with attendance at physician-based care. This suggests that individuals with cognitive impairment are at-risk for poor engagement with healthcare systems. Further, medical care was associated with possible treatment-related late-effects, indicating that survivors' current healthcare practices may be for the management, rather than prevention, of health conditions.
These results highlight the value of educating survivors about treatment-related risks prior to the onset of symptoms. Teaching content and practices may need to be modified to ensure comprehension by survivors with neurocognitive impairment. As long-term follow-up guidelines are revised periodically, it will be critical that survivors are informed of changes to their recommended healthcare evaluations. Additionally, it will be important to verify that survivors are aware of and understand the steps necessary to schedule medical appointments, as well as have the necessary support (i.e. reminders, family members) to attend these visits. Importantly, as neurocognitive late-effects change overtime,37 regular monitoring of cognitive function is critical, as education approaches and support systems for an individual may need to be modified based on declines in cognition. Future directions include evaluating adherence to community-based evaluations/interventions that are recommended during survivorship care. Further, as insurance was the strongest predictor of healthcare in the current study, it will be essential to track changes in survivors' healthcare utilization rates secondary to the implementation of the Affordable Care Act.
Acknowledgments
Funding Source: This work was supported by Cancer Center Support (CORE) grant No. CA21765 from the National Cancer Institute and by the American Lebanese Syrian Associated Charities (ALSAC)
Footnotes
Conflict of Interest: Nothing to disclose
References
- 1.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14(1):61–70. doi: 10.1038/nrc3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309(22):2371–81. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krull KR, Annett RD, Pan Z, et al. Neurocognitive functioning and health-related behaviours in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Eur J Cancer. 2011;47(9):1380–8. doi: 10.1016/j.ejca.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathan PC, Ness KK, Mahoney MC, et al. Screening and surveillance for second malignant neoplasms in adult survivors of childhood cancer: a report from the childhood cancer survivor study. Ann Intern Med. 2010;153(7):442–51. doi: 10.1059/0003-4819-153-7-201010050-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steele JR, Wall M, Salkowski N, et al. Predictors of risk-based medical follow-up: a report from the Childhood Cancer Survivor Study. J Cancer Surviv. 2013;7(3):379–91. doi: 10.1007/s11764-013-0280-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klosky JL, Cash DK, Buscemi J, et al. Factors influencing long-term follow-up clinic attendance among survivors of childhood cancer. J Cancer Surviv. 2008;2(4):225–32. doi: 10.1007/s11764-008-0063-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jhu E, Nowakowski J. Benchmarking Preventative Care Utilization. [accessed December 26, 2013];Millman Healthcare Reform Briefing Paper. 2011 Mar; Available from URL: http://publications.millman.com/publications/healthreform/pdfs/benchmarking-preventative-care-utilization.pdf.
- 8.Bersten AB, Hing E, Moss AJ, Allen KF, Siller AB, Tiggle RB. Health care in America: Trends in utilization. Hyattsville, Maryland: National Center for Health Statistics; 2003. [Google Scholar]
- 9.Jerant A, Fiscella K, Tancredi DJ, Franks P. Health insurance is associated with preventive care but not personal health behaviors. J Am Board Fam Med. 2013;26(6):759–67. doi: 10.3122/jabfm.2013.06.130054. [DOI] [PubMed] [Google Scholar]
- 10.Walsh EG, Wu B, Mitchell JB, Berkmann LF. Cognitive function and acute care utilization. J Gerontol B Psychol Sci Soc Sci. 2003;58(1):S38–S49. doi: 10.1093/geronb/58.1.s38. [DOI] [PubMed] [Google Scholar]
- 11.Alosco ML, Spitznagel MB, van DM, et al. Cognitive function and treatment adherence in older adults with heart failure. Psychosom Med. 2012;74(9):965–73. doi: 10.1097/PSY.0b013e318272ef2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denberg TD, Melhado TV, Coombes JM, et al. Predictors of nonadherence to screening colonoscopy. J Gen Intern Med. 2005;20(11):989–95. doi: 10.1111/j.1525-1497.2005.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Children's Oncology G. Long-term follow-up guidelines for survivors of childhood, adolescent and young adult cancers, Version 3.0. Arcadia, CA: Children's Oncology Group; Oct, 2008. [accessed December 26, 2013]. Available from URL www.survivorshipguidelines.org. [Google Scholar]
- 14.Howard JS, Mattacola CG, Howell DM, Lattermann C. Response shift theory: an application for health-related quality of life in rehabilitation research and practice. J Allied Health. 2011;40(1):31–8. [PubMed] [Google Scholar]
- 15.Barclay-Goddard R, King J, Dubouloz CJ, Schwartz CE. Building on transformative learning and response shift theory to investigate health-related quality of life changes over time in individuals with chronic health conditions and disability. Arch Phys Med Rehabil. 2012;93(2):214–20. doi: 10.1016/j.apmr.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Gavett R, Dunn JE, Stoddard A, Harty B, Weintraub S. The Cognitive Change in Women study (CCW): informant ratings of cognitive change but not self-ratings are associated with neuropsychological performance over 3 years. Alzheimer Dis Assoc Disord. 2011;25(4):305–11. doi: 10.1097/WAD.0b013e31820d8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011 May;56(5):825–36. doi: 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ojha RP, Oancea SC, Ness KK, et al. Assessment of potential bias from non-participation in a dynamic clinical cohort of long-term childhood cancer survivors: results from the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2013 May;60(5):856–64. doi: 10.1002/pbc.24348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 20.Conners CK. Conners' Continuous Performance Test II. North Tonawanda, NY: Multi-Health Systems; 2001. [Google Scholar]
- 21.Wechsler D. Wechsler Adult Intelligence Scale-Third Edition. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 22.Delis DC, Kramer JH, Kaplan E, et al. California Verbal Learning Test-Second Edition. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- 23.Strauss E, Sherman EM, Spreeen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 24.Derogatis LR. Brief Symptom Inventory 18: Administration, scoring and procedures manual. Minneapolis, MN: NCS Pearson; 2001. [Google Scholar]
- 25.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 26.Obes Res. Suppl 2. Vol. 6. National Institutes of Health; 1998. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report; pp. 51S–209S. [PubMed] [Google Scholar]
- 27.Johnson R, Horne B, Feltbower RG, Butler GE, Glaser AW. Hospital attendance patterns in long term survivors of cancer. Arch Dis Child. 2004;89(4):374–7. doi: 10.1136/adc.2002.021816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laing SS, Makambi K. Predicting regular breast cancer screening in African-American women with a family history of breast cancer. J Natl Med Assoc. 2008;100(11):1309–17. doi: 10.1016/s0027-9684(15)31510-8. [DOI] [PubMed] [Google Scholar]
- 29.Mackin RS, Arean PA. Cognitive and psychiatric predictors of medical treatment adherence among older adults in primary care clinics. Int J Geriatr Psychiatry. 2007;22(1):55–60. doi: 10.1002/gps.1653. [DOI] [PubMed] [Google Scholar]
- 30.Greenland S. Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. Am J Epidemiol. 2004 Aug 15;160(4):301–5. doi: 10.1093/aje/kwh221. [DOI] [PubMed] [Google Scholar]
- 31.Sakamoto Y, Ishiguro M, Kitagawa G. Akaike information criterion statistics. Dordrecht, The Netherlands: D. Reidel; 1986. [Google Scholar]
- 32.Burnham KP, Anderson DR. Model Selection and Multimodel Inference A Practical Information-Theorectic Approach. 2002:71. [Google Scholar]
- 33.Hajduk AM, Lemon SC, McManus DD, et al. Cognitive impairment and self-care in heart failure. Clin Epidemiol. 2013;5:407–16. doi: 10.2147/CLEP.S44560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maeda N, Horibe K, Kato K, Kojima S, Tsurusawa M. Survey of childhood cancer survivors who stopped follow-up physician visits. Pediatr Int. 2010;52(5):806–12. doi: 10.1111/j.1442-200X.2010.03158.x. [DOI] [PubMed] [Google Scholar]
- 35.Berger A, D E, Wittchen H, Morlock R, Edelsberg J, Oster G. Patterns of healthcare utilization in patients with generalized anxiety disorder in general practice in Germany. Eur J Psychiat. 2009;23:90–100. [Google Scholar]
- 36.Cox CL, Zhu L, Hudson MM, Steen BD, Robison LL, Oeffinger KC. Survivor typologies predict medical surveillance participation: the childhood cancer survivor study. Psychooncology. 2013;22(7):1534–42. doi: 10.1002/pon.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krull KR, Brinkman TM, Li C, et al. Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: a report from the St Jude lifetime cohort study. J Clin Oncol. 2013;31(35):4407–15. doi: 10.1200/JCO.2012.48.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kadan-Lottick NS, Zeltzer LK, Liu Q, et al. Neurocognitive functioning in adult survivors of childhood non-central nervous system cancers. J Natl Cancer Inst. 2010 Jun 16;102(12):881–93. doi: 10.1093/jnci/djq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hinkin CH, Hardy DJ, Mason KI, et al. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. Aids. 2004 Jan 1;18(Suppl 1):S19–25. doi: 10.1097/00002030-200418001-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirchhoff AC, Krull KR, Ness KK, et al. Physical, mental, and neurocognitive status and employment outcomes in the childhood cancer survivor study cohort. Cancer Epidemiol Biomarkers Prev. 2011 Sep;20(9):1838–49. doi: 10.1158/1055-9965.EPI-11-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]