Abstract
Rationale
The drug ±3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”, “molly”) is thought to produce pro-social effects and enhance social interaction. However, in most laboratory studies to date, the participants have been tested under non-social conditions, which may not simulate the effects the drug produces in more naturalistic social settings.
Methods
Healthy experienced MDMA users participated in three laboratory sessions in which they received MDMA (0.5 or 1.0 mg/kg or placebo; double blind). They were randomly assigned to one of three social conditions, in which they were tested alone (SOL; N=10), in the presence of a research assistant (RAP; N=11) or in the presence of another participant who also received the drug (OPP; N=11).
Results
As expected, MDMA increased heart rate and blood pressure, and produced positive subjective effects in all three groups. It also increased ratings of attractiveness of another person and increased social interaction in RAP and OPP. The social context affected certain responses to the drug. The effects of MDMA were greater in the OPP condition, compared to the SOL or RAP conditions, on measures of “feel drug”, “dizzy” and on cardiovascular measures. But responses to the drug on other measures, including social behavior, did not differ across the conditions.
Conclusions
These findings provide some support for the idea that drugs produce greater effects when they are used in the presence of other drug users. However, the influence of the social context was modest, and it remains to be determined whether other variables related to social context would substantially alter the effects of MDMA or other drugs.
Keywords: MDMA, social context, setting, mood, humans
Introduction
MDMA, or ecstasy, is a widely used recreational drug renowned for its prosocial effects, including feelings of sociability and interpersonal closeness (Kelly et al. 2006; Rodgers et al. 2006). The drug is commonly used in social settings, and MDMA users claim that they use the drug specifically to experience the prosocial effects (Bravo 2001; Sumnall et al. 2006). The interactions between this and other drugs and social environments are likely to be bi-directional: Drugs such as alcohol and stimulant drugs increase talking and social interaction (Higgins and Stitzer 1988; Lindfors and Lindman 1987; Marrone et al. 2010; Stitzer et al. 1981; Ward et al. 1997), and conversely, many drugs are experienced as more pleasurable in the presence of others (e.g., alcohol: (Doty and de Wit 1995; Kirkpatrick and de Wit 2013), diazepam (Evans et al. 1996), marijuana (Kelly et al. 1994), and d-amphetamine (de Wit et al. 1997). Despite the known role of social context on drug responses, relatively few controlled studies have examined these interactions in human volunteers.
The evidence for interactions between psychoactive drugs and social context is not limited to humans, but has also been reported in laboratory animals. There is an extensive early literature on the phenomenon of “aggregate toxicity”, showing that the presence of other animals increases the effects of stimulant drugs, including their toxicity (Mohrland and Craigmill 1978; Moore et al. 1965). Further, d-amphetamine produces more pronounced effects on dopamine and serotonin neurons in the brain in grouped, compared to isolated, animals (Chieu and Moore 1975, Lokiec et al, 1977; 1981). More recently, Theil et al (2008, 2009) showed that animals exhibited stronger conditioned place preference for cocaine or nicotine when the drug was tested in an environment that had been previously associated with the presence of other animals. Smith and colleagues (Smith and Pitts 2014; Strickland and Smith 2014) have shown that the presence of other drug-taking rats facilitates self-administration of cocaine, and certain drugs, including MDMA, appear to increase social behavior (Thompson et al. 2009).
Despite observations that drugs are commonly used in the presence of others, and despite the preclinical evidence that social context affects drug responses in laboratory animals, the role of social context on responses to drugs is poorly understood. Early studies reported mixed results with manipulations of social setting and responses to drugs such as alcohol or marijuana (Carlin et al. 1972; Fromme and Dunn 1992; Sher 1985). More recently, we have shown that alcohol produces more pleasurable subjective effects in the presence of others, and participants choose to take more when others are present (Doty and de Wit 1995; Kirkpatrick and de Wit 2013). Another important study has shown that alcohol also directly alters social bonding among young adults tested in social groups (Sayette et al. 2012). With another drug, MDMA, a comparison on findings from three laboratories suggested that responses to the drug were greater when a research assistant was in the experimental room compared to when participants were alone (Kirkpatrick et al. 2014a). However, to our knowledge there have been no systematic laboratory investigations of social contexts on the acute effects of MDMA. Furthermore, there have been few studies on how MDMA affects social interaction.
In the present study, we examined both the effects of social setting on responses to MDMA and the effects of MDMA on social interaction, by administering the drug under three conditions: in participants who were alone for the 4-hour laboratory sessions (i.e., a typical laboratory setting), in participants who were in the company of a research assistant (i.e., to determine effects of the simple presence of another person), and in participants who were in the company of other participants who also received the drug (i.e., to determine effects of other intoxicated individuals). We video-recorded the participants during the sessions to examine the effects of the drug on social interaction. We predicted that 1) compared to placebo, MDMA would increase cardiovascular measures and “positive” subjective effects in all three conditions; 2) the effects of MDMA on cardiovascular measures and positive subjective effects would be greater in the company of another individual; and 3) the effects of MDMA would be greater in the presence of other drug-treated individuals.
Methods
Participant Recruitment and Screening
Healthy young adult volunteers (aged 18–35) with moderate MDMA experience (4–80 lifetime uses) were recruited through posters, print and internet advertisements and word-of-mouth referrals. Candidates underwent a structured clinical psychiatric interview to exclude individuals with psychiatric disorders (American Psychiatric Association 1994) and completed a health questionnaire with detailed information on current and lifetime drug use. Participants complete the Michigan Alcohol Screening Test (MAST; Selzer 1975) to detect alcohol problems, and the Symptom Check List 90 Revised (SCL-90R; Derogatis 1999) to assess psychiatric symptoms. Further, participants received an electrocardiogram and a physical examination supervised by a physician. Inclusion criteria were high school diploma or equivalent; BMI between 19 and 30; and verbal fluency in English. Exclusion criteria were: history of adverse effects from ecstasy; past treatment for drug or alcohol problems or current substance dependence (DSM-IV criteria; American Psychiatric Association 2000); past year panic attacks or depressive, anxiety or eating disorder, history of psychotic or manic episodes (DSM-IV criteria; American Psychiatric Association 2000); medical conditions including diabetes insipidus; cardiovascular illness or high blood pressure, abnormal EKG, first degree relatives with heart disease; and pregnancy or lactation (females).
Orientation session
During an orientation visit, study procedures were explained and participants provided informed consent. They agreed not to use any alcohol for 24 hours before each session or other drugs for 48 hours before the sessions, and to abstain from drug use for 12 hours after the session. They were instructed to have normal sleep before the sessions, and to fast for two hours but use their normal amounts of caffeine. They were told that they might receive any of several drugs during the study, including a stimulant (such as MDMA or amphetamine), a sedative or a placebo. Participants were informed that the sessions would be videotaped. The study was approved by the University of Chicago Institutional Review Board.
Design
The study used a mixed between- and within-subject design. Participants were randomly assigned to one of three conditions: i. Solitary (SOL), in which completed laboratory sessions with minimal interaction with others, ii. Research Assistant Present (RAP), in which they completed sessions in the presence of a research assistant who was instructed to respond to social interactions by the participant but not to lead the interaction, and iii. Other Participant Present (OPP), in which participants completed sessions in the presence of one or two additional active participants who received the same dose level of MDMA or placebo during the same session. Participants in each condition completed three 5-hour sessions, in which they received a single capsule containing either MDMA (0.5 mg/kg MDMA, 1.0 mg/kg MDMA) or placebo under double blind conditions, in randomized order. During the sessions participants completed self-report questionnaires and tasks, and physiological measures were obtained.
Procedure
Each experimental session was conducted from 9am to 1:30pm. Upon arrival for each session, participants provided a breath sample to determine Breath Alcohol Level (BAL) (Alco-sensor III, Intoximeters, St. Louis, MO) or recent smoking (CO monitor). A urine sample was provided to test for recent use of methamphetamine, amphetamine, cocaine, cannabis metabolites, morphine, barbiturates, benzodiazepines, and phencyclidine (OnTrak TestSytik, Roche Diagnostics, Basel, Switzerland). Positive urine, saliva or breath tests resulted in rescheduling of the session. No sessions were rescheduled due to positive urine samples. Women were tested for pregnancy using a urinary hCG assay (Abbott Laboratories, Abbott Park, IL). None were positive. Pre-capsule blood pressure and heart rates were measured and sessions cancelled if either of these was too high.
After the compliance tests participants completed pre-capsule self-report questionnaires and physiological measures were obtained (see below). Then, they consumed a capsule containing placebo or MDMA (0.5, 1.0 mg/kg) under double-blind conditions. In the SOL condition, participants were tested individually and had only brief contact with a research assistant who came into the room for 2–4 minutes to obtain dependent measures at intervals during the session. In the RAP condition, a research assistant remained in the testing room with the participant for the duration of the session, conversing the interacting with the participant in as natural a manner as possible. The research assistant obtained cardiovascular measures at the required times, but left the room briefly when the participant completed mood questionnaires. In the OPP condition, participants were tested in groups of two or three, all of whom received the same drug on any single session. The groups were usually, but not always, comprised of the same individuals. A research assistant came into the room briefly to obtain cardiovascular measures. In all three conditions, participants completed self-report measures 30, 60, 90, 120, 210, and 240 minutes after taking the capsule, and heart rate and blood pressure were assessed at regular intervals. Between 60 and 180 min post capsule participants completed brief behavioral tasks that are not reported here. At times when participants were not engaged in tasks, they were free to read or watch TV or, in the social conditions, talk or play games. At 1:30 pm, participants received a snack, and were allowed to leave provided their heart rate and blood pressure had returned to within 10% of baseline values. Participants were telephoned the day after each session to monitor any residual drug effects. After the last session participants also attended a debriefing interview where they were told what drug they received, and why the study was conducted.
Physiological measures
Heart rate and blood pressure were measured at regular intervals throughout the sessions using portable monitors (Life Source, A&D, Tokyo, Japan).
Subjective Measures
Participants completed subjective effect questionnaires before and at regular intervals after capsule ingesting the capsule. The drug-effect questionnaire (DEQ) is a visual analogue questionnaire designed to assess the extent to which participants experienced the effects of the drugs: ‘Feel Drug’, ‘Feel High’, ‘Like Drug’, ‘Dislike Drug’, and ‘Want More’ (Fischman and Foltin 1991; Justice and de Wit 2000). Each item was presented with a 100-mm line labeled ‘not at all’ at one end and ‘extremely’ at the other end. They also completed a series of visual analog scales (VAS: 0 to 100 mm; not at all to extremely) that consisted of adjectives describing several MDMA-related mood effects (i.e., ‘I feel…’ ‘Anxious,’ ‘Dizzy,’ ‘Elated,’ ‘Restless,’ ‘Sedated,’ ‘Stimulated’) and “prosocial” effects (i.e., ‘I feel…’ ‘Confident,’ ‘Friendly,’ ‘Insightful,’ ‘Loving’, ‘Lonely,’ ‘Playful,’ ‘Sociable’).
Social Interaction Measures
Video recordings of social interaction
Participants in the RAP and OPP conditions were digitally recorded and the tapes were rated by two research assistants who were blind to the drug condition. Ratings included time spent “interacting” (i.e., playing games or talking: Haney et al. 2001; Kirkpatrick et al. 2012, 2013) or “not interacting” (i.e., reading, silently watching a movie, or sleeping). The subjects’ behavior was coded every 1.5 min throughout the 5-hour session, except at times were they were completing questionnaires or a study task. The dependent variables were the proportion of 1.5-min intervals during which participants were interacting or talking. The inter-rater reliability was over 90%.
Evaluations of the co-participant or research assistant and self
At the end of each session, participants completed three questionnaires to determine their perceptions of others. They were moved to separate rooms and completed the following questionnaires alone: the Interpersonal Attraction Questionnaire (AQ; McCroskey and McCain 1974), the Perceived Responsiveness Questionnaire (PRQ Other and PRQ Self; Reis 2003) and the Social Interaction Questionnaire (SIQ: aan het Rot et al. 2006). The AQ is a self-report questionnaire that assesses the perceived social attractiveness and physical attractiveness of another person, on a Likert scale (1–5). Participants in the SOL and RAP groups were instructed to rate the research assistant and participants in the OPP group rated their co-participants. The PRQ Other is a self-report questionnaire assessing the level of attention, interest, understanding, and empathy of another person (the co-participant or research assistant) on a Likert scale (1–7). The PRQ Self asked the same questions about the participant’s own level of responsiveness toward the co-participant or research assistant. On the SIQ participants rated their perception of their own levels of social affiliation, defined by agreeable and quarrelsome behaviors, and social power or status, defined by dominant and submissive behaviors.
Drugs
MDMA (0.5 or 1.0 mg/kg up to 125 mg) was prepared individually for each participant by the hospital pharmacist. The powder form of the drug was obtained from Dr. David Nichols of Purdue University, and placed in opaque size 00 capsules with lactose (USP) filler. Placebo capsules contained only lactose. These MDMA dose levels are low to moderate, relative to doses used in previous laboratory studies or recreationally outside the lab (Harris et al. 2002; Hysek et al. 2012, 2013; Kirkpatrick et al. 2014a, b). We chose relatively low doses of MDMA because of the possibility that the presence of others would increase responses to the drug, raising concerns about safety.
Data Analysis
First, we established that MDMA produced its prototypic effects, and investigated the effects of the drug under the three conditions (SOL, RAP, OPP). Cardiovascular and subjective-effects data were analyzed using multilevel linear models (MLMs). Independent (fixed) effects were Dose (placebo, 0.5 mg/kg, 1.0 mg/kg MDMA), Group (SOL, RAP, OPP), and Time of assessment (baseline and 30, 60, 90, 120, 210, 240 min post capsule administration).
Second, we assessed the effects of MDMA on objective and subjective measures of social behavior, using the video interaction data (for RAP and OPP only) and social questionnaire data (using all groups) using MLMs. Independent effects were Dose and Group.
For all analyses, significant main effects and Dose x Group interactions were followed by post hoc comparisons of the estimated marginal means. p Values were considered statistically significant at less than 0.05. We used comparison-wise Bonferroni corrections for multiple comparisons within each dependent measure.
RESULTS
Sample Characteristics
The participants were in their early twenties, most with partial college education and modest recreational drug use experience (Table 1). The three groups were similar in age, sex, education and drug use history.
Table 1.
Demographic characteristics of the three groups: SOL = solitary, RAP = research assistant present, and OPP = other participant present. Means (sem) or counts (percent of subjects) are shown. Quantities of use are calculated only for the participants who reported any use.
| SOL (N=10) | RAP (N=11) | OPP (N=12) | |
|---|---|---|---|
| Age (years) | 24.7 (2.7) | 25.7 (4.8) | 24.5 (3.3) |
| Education (years) | 14.4 (1.3) | 14.6 (1.3) | 15.5 (0.9) |
| Sex | |||
| Females | 2 (20%) | 3 (27%) | 4 (33%) |
| Males | 8 (80%) | 8 (73%) | 8 (67%) |
| Race | |||
| Caucasian | 7 (70%) | 6 (55%) | 9 (75%) |
| African | 1 (10%) | 3 (27%) | 2 (17%) |
| Hispanic | 1 (10%) | 2 (18%) | 1 (8%) |
| Asian | 1 (10%) | 0 | 0 |
| Caffeine | |||
| Daily Users | 6 | 7 | 8 |
| Cups/day | 1.7 (.52) | 1.9 (1.2) | 1.8 (.95) |
| Nicotine | |||
| Daily Users | 2 | 2 | 4 |
| Cigs/day | 6.3 (7.4) | 5.3 (2.5) | 2.4 (1.1) |
| Alcohol | |||
| Weekly (N) | 10 | 10 | 11 |
| Days/week | 2.1 (.72) | 2.8 (1.2) | 3.4 (1.6) |
| Drinks/drinking day | 4.6 (3.0) | 4.1 (2.4) | 2.9 (1.2) |
| MDMA | |||
| Lifetime use | 14.5 (22.2) | 18.4 (13.1) | 20.9 (21.5) |
| Cannabis | |||
| Ever users | 2 | 5 | 6 |
| Occasions/month | 7.5 (3.5) | 15.9 (10.6) | 6.1 (4.0) |
| Tranquilizers (recreational) | |||
| Ever used | 6 | 5 | 7 |
| Lifetime uses | 18.2 (40) | 2.2 (2.7) | 22.4 (24) |
| Stimulants (recreational) | |||
| Ever used | 9 | 7 | 9 |
| Lifetime uses | 34 (34) | 22 (35) | 29 (44) |
| Opiates (recreational) | |||
| Ever used | 8 | 3 | 7 |
| Lifetime uses | 19 (28) | 6.8 (5) | 33 (42) |
| Hallucinogens | |||
| Ever used | 7 | 9 | 11 |
| Lifetime uses | 8.9 (8.9) | 10.1 (12.9) | 21.2 |
Cardiovascular and subjective effects of MDMA
Overall, MDMA produced its typical dose-dependent subjective and cardiovascular effects, including increases in heart rate and blood pressure, and increases on most rating scales (Table 2; Supplementary Table 1). Peak effects occurred between 60 and 90 minutes after capsule administration.
Table 2.
Effects of MMDA and Group on systolic pressure and subjective effects.
| SOL (N=10) | RAP (N=11) | OPP (N=12) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| placebo | low MDMA | high MDMA | placebo | low MDMA | high MDMA | placebo | low MDMA | high MDMA | ||||||||||
| Systolic Pressure | −3.0 | (1.1) | 1.7 | (1.1) * | 7.8 | (1.5) *§ | −2.5 | (1.0) | 1.7 | (1.2) * | 8.0 | (1.4) *§ | 1.9 | (1.1) | 2.4 | (1.1) | 14.7 | (1.5) *§ |
| DEQ | ||||||||||||||||||
| Like Drug | 14.2 | (2.3) | 21.5 | (2.6) | 46.9 | (3.8) *§ | 22.9 | (2.9) | 29.3 | (3.7) | 42.9 | (3.6) *§ | 17.3 | (2.6) | 28.4 | (3.3) * | 49.4 | (3.9) *§ |
| Dislike Drug | 13.0 | (2.1) | 18.9 | (2.3) | 12.6 | (2.2) | 10.2 | (1.9) | 9.2 | (2.1) | 17.2 | (2.8) *§ | 8.1 | (1.5) | 18.6 | (3.1) * | 12.1 | (1.8) |
| Want More | 12.5 | (2.5) | 16.6 | (2.5) | 43.2 | (4.6) *§ | 17.8 | (3.1) | 22.8 | (3.4) | 32.1 | (4.0) *§ | 14.9 | (2.4) | 27.5 | (3.6) * | 32.7 | (3.7) * |
| VAS | ||||||||||||||||||
| Anxious | −2.1 | (1.0) | 0.6 | (2.1) | −3.5 | (2.0) | −7.4 | (2.3) | −3.3 | (1.7) | 5.5 | (2.6) *§ | −7.7 | (1.6) | −2.4 | (1.7) | 0.8 | (1.9) * |
| Elated | 4.9 | (1.6) | 1.0 | (1.4) | 13.6 | (2.8) | −1.2 | (2.3) | 4.4 | (1.6) | 19.3 | (2.9) | 1.4 | (1.9) | 5.8 | (1.7) | 19.0 | (2.8) |
| Insightful | 2.9 | (2.0) | 1.8 | (1.7) | 15.8 | (2.9) *§ | 3.8 | (1.9) | 7.5 | (1.7) | 13.4 | (1.9) * | 1.4 | (2.5) | 10.3 | (1.9) * | 13.7 | (2.9) * |
| Loving | 1.1 | (1.2) | 0.6 | (1.1) | 2.7 | (1.8) | −0.4 | (0.5) | −2.4 | (1.4) | 3.7 | (1.6) § | −8.2 | (2.2) | −7.7 | (2.1) | −0.4 | (1.2) * |
| Restless | −0.9 | (2.0) | 12.6 | (2.2) * | 6.6 | (3.4) | 1.6 | (2.2) | 4.6 | (2.3) | 17.5 | (3.4) *§ | 4.3 | (2.0) | 2.2 | (2.7) | 12.4 | (2.9) *§ |
| Sedated | 0.8 | (1.8) | 4.0 | (2.3) | 2.1 | (2.5) | 7.3 | (2.2) | −2.5 | (3.4) * | 2.4 | (2.2) | −4.4 | (2.4) | 7.1 | (2.6) * | −2.7 | (3.0) § |
| Stimulated | −1.0 | (0.9) | 6.8 | (1.6) * | 17.4 | (2.8) *§ | −1.5 | (2.3) | 4.8 | (1.9) | 24.5 | (3.5) *§ | −10.2 | (2.8) | 5.2 | (1.9) * | 22.0 | (3.8) *§ |
An * denotes significantly different than placebo (p < 0.05).
An § denotes high MDMA significantly different than low MDMA (p < 0.05).
Influence of social context of MDMA-related effects
Heart rate and blood pressure
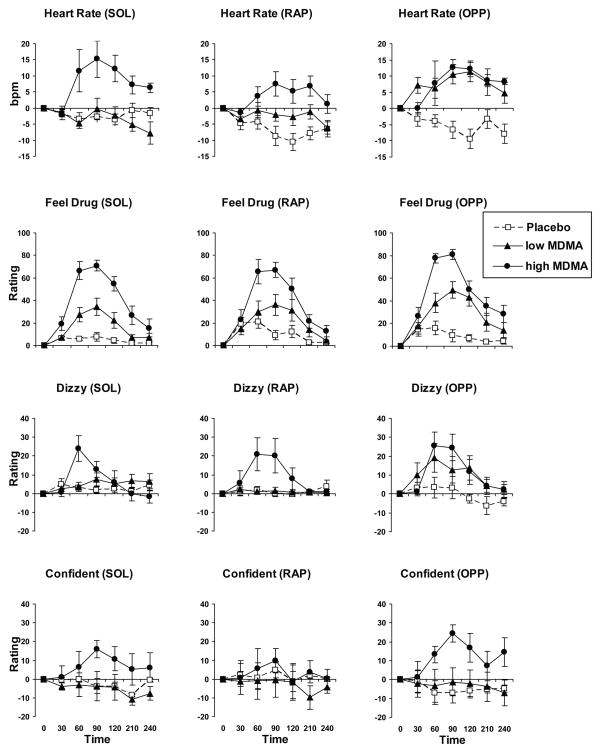
The effects of MDMA on heart rate and blood pressure varied across the three conditions. Whereas the higher dose (1.0 mg/kg) increased heart rate to a similar extent in all three experimental conditions, the lower dose (0.5 mg/kg) increased heart rate to a significantly greater extent in the OPP group, compared to both the RAP and SOL groups (Figure 1; Group x Dose interaction: F (4,580)=13.1, p<0.001; post hoc pairwise comparisons: p<0.001 for both comparisons). The higher dose also produced a greater increase in systolic blood pressure in the OPP group compared to the RAP and SOL groups (Supplementary Figure 1; Group x Dose interaction: F (4,580)=2.8, p<0.05; post hoc pairwise comparisons: p<0.01 for both comparisons).
Figure 1.
Effects of MDMA (“low MDMA” = 0.5 mg/kg, “high MDMA” = 1.0 mg/kg) and placebo on four measures in the three experimental conditions: SOL = solitary, RAP = research assistant present, and OPP = other participant present. Some effects of MDMA (0.5 mg/kg or 1.0 mg/kg) were greater in the OPP condition compared to the other conditions.
Subjective ratings
On mean ratings of “feel drug” across the entire session, the lower dose of MDMA produced greater effects in the OPP group compared to the SOL group, but at the higher dose the groups did not differ (Figure 1; Group x Dose interaction: F (4,579)=2.4, p<0.05; post hoc pairwise comparison: p<0.05). Similarly on ratings of “dizzy” the lower dose produced greater effects in the OPP group compared to the RAP group (Figure 1; Group x Dose interaction: F (4,579)=4.0, p<0.01; post hoc pairwise comparison: p<0.05). Finally, the higher dose of MDMA increased ratings of “confident” to a greater extent in the OPP compared to the RAP group (Figure 1; Group x Dose interaction: F (4,579)=5.4, p<0.001; post hoc pairwise comparison: p<0.05).
Table 2 and Supplementary Table 1 show that several other significant Group x Dose interactions were obtained. Individual post hoc tests within each dose level indicated that the OPP, RAP, and SOL groups did not significantly differ. However, post hoc tests within each group revealed several differences in dose response. For example, in OPP only, the low dose of MDMA increased ratings of ‘like drug’, ‘dislike drug’, ‘want more’, and ‘insightful’ compared to placebo.
MDMA-related effects on social behavior
Video recordings (RAP and OPP groups only)
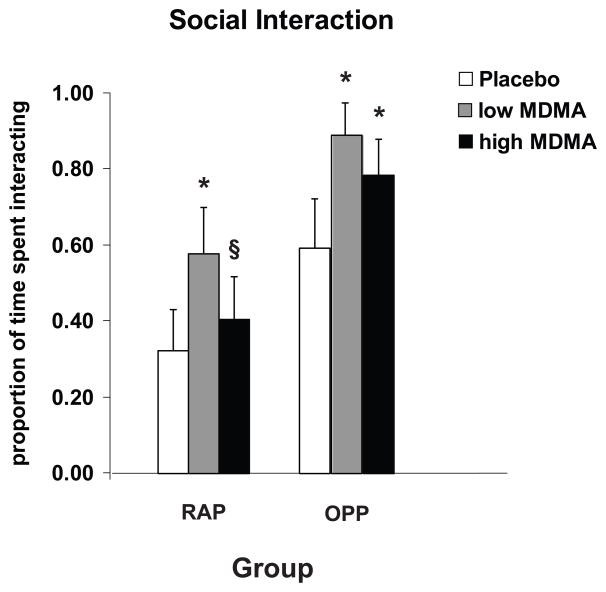
Both doses of MDMA increased social interaction in both groups, but this effect was most pronounced at the lower dose (Figure 2; Main Effect of Dose: F(2,253)=22.8, p<0.001). At the lower dose only, the effect of MDMA on social interaction was accounted for primarily by an increase in talking (Main Effect of Dose: F(2,253)=8.3, p<0.001; post hoc pairwise comparison: p<0.001). Regardless of drug condition, participants interacted more in the OPP group compared to the RAP group; there were no drug-related differences in social interaction across groups (data not shown; Main Effect of Group: F(1,20)=11.6, p<0.01).
Figure 2.
Effects of MDMA (0.5 mg/kg, 1.0 mg/kg or placebo) on the proportion of time participants engaged in social behavior, either interacting (verbal and nonverbal) or talking (RAP and OPP data combined). The participants’ activities were rated every 1.5 minutes across the 4-hour session by raters blind to the conditions. An * denotes significantly different than placebo (p < 0.05). An § denotes high MDMA significantly different than low MDMA (p < 0.05). There were no dose-related differences between the RAP and OPP groups.
End of session ratings of self and others (AQ, and PRQ Self and Other: all groups; SIQ: RAP and OPP conditions only)
Participants rated the other person in the room as more socially attractive following the larger MDMA dose compared to placebo regardless of group (AQ score mean ratings 1.0 mg/kg MDMA: 50.8±1.2 versus placebo: 48.2±1.2 averaged across the two conditions; Main Effect of Dose: F(2,57)=3.6, p<0.05; post hoc pairwise comparison: p<0.05). Additionally, participants in the OPP group reported more agreeable behaviors on the SIQ regardless of whether they got drug or not (mean ratings OPP: 6.5±0.5 versus RAP: 4.5±0.4 averaged across the two social groups; Main Effect of Group: F(1,18)=10.4, p<0.01). MDMA did not alter perceived emotional responsiveness, either for the PRQ Self or the Other.
Discussion
The present study was designed to investigate the effects of social conditions (i.e., presence of others) on the acute responses to MDMA. MDMA is typically used in highly social settings such as dance parties, or raves. In laboratory animals, as well, the effects of stimulant drugs are enhanced by the presence of other conspecifics, especially other animals that are also self-administering the drug. In the present study, MDMA produced its prototypical effects of increased heart rate and positive subjective effects in all three conditions, whether participants were alone, with a research assistant, or with other drug-treated participants. We found modest evidence for an enhancement of MDMA effects when participants received the drug with other co-participants. In particular, MDMA produced greater increases in cardiovascular measures and some subjective ratings when participants were tested with co-participants. We also found that the drug influenced the perception of others: MDMA increased ratings of attractiveness of the other person and increased social interaction. Our findings suggest that social context has a modest influence on responses to MDMA.
On some measures, the influence of the presence of other participants on responses to MDMA varied across the two doses. For most of the cardiovascular and subjective measures, the effects of MDMA were dose-dependent and linear, which is consistent with previous studies of the acute effects of MDMA (Bedi et al. 2010; Harris et al. 2002; Hysek and Liechti 2012; Kirkpatrick et al. 2014b; Tancer and Johanson 2003). However, in the OPP condition, the drug produced greater increases in heart rate at the lower dose and systolic blood pressure at the higher dose, compared to participants tested alone or with a research assistant present. The lower dose also produced greater ratings of feeling the drug and feeling dizzy in the OPP group. These data are consistent with results showing that a social context can enhance or intensify acute drug effects (de Wit et al. 1997; Doty and de Wit 1995; Evans et al. 1996; Kelly et al. 1994; Kirkpatrick and de Wit 2013). It is possible that the greater drug effects in OPP participants are related to an overall increase in activity related to social interaction. That is, the drug may increase social interaction, and the increase in social interaction may have effects on other measures. Separating these processes will be a challenge for future studies.
We observed differences in responses to MDMA in the OPP compared to the RAP group. The higher dose of MDMA produced greater feelings of confidence in the OPP group than the RAP group, and the lower MDMA dose increased ratings on feeling insightful, and drug wanting, liking, and disliking in the OPP group only. There are several possible reasons for these differences. First, they may be related to the number of individuals present in the room: in the RAP group there was just one additional person, and in the OPP group there were two or three. Second, they may be related to the responses to the drug in the other participants: This can only be determined in a carefully designed study where the drug state of the other co-participant is controlled (e.g., Kirkpatrick and de Wit 2013). Third, it is also possible that the participants’ knowledge that the research participant was a member of the staff – whereas the co-participants were also research volunteers – influenced participants’ responses. Although our data indicate the importance of the social context (Carlin et al. 1972; Kirkpatrick and de Wit 2013; Sher et al. 1985), they leave open the question of which variables influence the apparent social facilitation of the drug effect.
The study also provided information about the effects of MDMA on social behavior; the drug increased both objective and subjective measures of social behavior. MDMA increased the time the participants spent interacting and talking. The larger dose of MDMA also increased ratings of attractiveness of the other person (research assistant or other participant) in all three groups of participants. Overall, these findings are consistent with data from preclinical and human studies showing that MDMA enhances social processing (Bedi et al. 2009, 2010; Hysek et al. 2012; Kirkpatrick et al. 2014; Wardle et al. 2014) and social behavior, such as increased time spent interacting in rats (Ramos et al. 2013; Thompson et al. 2009), and increases in empathy and prosociality (Hysek et al. 2013). Surprisingly, the low dose of MDMA produced greater levels of interaction than the larger dose, whereas others have reported that lower doses of MDMA (e.g., 75 mg) produce less empathogenic effects and smaller increases in oxytocin levels than larger doses (e.g., 125 mg: Schmid et al. 2014). This nonlinear dose response on measures of social interaction remains to be investigated. The effects of MDMA on social interaction appear to be similar to effects of several other drugs, including alcohol and other stimulant drugs (Higgins and Stitzer 1988; Lindfors and Lindman 1987; Marrone et al. 2010; Stitzer et al. 1981; Ward et al. 1997). Whether specific aspects of the pro-social effects of MDMA distinguish it from other drugs remains to be determined.
The current results should be interpreted in the context of at least three limitations. First, our study was small (N=32 across three groups) and thus we may not have had the power to detect subtler drug response differences between the groups. Second, in the OPP condition participants were arbitrarily matched with other co-participants, based mainly on availability. It is possible that the characteristics of the partner influenced both subjective drug response and sociability. For example, it is possible that the personalities of the participants could mediate the drug experience for each individual, creating positive experiences in some and negative experiences in others. Although participants were randomly assigned participants to groups to minimize this type of bias, future studies might assess and systematically evaluate the influence of partner characteristics (i.e., friends vs strangers) on drug response. Another limitation relates to the social contexts that we created and the activities that the participants could engage in. For instance, we allowed participants to watch movies, which may have confounded the observed drug effect by influencing their mood states and altering the social interactions in a number of ways, including possibly reducing the time spent talking. Finally, our laboratory environment differs from naturalistic social contexts in which MDMA is used, and future studies might investigate the drug under more naturalistic social conditions.
In conclusion, we found modest evidence that the effects of MDMA were influenced by a social context. We found that the presence of other intoxicated participants increased cardiovascular responses and enhanced some subjective responses to MDMA. However, for the majority of measures the groups’ drug responses did not differ meaningfully, suggesting that the social contexts used in the present study produced modest influences on drug response. Additionally, we found that MDMA increased social interaction, and ratings of the attractiveness of another person in the room. Overall, these findings further highlight the potential influence of social factors in acute drug effects and may partially explain why MDMA is used in a social context. Future studies may examine the influence of a range of more naturalistic social settings and the extent to which socially facilitated alterations in subjective states influence MDMA consumption.
Supplementary Material
Acknowledgments
Supported by DA02812 and DA026570 (Harriet de Wit PI).
This research was supported by R21 DA026570 and R01 DA02812. The authors thank Matthew Baggott for advice, Jonathan Solamillo and Nicole Noga for excellent technical assistance, and Emmanuel Semmes for pharmacy support.
Footnotes
Conflict of Interest Statement: The authors declare no conflicts of interest.
References
- aan het Rot M, Moskowitz DS, et al. Social behaviour and mood in everyday life: the effects of tryptophan in quarrelsome individuals. J Psychiatry Neurosci. 2006;31:253–62. [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, D.C: American Psychiatric Association; 2000. text revision. [Google Scholar]
- Bedi G, Hyman D, de Wit H. Is ecstasy an “empathogen”? Effects of +/−3,4-methylenedioxymethamphetamine on prosocial feelings and identification of emotional states in others. Biol Psychiatry. 2010;68(12):1134–1140. doi: 10.1016/j.biopsych.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Phan KL, Angstadt M, de Wit H. Effects of MDMA on sociability and neural response to social threat and social reward. Psychopharmacology (Berl) 2009;207(1):73–83. doi: 10.1007/s00213-009-1635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos PA, Panksepp J, Bluthe RM, Honk JV. Acute effects of steroid hormones and neuropeptides on human social-emotional behavior: A review of single administration studies. Front Neuroendocrinol. 2011 doi: 10.1016/j.yfrne.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Bravo GL. What does MDMA feel like? In: Holland J, editor. Ecstasy: The complete guide: A comprehensive look at the risks and benefits of MDMA. Rochester, VT, US: Park Street Press; 2001. pp. 21–28. [Google Scholar]
- Carlin AS, Bakker CB, Halpern L, Post RD. Social facilitation of marijuana intoxication: impact of social set and pharmacological activity. J Abnorm Psychol. 1972;80:132–140. doi: 10.1037/h0033317. [DOI] [PubMed] [Google Scholar]
- Chieu CC, Moore KE. D-amphetamine-induced release of “newly synthesized” and “stored” dopamine from the caudate nucleus in vivo. J Pharmacol Exp Ther. 1975;192:642–653. [PubMed] [Google Scholar]
- Derogatis LR, Savitz KL. The SCL-90-R, Brief Symptom Inventory, and Matching Clinical Rating Scales The use of psychological testing for treatment planning and outcomes assessment. 2. Mahwah, NJ, US: Lawrence Erlbaum Associates Publishers; 1999. pp. 679–724. [Google Scholar]
- de Wit H, Clark M, Brauer LH. Effects of d-amphetamine in grouped versus isolated humans. Pharmacol Biochem Behav. 1997;57(1–2):333–340. doi: 10.1016/s0091-3057(96)00316-4. [DOI] [PubMed] [Google Scholar]
- Doty P, de Wit H. Effect of setting on the reinforcing and subjective effects of ethanol in social drinkers. Psychopharmacology (Berl) 1995;118(1):19–27. doi: 10.1007/BF02245245. [DOI] [PubMed] [Google Scholar]
- Evans SM, Griffiths RR, de Wit H. Preference for diazepam, but not buspirone, in moderate drinkers. Psychopharmacology. 1996;123(2):154–163. doi: 10.1007/BF02246172. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Foltin RW. Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. Br J Addict. 1991;86:1563–70. doi: 10.1111/j.1360-0443.1991.tb01749.x. [DOI] [PubMed] [Google Scholar]
- Fromme K, Dunn ME. Alcohol expectancies, social and environmental cues as determinants of drinking and perceived reinforcement. Addictive Behaviors. 1992;17:167–177. doi: 10.1016/0306-4603(92)90021-m. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Hart CL, Foltin RW, Fischman MW. Bupropion SR worsens mood during marijuana withdrawal in humans. Psychopharmacology. 2001;155:171–179. doi: 10.1007/s002130000657. [DOI] [PubMed] [Google Scholar]
- Harris DS, Baggott M, Mendelson JH, Mendelson JE, Jones RT. Subjective and hormonal effects of 3, 4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology. 2002;162:396–405. doi: 10.1007/s00213-002-1131-1. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Stitzer ML. Effects of alcohol on speaking in isolated humans. Psychopharmacol (Berl) 1988;95:189–194. doi: 10.1007/BF00174508. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Domes G, Liechti ME. MDMA enhances “mind reading” of positive emotions and impairs “mind reading” of negative emotions. Psychopharmacology (Berl) 2012;222(2):293–302. doi: 10.1007/s00213-012-2645-9. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Schmid Y, Simmler LD, Domes G, Heinrichs M, Eisenegger C, Liechti ME. MDMA enhances emotional empathy and prosocial behavior. Social Cog and Affective Neuro. 2013 doi: 10.1093/scan/nst161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice AJ, De Wit H. Acute effects of d-amphetamine during the early and late follicular phases of the menstrual cycle in women. Pharmacol Biochem Behav. 2000;66:509–515. doi: 10.1016/s0091-3057(00)00218-5. [DOI] [PubMed] [Google Scholar]
- Kelly BC, Parsons JT, Wells BE. Prevalence and predictors of club drug use among club-going young adults in New York City. J Urban Health. 2006;83:884–895. doi: 10.1007/s11524-006-9057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TH, Foltin RW, Mayr MT, Fischman MW. Effects of 9-tetrahydrocannabinol and social context on marijuana self-administration by humans. Pharmacology Biochemistry and Behavior. 1994;49(3):763–768. doi: 10.1016/0091-3057(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick MG, Gunderson EW, Perez AY, Haney M, Foltin RW, Hart CL. A direct comparison of the behavioral and physiological effects of methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology (Berl) 2012;219:109–122. doi: 10.1007/s00213-011-2383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, de Wit H. In the company of others: social factors alter acute alcohol effects. Psychopharmacology (Berl) 2013;230:215–226. doi: 10.1007/s00213-013-3147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Baggott MJ, Mendelson JE, Galloway GP, Liechti ME, Hysek CM, de Wit H. MDMA effects consistent across laboratories. Psychopharmacology. 2014a:1–7. doi: 10.1007/s00213-014-3528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Lee R, Wardle MC, Jacob S, de Wit H. Effects of MDMA and Intranasal Oxytocin on Social and Emotional Processing. Neuropsychopharmacology. 2014b;39:1654–63. doi: 10.1038/npp.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindfors B, Lindman R. Alcohol and previous acquaintance: mood and social interactions in small groups. Scand J Psychol. 1987;28(3):211–219. doi: 10.1111/j.1467-9450.1987.tb00757.x. [DOI] [PubMed] [Google Scholar]
- Lokiec F, Jacquot C, Cohen Y. Cageing density and dopamine striatal elimination after amphetamine in the rat. Psychopharmacology (Berl) 1981;73(4):402–403. doi: 10.1007/BF00426476. [DOI] [PubMed] [Google Scholar]
- Lokiec F, Jacquot C, Rapin JR, Cohen Y. Effects of amphetamine on brain biogenic amines in isolated and aggregated rats Eur. J Pharmacol. 1977;44:391–395. doi: 10.1016/0014-2999(77)90314-4. [DOI] [PubMed] [Google Scholar]
- Marrone GF, Pardo JS, Krauss RM, Hart CL. Amphetamine analogs methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA) differentially affect speech. Psychopharmacol (Berl) 2010;208:169–177. doi: 10.1007/s00213-009-1715-0. [DOI] [PubMed] [Google Scholar]
- McCroskey JC, McCain TA. The measurement of interpersonal attraction. Speech Monographs. 1974;41:261–266. [Google Scholar]
- Mohrland JS, Craigmill AL. The effect of aggregation on the lethality of morphine in mice Arch. Int Pharmacodyn Ther. 1978;236:252–265. [PubMed] [Google Scholar]
- Moore KE, Sawdy LC, Shaul SR. Effects of d-amphetamine on blood glucose and tissue glycogen levels of isolated and aggregated mice. Biochem Pharmacol. 1965;14:197–204. doi: 10.1016/0006-2952(65)90183-8. [DOI] [PubMed] [Google Scholar]
- Ramos L, Hicks C, Kevin R, Caminer A, Narlawar R, Kassiou M, et al. Acute Prosocial Effects of Oxytocin and Vasopressin When Given Alone or in Combination with 3,4-Methylenedioxymethamphetamine in Rats: Involvement of the V1A Receptor. Neuropsychopharmacology. 2013;38(11):2249–2259. doi: 10.1038/npp.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis HT. A self-report measure of perceived partner responsiveness. University of Rochester; 2003. [Google Scholar]
- Rodgers J, Buchanan T, Pearson C, Parrott AC, Ling J, Hefferman TM, et al. Differential experiences of the psycho-biological sequelae of ecstasy use: quantitative and qualitative data from an internet study. Psychopharmacology (Berl) 2006;20:437–446. doi: 10.1177/0269881105058777. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Creswell KG, Dimoff JD, Fairbairn CE, Cohn JF, Heckman BW, Moreland RL. Alcohol and Group Formation A Multimodal Investigation of the Effects of Alcohol on Emotion and Social Bonding. Psychological science. 2012 doi: 10.1177/0956797611435134. 0956797611435134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid Y, Hysek CM, Simmler LD, Crockett MJ, Quednow BB, Liechti ME. Differential effects of MDMA and methylphenidate on social cognition. Journal of Psychopharmacology. 2014;28:847–856. doi: 10.1177/0269881114542454. [DOI] [PubMed] [Google Scholar]
- Selzer ML, Vinokur A, van Rooijen L. A self-administered Short Michigan Alcoholism Screening Test (SMAST) Journal of Studies on Alcohol. 1975;36:117–126. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- Sher KJ. Subjective effects of alcohol: the influence of setting and individual differences in alcohol expectancies. J Stud Alcohol. 1985;46:137–146. doi: 10.15288/jsa.1985.46.137. [DOI] [PubMed] [Google Scholar]
- Shintel H, et al. Accentuate the negative, eliminate the positive? Individual differences in attentional bias to positive and negative information. 47th Annual Meeting of the Psychonomic Society.2006. [Google Scholar]
- Smith MA, Pitts EG. Social preference and drug self-administration: a preclinical model of social choice within peer groups. Drug Alcohol Depend. 2014;135:140–5. doi: 10.1016/j.drugalcdep.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitzer ML, Griffiths RR, et al. Human social conversation: effects of ethanol, secobarbital and chlorpromazine. Pharmacol Biochem Behav. 1981;14(3):353–360. doi: 10.1016/0091-3057(81)90402-0. [DOI] [PubMed] [Google Scholar]
- Strickland JC, Smith MA. The effects of social contact on drug use: behavioral mechanisms controlling drug intake. Exp Clin Psychopharmacol. 2014;22(1):23–24. doi: 10.1037/a0034669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumnall HR, Cole JC, Jerome L. The varieties of ecstatic experience: an exploration of the subjective experiences of ecstasy. Psychopharmacology (Berl) 2006;20:437–446. doi: 10.1177/0269881106060764. [DOI] [PubMed] [Google Scholar]
- Tancer M, Johanson CE. Reinforcing, subjective, and physiological effects of MDMA in humans: a comparison with d-amphetamine and mCPP. Drug and alcohol dependence. 2003;72:33–44. doi: 10.1016/s0376-8716(03)00172-8. [DOI] [PubMed] [Google Scholar]
- Thiel KJ, Okun AC, Neisewander JL. Social reward-conditioned place preference: a model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Depend. 2008;96(3):202–12. doi: 10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Neisewander JL. Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology (Berl) 2009;204(3):391–402. doi: 10.1007/s00213-009-1470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MR, Hunt GE, McGregor IS. Neural correlates of MDMA (“Ecstasy”)-induced social interaction in rats. Soc Neurosci. 2009;4(1):60–72. doi: 10.1080/17470910802045042. [DOI] [PubMed] [Google Scholar]
- Ward AS, Kelly TH, Foltin RW, Fischman MW. Effects of d-amphetamine on task performance and social behavior of humans in a residential laboratory. Experimental and clinical psychopharmacology. 1997;5(2):130. doi: 10.1037//1064-1297.5.2.130. [DOI] [PubMed] [Google Scholar]
- Wardle MC, de Wit H. MDMA alters emotional processing and facilitates positive social interaction. Psychopharmacology (Berl) 2014;231:1–11. doi: 10.1007/s00213-014-3570-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.