Abstract
Background
The purpose of this study was to compare longitudinal trajectories of maximal aerobic capacity in children with sickle cell anemia (SCA) and matched healthy controls, and explore whether these trajectories were associated with selected physiologic variables.
Procedures
Children with SCA (n=33) and healthy controls (n=30) matched at baseline for race, sex, Tanner stage, height, and weight completed three consecutive annual fitness assessments (VO2peak). Data were compared between the groups at each time point and within groups over time. Change in VO2peak between the two groups over time was assessed using a linear mixed model with age, sex, fat-free mass (FFM), Tanner stage, and hemoglobin (Hgb) concentration as covariates.
Results
At baseline, children with SCA had significantly lower Hgb concentration (8.9 vs. 13.7 g/dL, p<0.001) and relative VO2peak (24.2 vs. 27.9 ml/kg/min, p=0.006) than healthy controls. Over time, children with SCA had smaller increases than healthy controls in VO2peak (−0.1 and +4.9 ml/kg/min, p<0.001), Tanner stage at year 2 (15% and 66% Tanner 4, p<0.001), and FFM (+4.0 and +6.8 kg, p=0.02). Changes in Hgb concentration did not differ between groups (+0.03 and +0.09 g/dL, p=1.0). After adjusting for age, sex, Tanner stage, FFM, and Hgb concentration the differences in change in VO2peak over time remained significant (p<0.001).
Conclusion
Children with SCA demonstrate lower relative VO2peak compared to healthy children and the difference increases over time. The difference in VO2peak trajectories between the two groups during puberty remains significant after adjusting for age, sex, FFM, Tanner stage, and Hgb concentration.
Keywords: sickle cell anemia, aerobic capacity, VO2 max, Tanner stage, adolescence
INTRODUCTION
The clinical manifestations of sickle cell anemia (SCA) have a tremendous impact on an affected child’s overall health and quality of life.(1) Complications of SCA range from hemolytic anemia, acute chest syndrome, and stroke to end-organ damage affecting lung, cardiac and renal function over time.(1) The impact of the disease on physical functioning and fitness may also be significant; previous studies have demonstrated that exercise limitation is prevalent among adults with SCA and presumed secondary to anemia or cardiopulmonary disease.(2, 3) In cross-sectional studies, fitness levels defined as peak aerobic capacity (VO2peak), measured as the maximum amount of oxygen that can be utilized by the body per unit of time during incremental exercise, are lower in children with SCA compared to healthy control children.(4, 5) In a single study of longitudinal fitness changes in children with SCA, relative work capacity (W/kg/min) was found to decrease over time, but was not compared to healthy children.(6)
There is a paucity of research that explores the determinants of physical activity limitations in children with SCA.(7) Among the physiologic correlates of VO2peak in healthy populations, fat free mass (FFM) is increasingly recognized as an important factor in both adults and children.(8-11) FFM may influence aerobic capacity through augmentation of the skeletal muscle pump and venous return, and it has been suggested that FFM may account for observed sex differences in VO2peak.(12-14) Among children, the relationship between FFM and VO2peak may be dependent on pubertal maturity, as both increase during adolescence.(15, 16) It has also been shown that FFM is reduced and pubertal development is delayed in children with SCA.(17-19) Although FFM and pubertal development are correlated with fitness in healthy children, their association with overall fitness or change in fitness over time in children with SCA has not been reported.
Identifying longitudinal differences in fitness between children with SCA and healthy controls, as well as the physiologic predictors of these differences, may provide opportunities for interventions aimed at improving overall fitness and quality of life. We are not aware of any studies, however, which have prospectively compared the longitudinal changes in fitness in children with SCA to healthy controls. Thus, the primary objective of this study was to compare baseline and 2-year change in VO2peak between pubertal children with SCA and matched healthy controls. The secondary objective was to explore the associations of FFM, Tanner stage, and hemoglobin concentration with changes in VO2peak over a 2-year period in this study population.
METHODS
Study Participants
Thirty-four African-American children with Hgb SS disease (19 males, 15 females, age 10-13 years old) were identified for participation in the study at the Pediatric Sickle Cell Clinic at Vanderbilt in Nashville, TN and at the MidSouth Sickle Cell Clinic in Memphis, TN from June 2001 to January 2006. Additionally, 34 African-American children from Nashville who did not carry the sickle cell (Hgb S) trait or any other hemoglobin (Hgb) variants were matched for sex, Tanner stage, and approximate height, weight, and fat mass to serve as a control group for the study. The presence or absence of Hgb SS disease was confirmed through Hgb electrophoresis in all participants. Exclusion criteria included vaso-occlusive pain within the two months prior to screening, chronic transfusion therapy, hydroxyurea therapy at baseline, pregnancy, or the presence of any apparent metabolic, skeletal, hepatic, or renal dysfunction based on prior laboratory or radiological evaluation. Given the exclusion of patients on hydroxyurea or chronic transfusion therapy, there were no children with disease characterized by stroke, multiple episodes of acute chest syndrome, or greater than 3 hospitalizations per year due to acute pain. Self-reported complications occurring between the study visits were recorded. Children and their parents or guardians received written information, verbal explanation about the nature and purpose of the study, and signed informed assent or consent according to the Declaration of Helsinki. The form was approved by the Vanderbilt University School of Medicine and Meharry Medical College Institutional Review Boards for procedures to be performed at the Clinical Research Center (CRC) at Vanderbilt University.
Procedures
Children were evaluated at baseline and annually for 2 years. Prior to participation in the study, children gave a medical history and underwent a complete physical examination. Pubertal development (Tanner stage) was assessed on physical examination at baseline, then by self-assessment questionnaire at year 1 and year 2.(20-22)
Anthropometrics and Body Composition
Body weight was measured to the nearest 0.05 kg with a monthly calibrated digital scale (Detecto-Medic, Detecto Scales, Inc., Northbrook, IL) with the participants wearing minimal clothing and no shoes. Height was measured using a wall-mounted stadiometer that was calibrated upon wall installation and recalibrated yearly (Perspective Enterprises, Portage, MI). Fat mass, FFM, and bone mineral density (BMD) were determined by dual energy X-ray absorptiometry (DXA, Lunar Prodigy, GE Medical Systems, Madison WI, children software, version 9.15) as previously described.(23) Our laboratory’s intraassay coefficient of variation for percent of FM using DXA is 0.79 ± 0.49%.
Aerobic(VO2peak) Testing
Peak aerobic capacity was measured using a modified treadmill exercise testing protocol in which participating children were asked to complete 2-minute stages of walking or running with increasing treadmill speed without incline, starting at 2 mph and increasing by 1 mph with each stage until volitional fatigue.(24) Participants were asked to avoid vigorous physical activity for 24 hours prior to exercise testing. Each participant received a full description of the exercise test procedure and was familiarized with Borg’s rating of perceived exertion (RPE) 20 point category ratio scale (0-20) using a visual scale.6 During the test, heart rate (HR) was measured using electrocardiography. Children received verbal encouragement throughout the test. Breath-by-breath oxygen consumption and carbon dioxide production were measured using a Med-Graphics Ultima Series system (Medical Graphics Corp., St. Paul, MN, USA), and processed and analyzed with the BreezeSuite software Version 6.4.023 (St. Paul, MN, USA). Ten seconds before the end of the 2nd minute of each stage, HR and rate of perceived exertion (RPE) were recorded. Upon completion of the exercise test, HR, BP, and RPE were recorded, and the participant continued to walk at a 2 mph for 2 minutes. After the cool-down, HR and BP were assessed to ensure these values returned to near-baseline levels.
Blood Collection and Analytical Procedures
Blood was drawn by venipuncture into EDTA tubes and used for measurements of hematological parameters that included whole blood Hgb concentration, packed cell volume, white blood cell count, reticulocyte count, and platelet count. The measurements were performed at Vanderbilt University Hospital Laboratory, while plasma albumin was measured at Vanderbilt’s Clinical Research Center Core Laboratory. All assays were performed using standard methodologies.
Statistical Analysis
Baseline anthropometric and physiologic data were grouped by disease (SCA, healthy controls). Continuous and categorical variables were compared between groups using a Wilcoxon Rank-Sum test and a Pearson chi-square test, respectively. VO2peak, FFM, and Hgb concentration were compared between groups at baseline, year 1, and year 2 using a Wilcoxon Rank-Sum test and within disease groups across time (baseline vs. year 2) using a Wilcoxon Signed-Rank test. The differences in change over time (baseline vs. year 2) between groups for VO2peak, FFM, and Hgb were compared using a Wilcoxon Rank-Sum test. Tanner stage was compared between groups (SCA, healthy controls) at baseline, year 1 and year 2 and within groups across time (baseline vs. year 2) using a Pearson chi-square test. P values less than 0.05 were considered statistically significant, and all tests were two-tailed.
To determine if there were any longitudinal differences in the VO2peak over the 2-year study period between the SCA and control groups, we used a linear mixed model to regress VO2peak on visits (baseline, year 1, and year 2), group (SCA or control), and the interaction between visits and group. The linear mixed model is essentially a linear regression model that uses random effects (intercepts) to account for correlation due to repeated measures. A second linear mixed model was developed to evaluate longitudinal differences in VO2peak after adjusting for selected physiologic and demographic variables. The model included effects for group, visits, visits and group interaction, age, sex, Tanner stage, FFM, and Hgb concentration. A third linear model evaluated interactions of selected variables with group and visits to determine whether differences in VO2peak between groups varied by sex, FFM, Tanner stage, or Hgb concentration.
RESULTS
Baseline characteristics
One female participant from the SCA group and 4 males from the healthy control group dropped out of the study before the baseline visit, yielding groups of 33 (19 male, 14 female) and 30 (15 male, 15 female) participants, respectively. Baseline characteristics for children with SCA and healthy controls, and differences between the groups are shown in Table 1. At baseline, children with SCA were significantly older, had significantly lower Hgb concentration and higher resting heart rate than healthy controls. No significant differences between the groups were found in Tanner stage, height, weight or FFM. Children with SCA had significantly lower relative VO2peak than healthy controls (24.2±4.5 vs. 27.9±5.6 ml/kg/min, p=0.006).
Table I.
Baseline characteristics of children with sickle cell anemia and healthy control children matched for race, sex, Tanner stage, weight, and height.a
| Healthy Control (n=30) |
Sickle Cell Anemia (n=33) |
p | |
|---|---|---|---|
| Age (years) | 10.2 ± 1.0 | 11.4 ± 1.4 | < 0.001c |
| Sex | 0.55b | ||
| Male (n, percent) | 15 (50) | 19 (58) | |
| Female (n, percent) | 15 (50) | 14 (42) | |
| Tanner Stage | 0.28b | ||
| 2 (n, percent) | 28 (93) | 28 (85) | |
| 3 (n, percent) | 2 (7) | 5 (15) | |
| Height (cm) | 144 ± 7 | 145 ± 12 | 0.78c |
| Weight (kg) | 38.9 ± 7.5 | 38.1 ± 8.4 | 0.61c |
| FFM (kg) | 29.9 ± 4.8 | 29.7 ± 5.3 | 0.84c |
| Hemoglobin (g/dL) | 13.7 ± 1.3 | 8.9 ± 1.2 | < 0.001c |
| White blood cell count (103/μl) | 5.6 ± 1.4 | 12.1 ± 4.8 | < 0.001c |
| Platelet count (103/μl) | 293 ± 63 | 440 ± 164 | < 0.001c |
| Reticulocyte count (%) | 1.3 ± 0.5 | 11.3 ±7.3 | < 0.001c |
| Albumin (g/dL) | 4.2 ± 0.23 | 4.37 ± 0.37 | 0.058c |
| Resting heart rate (beats/min) | 84.5 ± 6.3 | 89.4 ± 6.1 | 0.001c |
Data are presented as mean and standard deviation or percentage and number
Difference between children with SCA and healthy control group (Pearson chi-square test)
Difference between children with SCA and healthy control group (Wilcoxon Rank-Sum test)
Longitudinal changes in Tanner stage, FFM, and Hgb concentration
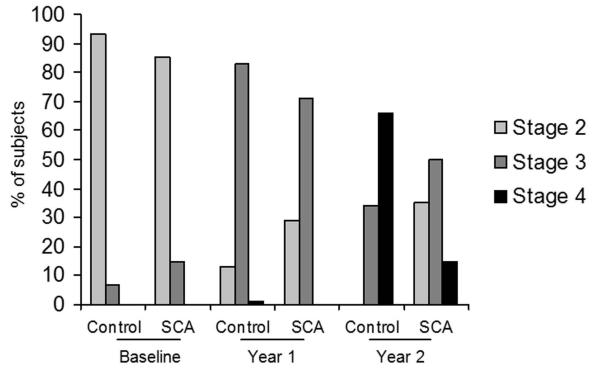
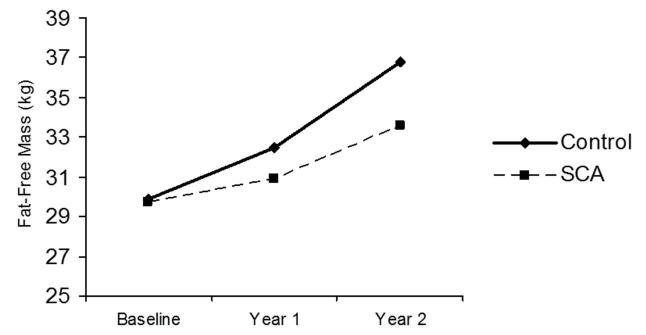
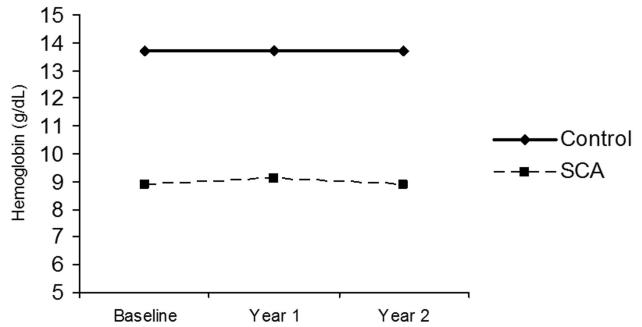
All children with SCA (n=33) and healthy controls (n=30) completed the 3 separate yearly evaluations. The changes in Tanner stage, FFM, and Hgb concentration over time are shown in Figures 1, 2, and 3, respectively. Children from both groups demonstrated increases in Tanner stage over time, but a significantly smaller percentage of children with SCA transitioned to Tanner stage 4 by year 2 than healthy controls (15% v. 66%, p<0.001; Figure 1). FFM increased significantly between baseline and year 2 in both the SCA group (29.6±5.3 and 33.6±5.7 kg, p<0.001), and healthy control group (29.9±4.8 v. 36.8±6.8 kg, p<0.001), but children with SCA had a significantly smaller change in FFM over time compared to healthy controls (+3.99 kg and +6.81 kg, respectively, p=0.02). Children with SCA continued to have significantly lower Hgb concentration at year 2 compared to controls (8.9 vs. 13.7 g/dL, p<0.001). No differences in Hgb were found over time in either group and the change over time did not differ between groups (+0.089 and +0.029 g/dL, p=1.0; Figure 3).
Figure 1. Changes in Tanner stage during the study (2 years) among children with and without sickle cell anemia (SCA).
The results are expressed as percentage of participants in each group at Tanner stages (2, 3, or 4) at each time point. More healthy control children progressed to Tanner stage 4 by year 2 than children with SCA.
Figure 2. Changes in fat-free mass (FFM) during the study (2 years) among children with and without sickle cell anemia.
Both groups demonstrated significant increases in FFM over time. Children with SCA show significantly smaller increases over time compared to healthy controls.
Figure 3. Changes in hemoglobin over time among children with and without sickle cell anemia.
Children with SCA have significantly lower hemoglobin concentration at each time point and no differences are noted over time within either group.
Longitudinal changes in VO 2peak
Absolute VO2peak increased significantly over time in healthy controls from 1.07 to 1.64 L/min (p<0.001) and in children with SCA from 0.92 to 1.15 L/min (p<0.001). However, the mean increase among children with SCA was significantly smaller than in healthy controls (+0.23 and +0.56 L/min, respectively, p<0.001).
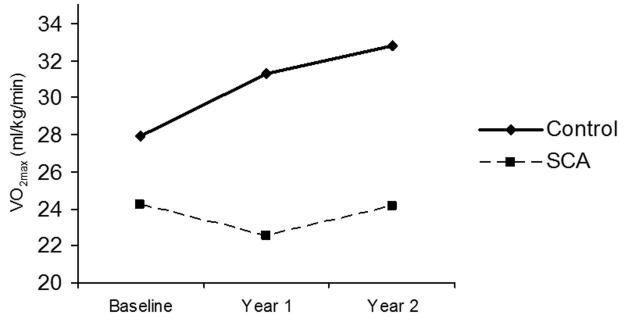
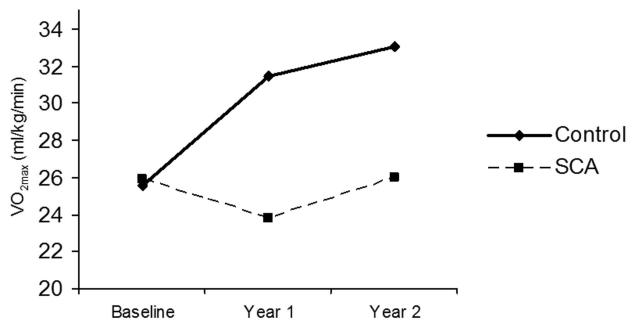
Unadjusted changes in relative VO2peak over time for both groups are shown in Figure 4. Children with SCA did not show any significant change in VO2peak over the 2-year study period (24.2±1.8 to 24.1±1.9 ml/kg/min, p=0.78). In contrast, healthy control children demonstrated a significant increase in VO2peak during the same period (27.9±1.9 to 32.8±2.0 ml/kg/min, p<0.001). The difference in relative VO2peak change over time between the SCA and healthy control groups was significant (−0.11 and +4.92 ml/kg/min, respectively, p=0.001).
Figure 4. Unadjusted changes in VO2peak over time among children with and without sickle cell anemia.
Children with sickle cell anemia have significantly lower baseline VO2peak and significantly reduced change in VO2peak over time compared to controls.
Determinants of VO2peak
After adjusting for, sex, age, Tanner stage, FFM, and Hgb concentration, baseline VO2peak in children with SCA and healthy controls was no longer significant (27.1±3.1 vs. 27.1±2.9 ml/kg/min, p = 0.99), but controls still demonstrated significantly greater VO2peak at year 2 (32.5±3.9 vs. 26.8±3.7 ml/kg/min, p = 0.032; Figure 5). Further analysis demonstrated that the adjusted differences in relative VO2peak changes over time between the groups remained significant (−0.24 and +5.35 ml/kg/min, respectively, p<0.001; Figure 5). No significant interactions of sex, FFM, Tanner stage, or Hgb concentration with change in relative VO2peak over time were noted (p>0.05 for all).
Figure 5. Changes in VO2peak over time among children with and without sickle cell anemia adjusted for age, gender, FFM, Tanner stage, and hemoglobin.
Baseline differences in VO2peak between children with and without sickle cell anemia are no longer evident, but children with SCA continue to have significantly reduced VO2peak over time.
DISCUSSION
The major finding of this study is that during a 2-year period of pubertal development, children with SCA demonstrated significantly smaller change in VO2peak than healthy control children matched at baseline for sex, race, height, weight, Tanner stage, and FFM. While our study confirms previous findings that prepubertal children with SCA have significantly lower VO2peak level than healthy control children, we report that during puberty children with SCA showed no change in relative VO2peak (ml/kg/min) while healthy controls demonstrated significant increases over the 2-year period.
Our results in healthy controls are in agreement with studies reporting increases in relative VO2peak in pubertal children until mid-adolescence, with a plateau or decrease thereafter.(25-30) However, other studies have suggested that relative VO2peak values remain stable during adolescence.(10, 16, 31) Nevertheless, despite being matched for Tanner stage, weight, FFM, and height children with SCA demonstrated a significantly different VO2peak trajectory characterized by unchanged relative VO2peak values throughout the study period.
In this study we also evaluated differences in Tanner stage, FFM, and Hgb concentration over time between the groups. Although the majority of children in both groups were initially at Tanner 2 stage, only 15% of children with SCA progressed to Tanner 4 by year 2, compared to 66% of healthy control children. These results are in line with previous findings reported by our group and others showing that a delay in pubertal development in children with SCA is associated with the degree of anemia.(17, 19) Similarly, while by design no significant differences in FFM between the groups were present at baseline, children with SCA demonstrated significantly smaller FFM increases during the study course compared to healthy controls. For example, Zemel et al(17) clearly demonstrated that attenuated FFM gains among children with SCA over time are a function of delayed pubertal development.
Previous studies in healthy children found significant associations between VO2peak increase and pubertal development and suggested that changes in muscle mass may drive increases in VO2peak throughout adolescence by augmenting venous return.(9, 32, 33) Although our results demonstrate that Tanner stage transition and FFM increased more rapidly among healthy adolescents than those with SCA, the differences in the change in VO2peak over time between groups persisted after including these variables in the adjusted model. It is possible that the persistent between-group difference in VO2peak change was due to clinical disease progression in children with SCA that we did not identify and account for during the study (e.g., cardiopulmonary disease). It is also possible that the sample size did not yield sufficient power to identify significant associations among the included variables. Thus, studies designed to explain mechanisms that underlie the longitudinal differences in VO2peak trajectory between children with and without SCA are warranted.
Anemia has been consistently suggested as a likely contributor to decreased VO2peak among adults and children with SCA.(4, 5, 7, 34, 35) In prior evaluations of maximal aerobic capacity, children with SCA had increased pulmonary blood flow and decreased maximal arteriovenous oxygen difference compared to healthy children, both of which were attributed to anemia.(4, 36) In several cross-sectional studies, anemia has been associated with decreased VO2peak as well as weight-adjusted 6-minute walk time in children with SCA undergoing cardiopulmonary testing.(5, 7) Finally, anemia has been suggested as a causative factor in exercise intolerance in adults with SCA, although it has not been fully distinguished from the contributions of cumulative myocardial dysfunction, pulmonary parenchymal disease, or pulmonary vascular disease.(3, 35, 37, 38) In this study, Hgb concentration was lower in children with SCA than in healthy control children at all 3 time points, as expected, but did not change during the study period while the difference in VO2peak between groups increased significantly.
The secondary objective of this study was to compare changes in VO2peak over time before and after adjusting for age, sex, Tanner stage, FFM, and Hgb concentration. We found that while the significant baseline difference in VO2peak between the groups that was present in our unadjusted model was no longer evident after the model adjustment, the magnitude of the longitudinal differences remained unchanged.
This study has several limitations. First, the study was powered to characterize predictors of longitudinal changes in VO2peak in SCA rather than fully elucidate the physiologic causes of exercise limitation in children with SCA. Thus, the study sample did not provide statistical power to test all clinical variables tested in the study or other variables previously shown to be associated with VO2peak in children, such as cardiac output, blood volume, or ventilatory efficiency. In addition, we did not evaluate the potential contribution of red blood cell sickling during exercise as a potential limiting factor, nor did we account for differences in habitual physical activity between the groups. We did not examine the impact of SCA-related complications after the initial screening, which may have affected subsequent fitness and exercise performance of participating children. Tanner staging in year 1 and 2 was self-reported; however, we did not note any regress in the Tanner staging trajectory. In addition, the staging results were in line with our previous studies of children with SCA and other studies reporting the relationship between Tanner stage and age in both children with SCA and healthy children. Another limitation is that we followed our participants for only 2 years. Including the entire pubertal development period would have provided additional results. Finally, we conducted the study at one site with a regional referral base and the results may not be generalizable to other SCA populations.
CONCLUSIONS
Children with SCA demonstrated lower VO2peak compared to sex, gender, race, Tanner stage, and FFM matched healthy controls. During a 2-year follow-up period, VO2peak in children with SCA remained unchanged but it significantly increased in healthy controls. The differences in change in VO2peak between the groups over time were not explained by sex, Tanner stage, FFM, or Hgb concentration. Further research to identify the etiological factors responsible for lower VO2peak in SCA may guide interventions designed to improve both functional capacity and quality of life in children with SCA.
ACKNOWLEDGEMENTS
We acknowledge the contribution of Andrea Buchholz and Kay Hongu from the Energy Balance Laboratory as well as the Clinical Research Center at Vanderbilt University Medical Center for help with conducting the study. This study was supported in part by R01 HL082988, the National Center for Advancing Translational Sciences of the National Institute of Health under Award Number UL1 TR000445, and Vanderbilt Diabetes Research Center grant DK20593.
Funding source: This study was supported in part by R01 HL082988, the National Center for Advancing Translational Sciences of the National Institute of Health under Award Number UL1 TR000445, and Vanderbilt Diabetes Research Center grant DK20593.
Footnotes
Conflicts of Interest Statement: The authors have no relevant conflicts of interest or financial relationships to disclose.
Contributors Statement Andrew M. Watson conceptualized and designed the study data analysis and data interpretation, drafted the manuscript, and approved the final manuscript as submitted. Robert Liem conceptualized and designed the data analysis, reviewed and revised the manuscript and approved the final manuscript as submitted. Zengqi Lu and Ben Saville analyzed and interpreted the data, reviewed the manuscript and approved the final manuscript as submitted. Sari Acra contributed to the study design, reviewed and revised the manuscript and approved the final manuscript as submitted. Sadhna Shankar conceptualized and designed the study, reviewed and revised the manuscript and approved the final manuscript as submitted. Maciej Buchowski conceptualized and designed the study, contributed to data analysis and interpretation, reviewed and revised the manuscript and approved the final manuscript as submitted.
REFERENCES
- 1.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376:2018–31. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- 2.Braden DS, Covitz W, Milner PF. Cardiovascular function during rest and exercise in patients with sickle-cell anemia and coexisting alpha thalassemia-2. Am J Hematol. 1996;52:96–102. doi: 10.1002/(SICI)1096-8652(199606)52:2<96::AID-AJH5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 3.Callahan LA, Woods KF, Mensah GA, Ramsey LT, Barbeau P, Gutin B. Cardiopulmonary responses to exercise in women with sickle cell anemia. Am J Respir Crit Care Med. 2002;165:1309–16. doi: 10.1164/rccm.2002036. [DOI] [PubMed] [Google Scholar]
- 4.Pianosi P, D’Souza SJ, Esseltine DW, Charge TD, Coates AL. Ventilation and gas exchange during exercise in sickle cell anemia. Am Rev Respir Dis. 1991;143:226–30. doi: 10.1164/ajrccm/143.2.226. [DOI] [PubMed] [Google Scholar]
- 5.Liem RI, Nevin MA, Prestridge A, Young LT, Thompson AA. Functional capacity in children and young adults with sickle cell disease undergoing evaluation for cardiopulmonary disease. Am J Hematol. 2009;84:645–9. doi: 10.1002/ajh.21507. [DOI] [PubMed] [Google Scholar]
- 6.Alpert BS, Dover EV, Strong WB, Covitz W. Longitudinal exercise hemodynamics in children with sickle cell anemia. Am J Dis Child. 1984;138:1021–4. doi: 10.1001/archpedi.1984.02140490021005. [DOI] [PubMed] [Google Scholar]
- 7.van Beers EJ, van der Plas MN, Nur E, Bogaard HJ, van Steenwijk RP, Biemond BJ, Bresser P. Exercise tolerance, lung function abnormalities, anemia, and cardiothoracic ratio in sickle cell patients. Am J Hematol. 2014;89:819–24. doi: 10.1002/ajh.23752. [DOI] [PubMed] [Google Scholar]
- 8.Toth MJ, Gardner AW, Ades PA, Poehlman ET. Contribution of body composition and physical activity to age-related decline in peak VO2 in men and women. J Appl Physiol (1985) 1994;77:647–52. doi: 10.1152/jappl.1994.77.2.647. [DOI] [PubMed] [Google Scholar]
- 9.Hunt BE, Davy KP, Jones PP, DeSouza CA, Van Pelt RE, Tanaka H, Seals DR. Role of central circulatory factors in the fat-free mass-maximal aerobic capacity relation across age. Am J Physiol. 1998;275:H1178–82. doi: 10.1152/ajpheart.1998.275.4.H1178. [DOI] [PubMed] [Google Scholar]
- 10.Janz KF, Burns TL, Witt JD, Mahoney LT. Longitudinal analysis of scaling VO2 for differences in body size during puberty: the Muscatine Study. Med Sci Sports Exerc. 1998;30:1436–44. doi: 10.1097/00005768-199809000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Batterham AM, Vanderburgh PM, Mahar MT, Jackson AS. Modeling the influence of body size on V(O2) peak: effects of model choice and body composition. J Appl Physiol (1985) 1999;87:1317–25. doi: 10.1152/jappl.1999.87.4.1317. [DOI] [PubMed] [Google Scholar]
- 12.Dencker M, Thorsson O, Karlsson MK, Linden C, Eiberg S, Wollmer P, Andersen LB. Gender differences and determinants of aerobic fitness in children aged 8-11 years. Eur J Appl Physiol. 2007;99:19–26. doi: 10.1007/s00421-006-0310-x. [DOI] [PubMed] [Google Scholar]
- 13.Davis JA, Wilson LD, Caiozzo VJ, Storer TW, Pham PH. Maximal oxygen uptake at the same fat-free mass is greater in men than women. Clin Physiol Funct Imaging. 2006;26:61–6. doi: 10.1111/j.1475-097X.2005.00647.x. [DOI] [PubMed] [Google Scholar]
- 14.Vinet A, Mandigout S, Nottin S, Nguyen L, Lecoq AM, Courteix D, Obert P. Influence of body composition, hemoglobin concentration, and cardiac size and function of gender differences in maximal oxygen uptake in prepubertal children. Chest. 2003;124:1494–9. doi: 10.1378/chest.124.4.1494. [DOI] [PubMed] [Google Scholar]
- 15.Geithner CA, Thomis MA, Vanden Eynde B, Maes HH, Loos RJ, Peeters M, Claessens AL, Vlietinck R, Malina RM, Beunen GP. Growth in peak aerobic power during adolescence. Med Sci Sports Exerc. 2004;36:1616–24. doi: 10.1249/01.mss.0000139807.72229.41. [DOI] [PubMed] [Google Scholar]
- 16.Krahenbuhl GS, Skinner JS, Kohrt WM. Developmental aspects of maximal aerobic power in children. Exerc Sport Sci Rev. 1985;13:503–38. [PubMed] [Google Scholar]
- 17.Zemel BS, Kawchak DA, Ohene-Frempong K, Schall JI, Stallings VA. Effects of delayed pubertal development, nutritional status, and disease severity on longitudinal patterns of growth failure in children with sickle cell disease. Pediatr Res. 2007;61:607–13. doi: 10.1203/pdr.0b013e318045bdca. [DOI] [PubMed] [Google Scholar]
- 18.Barden EM, Kawchak DA, Ohene-Frempong K, Stallings VA, Zemel BS. Body composition in children with sickle cell disease. Am J Clin Nutr. 2002;76:218–25. doi: 10.1093/ajcn/76.1.218. [DOI] [PubMed] [Google Scholar]
- 19.Rhodes M, Akohoue SA, Shankar SM, Fleming I, Qi An A, Yu C, Acra S, Buchowski MS. Growth patterns in children with sickle cell anemia during puberty. Pediatr Blood Cancer. 2009;53:635–41. doi: 10.1002/pbc.22137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanner JM. In: Growth at adolescence : with a general consideration of the effects of hereditary and environmental factors upon growth and maturation from birth to maturity. 2nd ed. Thomas CC, editor. Springfield, Ill.: 1962. p. xiii.p. 325. [15] leaves of plates p. [Google Scholar]
- 21.Buchowski MS, Chen KY, Byrne D, Wang WC. Equation to estimate resting energy expenditure in adolescents with sickle cell anemia. Am J Clin Nutr. 2002;76:1335–44. doi: 10.1093/ajcn/76.6.1335. [DOI] [PubMed] [Google Scholar]
- 22.Buchowski MS, Townsend KM, Williams R, Chen KY. Patterns and energy expenditure of free-living physical activity in adolescents with sickle cell anemia. J Pediatr. 2002;140:86–92. doi: 10.1067/mpd.2002.120689. [DOI] [PubMed] [Google Scholar]
- 23.Buchholz AC, Majchrzak KM, Chen KY, Shankar SM, Buchowski MS. Use of air displacement plethysmography in the determination of percentage of fat mass in african american children. Pediatr Res. 2004;56:47–54. doi: 10.1203/01.PDR.0000130477.05324.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruce RA. Exercise testing of patients with coronary heart disease. Principles and normal standards for evaluation. Ann Clin Res. 1971;3:323–32. [PubMed] [Google Scholar]
- 25.Andersen KL, Seliger V, Rutenfranz J, Nesset T. Physical performance capacity of children in Norway. V. The influence of social isolation on the rate of growth in body size and composition and on the achievement in lung function and maximal aerobic power of children in a rural community. Eur J Appl Physiol Occup Physiol. 1980;45:155–66. doi: 10.1007/BF00421323. [DOI] [PubMed] [Google Scholar]
- 26.Davies CTM. Maximum Aerobic Power in Relation to Body Composition in Healthy, Sedentary Adults. Human Biology. 1972;44:127. [PubMed] [Google Scholar]
- 27.Eisenmann JC, Laurson KR, Welk GJ. Aerobic fitness percentiles for U.S. adolescents. Am J Prev Med. 2011;41:S106–10. doi: 10.1016/j.amepre.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Eriksson O, Saltin B. Muscle metabolism during exercise in boys aged 11 to 16 years compared to adults. Acta Paediatr Belg. 1974;28(suppl):257–65. [PubMed] [Google Scholar]
- 29.Hermansen L, Oseid S. Direct and indirect estimation of maximal oxygen uptake in pre pubertal boys. Acta Paediatr Scand Suppl. 1971;217:18–23. doi: 10.1111/j.1651-2227.1971.tb05684.x. [DOI] [PubMed] [Google Scholar]
- 30.Yamaji K, Miyashita M. Oxygen transport system during exhaustive exercise in Japanese boys. Eur J Appl Physiol Occup Physiol. 1977;36:93–9. doi: 10.1007/BF00423116. [DOI] [PubMed] [Google Scholar]
- 31.Armstrong N, Welsman JR. Assessment and interpretation of aerobic fitness in children and adolescents. Exerc Sport Sci Rev. 1994;22:435–76. [PubMed] [Google Scholar]
- 32.Rowland T, Goff D, Martel L, Ferrone L. Influence of cardiac functional capacity on gender differences in maximal oxygen uptake in children. Chest. 2000;117:629–35. doi: 10.1378/chest.117.3.629. [DOI] [PubMed] [Google Scholar]
- 33.Convertino VA. Blood volume response to physical activity and inactivity. American Journal of the Medical Sciences. 2007;334:72–9. doi: 10.1097/MAJ.0b013e318063c6e4. [DOI] [PubMed] [Google Scholar]
- 34.Chaudry RA, Bush A, Rosenthal M, Crowley S. The impact of sickle cell disease on exercise capacity in children. Chest. 2013;143:478–84. doi: 10.1378/chest.12-0611. [DOI] [PubMed] [Google Scholar]
- 35.Miller GJ, Serjeant GR, Sivapragasam S, Petch MC. Cardio-pulmonary responses and gas exchange during exercise in adults with homozygous sickle-cell disease (sickle-cell anaemia) Clin Sci. 1973;44:113–28. doi: 10.1042/cs0440113. [DOI] [PubMed] [Google Scholar]
- 36.Pianosi P, D’Souza SJ, Charge TD, Beland MJ, Esseltine DW, Coates AL. Cardiac output and oxygen delivery during exercise in sickle cell anemia. Am Rev Respir Dis. 1991;143:231–5. doi: 10.1164/ajrccm/143.2.231. [DOI] [PubMed] [Google Scholar]
- 37.Martins WD, Lopes HF, Consolim-Colombo FM, Gualandro SDM, Arteaga-Fernandez E, Mady C. Cardiovascular autonomic dysfunction in sickle cell anemia. Autonomic Neuroscience-Basic & Clinical. 2012;166:54–9. doi: 10.1016/j.autneu.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 38.Gladwin MT, Sachdev V. Cardiovascular Abnormalities in Sickle Cell Disease. J Am Coll Cardiol. 2012;59:1123–33. doi: 10.1016/j.jacc.2011.10.900. [DOI] [PMC free article] [PubMed] [Google Scholar]