Abstract
Context
Clinical efforts to repair damaged articular cartilage (AC) currently face major obstacles due to limited intrinsic repair capacity of the tissue and unsuccessful biological interventions. This highlights a need for better therapeutic strategies.
Evidence Acquisition
Relevant articles were identified through a search of the PubMed database from January 1956 to August 2014 using the following keywords: articular cartilage repair, stem cell, cartilage tissue-engineering, synovium, and NFAT.
Evidence Synthesis
In both animals and humans, AC defects that penetrate into the subchondral bone marrow are mainly filled with fibrocartilaginous tissue through the differentiation of bone marrow mesenchymal stem cells (MSCs), followed by degeneration of repaired cartilage and osteoarthritis. Cell therapy and tissue engineering techniques using culture-expanded chondrocytes, bone marrow MSCs, or pluripotent stem cells with chondroinductive growth factors may generate cartilaginous tissue in AC defects but do not form hyaline cartilage-based articular surface because repair cells often lose chondrogenic activity or result in chondrocyte hypertrophy. The new evidence that AC and synovium develop from the same pool of precursors with similar gene profiles and that synovium-derived chondrocytes have stable chondrogenic activity has promoted use of synovium as a new cell source for AC repair. The recent finding that NFAT1 and NFAT2 transcription factors inhibit chondrocyte hypertrophy and maintain metabolic balance in AC is a significant advance in the field of AC repair.
Conclusions
The use of synovial MSCs and discovery of upstream transcriptional regulators that help maintain the AC phenotype have opened new avenues to improve the outcome of AC regeneration.
Keywords: Articular cartilage repair, Stem cell, Cartilage tissue-engineering, Synovium, Post-traumatic osteoarthritis, NFAT
Introduction
An acute cartilage or osteochondral defect may be caused by a comminuted or displaced intra-articular fracture, while a chronic articular cartilage (AC) defect is often a result of AC degradation during the progression of osteoarthritis (OA). Another cause of osteochondral defects that is relatively rare is osteochondritis dissecans (OCD), a joint disease with osteonecrosis of the subchondral bone usually linked to antecedent trauma, which occurs most often in the knee of young men and athletes1–3. The link between AC damage and OA is undeniable, making the pursuit of clinical advancement in the area of cartilage regeneration of paramount importance. Unlike spontaneous OA, which mostly affects middle-aged and older populations, cartilage injury-induced post-traumatic OA (PTOA) often affects younger adults for whom desirable treatment is to preserve the function of the original joint by regenerating damaged AC instead of joint replacement or arthrodesis. This highlights a great need for earlier, less invasive treatment modalities for both acute and chronic AC lesions.
Many new lines of treatment for AC defects have become available over the past 5 decades with even more animal models on the verge of clinical trial, yet our understanding of how AC heals remains insufficient to support any given line of therapy over another. Most cartilage repair techniques have been based on a postulate that a substance, such as a graft, scaffold, or mesenchymal-cell-rich blood clot, must be interposed in order for an AC defect to be repaired. This is based on many years of success gained from the general art of using grafts to fill defects in the skin and bone. Unfortunately, grafting techniques for AC regeneration have not been as successful as for skin or bone regeneration.
The major breakthroughs in AC repair began in 1959 when Pridie published his drilling method for AC resurfacing in osteoarthritic knee joints noting that accessing the underlying bone marrow led to a clot formation which had the potential to form cartilage4. This procedure was refined in the 1980’s by Steadman et al. who coined the term microfracture as a method of accessing the bone marrow with a bone pick without the potentially harmful effects of drilling. A clinical follow-up revealed that 80% of the patients had significant improvement in joint function and pain5. However, it has become clear that the fibrocartilage-like repair tissue with hypertrophic chondrocytes generated by the bone marrow stimulation procedure was less than optimal for long term outcomes6, 7.
Osteochondral allografting was also being used during this time period and remains in use today for the treatment of large cartilage defects in young, high-demand patients in whom total joint arthroplasty was a poor option. Transplantation of mature hyaline cartilage into the affected area is an advantage of the procedure. However, disease transmission, immunological response, and the long-term viability of transplanted allografts are concerns with any allografting procedure. Graft nonunion and fragmentation may occur from months to years after the procedure8, 9. Osteochondral autografting (mosaicplasty) affords the same advantages without the risk of disease transmission or immunologic response, but it is limited by donor site availability and morbidity. Short- (<5 years) and medium-term (5–9 years) clinical outcomes showed that patients with osteochondral defects treated with mosaicplasty maintain a superior level of athletic activity compared with those treated with microfracture. However, long-term (>10 years) clinical outcome after mosaicplasty varies greatly depending on the age, gender, and size of the lesions10, 11.
In 1987, it was reported that chondrocytes could be cultured and implanted into chondral defects which had not disrupted the subchondral bone12. Soon thereafter Brittburg and Peterson et al. published their first case series describing a new method of treatment termed autologous cartilage transplantation, later referred to as autologous chondrocyte implantation (ACI)13. Subsequent follow up studies, however, have failed to demonstrate a significant difference in structural repair at 24 months in randomized controlled clinical trials comparing ACI to microfracture14–17.
Tissue engineering techniques for cartilage or osteochondral repair have gained a significant amount of interest over the past two decades. This technology involves three main components: biomaterial-based scaffolding, a cell source, and growth or differentiation factors. Scaffolds for repair of osteochondral defects may be fabricated with natural (e.g, collagen) or synthetic materials18–21. Cell sources include isolated autologous chondrocytes, minced autologous cartilage, multipotent stem cells (e.g., bone marrow-, muscle-, synovium-, or adipose-derived mesenchymal cells), pluripotent stem cells, and induced pluripotent stem cells (iPSC)16, 18, 19, 22–26. Chondroinductive growth factors mainly consist of members of the transforming growth factor-β (TGF- β) superfamily, insulin-like growth factor-1 (IGF-1), and specific members of fibroblast growth factor (FGF) family. These growth factors have been used for stimulating chondrogenic differentiation of stem cells in cell culture or through controlled release, gene transduction/delivery, or nanoparticle delivery16, 25, 27–30. Bioreactors are utilized to enhance nutrient delivery and provide mechanical stimulation to tissue-engineered cartilage constructs ex vivo prior to in vivo implantation.
While cell-based therapies (e.g., microfracture, ACI) are already in clinical use for promotion of AC repair, none of these options have been proven successful in restoring the original AC structure with hyaline cartilage in humans16, 17. Clinicians and scientists are striving for a better understanding of cartilage healing process in order to develop more reliable methods of AC repair. Here, we review the recent advances in cell-based therapies for AC repair, with a focus on the latest development in synovial MSCs as a cell source and novel transcription factors that may serve as potential upstream regulators for maintaining the permanent hyaline cartilage phenotype of healing AC and preventing PTOA.
Current challenges
Cartilage remains one of the most difficult tissues to heal. Several approaches including tissue engineering have been developed in the past decades to regenerate damaged AC; however, none of these approaches have been proven to effectively produce a repair tissue with the same or similar mechanical and functional characteristics of the native AC. At a cellular level the challenges we currently face in AC regeneration fall into at least two major categories:
Chondrocyte differentiation problems including insufficient chondrogenic differentiation, chondrocyte dedifferentiation, and chondrocyte hypertrophy: Although chondroinductive growth factors may induce the differentiation of various stem cells into chondrocytes, the induction process may not be sufficient to produce functional chondrocytes. Autologous chondrocytes have shown the most promise in this regard but may undergo dedifferentiation to fibroblast-like cells during the ex vivo expansion or in vivo repair process. As a result, an AC defect site may be filled with fibrous tissue or fibrocartilage-like repair tissue instead of the desirable articular cartilage containing hyaline cartilage that is uniquely organized into a complex, layered structure and physiologically tightly regulated. One of the key limitations to engineered cartilage tissues is that it is amorphous and lacks the 3-dimensional organization and structural properties of native articular cartilage, thereby rendering it susceptible to physical and physiological stresses. On the other hand, it has been observed that bone marrow MSCs have an intrinsic differentiation program reminiscent of endochondral bone formation31. Some repair chondrocytes may undergo hypertrophic differentiation, followed by matrix calcification, vascular invasion, and endochondral ossification leading to new bone formation in an AC defect site. Because of these drawbacks researchers are searching for better repair techniques which can induce differentiation of stem cells into functional, matrix producing articular chondrocytes with less potential for dedifferentiation or hypertrophic differentiation.
Cartilage homeostasis problems characterized by imbalanced anabolic and catabolic cellular activity of repair cells: In the acute post-traumatic phase, joint trauma may lead to suppression of collagen and proteoglycan synthesis in articular cartilage. Remaining viable cells in joint tissues may respond to the injury with enhanced synthetic activity and overexpression of matrix-degrading enzymes and inflammatory mediators. During the healing of AC defects, cytokines and enzymes released by synoviocytes and chondrocytes in and around the repair tissue are required in order to initiate the repair process and eventually integrate the repair tissue within the defect. However, overexpression of catabolic factors may cause an imbalance between anabolic and catabolic activities at the defect site, leading to cartilage degradation, failed repair, and subsequent PTOA2, 32. Therefore, the chondrocyte homeostasis in the defect is critical for the quality of healing cartilage and the integration of repair cartilage with the existing AC and subchondral bone. In addition, articular chondrocytes respond physiologically to both chemical 33–35 and mechanical 36–39 stimuli. This responsiveness could explain in part the late degradation of repair tissue which is initially hyaline-like but degenerates over time.
In order to overcome these challenges, researchers have been searching for new cell sources for AC repair by studying the link between development and regeneration of AC and exploring key upstream factors that can maintain AC homeostasis by regulating both anabolic and catabolic activities of articular chondrocytes.
The link between development and regeneration
While much focus has been placed on the central inductive postulate of filling an AC defect with a repair material (e.g., grafting technique, bone marrow stimulation), less attention has been paid to the more basic deductive thought that the art of AC regeneration might link to the processes of joint development. Theoretically, if we could influence the body to repeat the processes of development in the setting of an injury, we would cure the problem of AC injury. In order to do this several questions must be answered, however. First, it is necessary to have a better understanding of where the cells for the formation of joint tissues come from. Second, it is important to investigate the specific processes of articular cartilage formation. Many authors both from the remote past as well as the near present have contributed to this study.40–47 Each facet of joint development constitutes an item of extensive discussion throughout the literature.40–43, 46–53 Here we will take a deeper look into some of these areas, with a focus on the formation of the subchondral bone, articular cartilage, and synovium.
Development of the secondary ossification center and subchondral bone
The mid-shaft (diaphysis) of a long bone develops by endochondral ossification through the development of the primary ossification center (POC). The bone tissue at the ends of the developing POC constitutes the metaphysis54.
The cartilaginous epiphysis begins to take shape at each end of the diaphysis just before or after birth (depending on the specific epiphysis) both in humans and mammalian animals54, 55. The secondary ossification center (SOC) is formed in the proximal and distal ends of the cartilage model shortly after birth. The initial structural change in the development of the SOC is that chondrocytes within the center of the epiphyseal cartilage become hypertrophic. The matrix adjacent to the hypertrophic chondrocytes then mineralizes and is invaded by vessels of the cartilage canals carrying mesenchymal cells and preosteoblasts54–56.
Early in the postnatal phase, much of the epiphyseal cartilage is replaced by bone and bone marrow via endochondral ossification. Continuous ossification leads to the expansion of trabecular bone and formation of the subchondral bone plate54, 55. Taken together, these developmental studies have confirmed that both POC and SOC including the subchondral bone plate and bone marrow develop through the endochondal sequence of ossification.
Development of the articular cartilage and synovium
During the late-stage of long bone development, the SOC grows outward, and the surrounding epiphyseal cartilage becomes thinner54, 55. This raises the question of whether or not the articular surface represents a remnant epiphyseal cartilage which has undergone transformation to permanent cartilage, or perhaps the remaining epiphyseal cartilage is resorbed and replaced by a new tissue which forms the articular surface.
Both human and animal studies suggest that a special cell population called “interzone cells” may be involved in the formation of synovial joint. Gardner et al. proposed that the formation of three-layered interzones begins in most joints during the fetal period in humans44. Holder reported that removal of the interzone area of tissue results in fusion of bone segment with no sign of joint formation in chicks41.
A study of rat joint formation by Mitrovic suggested that interzone cells are responsible for formation of joint tissues and structures, including articular cartilage, ligaments, and synovial lining, while the joint capsule appears to be derived from a distinct condensation57. Subsequent studies suggested that interzone cells from the outer layers differentiate into chondrocytes early in embryogenesis and become incorporated into the epiphysis, thus contributing to initial lengthening of the anlagen. A subset of interzone cells from the intermediate layer become articular chondrocytes and other intra-articular tissue cells42, 58, 59. Recent genome-wide gene expression analyses on interzone cells isolated from mouse embryos at 15.5 days supports this conclusion by showing a higher gene expression level relevant to chondrocyte hypertrophy and endochondral ossification in the outer layer than the intermediate layer60. Other studies, however, seem to support the idea that the interzone may originate from a distinctly separate subpopulation of cells, which are different from those predestined for endochondral ossification of the diaphysis48, 61. The potential regulatory factors for interzone cell differentiation and joint formation have been described in a comprehensive review48.
The role of chondrogenic progenitor cells in postnatal development of AC
Hunziker et al. examined postnatal development and maturation of AC in rabbits from the first to eighth month. They concluded that AC is reorganized by a process of tissue resorption and neoformation, rather than by internal remodeling62. However, the origin of the chondrogenic progenitor cells was not elucidated in that study.
Simkin later proposed that articular chondrocyte stem cells originate in an area he refers to as the “marginal transitional zone” where the articular cartilage meets synovium and periosteum at the peripheral margins of the joint63. In mature AC, mesenchymal stem cells continue to arrive at the joint margins and then descend into the deeper zones but no further division occurs. The resultant depot for apoptotic chondrocyte debris forms the histological feature of the tidemark between uncalcified and calcified cartilage63. This hypothesis is particularly interesting as it seems to parallel the findings regarding the origin and travel of interzone cells, suggesting that the embryonic pattern of AC development continues throughout postnatal development and even into adulthood. However, experimental analyses are required to validate these hypotheses.
While adult AC is considered an avascular, aneural, and alymphatic tissue with little capacity for self-repair after injury, several studies have identified chondrogenic progenitor cells in the superficial zone of normal and osteoarthritic AC in animals and humans51, 62, 64–67. However, the function of the progenitor cells in AC repair during the adult stage needs to be further elucidated.
Although enormous amounts of debate persist and questions about whether the AC is contrived entirely of interzone cells or formed by other cell populations are still open for discussion, it is clear by now that the SOC and subchondral bone including its bone marrow are developed via the endochondral sequence of ossification. It is also clear that normal epiphyseal cartilage is transient/temporal cartilage in which chondrocytes undergo hypertrophic differentiation and endochondral ossification. In contrast, the differentiation of articular chondrocytes in normal AC (permanent cartilage) is halted at the matrix producing stage, and they do not undergo hypertrophic changes or endochondral ossification. The proposed tissue origins of AC, synovium, and subchondral bone are illustrated in Figure 1.
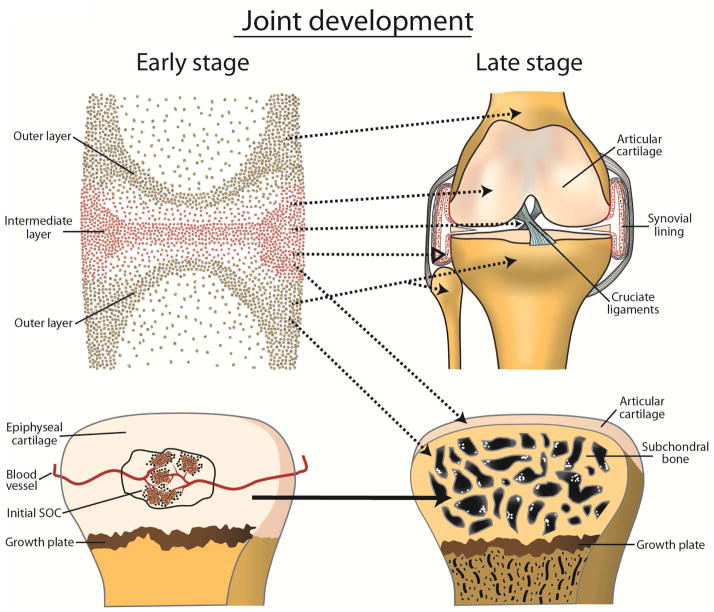
Figure 1.
A diagram showing proposed mechanisms for the development of major joint tissues. Upper panels: The interzone is distinguishable into a central intermediate zone and two outer layers contiguous to the epiphyseal ends. Interzone cells from the intermediate layer contribute to the formation of AC, synovial lining, and intra-articular ligaments. Interzone cells from the outer layers differentiate into chondrocytes and become incorporated into the epiphysis, which undergoes endochondral ossification. Dotted arrows indicate that further elucidation is required. Lower panels: The development of the secondary ossification center (SOC) begins with the formation of cartilage canal containing blood vessels, followed by chondrocyte hypertrophy and endochondral ossification in the center of the epiphyseal cartilage.
Synovium-derived mesenchymal stem cells (MSCs) for AC repair
The chondrogenic potential of synovium-derived MSCs and their application in AC repair have been studied in vitro and in vivo68–75. An early in vitro study demonstrated that human multipotent MSCs can be isolated from the synovial membrane of knee joints. These cells have the ability to proliferate extensively in culture and maintain their multilineage differentiation potential in cultures, establishing their progenitor cell nature76. Subsequent studies revealed that human synovial MSCs have greater expansion and chondrogenic ability in vitro than MSCs from bone marrow, periosteum, muscle, and adipose tissue77. The weight of cartilaginous pellets from cultured mouse synovial MSCs is significantly greater than that from cultured bone marrow MSCs68. Extracellular matrix deposited by synovial MSCs delays replicative senescent chondrocyte dedifferentiation and enhances redifferentiation73.
Another important rationale for the use of synovial MSCs for AC repair is that synovial MSC-derived chondrocytes and articular chondrocytes share similar gene expression profile. Synovial MSCs-mediated tissue engineered cartilage matrix is deposited with collagen-II and aggrecan but not collagen-I or collagen-X and is mechanically similar to articular cartilage. Moreover, synovial MSCs express a specific proteoglycan (superficial zone protein), a functional characteristic of progenitor cells in the superficial zone of AC. Gene expression profiles revealed that chondrogenic progenitor cells from the superficial zone of AC and synovial cells are closely related67, 77–80. Thus, synovial MSCs may be particularly useful in regenerating the superficial layer of AC.
AC or osteochondral repair with synovial MSCs has also been demonstrated in animal studies. Transplantation of synovial MSCs into full-thickness osteochondral defects of adult rabbits resulted in cartilage formation in the defect but some transplanted MSCs differentiated into bone cells in the deep zone, suggesting that synovial MSCs may differentiate into different lineage cells according to local microenvironments81. Several more recent animal studies further confirmed the repair process of AC defects using synovium-derived MSCs with or without scaffolds69, 71, 72, 74.
In 2011, Sekiya et al. reported arthroscopic transplantation of synovial MSCs for the treatment of AC defects in humans. Regeneration of cartilage, reduction in defects size, and improvement of symptoms were observed in most patients over the 3-year study82.
Synovial response to AC damage
Hunziker et al. studied partial-thickness AC defects (without disruption of subchondral bone) in adult rabbit knees and found that the source of cells for repair was either within the synovium or in the subsynovial space83. It was suggested that these cells traveled along the articular surface until they found their way into the defect. These findings would not refute evidence supporting the idea that stem cells reside in the articular surface but it would clarify where they originate and, furthermore, disclose where they may be housed in adulthood. Kurth et al. reported the existence of resident MSCs in the knee joint synovium that undergo proliferation and chondrogenic differentiation following joint-surface injury in mice70.
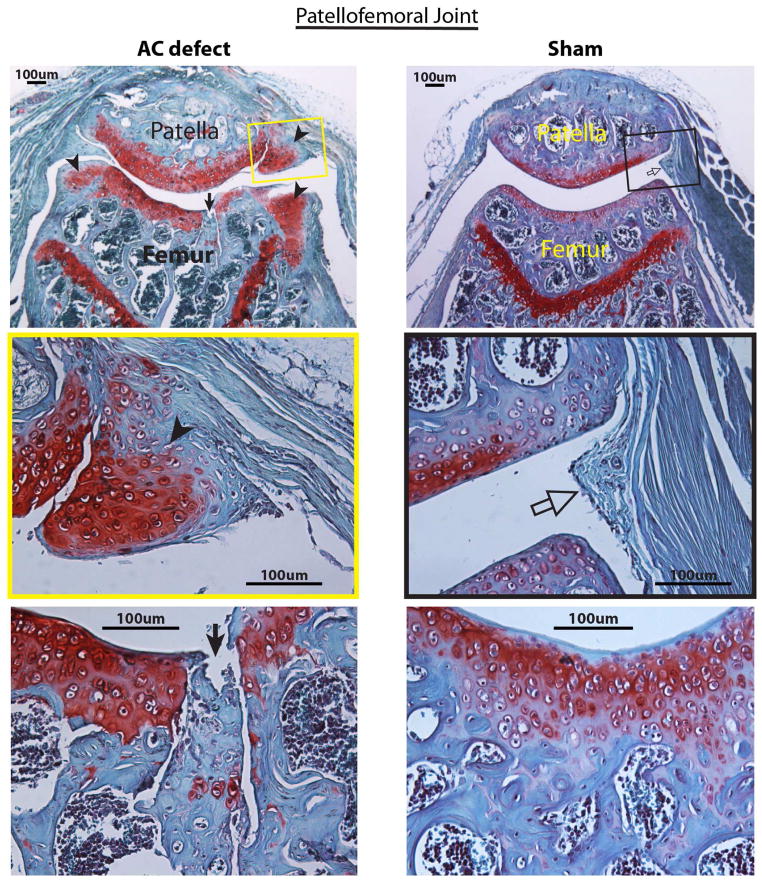
Most recently, we observed chondrogenic differentiation of MSCs in the synovium after the creation of osteochondral defects in the patellofemoral joint groove of adult mice. Cartilage formation is even more abundant in the synovium than in the AC defect in which the repair cells are derived from subchondral bone marrow (Figure 2, unpublished data).
Figure 2.
Photomicrographs of a representative mouse patellofemoral joint with a osteochondral defect created in the patellar groove of the distal femur (left) and a mouse patellofemoral joint received sham surgery (arthrotomy only, right) at 6 weeks after surgery. Cartilage is stained in red. Six mice from each group were evaluated at this time point. Top left: A patellofemoral joint with an osteochondral defect (arrow) and chondrocyte differentiation in the synovium that is attached to the joint margins (arrowheads). Middle left: A micrograph enlarged from the yellow box in the top left panel shows the differentiation of synovial cells into chondrocytes (arrowhead) forming new cartilage in the synovial plica. Bottom left: A micrograph with higher magnification shows that the osteochondral defect (arrow) is filled with new cartilage cells (red) in the lower portion of the defect and fibrous tissue in the upper defect. Top right: A patellofemoral joint that received sham surgery shows a normal synovial plica (open arrow in the black box). Middle right: A normal synovial plica (open arrow) enlarged from the black box in the top right panel shows normal synovial lining and subsynovial fibrous tissue. Bottom right: A patellar groove of the distal femur without an osteochondral defect shows essentially normal articular cartilage and subchondral bone. Safranin-O and fast green staining, counterstained with haematoxylin.
Regulation of adult AC homeostasis: the role of NFAT signaling
Imbalance of metabolic activities at the AC defect site
Proper balance of anabolic and catabolic activities is critical for the maintenance of AC integrity and the regeneration of AC damage. PTOA occurs when the equilibrium between breakdown and repair of the joint tissues becomes unbalanced84–86. A chondral or osteochondral defect may occur after severe joint injuries such as displaced articular fractures. Even with the best current care of joint injuries, such as anatomic reduction and rigid fixation of intra-articular fractures and reconstruction of ruptured ligaments with successful restoration of joint biomechanics, the risk of PTOA after joint injuries ranges from 20% to more than 50%2, 87. These clinical studies suggest that biological factors may be involved in the development of PTOA.
Immediate effects of joint trauma include structural damage to joint tissues, hemarthrosis, and death of articular chondrocytes32, 88. The lubricating properties of the synovial fluid is compromised as a result of the dilution of synovial fluid by intra-articular bleeding and plasma extravasation, leading to lower concentrations of hyaluronic acid and lubricant. In the acute post-traumatic phase, joint trauma may lead to suppression of collagen and proteoglycan synthesis in articular cartilage. Remaining viable cells in joint tissues may respond to the injury with enhanced synthetic activity and overexpression of matrix-degrading enzymes and inflammatory mediators89–91. Initial cell necrosis is followed by a subsequent spreading of cell death mediated by apoptotic mechanisms, which occurs beyond the initial area into surrounding unimpacted regions. During the healing of AC damage, cytokines and enzymes released by synoviocytes and chondrocytes in and around the repair tissue may cause an imbalance between anabolic and catabolic activities, leading to cartilage degradation and subsequent PTOA88, 91. Therefore, the chondrocyte homeostasis in the healing defect is critical for the quality of healing cartilage and the integration of repair cartilage with the original AC and subchondral bone.
NFAT1 and NFAT2 regulate metabolic activities of articular chondrocytes and suppress chondrocyte hypertrophy
NFAT (nuclear factor of activated T cells) is a family of transcription factors originally identified as regulators of gene transcription in response to T-cell receptor-mediated signals in lymphocytes. Currently, five members of the NFAT family have been identified: NFAT1 (NFATc2/NFATp), NFAT2 (NFATc1/NFATc), NFAT3 (NFATc4), NFAT4 (NFATc3/NFATx), and NFAT5. With the exception of NFAT5, which is ubiquitously expressed and activated in response to osmotic stress, nuclear translocation and activation of NFAT1-4 proteins are induced by the Ca2+-calmodulin-dependent phosphatase calcineurin 92–95. Early studies reported NFAT1 as a regulator of the expression of cytokine genes during the immune response; mice lacking NFAT1 displayed an enhanced immune response96, 97. However, the in vitro effects of specific NFAT members on chondrocyte function have been controversial. An early study suggested that NFAT4 induces chondrogenesis, which is an anabolic effect98, while other studies reported that NFAT1 promotes ADAMTS-4 expression and NFAT2 (NFATc1) activates ADMTS-9 in chondrocytes, which are catabolic effects99, 100.
Our recent in vivo studies demonstrated that mice lacking NFAT1 exhibit normal skeletal development but display most of the features of human OA in adults101, 102. Expression of multiple pro-inflammatory cytokines (e.g., IL-1β, IL-6, IL-17α) and matrix-degrading proteinases (e.g., MMP1a, MMP13, ADMTS5) is significantly up-regulated in AC and synovium of adult Nfat1−/− mice, while expression of specific anabolic factors such as BMP-2, -5, -7, -10, -11, -12, and -13 as well as IGF-1, TGF-β1, -β2, and -β3 is significantly down-regulated in the AC of adult Nfat1−/− mice101, 102. NFAT1 binding sites were identified in the genes for specific catabolic and anabolic factors, such as IL-1β, TNF-α, MMP-13, ADMTS-5, BMP-7, TGF-β1, and Collagen-2, -9, 10, and -11. Our chromatin immunoprecipitation (ChIP) assays have confirmed the binding of NFAT1 protein to the promoter of these genes in articular chondrocytes of adult mice103. These new findings suggest that NFAT1 regulates the expression of multiple catabolic and anabolic molecules in AC and is a key transcription factor for maintaining the homeostasis of AC in adult mice. Nfat1 deficiency causes OA mainly due to an imbalance between catabolic and anabolic activities of articular chondrocytes with dysfunction of peri-articular tissue cells, particularly synovial cells.
A more recent study by Greenblatt et al. supports our conclusion. The authors investigated the role of NFATc1 (NFAT2) and NFATc3 (NFAT4) in AC biology104. NFATc1 was previously identified as a regulator of cardiac development and osteoclast differentiation105, 106. They found that Nfatc1 mRNA expression is reduced in lesional AC from human OA patients. Since cartilage-specific Nfatc1 mutant (Nfatc1col2) or Nfatc3 mutant (Nfatc3col2) mice did not display any phenotypic differences to wild type mice, Nfatc1col2 or Nfatc3col2 mice were intercrossed with Nfatc2 (Nfat1) null allele to generate double mutant mice. Nfatc2−/−Nfatc3 col2 mice displayed no additional abnormalities beyond those seen in Nfatc2−/− mice, whereas Nfatc2−/−Nfatc1 col2 mice displayed severe cartilage degradation with subluxation of the elbow at 1 week of age and of the metatarsals at 3 weeks of age. At the molecular level, these double mutant mice exhibited increased expression of genes encoding many matrix-degrading proteinases, along with the hypertrophic chondrocyte marker collagen X. At the same time, expression of Sox9 and lubricin were reduced in the Nfatc2−/−Nfatc1col2 mutants104. These results suggest that NFAT1 may play a more important role than NFAT2 in the maintenance of AC homeostasis and prevention of OA.
To evaluate the effect of NFAT1 deficiency on the healing of AC, the authors of this review have recently developed a new mouse model of cartilage repair by surgical creation of an osteochondral defect in the patellar groove of the distal femur of Nfat1−/− and wild-type (WT) mice. Although oteochondral defects were filled with repair cartilaginous tissue in both WT and Nfat1−/− mice, more hypertrophic chondrocytes and endochondral ossification were observed in Nfat1−/− defects than in WT defects. The expression of mRNA for type-10 collagen and specific pro-inflammatory cytokines and matrix-degrading proteinases was up-regulated in Nfat1−/− defects compared to WT defects. At 26 weeks after surgery, WT mice showed mild to moderate early-stage OA in the patellofemoral joints, while Nfat1−/− mice displayed severe late-stage OA in the patellofemoral joints with segmentation of repair tissue and severe incongruity of the articular surfaces107. In addition, more severe osteoarthritic cartilage lesions were seen in the knee joints of Nfat1−/− mice than WT mice after destabilizing the medial meniscus108.
These in vivo studies have provided strong evidence that NFAT1 suppresses chondrocyte hypertrophy and catabolic metabolism during the healing of cartilage lesions, thereby attenuating the progression of PTOA. The proposed mechanisms by which NFAT1 suppresses the development of OA are illustrated in Figure 3.
Figure 3.
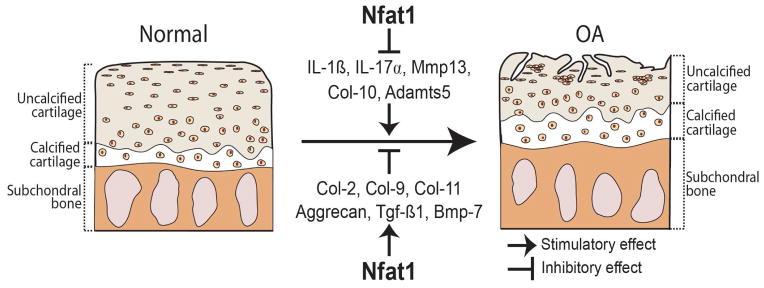
An illustration demonstrates that catabolic and anabolic factors that may be responsible for the development of OA and the possible role of NFAT1 in preventing the initiation or attenuating the progression of OA.
Future perspectives
Although many strategies could improve the outcome of AC repair, our perspectives will focus on cell-based repair of AC and osteochondral lesions.
Optimization of scaffolds and mechanical loading to improve cell migration, proliferation and differentiation
Development of novel scaffolds that mimic the inherent gradient structure of healthy osteochondral tissue might improve cellular activity in tissue engineering-mediated AC repair. For example, a gradient scaffold may consist of a bone layer composed of type I collagen and beta-tricalcium phosphate (TCP) or hydroxyapatite (HA), an intermediate layer composed of type I collagen, type II collagen and TCP/HA, and a cartilaginous region composed of type II collagen and hyaluronic acid109–111. Refinement of the chemical and material properties of scaffolds may improve the biological cues required for infiltration and proliferation of MSCs or chondrocytes in scaffolds, while the biomechanical properties of an optimized scaffold may provide an environment to promote differentiation of stem cells towards the required lineage in each region. The inclusion of bioactive factors in gradient-based scaffolding may further improve the outcome of osteochondral defect repair.
Mechanical factors play a significant role in the maintenance of chondrocyte phenotype as chondrocytes are known to lose their chondrocyte specific phenotype when removed from their native ECM for monolayer culture, resembling prechondrocytic MSCs but regain chondrocyte phenotype when placed into a three-dimensional culture (i.e. agarose gel) for continued culture112–114. Chondrocytes harvested from adult, human articular cartilage do not demonstrate the same need for chemical induction in order to form new articular cartilage; however, they do seem to possess the same propensity to progress into the transient phenotype in monolayer culture12. Appropriate mechanical loading on the joint with healing AC may be beneficial to the formation of hyaline cartilage and congruity of articular surfaces.
Synovial MSCs as a cell source for AC repair
Perhaps the deficiencies we have encountered so far with bone marrow stimulation techniques are due to the fact that bone marrow MSCs may not be the best cell source or may require specific modulation for the healing of AC damage. Future directions would include a deeper look into the potential function of synovial MSCs, thereby discovering functional distinctions they may have from bone marrow MSCs and mature articular chondrocytes.
As described above, synovial MSCs could be a new cell source for better AC repair because synovium and AC develop from the same pool of precursor cells, synovium is attached to AC in adulthood and synovial MSCs actively respond to AC damage, the gene profile of AC cells more closely matches that of synovial cells than bone marrow MSCs, and chondrogenic potential of synovial cells for AC repair has been demonstrated in animal models and preliminary clinical trials. Some techniques need to be further refined. For example, two different types of synoviocyte cells, macrophage-like (type A) and fibroblast-like (type-B) cells, should be distinguished by specific techniques. Type A cells function in innate and adaptive immunity, while type B cells contribute to the formation of synovial fluid and are believed to be the source of synovial MSCs70, 115, 116. Reproducible methods for isolation and identification of specific type B synoviocytes from experimental animals and humans need to be optimized.
Use of upstream regulators of chondrocyte differentiation and cartilage homeostasis
The molecular mechanisms that lead to regeneration and maintenance of AC structure and function would be of tremendous therapeutic value, especially noting that degenerating repair cartilage seems to demonstrate the hypertrophic and endochondral phenotype (i.e. type X collagen expression)31. Tissue engineering offers the possibility of promoting anabolic and inhibiting catabolic activity in AC repair by adding an anabolic growth factor or anti-catabolic agent. One of the major reasons for the failure of cartilage tissue engineering would be that multiple anabolic and catabolic factors are involved in the healing of cartilage lesions 88–91; thus, one or more chondrogenic growth factors currently used for cartilage tissue engineering is unlikely to sufficiently modulate the healing process in long-term. Furthermore, OA is a multifactorial disease; genetic modifications of one of susceptible factors may precipitate OA-like changes in mice. Many factors are involved in the pathogenesis of OA, including aging, genetic factors, matrix-degrading proteinases, pro- and anti-inflammatory cytokines, growth factors, and hormones. Therefore, upstream regulatory factors such as transcription factors that regulate multiple anabolic and catabolic molecules would be more desirable for the regeneration of AC and prevention of PTOA.
A number of transcription factors have been reported to play a role in chondrocyte differentiation and cartilage homeostasis during the development and adulthood. SOX9 is critical for chondrocyte differentiation and cartilage morphogenesis during skeletal development117. SOX9 and SOX trio (SOX-5, 6, and-9) may promote cartilage repair in osteochondral defects118. However, overexpression of SOX9 is unable to restore the chondrocyte phenotype in dedifferentiated osteoarthritic chondrocytes119, and postnatal inactivation of Sox9 in mouse cartilage does not result in OA by 18 months of age120. RUNX family proteins, RUNX1-3, play important roles in skeletal development and repair. RUNX1 is required for differentiation of chondroprogenitor cells and promotes cartilaginous callus formation during fracture healing121. RUNX2 (Cbfa1) is required for chondrocyte maturation and osteoblast differentiation, and deletion of RUNX2 results in a complete lack of bone formation122, 123. RUNX2 enhances subchondral bone formation during the healing of osteochondral defects124. RUNX3 regulates chondrocyte differentiation and promotes cartilage formation during fracture healing125. RUNX3-deficient mice display severe limb ataxia126. Somatic deletion of the β-catenin (a key transcriptional activator of the canonical Wnt pathway) gene results in lethality before formation of the skeletal elements127. Conditional deletion of the β-catenin gene in early mesenchymal progenitor cells leads to enhanced chondrogenesis 128. Both gain- and loss-of-function of β-catenin in AC resulted in similar OA-like phenotypes129, 130. Beta-catenin expression is up-regulated in AC of young adult Nfat1−/− mice, at which time some Nfat1−/− hip joints began to show early OA-like changes102. The role of increased beta-catenin in Nfat1 deficiency-induced OA remains to be elucidated. C-maf plays a role in both chondrocyte differentiation and homeostasis 131, 132.
Table 1 summarizes the roles of above-mentioned transcription factors and NFAT1-2 in cartilage biology and pathology. Except NFAT1, global or conditional gene deletion of all these factors results in severe developmental defects in the skeletal system. NFAT1 appears to be one of the few, if not the sole, transcription factors that specifically regulate the function of articular chondrocytes in the adult, but not in the developmental stage. Since transcription factors usually serve as upstream regulators of multiple catabolic and anabolic genes, appropriate use of a specific transcription factor could be more effective than that of a single anabolic growth factor or anti-catabolic cytokine for the regeneration of AC and prevention of PTOA.
Table 1.
Selected transcription factors (TF) in chondrocyte differentiation and skeletal homeostasis/repair
| TF | Developmental defect in skeleton by mutation | Role in adult cartilage/bone | Ref #* |
|---|---|---|---|
| SOX9 | Yes. Required for chondrocyte differentiation and cartilage formation | Promoting anabolic activity and repair of AC | 117–120 |
| RUNX1 | Yes. Required for differentiation of chondroprogenitor cells into the chondrogenic lineage | Promoting cartilaginous callus formation during fracture healing | 121 |
| RUNX2 (Cbfa1) | Yes. Required for chondrocyte maturation and osteoblast differentiation | Enhancing subchondral bone formation in osteochondral repair | 122–124 |
| RUNX3 | Yes. Regulating chondrocyte terminal differentiation, limb ataxia in Runx3-deficient mice | Promoting cartilage formation during fracture healing | 125–126 |
| β-cat** | Yes. Required for skeletal development, promoting osteogenesis and inhibiting chondrogenesis | Regulating homeostasis of AC and bone formation | 127–130 |
| c-Maf | Yes. Required for normal chondrocyte differentiation | Activating MMP-13 gene expression in OA AC | 131–132 |
| NFATc1 (NFAT2) | Yes. Required for cardiac development, defective joint formation in double mutant mice lacking NFATs c1 and c2 in cartilage | Regulating chondrocyte function, bone formation, and terminal differentiation of osteoclasts in bone marrow cells | 104–106 |
| NFATc2 (NFAT1) | No. Not required for skeletal development, no developmental defects in skeleton of Nfat1-deficient mice | Suppressing OA, chondrocyte hypertrophy in AC, and PTOA after cartilage injury in Nfat1-deficient mice | 101–104, 107–108 |
Ref # = Reference number cited in this article
β-cat = β-catenin
Acknowledgments
This work was supported by the U.S. National Institutes of Health (NIH) grant R01 AR059088 (to J. Wang), U.S. Department of Defense medical research grant W81XWH-12-1-0304 (to J. Wang), the Mary A. & Paul R. Harrington Distinguished Professorship Endowment (to J. Wang), and the Asher Orthopedic Research Endowment. The authors thank Professor Maurizio Pacifici (Children’s Hospital of Philadelphia and the University of Pennsylvania, PA, USA) for his comments on the joint development studies and Mr. Andrew Miller and Mr. Brian Egan for graphic and editorial assistance.
Footnotes
Author Contributions
Both authors searched the literature, summarized the results, and wrote the manuscript.
Competing Interest Statement
The authors have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bekkers JE, Inklaar M, Saris DB. Treatment selection in articular cartilage lesions of the knee: a systematic review. Am J Sports Med. 2009;37 (Suppl 1):148S–55S. doi: 10.1177/0363546509351143. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DD, Chubinskaya S, Guilak F, Martin JA, Oegema TR, Olson SA, et al. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res. 2011;29:802–9. doi: 10.1002/jor.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grimm NL, Weiss JM, Kessler JI, Aoki SK. Osteochondritis Dissecans of the Knee: Pathoanatomy, Epidemiology, and Diagnosis. Clin Sports Med. 2014;33:181–8. doi: 10.1016/j.csm.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Pridie KH. A method of resurfacing osteoarthritic knee joints. Journal of Bone and Joint Surgery [Br] 1959;41-B:618–9. [Google Scholar]
- 5.Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19:477–84. doi: 10.1053/jars.2003.50112. [DOI] [PubMed] [Google Scholar]
- 6.Frisbie DD, Oxford JT, Southwood L, Trotter GW, Rodkey WG, Steadman JR, et al. Early events in cartilage repair after subchondral bone microfracture. Clin Orthop Relat Res. 2003:215–27. doi: 10.1097/00003086-200302000-00031. [DOI] [PubMed] [Google Scholar]
- 7.Kaul G, Cucchiarini M, Remberger K, Kohn D, Madry H. Failed cartilage repair for early osteoarthritis defects: a biochemical, histological and immunohistochemical analysis of the repair tissue after treatment with marrow-stimulation techniques. Knee Surg Sports Traumatol Arthrosc. 2012;20:2315–24. doi: 10.1007/s00167-011-1853-x. [DOI] [PubMed] [Google Scholar]
- 8.Gross AESE, Falk J, Falk R, Langer F. The allotransplantation of partial joints in the treatment of osteoarthritis of the knee. Clin Orthop Relat Res. 1975;108:7–14. doi: 10.1097/00003086-197505000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Sherman SL, Garrity J, Bauer K, Cook J, Stannard J, Bugbee W. Fresh osteochondral allograft transplantation for the knee: current concepts. J Am Acad Orthop Surg. 2014;22:121–33. doi: 10.5435/JAAOS-22-02-121. [DOI] [PubMed] [Google Scholar]
- 10.Krych AJ, Harnly HW, Rodeo SA, Williams RJ., 3rd Activity levels are higher after osteochondral autograft transfer mosaicplasty than after microfracture for articular cartilage defects of the knee: a retrospective comparative study. J Bone Joint Surg Am. 2012;94:971–8. doi: 10.2106/JBJS.K.00815. [DOI] [PubMed] [Google Scholar]
- 11.Solheim E, Hegna J, Oyen J, Harlem T, Strand T. Results at 10 to 14 years after osteochondral autografting (mosaicplasty) in articular cartilage defects in the knee. Knee. 2013;20:287–90. doi: 10.1016/j.knee.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Grande DA, Singh IJ, Pugh J. Healing of experimentally produced lesions in articular cartilage following chondrocyte transplantation. Anat Rec. 1987;218:142–8. doi: 10.1002/ar.1092180208. [DOI] [PubMed] [Google Scholar]
- 13.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–95. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 14.Knutsen G, Drogset JO, Engebretsen L, Grontvedt T, Isaksen V, Ludvigsen TC, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89:2105–12. doi: 10.2106/JBJS.G.00003. [DOI] [PubMed] [Google Scholar]
- 15.Bentley G, Biant LC, Carrington RW, Akmal M, Goldberg A, Williams AM, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85:223–30. doi: 10.1302/0301-620x.85b2.13543. [DOI] [PubMed] [Google Scholar]
- 16.Mollon B, Kandel R, Chahal J, Theodoropoulos J. The clinical status of cartilage tissue regeneration in humans. Osteoarthritis Cartilage. 2013;21:1824–33. doi: 10.1016/j.joca.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 17.Benneker LM, Cucchiarini M, Madry H, Guilak F, Saris DB, Stoddart MJ, et al. A vision on the future of articular cartilage repair. Eur Cell Mater. 2014;27:12–6. doi: 10.22203/ecm.v027sa03. [DOI] [PubMed] [Google Scholar]
- 18.Chiang H, Liao CJ, Hsieh CH, Shen CY, Huang YY, Jiang CC. Clinical feasibility of a novel biphasic osteochondral composite for matrix-associated autologous chondrocyte implantation. Osteoarthritis Cartilage. 2013;21:589–98. doi: 10.1016/j.joca.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Cui L, Wu Y, Cen L, Zhou H, Yin S, Liu G, et al. Repair of articular cartilage defect in non-weight bearing areas using adipose derived stem cells loaded polyglycolic acid mesh. Biomaterials. 2009;30:2683–93. doi: 10.1016/j.biomaterials.2009.01.045. [DOI] [PubMed] [Google Scholar]
- 20.Levingstone TJ, Matsiko A, Dickson GR, O’Brien FJ, Gleeson JP. A biomimetic multi-layered collagen-based scaffold for osteochondral repair. Acta Biomater. 2014 doi: 10.1016/j.actbio.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Li YY, Cheng HW, Cheung KM, Chan D, Chan BP. Mesenchymal stem cell-collagen microspheres for articular cartilage repair: Cell density and differentiation status. Acta Biomater. 2014 doi: 10.1016/j.actbio.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Cheng A, Hardingham TE, Kimber SJ. Generating Cartilage Repair from Pluripotent Stem Cells. Tissue Eng Part B Rev. 2013 doi: 10.1089/ten.teb.2012.0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diekman BO, Christoforou N, Willard VP, Sun H, Sanchez-Adams J, Leong KW, et al. Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2012;109:19172–7. doi: 10.1073/pnas.1210422109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ko JY, Kim KI, Park S, Im GI. In vitro chondrogenesis and in vivo repair of osteochondral defect with human induced pluripotent stem cells. Biomaterials. 2014;35:3571–81. doi: 10.1016/j.biomaterials.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Mifune Y, Matsumoto T, Takayama K, Ota S, Li H, Meszaros LB, et al. The effect of platelet-rich plasma on the regenerative therapy of muscle derived stem cells for articular cartilage repair. Osteoarthritis Cartilage. 2013;21:175–85. doi: 10.1016/j.joca.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 26.Cole BJ, Farr J, Winalski CS, Hosea T, Richmond J, Mandelbaum B, et al. Outcomes After a Single-Stage Procedure for Cell-Based Cartilage Repair: A Prospective Clinical Safety Trial With 2-year Follow-up. Am J Sports Med. 2011;39:1170–9. doi: 10.1177/0363546511399382. [DOI] [PubMed] [Google Scholar]
- 27.Brunger JM, Huynh NP, Guenther CM, Perez-Pinera P, Moutos FT, Sanchez-Adams J, et al. Scaffold-mediated lentiviral transduction for functional tissue engineering of cartilage. Proc Natl Acad Sci U S A. 2014;111:E798–806. doi: 10.1073/pnas.1321744111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi J, Zhang X, Zhu J, Pi Y, Hu X, Zhou C, et al. Nanoparticle delivery of the bone morphogenetic protein 4 gene to adipose-derived stem cells promotes articular cartilage repair in vitro and in vivo. Arthroscopy. 2013;29:2001–11. e2. doi: 10.1016/j.arthro.2013.09.076. [DOI] [PubMed] [Google Scholar]
- 29.Tuan RS, Chen AF, Klatt BA. Cartilage regeneration. J Am Acad Orthop Surg. 2013;21:303–11. doi: 10.5435/JAAOS-21-05-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barr L, Getgood A, Guehring H, Rushton N, Henson FM. The effect of recombinant human fibroblast growth factor-18 on articular cartilage following single impact load. J Orthop Res. 2014;32:923–7. doi: 10.1002/jor.22622. [DOI] [PubMed] [Google Scholar]
- 31.Somoza RA, Welter JF, Correa D, Caplan AI. Chondrogenic differentiation of Mesenchymal Stem Cells: challenges and unfulfilled expectations. Tissue Eng Part B Rev. 2014 doi: 10.1089/ten.teb.2013.0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lotz MK, Kraus VB. New developments in osteoarthritis. Posttraumatic osteoarthritis: pathogenesis and pharmacological treatment options. Arthritis Res Ther. 2010;12:211. doi: 10.1186/ar3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldring MB, Otero M, Plumb DA, Dragomir C, Favero M, El Hachem K, et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur Cell Mater. 2011;21:202–20. doi: 10.22203/ecm.v021a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forriol F. Growth factors in cartilage and meniscus repair. Injury. 2009;40 (Suppl 3):S12–6. doi: 10.1016/S0020-1383(09)70005-1. [DOI] [PubMed] [Google Scholar]
- 35.Schroeppel JP, Crist JD, Anderson HC, Wang J. Molecular regulation of articular chondrocyte function and its significance in osteoarthritis. Histol Histopathol. 2011;26:377–94. doi: 10.14670/HH-26.377. [DOI] [PubMed] [Google Scholar]
- 36.Dell’Accio F, De Bari C, Luyten FP. Microenvironment and phenotypic stability specify tissue formation by human articular cartilage-derived cells in vivo. Exp Cell Res. 2003;287:16–27. doi: 10.1016/s0014-4827(03)00036-3. [DOI] [PubMed] [Google Scholar]
- 37.Backus JD, Furman BD, Swimmer T, Kent CL, McNulty AL, Defrate LE, et al. Cartilage viability and catabolism in the intact porcine knee following transarticular impact loading with and without articular fracture. J Orthop Res. 2011;29:501–10. doi: 10.1002/jor.21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding L, Heying E, Nicholson N, Stroud NJ, Homandberg GA, Buckwalter JA, et al. Mechanical impact induces cartilage degradation via mitogen activated protein kinases. Osteoarthritis Cartilage. 2010;18:1509–17. doi: 10.1016/j.joca.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun HB. Mechanical loading, cartilage degradation, and arthritis. Ann N Y Acad Sci. 2010;1211:37–50. doi: 10.1111/j.1749-6632.2010.05808.x. [DOI] [PubMed] [Google Scholar]
- 40.Lamb KJ, Lewthwaite JC, Bastow ER, Pitsillides AA. Defining boundaries during joint cavity formation: going out on a limb. Int J Exp Pathol. 2003;84:55–67. doi: 10.1046/j.1365-2613.2003.00338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holder N. An experimental investigation into the early development of the chick elbow joint. J Embryol Exp Morphol. 1977;39:115–27. [PubMed] [Google Scholar]
- 42.Ito MM, Kida MY. Morphological and biochemical re-evaluation of the process of cavitation in the rat knee joint: cellular and cell strata alterations in the interzone. J Anat. 2000;197(Pt 4):659–79. doi: 10.1046/j.1469-7580.2000.19740659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Archer CW, Dowthwaite GP, Francis-West P. Development of synovial joints. Birth Defects Res C Embryo Today. 2003;69:144–55. doi: 10.1002/bdrc.10015. [DOI] [PubMed] [Google Scholar]
- 44.Gardner E, Gray DJ, O’Rahilly R. The prenatal development of the skeleton and joints of the human foot. J Bone Joint Surg Am. 1959;41-A:847–76. [PubMed] [Google Scholar]
- 45.Pacifici M, Koyama E, Shibukawa Y, Wu C, Tamamura Y, Enomoto-Iwamoto M, et al. Cellular and molecular mechanisms of synovial joint and articular cartilage formation. Ann N Y Acad Sci. 2006;1068:74–86. doi: 10.1196/annals.1346.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pitsillides AA, Ashhurst DE. A critical evaluation of specific aspects of joint development. Dev Dyn. 2008;237:2284–94. doi: 10.1002/dvdy.21654. [DOI] [PubMed] [Google Scholar]
- 47.Roddy KA, Prendergast PJ, Murphy P. Mechanical influences on morphogenesis of the knee joint revealed through morphological, molecular and computational analysis of immobilised embryos. PLoS One. 2011;6:e17526. doi: 10.1371/journal.pone.0017526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pacifici M, Koyama E, Iwamoto M. Mechanisms of synovial joint and articular cartilage formation: recent advances, but many lingering mysteries. Birth Defects Res C Embryo Today. 2005;75:237–48. doi: 10.1002/bdrc.20050. [DOI] [PubMed] [Google Scholar]
- 49.Bland YS, Ashhurst DE. Development and ageing of the articular cartilage of the rabbit knee joint: distribution of the fibrillar collagens. Anat Embryol (Berl) 1996;194:607–19. doi: 10.1007/BF00187473. [DOI] [PubMed] [Google Scholar]
- 50.Hartmann C, Tabin CJ. Wnt-14 plays a pivotal role in inducing synovial joint formation in the developing appendicular skeleton. Cell. 2001;104:341–51. doi: 10.1016/s0092-8674(01)00222-7. [DOI] [PubMed] [Google Scholar]
- 51.Hayes AJ, MacPherson S, Morrison H, Dowthwaite G, Archer CW. The development of articular cartilage: evidence for an appositional growth mechanism. Anat Embryol (Berl) 2001;203:469–79. doi: 10.1007/s004290100178. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Q, Cigan AD, Marrero L, Lopreore C, Liu S, Ge D, et al. Expression of doublecortin reveals articular chondrocyte lineage in mouse embryonic limbs. Genesis. 2011;49:75–82. doi: 10.1002/dvg.20702. [DOI] [PubMed] [Google Scholar]
- 53.Peinado Cortes LM, Vanegas Acosta JC, Garzon Alvarado DA. A mechanobiological model of epiphysis structures formation. J Theor Biol. 2011;287:13–25. doi: 10.1016/j.jtbi.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 54.Ross MH, Pawlina W. Bone. In: Ross MH, Pawlina W, editors. Histology: a Text and Atlas. Philadelphia: Lippincott Williams & Wilkins; 2011. pp. 218–53. [Google Scholar]
- 55.Rivas R, Shapiro F. Structural stages in the development of the long bones and epiphyses: a study in the New Zealand white rabbit. J Bone Joint Surg Am. 2002;84-A:85–100. doi: 10.2106/00004623-200201000-00013. [DOI] [PubMed] [Google Scholar]
- 56.Floyd WE, 3rd, Zaleske DJ, Schiller AL, Trahan C, Mankin HJ. Vascular events associated with the appearance of the secondary center of ossification in the murine distal femoral epiphysis. J Bone Joint Surg Am. 1987;69:185–90. [PubMed] [Google Scholar]
- 57.Mitrovic D. Development of the diarthrodial joints in the rat embryo. Am J Anat. 1978;151:475–85. doi: 10.1002/aja.1001510403. [DOI] [PubMed] [Google Scholar]
- 58.Archer CW, Morrison H, Pitsillides AA. Cellular aspects of the development of diarthrodial joints and articular cartilage. J Anat. 1994;184 (Pt 3):447–56. [PMC free article] [PubMed] [Google Scholar]
- 59.Lizarraga G, Lichtler A, Upholt WB, Kosher RA. Studies on the role of Cux1 in regulation of the onset of joint formation in the developing limb. Dev Biol. 2002;243:44–54. doi: 10.1006/dbio.2001.0559. [DOI] [PubMed] [Google Scholar]
- 60.Jenner F, Ijpma A, Cleary M, Heijsman D, Narcisi R, van der Spek PJ, et al. Differential Gene Expression of the Intermediate and Outer Interzone layers of developing articular cartilage in murine embryos. Stem Cells Dev. 2014 doi: 10.1089/scd.2013.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koyama E, Shibukawa Y, Nagayama M, Sugito H, Young B, Yuasa T, et al. A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev Biol. 2008;316:62–73. doi: 10.1016/j.ydbio.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hunziker EB, Kapfinger E, Geiss J. The structural architecture of adult mammalian articular cartilage evolves by a synchronized process of tissue resorption and neoformation during postnatal development. Osteoarthritis Cartilage. 2007;15:403–13. doi: 10.1016/j.joca.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 63.Simkin PA. A biography of the chondrocyte. Ann Rheum Dis. 2008;67:1064–8. doi: 10.1136/ard.2007.084574. [DOI] [PubMed] [Google Scholar]
- 64.Dowthwaite GP, Bishop JC, Redman SN, Khan IM, Rooney P, Evans DJ, et al. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117:889–97. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- 65.Grogan SP, Miyaki S, Asahara H, D’Lima DD, Lotz MK. Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res Ther. 2009;11:R85. doi: 10.1186/ar2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Williams R, Khan IM, Richardson K, Nelson L, McCarthy HE, Analbelsi T, et al. Identification and clonal characterisation of a progenitor cell sub-population in normal human articular cartilage. PLoS One. 2010;5:e13246. doi: 10.1371/journal.pone.0013246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou C, Zheng H, Seol D, Yu Y, Martin JA. Gene expression profiles reveal that chondrogenic progenitor cells and synovial cells are closely related. J Orthop Res. 2014 doi: 10.1002/jor.22641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Futami I, Ishijima M, Kaneko H, Tsuji K, Ichikawa-Tomikawa N, Sadatsuki R, et al. Isolation and characterization of multipotential mesenchymal cells from the mouse synovium. PLoS One. 2012;7:e45517. doi: 10.1371/journal.pone.0045517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ito S, Sato M, Yamato M, Mitani G, Kutsuna T, Nagai T, et al. Repair of articular cartilage defect with layered chondrocyte sheets and cultured synovial cells. Biomaterials. 2012;33:5278–86. doi: 10.1016/j.biomaterials.2012.03.073. [DOI] [PubMed] [Google Scholar]
- 70.Kurth TB, Dell’accio F, Crouch V, Augello A, Sharpe PT, De Bari C. Functional mesenchymal stem cell niches in adult mouse knee joint synovium in vivo. Arthritis Rheum. 2011;63:1289–300. doi: 10.1002/art.30234. [DOI] [PubMed] [Google Scholar]
- 71.Lee JC, Min HJ, Park HJ, Lee S, Seong SC, Lee MC. Synovial membrane-derived mesenchymal stem cells supported by platelet-rich plasma can repair osteochondral defects in a rabbit model. Arthroscopy. 2013;29:1034–46. doi: 10.1016/j.arthro.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 72.Lee JI, Sato M, Kim HW, Mochida J. Transplantatation of scaffold-free spheroids composed of synovium-derived cells and chondrocytes for the treatment of cartilage defects of the knee. Eur Cell Mater. 2011;22:275–90. doi: 10.22203/ecm.v022a21. discussion 90. [DOI] [PubMed] [Google Scholar]
- 73.Pei M, He F. Extracellular matrix deposited by synovium-derived stem cells delays replicative senescent chondrocyte dedifferentiation and enhances redifferentiation. J Cell Physiol. 2012;227:2163–74. doi: 10.1002/jcp.22950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shimomura K, Moriguchi Y, Ando W, Nansai R, Fujie H, Hart DA, et al. Osteochondral Repair Using a Scaffold-Free Tissue-Engineered Construct Derived from Synovial Mesenchymal Stem Cells and a Hydroxyapatite-Based Artificial Bone. Tissue Eng Part A. 2014 doi: 10.1089/ten.tea.2013.0414. [DOI] [PubMed] [Google Scholar]
- 75.Shintani N, Siebenrock KA, Hunziker EB. TGF-ss1 enhances the BMP-2-induced chondrogenesis of bovine synovial explants and arrests downstream differentiation at an early stage of hypertrophy. PLoS One. 2013;8:e53086. doi: 10.1371/journal.pone.0053086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–42. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 77.Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–9. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 78.Kurth T, Hedbom E, Shintani N, Sugimoto M, Chen FH, Haspl M, et al. Chondrogenic potential of human synovial mesenchymal stem cells in alginate. Osteoarthritis Cartilage. 2007;15:1178–89. doi: 10.1016/j.joca.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 79.Pei M, He F, Kish VL, Vunjak-Novakovic G. Engineering of functional cartilage tissue using stem cells from synovial lining: a preliminary study. Clin Orthop Relat Res. 2008;466:1880–9. doi: 10.1007/s11999-008-0316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schumacher BL, Hughes CE, Kuettner KE, Caterson B, Aydelotte MB. Immunodetection and partial cDNA sequence of the proteoglycan, superficial zone protein, synthesized by cells lining synovial joints. J Orthop Res. 1999;17:110–20. doi: 10.1002/jor.1100170117. [DOI] [PubMed] [Google Scholar]
- 81.Koga H, Muneta T, Ju YJ, Nagase T, Nimura A, Mochizuki T, et al. Synovial stem cells are regionally specified according to local microenvironments after implantation for cartilage regeneration. Stem Cells. 2007;25:689–96. doi: 10.1634/stemcells.2006-0281. [DOI] [PubMed] [Google Scholar]
- 82.Sekiya I, Muneta T, Koga H, Nimura A, Morito T, Shimaya M, et al. Articular cartilage regeneration with synovial mesenchymal stem cells. Clin Calcium. 2011;21:879–89. [PubMed] [Google Scholar]
- 83.Hunziker EB, Rosenberg LC. Repair of partial-thickness defects in articular cartilage: cell recruitment from the synovial membrane. J Bone Joint Surg Am. 1996;78:721–33. doi: 10.2106/00004623-199605000-00012. [DOI] [PubMed] [Google Scholar]
- 84.Fini M, Pagani S, Giavaresi G, De Mattei M, Ongaro A, Varani K, et al. Functional tissue engineering in articular cartilage repair: is there a role for electromagnetic biophysical stimulation? Tissue Eng Part B Rev. 2013;19:353–67. doi: 10.1089/ten.TEB.2012.0501. [DOI] [PubMed] [Google Scholar]
- 85.Fukui N, Purple CR, Sandell LJ. Cell biology of osteoarthritis: the chondrocyte’s response to injury. Curr Rheumatol Rep. 2001;3:496–505. doi: 10.1007/s11926-001-0064-8. [DOI] [PubMed] [Google Scholar]
- 86.Mueller MB, Tuan RS. Anabolic/Catabolic balance in pathogenesis of osteoarthritis: identifying molecular targets. Pm r. 2011;3:S3–11. doi: 10.1016/j.pmrj.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 87.Marsh JL, Buckwalter J, Gelberman R, Dirschl D, Olson S, Brown T, et al. Articular fractures: does an anatomic reduction really change the result? J Bone Joint Surg Am. 2002;84-a:1259–71. [PubMed] [Google Scholar]
- 88.Szczodry M, Coyle CH, Kramer SJ, Smolinski P, Chu CR. Progressive chondrocyte death after impact injury indicates a need for chondroprotective therapy. Am J Sports Med. 2009;37:2318–22. doi: 10.1177/0363546509348840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.DiMicco MA, Patwari P, Siparsky PN, Kumar S, Pratta MA, Lark MW, et al. Mechanisms and kinetics of glycosaminoglycan release following in vitro cartilage injury. Arthritis Rheum. 2004;50:840–8. doi: 10.1002/art.20101. [DOI] [PubMed] [Google Scholar]
- 90.Lee JH, Fitzgerald JB, Dimicco MA, Grodzinsky AJ. Mechanical injury of cartilage explants causes specific time-dependent changes in chondrocyte gene expression. Arthritis Rheum. 2005;52:2386–95. doi: 10.1002/art.21215. [DOI] [PubMed] [Google Scholar]
- 91.Kramer WC, Hendricks KJ, Wang J. Pathogenetic mechanisms of posttraumatic osteoarthritis: opportunities for early intervention. Int J Clin Exp Med. 2011;4:285–98. [PMC free article] [PubMed] [Google Scholar]
- 92.Esensten JH, Tsytsykova AV, Lopez-Rodriguez C, Ligeiro FA, Rao A, Goldfeld AE. NFAT5 binds to the TNF promoter distinctly from NFATp, c, 3 and 4, and activates TNF transcription during hypertonic stress alone. Nucleic Acids Res. 2005;33:3845–54. doi: 10.1093/nar/gki701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Masuda ES, Naito Y, Tokumitsu H, Campbell D, Saito F, Hannum C, et al. NFATx, a novel member of the nuclear factor of activated T cells family that is expressed predominantly. Mol Cell Biol. 1995;15:2697–706. doi: 10.1128/mcb.15.5.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McCaffrey PG, Luo C, Kerppola TK, Jain J, Badalian TM, Ho AM, et al. Isolation of the cyclosporin-sensitive T cell transcription factor NFATp. Science. 1993;262:750–4. doi: 10.1126/science.8235597. [DOI] [PubMed] [Google Scholar]
- 95.Hoey T, Sun YL, Williamson K, Xu X. Isolation of two new members of the NF-AT gene family and functional characterization of the NF-AT proteins. Immunity. 1995;2:461–72. doi: 10.1016/1074-7613(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 96.Hodge MR, Ranger AM, Charles de la Brousse F, Hoey T, Grusby MJ, Glimcher LH. Hyperproliferation and dysregulation of IL-4 expression in NF-ATp-deficient mice. Immunity. 1996;4:397–405. doi: 10.1016/s1074-7613(00)80253-8. [DOI] [PubMed] [Google Scholar]
- 97.Xanthoudakis S, Viola JP, Shaw KT, Luo C, Wallace JD, Bozza PT, et al. An enhanced immune response in mice lacking the transcription factor NFAT1. Science. 1996;272:892–5. doi: 10.1126/science.272.5263.892. [DOI] [PubMed] [Google Scholar]
- 98.Tomita M, Reinhold MI, Molkentin JD, Naski MC. Calcineurin and NFAT4 induce chondrogenesis. J Biol Chem. 2002;277:42214–8. doi: 10.1074/jbc.C200504200. [DOI] [PubMed] [Google Scholar]
- 99.Thirunavukkarasu K, Pei Y, Moore TL, Wang H, Yu XP, Geiser AG, et al. Regulation of the human ADAMTS-4 promoter by transcription factors and cytokines. Biochem Biophys Res Commun. 2006;345:197–204. doi: 10.1016/j.bbrc.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 100.Yaykasli KO, Oohashi T, Hirohata S, Hatipoglu OF, Inagawa K, Demircan K, et al. ADAMTS9 activation by interleukin 1 beta via NFATc1 in OUMS-27 chondrosarcoma cells and in human chondrocytes. Mol Cell Biochem. 2009;323:69–79. doi: 10.1007/s11010-008-9965-4. [DOI] [PubMed] [Google Scholar]
- 101.Rodova M, Lu Q, Li Y, Woodbury BG, Crist JD, Gardner BM, et al. Nfat1 regulates adult articular chondrocyte function through its age-dependent expression mediated by epigenetic histone methylation. J Bone Miner Res. 2011;26:1974–86. doi: 10.1002/jbmr.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang J, Gardner BM, Lu Q, Rodova M, Woodbury BG, Yost JG, et al. Transcription factor Nfat1 deficiency causes osteoarthritis through dysfunction of adult articular chondrocytes. J Pathol. 2009;219:163–72. doi: 10.1002/path.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang M, Lu Q, Miller A, Theleman C, Wang J. NFAT1 is an upstream regulator of specific anabolic and catabolic genes in mouse articular cartilage. J Bone Miner Res. 2014;29:S235. [Google Scholar]
- 104.Greenblatt MB, Ritter SY, Wright J, Tsang K, Hu D, Glimcher LH, et al. NFATc1 and NFATc2 repress spontaneous osteoarthritis. Proc Natl Acad Sci U S A. 2013;110:19914–9. doi: 10.1073/pnas.1320036110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.de la Pompa JL, Timmerman LA, Takimoto H, Yoshida H, Elia AJ, Samper E, et al. Role of the NF-ATc transcription factor in morphogenesis of cardiac valves and septum. Nature. 1998;392:182–6. doi: 10.1038/32419. [DOI] [PubMed] [Google Scholar]
- 106.Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 107.Caldwell KL, Lu Q, Feng Y, Barnthouse NC, Zhang M, Miller AH, Wang J. Nfat1 deficiency promotes progression of post-traumatic osteoarthritis through enhancement of chondrocyte hypertrophy and overexpression of proinflammatory cytokines in repair cartilage. Orthopaedic Research Society annual meeting transactions. 2014;60:0334. [Google Scholar]
- 108.Kramer WC, Lu Q, Wang J. Transcription factor Nfat1 deficiency: A risk factor for progression of posttraumatic osteoarthritis in mice. Orthpaedic Research Society annual meeting transactions. 2012;58:0790. [Google Scholar]
- 109.Dormer NH, Singh M, Zhao L, Mohan N, Berkland CJ, Detamore MS. Osteochondral interface regeneration of the rabbit knee with macroscopic gradients of bioactive signals. J Biomed Mater Res A. 2012;100:162–70. doi: 10.1002/jbm.a.33225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Levingstone TJ, Matsiko A, Dickson GR, O’Brien FJ, Gleeson JP. A biomimetic multi-layered collagen-based scaffold for osteochondral repair. Acta Biomater. 2014;10:1996–2004. doi: 10.1016/j.actbio.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 111.Mohan N, Dormer NH, Caldwell KL, Key VH, Berkland CJ, Detamore MS. Continuous gradients of material composition and growth factors for effective regeneration of the osteochondral interface. Tissue Eng Part A. 2011;17:2845–55. doi: 10.1089/ten.tea.2011.0135. [DOI] [PubMed] [Google Scholar]
- 112.Schulze-Tanzil G. Activation and dedifferentiation of chondrocytes: implications in cartilage injury and repair. Ann Anat. 2009;191:325–38. doi: 10.1016/j.aanat.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 113.Schulze-Tanzil G, de Souza P, Villegas Castrejon H, John T, Merker HJ, Scheid A, et al. Redifferentiation of dedifferentiated human chondrocytes in high-density cultures. Cell Tissue Res. 2002;308:371–9. doi: 10.1007/s00441-002-0562-7. [DOI] [PubMed] [Google Scholar]
- 114.Schulze-Tanzil G, Mobasheri A, de Souza P, John T, Shakibaei M. Loss of chondrogenic potential in dedifferentiated chondrocytes correlates with deficient Shc-Erk interaction and apoptosis. Osteoarthritis Cartilage. 2004;12:448–58. doi: 10.1016/j.joca.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 115.Vandenabeele F, De Bari C, Moreels M, Lambrichts I, Dell’Accio F, Lippens PL, et al. Morphological and immunocytochemical characterization of cultured fibroblast-like cells derived from adult human synovial membrane. Arch Histol Cytol. 2003;66:145–53. doi: 10.1679/aohc.66.145. [DOI] [PubMed] [Google Scholar]
- 116.Edwards JC. The nature and origins of synovium: experimental approaches to the study of synoviocyte differentiation. J Anat. 1994;184 (Pt 3):493–501. [PMC free article] [PubMed] [Google Scholar]
- 117.Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Sox9 is required for cartilage formation. Nat Genet. 1999;22:85–9. doi: 10.1038/8792. [DOI] [PubMed] [Google Scholar]
- 118.Lee JM, Im GI. SOX trio-co-transduced adipose stem cells in fibrin gel to enhance cartilage repair and delay the progression of osteoarthritis in the rat. Biomaterials. 2012;33:2016–24. doi: 10.1016/j.biomaterials.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 119.Kypriotou M, Fossard-Demoor M, Chadjichristos C, Ghayor C, de Crombrugghe B, Pujol JP, et al. SOX9 exerts a bifunctional effect on type II collagen gene (COL2A1) expression in chondrocytes depending on the differentiation state. DNA Cell Biol. 2003;22:119–29. doi: 10.1089/104454903321515922. [DOI] [PubMed] [Google Scholar]
- 120.Henry SP, Liang S, Akdemir KC, de Crombrugghe B. The postnatal role of Sox9 in cartilage. J Bone Miner Res. 2012;27:2511–25. doi: 10.1002/jbmr.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Soung do Y, Talebian L, Matheny CJ, Guzzo R, Speck ME, Lieberman JR, et al. Runx1 dose-dependently regulates endochondral ossification during skeletal development and fracture healing. J Bone Miner Res. 2012;27:1585–97. doi: 10.1002/jbmr.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Enomoto H, Enomoto-Iwamoto M, Iwamoto M, Nomura S, Himeno M, Kitamura Y, et al. Cbfa1 is a positive regulatory factor in chondrocyte maturation. J Biol Chem. 2000;275:8695–702. doi: 10.1074/jbc.275.12.8695. [DOI] [PubMed] [Google Scholar]
- 123.Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–71. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 124.Needham CJ, Shah SR, Dahlin RL, Kinard LA, Lam J, Watson BM, et al. Osteochondral tissue regeneration through polymeric delivery of DNA encoding for the SOX trio and RUNX2. Acta Biomater. 2014;10:4103–12. doi: 10.1016/j.actbio.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wigner NA, Soung do Y, Einhorn TA, Drissi H, Gerstenfeld LC. Functional role of Runx3 in the regulation of aggrecan expression during cartilage development. J Cell Physiol. 2013;228:2232–42. doi: 10.1002/jcp.24396. [DOI] [PubMed] [Google Scholar]
- 126.Levanon D, Bettoun D, Harris-Cerruti C, Woolf E, Negreanu V, Eilam R, et al. The Runx3 transcription factor regulates development and survival of TrkC dorsal root ganglia neurons. Embo j. 2002;21:3454–63. doi: 10.1093/emboj/cdf370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht KRK. Lack of beta-catenin affects mouse development at gastrulation. Development. 1995;121:3529–37. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- 128.Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8:727–38. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 129.Zhu M, Chen M, Zuscik M, Wu Q, Wang YJ, Rosier RN, et al. Inhibition of beta-catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis Rheum. 2008;58:2053–64. doi: 10.1002/art.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C, et al. Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J Bone Miner Res. 2009;24:12–21. doi: 10.1359/JBMR.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li T, Xiao J, Wu Z, Qiu G, Ding Y. Transcriptional activation of human MMP-13 gene expression by c-Maf in osteoarthritic chondrocyte. Connect Tissue Res. 2010;51:48–54. doi: 10.3109/03008200902989104. [DOI] [PubMed] [Google Scholar]
- 132.MacLean HE, Kim JI, Glimcher MJ, Wang J, Kronenberg HM, Glimcher LH. Absence of transcription factor c-maf causes abnormal terminal differentiation of hypertrophic chondrocytes during endochondral bone development. Dev Biol. 2003;262:51–63. doi: 10.1016/s0012-1606(03)00324-5. [DOI] [PubMed] [Google Scholar]