Abstract
OBJECTIVES
To determine whether a previously developed and externally validated equation utilizing common variables (demographics and weight) that are important determinants of muscle mass to estimate 24-hour urine creatinine excretion rate (eCER) is associated with muscle mass and whether spot urine creatinine (UCr) provides similar estimates of muscle mass.
DESIGN
Observational, cross-sectional cohort study
SETTING
The Rancho Bernardo Study, San Diego, California
SUBJECTS
1371 Caucasian, middle class, community-dwelling older adults
INTERVENTION
Morning spot UCr and fat free mass (FFM) by dual energy x-ray absorptiometry were measured. eCER was calculated: eCER (mg/day) = 879.89+ 12.51*weight (kg) − 6.19*age + 34.51 if black − 379.42 if female. Pearson correlation coefficients and linear regression were used to determine strengths of association of eCER and spot UCr with FFM.
RESULTS
Mean age was 70 years, and 58% were women. eCER was strongly correlated with FFM (r = 0.95, p<0.001), a correlation that was superior to that of spot UCr (r = 0.40, p<0.001).
CONCLUSION
An equation incorporating age, weight, sex, and race to estimate eCER is highly correlated with FFM in community-dwelling older persons and provides a more precise estimate than spot UCr. A simple screening tool for sarcopenia in older persons may allow interventions to maintain or improve muscle mass. Future studies should evaluate whether eCER predicts sarcopenia-related frailty and mortality in older persons.
Keywords: sarcopenia, fat free mass, urine creatinine excretion
INTRODUCTION
Sarcopenia is the progressive, generalized loss of skeletal muscle mass (1). Loss of muscle mass is an important complication of aging (2), (3), (4) and chronic disease, (5), (6), (7) and has been associated with mortality, (8), (9) functional decline, (3), (4) frailty, (10) fracture risk, (11) and decreased quality of life. Several studies have shown that resistance exercise training improves muscle strength, even with interventions as short as 12 weeks, (12) and as infrequent as one session per week (13). Furthermore, a recent randomized controlled clinical trial demonstrated that exercise interventions that preserved and improved muscle mass also improved functional capacity in older community-dwelling women (14). An accurate and affordable screening tool for sarcopenia would allow clinicians to identify older patients at risk for sarcopenia who might benefit from interventions to maintain or increase muscle mass.
Measures of muscle mass commonly used in research studies include dual-energy x-ray absorptiometry (DXA) scans, bioelectric impedance, and computed tomography; these may not be routinely available, feasible, or cost-effective for screening older adults. 24-hour urine creatinine excretion rate is an established marker of muscle mass (15). Because creatinine is produced by muscle at a continuous rate, and nearly exclusively excreted from the body through urine, the amount of creatinine excretion per day is highly correlated with the total muscle mass (15). We have previously shown that low 24-hour urine creatinine excretion rate is associated with greater mortality risk in outpatients with stable coronary artery disease, independent of adiposity and other traditional cardiovascular risk factors (16). However, collection of 24-hour urine creatinine is often unreliable due to collection errors in the outpatient setting, and is not obtained in most primary care or geriatric practices. We have previously developed and externally validated an equation utilizing common variables that are important determinants of muscle mass (age, sex, race, and body weight) to estimate 24-hour urinary creatinine excretion rate (eCER) (17). Here, we hypothesized that this equation, which can be estimated without requiring a 24-hour urine collection, might serve as a useful marker of muscle mass. It is also possible that spot urine creatinine (UCr) could provide similar useful information about muscle mass. UCr can be measured on a random urine sample, which is easily collected and inexpensive and has also been shown to correlate with lean body mass (18). If either of these methods accurately estimates muscle mass, it might provide a practical means of identifying older patients with early sarcopenia at risk for morbidity and mortality. In this study, we compare the ability of eCER (from the previously derived estimating equation) (17) and spot UCr to estimate fat free mass (FFM) in a cohort of older, community-dwelling adults.
METHODS
The Rancho Bernardo Study, a cohort of Caucasian, middle class, community-dwelling adults living in a southern California suburb, was established in 1972. Between 1992 and 1996, 82% of surviving community-dwelling participants (n=1781) attended a follow-up visit, completed a medical evaluation including a DXA scan, and provided blood and spot urine samples. After excluding participants on diuretics (n=294), given their known urine diluting effect, and those missing DXA scan data (n=102) or spot urine specimens (n=14), the remaining 1371 participants (573 men and 798 women) are the subject of this report. The University of California, San Diego Human Subjects Protection Program approved this study and all participants gave written informed consent.
Standard self-administered questionnaires were used to measure age, education, health habits (smoking, alcohol consumption, and exercise), medical history, and current medications. Height and weight were measured using a regularly calibrated stadiometer and balance-beam scale with participants wearing light clothing and no shoes. Body mass index was calculated as weight (kilograms) divided by height (meters) squared. Systolic and diastolic blood pressures were measured twice in the seated position after a 5-minute rest by a trained nurse using the Hypertension Detection and Follow-up Program protocol (19). A blood sample was obtained by venipuncture after a requested 12-hour overnight fast. Serum creatinine was measured by the Jaffe method at SmithKline Beecham Clinical Laboratories (King of Prussia, Pennsylvania). Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease equation (20). A single, clean-catch, untimed morning urine sample (usually the second void) was collected. Urine samples were shipped to the National Institutes of Health laboratory in Phoenix, Arizona and creatinine (mg/dL) was measured by the kinetic alkaline picrate method using the Ciba-Corning Express (Corning, Medfield, Massachusetts).
The eCER was calculated based on the equation developed and externally validated in the CKD Epidemiology collaboration, which provides units of estimated mg of creatinine excreted in the urine per day. This equation uses clinical variables only, and does not require a 24 hour urine sample: (17) eCER (mg/day) = 879.89 + 12.51*weight (kg) – 6.19*age + 34.51 if black − 379.42 if female.
FFM was measured by DXA on the Hologic QDR 4500 (Hologic, Waltham, MA) using a standard adult whole body scan mode which excludes the head (21). The DXA instrument was standardized by a Hologic-designed whole body phantom that included six white high-density polyethylene rectangles, and a sheet of polyvinylchloride bonded to a high-density polyethylene rectangle to mimic fat mass. DXA is currently the preferred method for measuring muscle mass (1).
Pearson correlation coefficients and mean regression lines and 95% limits of agreement plotting eCER vs. FFM and spot UCr vs. FFM were used to determine the strength of the association of each measure with FFM. Analyses were repeated after stratification by sex to assess for possible sex differences in the associations. Analyses were conducted using Stata, Version 11.0 (Stata corporation, College Station, Texas), SPSS Version 18.0 (SPSS Inc., Chicago, Illinois) and SAS Version 9.2 (Cary, North Carolina).
RESULTS
Mean (SD) age was 70 (11) years, 58% were women, and mean (SD) eGFR was 69 (16) ml/min/1.73m2. Baseline characteristics for all participants, and stratified by sex, are shown in Table 1. Overall mean (SD) values for FFM, eCER, and spot UCr were 47 (11) kg, 1107 (339) mg/day, and 94 (55) mg/dL, respectively. Men were significantly heavier than women, with greater BMI, waist circumference and FFM and had higher serum Cr, eGFR, spot UCr, and eCER than women (p<0.001 for each). Men were also more likely to report exercising three or more times per week (p=0.04) than women, with no significant differences in current smoking or heavy alcohol use (Table 1).
Table 1.
Baseline Characteristics of Participants of the Rancho Bernardo Study, 1992–1996
| All (n=1371) |
Men (n=573) |
Women (n=798) |
p value | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age (years) | 70.4 ± 11.3 | 70.5 ± 11.1 | 70.2 ± 11.5 | 0.63 |
| Weight (kg) | 70.6 ± 14.2 | 80.2 ± 12.0 | 63.7 ± 11.3 | <0.001 |
| Body mass index (kg/m2) | 25.3 ± 3.8 | 26.2 ± 3.4 | 24.6 ± 3.9 | <0.001 |
| Waist (cm) | 85.0 ± 12.6 | 94.4 ± 9.2 | 78.3 ± 10.1 | <0.001 |
| Fat free mass by DXA (kg) | 46.7 ± 10.9 | 57.3 ± 7.5 | 39.0 ± 4.7 | <0.001 |
| Serum creatinine (mg/dL) | 1.01 ± 0.22 | 1.14 ± 0.22 | 0.92 ± 0.17 | <0.001 |
| Estimated glomerular filtration rate (mL/min/1.73m2) | 68.8 ± 15.5 | 70.6 ± 15.8 | 67.5 ± 15.1 | <0.001 |
| Urine creatinine (mg/dL) | 94.0 ± 54.6 | 118.2 ± 57.3 | 76.7 ± 45.2 | <0.001 |
| Estimated 24-hour creatinine excretion (mg/day) | 1107 ± 339 | 1447 ± 185 | 863 ± 174 | <0.001 |
| n (%) | n (%) | n (%) | ||
| Diabetes | 175 (13%) | 86 (15%) | 89 (11%) | 0.05 |
| Current Smokers | 103 (8%) | 41 (7%) | 62 (8%) | 0.67 |
| Exercise 3 or more times per week | 993 (72%) | 431 (75%) | 562 (70%) | 0.04 |
| Heavy alcohola | 200 (15%) | 93 (16%) | 107 (13%) | 0.14 |
Heavy alcohol was defined as > 181.0 g/week for men and >120.5 g/week for women.
Table 2 shows Pearson correlation coefficients for FFM with eCER, spot UCr and pertinent covariates for the population as a whole and stratified by sex. FFM was inversely correlated with age and positively correlated with weight. In unadjusted analyses, eCER was strongly correlated with FFM (r = 0.95, p<0.001); a correlation that was much stronger than that of spot UCr with FFM (r = 0.40, p<0.001). The eCER and FFM correlation was stronger than that of any other available anthropometric measure, including weight, body mass index, or waist girth. Furthermore, the association of eCER with FFM differed by sex (r=0.91 in men and 0.77 in women, p interaction < 0.001).
Table 2.
Pearson Correlation Coefficients of Fat Free Mass and Other Baseline Characteristics
| Fat Free Mass by DXA | |||
|---|---|---|---|
| All (n=1371) | Men (n=573) | Women (n=798) | |
| Estimated 24-hour creatinine excretion | 0.95 (<0.001) | 0.91 (<0.001) | 0.77 (<0.001) |
| Urine creatinine | 0.40 (<0.001) | 0.20 (<0.001) | 0.11 (<0.001) |
| Age | −0.21 (<0.001) | −0.47 (<0.001) | −0.34 (<0.001) |
| Weight | 0.86 (<0.001) | 0.90 (<0.001) | 0.78 (<0.001) |
| Body Mass Index | 0.48 (<0.001) | 0.68 (<0.001) | 0.54 (<0.001) |
| Waist | 0.77 (<0.001) | 0.61 (<0.001) | 0.58 (<0.001) |
| Serum creatinine | 0.41 (<0.001) | −0.07 (0.10) | 0.09 (0.01) |
| Estimated glomerular filtration rate | 0.10 (<0.001) | 0.11 (0.01) | − 0.05 (0.14) |
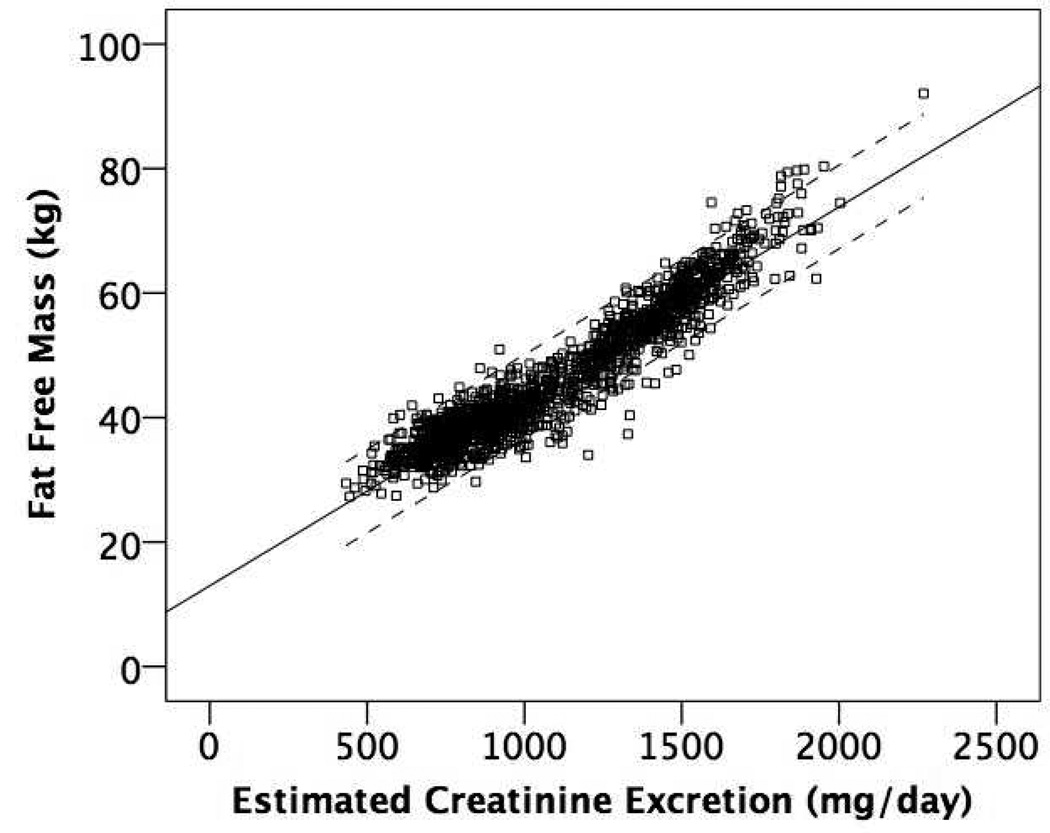
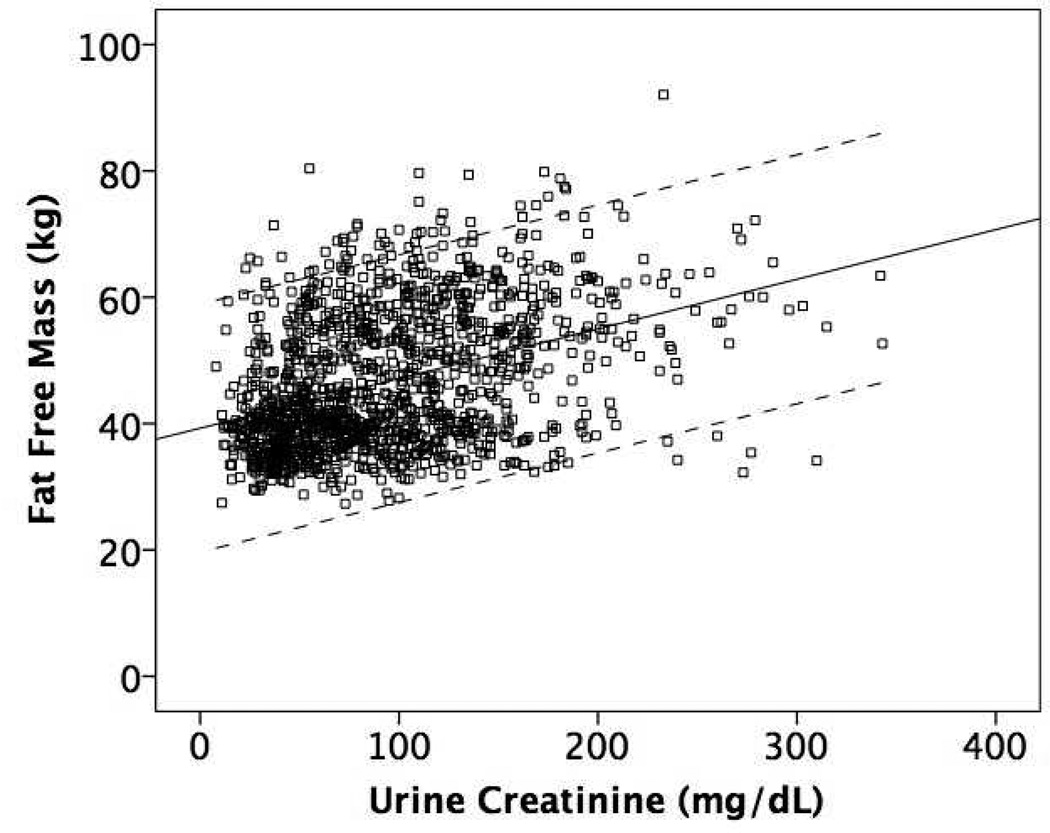
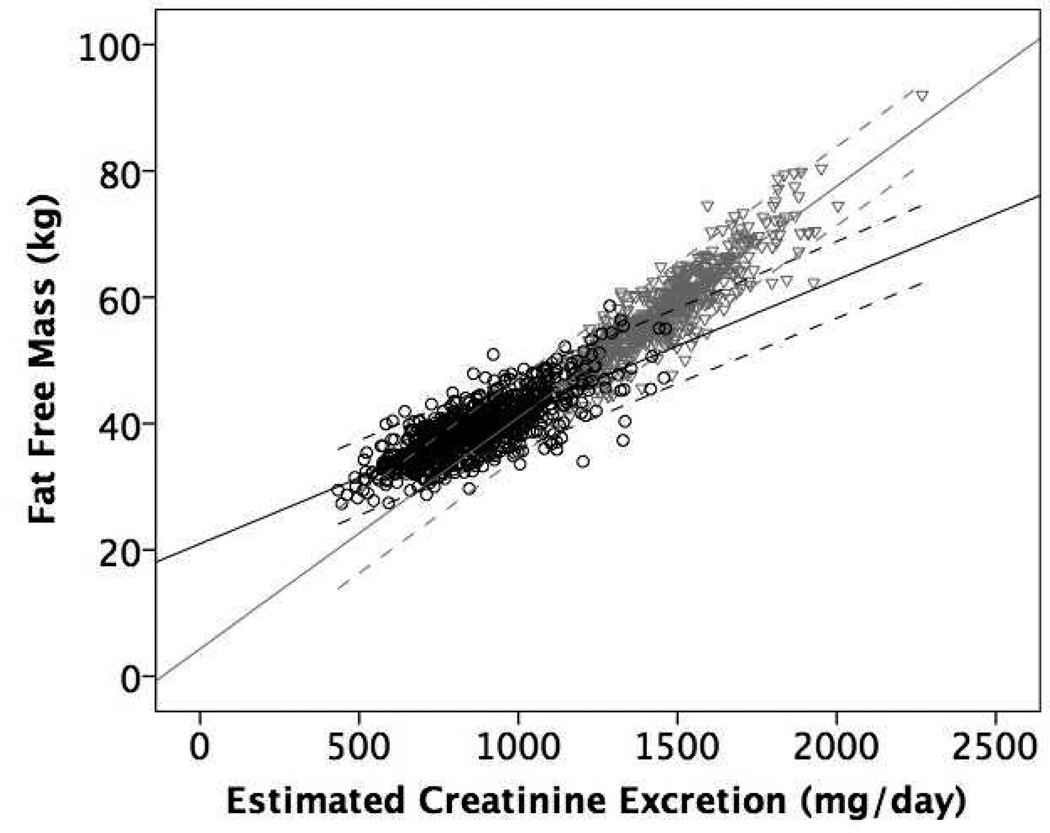
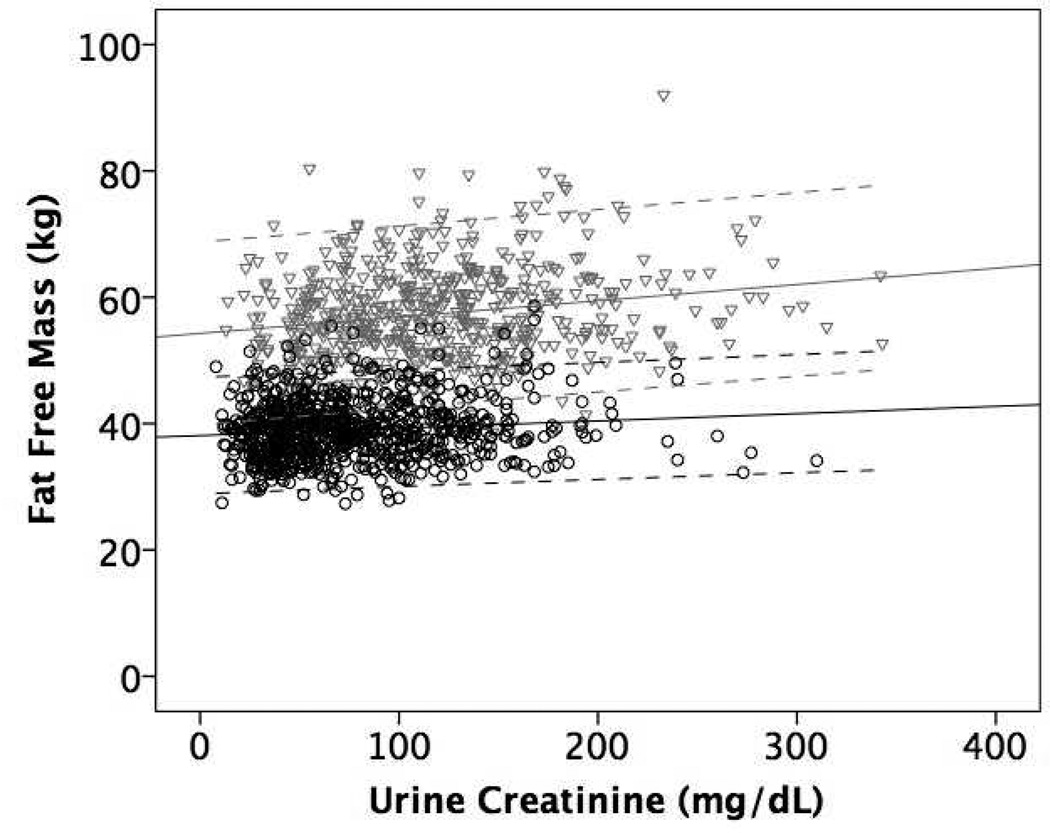
Figure 1 shows the mean regression lines and 95% limits of agreement for the association of eCER (panel A) and spot UCr (panel B) with FFM in all participants. The slope of the mean regression line was steeper and the 95% limits of agreement were narrower for eCER compared to spot UCr, demonstrating that eCER had greater accuracy and precision for FFM. Figure 2 shows mean regression lines and 95% limits of agreement for the association of eCER (panel A) and spot UCr (panel B) with FFM, stratified by sex. The slope of the mean regression line for eCER with FFM was steeper in men than in women (Figure 2A). The slopes of the mean regression lines for spot UCr with FFM were similar in men and women, but for every level of spot UCr, FFM was lower in women than in men (Figure 2B).
Figure 1.
Mean regression lines and 95% limits of agreement for A) Estimated 24-hour creatinine excretion (eCER) and B) spot urine creatinine (UCr) with fat free mass (FFM) by dual x-ray absorptiometry (DXA) All (n=1371)
A) Regression equation: FFM (kg) = 13.0 + 0.03*eCER (mg/day)
B) Regression equation: FFM (kg) = 39.3 + 0.08* spot UCr (mg/dL)
Figure 2.
Mean regression lines and 95% limits of agreement for A) Estimated 24-hour creatinine excretion (eCER) and B) spot urine creatinine (UCr) with fat free mass (FFM) by dual x-ray absorptiometry (DXA) in Men (n=573) in gray triangles and Women (n=798) in black circles
A) Regression equation: Men FFM (kg) = 4.3 + 0.04*CER (mg/day); Women FFM = 21.0 + 0.02*CER (mg/day)
B) Regression equation: Men FFM (kg) = 54.3 + 0.03* spot UCr (mg/dL); Women FFM (kg) = 37.9 + 0.015* spot UCr (mg/dL)
DISCUSSION
We found that an equation incorporating age, weight, sex, and race to estimate CER was strongly correlated with FFM and was much more precise than spot UCr in a cohort of community-dwelling older men and women. The eCER equation was more strongly associated with FFM in men than in women. Because the eCER equation uses only weight and demographic information, and because it appears to precisely estimate FFM in older adults, it may provide a useful means to estimate FFM in the general population including in primary care and geriatric outpatient settings, and could be readily applied even in resource limited healthcare settings.
Estimating FFM without the use of DXA would offer an inexpensive method to screen for sarcopenia in older adults. The ideal screening test should detect a high proportion of those with early sarcopenia, be safe to administer, reasonable in cost, widely available, and have the potential to lead to improved health outcomes (22). Sarcopenia is common, affecting 7% and 10%, respectively, of community-dwelling men and women over the age of 60, (3) and prevalence increases sharply with advancing age (2). Sarcopenia is associated with morbidity, increased risk of risk of falls, loss of independent living, and functional decline (3), (4). Moreover, sarcopenia may be reversible, as several studies have shown that resistance exercise training may improve muscle strength; (12), (13), (23) the Senior Fitness and Prevention Study demonstrated that an 18-month high intensity exercise program resulted in significant improvements in lean body mass, and functional ability (as measured by enhanced isometric maximum trunk-extensor leg press strength, timed up-and-go test, and aerobic fitness) and reductions in abdominal and total fat (14). Despite these findings, screening for sarcopenia has not been widely implemented, and may often be initiated only when severe sarcopenia and frailty become clinically apparent and when interventions may be more difficult and less effective. Barriers to screening may include lack of a consistent criteria for sarcopenia and lack of access to DXA, bioelectrical impedance, or computed tomography imaging, expense, or risk of radiation exposure. Thus, simple, non-invasive screening tools, such as the eCER equation, might help identify older persons at risk for sarcopenia, or those with early muscle loss, in whom additional screening may be more cost-effective, or among whom early intervention may ultimately improve muscle mass and quality of life.
Another potential application for estimating muscle mass is in the appropriate dosing of therapeutics in older persons. With advancing age, body composition changes and the altered proportion of muscle and fat can influence the volume of distribution and clearance of many compounds (24). Age-related changes in pharmacokinetics and pharmacodynamics, together with co-morbidity and polypharmacy, put older adults at greater risk for adverse drug reactions, and resulting morbidity, mortality, and costs (24). Simple, rapid, non-invasive, and accurate assessment of muscle mass may ultimately allow for safer drug dosing, or may identify individuals who require closer monitoring for side effects.
To our knowledge, this is the first study to evaluate an equation to estimate FFM in community-living older persons, however, prior studies have used equations to estimate lean body mass in other settings with similar accuracy (25), (26), (27). In a study of maintenance hemodialysis patients, Noori and colleagues found that regression equations based on either serum creatinine or mid-arm muscle circumference and demographic variables (sex, height, weight, and age) accurately estimated lean body mass (r2 = 0.86 for both (26). However, in dialysis patients, higher serum creatinine is predominantly a marker of muscle mass since most have no residual kidney function. In contrast, the main determinant of serum creatinine in the general population is kidney function; it is unlikely that the equation developed by Noori would be generalizable to the community-living elders evaluated in our study. Another study by Yavari and colleagues demonstrated that stepwise linear regression models incorporating age, weight, height, waist circumference, and hip circumference predicted FFM by DXA with good accuracy (r2 = 0.92) in a cohort of middle-aged overweight women (mean age 56, mean BMI 29 kg/m2)(27). A Canadian study developed an equation to estimate muscle mass using height, upper thigh, calf, and arm girth in two groups of older male cadavers with similar accuracy (r2 = 0.96).(25) As these equations were developed in select cohorts, their generalizability and performance relative to eCER in community-dwelling older adults is presently unknown.
We found that the eCER equation was more strongly associated with FFM in men than in women. The reasons for this sex difference are not clear. The eCER equation was developed in a population of participants from the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI), of whom 42% were women (17). No sex interaction was observed when the equation was developed and validated in this population comprised from three kidney disease trials. It is possible that the sex differences seen in our study were due to differences in study settings—participants of CKD-EPI collaboration had kidney disease (median eGFR 50 mL/min/1.73m2) and were younger (mean age 46) than participants in the Rancho Bernardo cohort, however weight and BMI were similar in the two cohorts (17). The sex differences should be evaluated and confirmed elsewhere, however until then, the eCER equation may be a better estimate of FFM in men than in women.
Strengths of this study include that the eCER equation was developed and previously validated in a different, diverse population of more than 3000 participants (17). Participants in the present study had measurements of FFM by DXA, urine creatinine, and multiple measures of anthropometry concurrently. DXA has been shown to be an accurate method to estimate FFM and was therefore selected as the gold standard for this study (21).
The study also has important limitations. The Rancho Bernardo Study participants are all Caucasian, lean, and middle- to upper middle class. 24-hour urine collections were not available in this cohort to validate the eCER equation as a marker for measured creatinine excretion. Although eCER was highly correlated with measured creatinine excretion rate and was externally validated in our prior studies, most individuals in these prior studies had kidney disease. Nonetheless, the high correlation observed between eCER and FFM by DXA suggest that eCER likely closely approximates daily creatinine excretion and is proportional to muscle mass in this cohort of older adults as well.
In conclusion, a simple equation incorporating body weight and demographic variables is highly correlated with FFM in community-living older persons. The equation has greater precision than spot UCr concentration alone. Future studies should evaluate whether eCER provides a simple method to predict frailty and mortality in older persons, and whether the observed sex difference is reproducible in other settings.
PRACTICAL APPLICATION
If found to correlate with health outcomes such as mortality, falls or fractures, or drug-related side effects, eCER may provide a cost-effective and readily available tool to identify older persons at greatest risk for sarcopenia-related health outcomes and those most appropriate for interventions to reverse sarcopenia such as resistive exercise interventions.
Acknowledgments
SUPPORT AND FINANCIAL DISCLOSURE DECLARATION: Dr. Ix is supported by grant R01HL096851 from the National Heart, Lung, and Blood Institute (NHLBI). This work is also supported by R21 HL089622 to Dr. Wassel, Award 0930073N from the American Heart Association to Dr. Laughlin, grant K23 DK091521 from the National Institute of Health to Dr. Rifkin, and AG07181, AG028507 from the National Institute on Aging and DK31801 from the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) to Dr. Barrett-Connor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The sponsors played no role in the design, execution, analysis and interpretation of data, or writing of the study.
REFERENCES
- 1.Burton LA, Sumukadas D. Optimal management of sarcopenia. Clin Interv Aging. 2010;5:217–228. doi: 10.2147/cia.s11473. Epub 2010/09/21. PubMed PMID: 20852669; PubMed Central PMCID: PMC2938029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castillo EM, Goodman-Gruen D, Kritz-Silverstein D, Morton DJ, Wingard DL, Barrett-Connor E. Sarcopenia in elderly men and women: the Rancho Bernardo study. Am J Prev Med. 2003;25(3):226–231. doi: 10.1016/s0749-3797(03)00197-1. Epub 2003/09/26. PubMed PMID: 14507529. [DOI] [PubMed] [Google Scholar]
- 3.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50(5):889–896. doi: 10.1046/j.1532-5415.2002.50216.x. Epub 2002/05/25. PubMed PMID: 12028177. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–763. doi: 10.1093/oxfordjournals.aje.a009520. Epub 1998/04/29. PubMed PMID: 9554417. [DOI] [PubMed] [Google Scholar]
- 5.Oreopoulos A, Ezekowitz JA, McAlister FA, Kalantar-Zadeh K, Fonarow GC, Norris CM, et al. Association between direct measures of body composition and prognostic factors in chronic heart failure. Mayo Clin Proc. 2010;85(7):609–617. doi: 10.4065/mcp.2010.0103. Epub 2010/07/02. PubMed PMID: 20592169; PubMed Central PMCID: PMC2894716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price SR, Gooch JL, Donaldson SK, Roberts-Wilson TK. Muscle atrophy in chronic kidney disease results from abnormalities in insulin signaling. J Ren Nutr. 2010;20(5 Suppl):S24–S28. doi: 10.1053/j.jrn.2010.05.007. Epub 2010/09/10. PubMed PMID: 20797566; PubMed Central PMCID: PMC2937009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L, Zhou M, Smith M, Yang G, Peto R, Wang J, et al. Body mass index and chronic obstructive pulmonary disease-related mortality: a nationally representative prospective study of 220,000 men in China. Int J Epidemiol. 2010;39(4):1027–1036. doi: 10.1093/ije/dyq051. Epub 2010/04/20. PubMed PMID: 20400495. [DOI] [PubMed] [Google Scholar]
- 8.Kimyagarov S, Klid R, Levenkrohn S, Fleissig Y, Kopel B, Arad M, et al. Body mass index (BMI), body composition and mortality of nursing home elderly residents. Arch Gerontol Geriatr. 2010;51(2):227–230. doi: 10.1016/j.archger.2009.10.013. Epub 2009/11/27. PubMed PMID: 19939476. [DOI] [PubMed] [Google Scholar]
- 9.Landi F, Liperoti R, Fusco D, Mastropaolo S, Quattrociocchi D, Proia A, et al. Sarcopenia and mortality among older nursing home residents. J Am Med Dir Assoc. 2012;13(2):121–126. doi: 10.1016/j.jamda.2011.07.004. Epub 2011/08/23. PubMed PMID: 21856243. [DOI] [PubMed] [Google Scholar]
- 10.Gielen E, Verschueren S, O'Neill TW, Pye SR, O'Connell MD, Lee DM, et al. Musculoskeletal frailty: a geriatric syndrome at the core of fracture occurrence in older age. Calcif Tissue Int. 2012;91(3):161–177. doi: 10.1007/s00223-012-9622-5. Epub 2012/07/17. PubMed PMID: 22797855. [DOI] [PubMed] [Google Scholar]
- 11.Lang T, Cauley JA, Tylavsky F, Bauer D, Cummings S, Harris TB. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res. 2010;25(3):513–519. doi: 10.1359/jbmr.090807. Epub 2010/04/28. PubMed PMID: 20422623; PubMed Central PMCID: PMC3153392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown AB, McCartney N, Sale DG. Positive adaptations to weight-lifting training in the elderly. J Appl Physiol. 1990;69(5):1725–1733. doi: 10.1152/jappl.1990.69.5.1725. Epub 1990/11/01. PubMed PMID: 2272965. [DOI] [PubMed] [Google Scholar]
- 13.Taaffe DR, Duret C, Wheeler S, Marcus R. Once-weekly resistance exercise improves muscle strength and neuromuscular performance in older adults. J Am Geriatr Soc. 1999;47(10):1208–1214. doi: 10.1111/j.1532-5415.1999.tb05201.x. Epub 1999/10/16. PubMed PMID: 10522954. [DOI] [PubMed] [Google Scholar]
- 14.Kemmler W, von Stengel S, Engelke K, Haberle L, Mayhew JL, Kalender WA. Exercise, body composition, and functional ability: a randomized controlled trial. Am J Prev Med. 2010;38(3):279–287. doi: 10.1016/j.amepre.2009.10.042. Epub 2010/02/23. PubMed PMID: 20171529. [DOI] [PubMed] [Google Scholar]
- 15.Heymsfield SB, Arteaga C, McManus C, Smith J, Moffitt S. Measurement of muscle mass in humans: validity of the 24-hour urinary creatinine method. Am J Clin Nutr. 1983;37(3):478–494. doi: 10.1093/ajcn/37.3.478. Epub 1983/03/01. PubMed PMID: 6829490. [DOI] [PubMed] [Google Scholar]
- 16.Ix JH, de Boer IH, Wassel CL, Criqui MH, Shlipak MG, Whooley MA. Urinary creatinine excretion rate and mortality in persons with coronary artery disease: the Heart and Soul Study. Circulation. 2010;121(11):1295–1303. doi: 10.1161/CIRCULATIONAHA.109.924266. Epub 2010/03/10. PubMed PMID: 20212276; PubMed Central PMCID: PMC2844485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ix JH, Wassel CL, Stevens LA, Beck GJ, Froissart M, Navis G, et al. Equations to estimate creatinine excretion rate: the CKD epidemiology collaboration. Clin J Am Soc Nephrol. 2011;6(1):184–191. doi: 10.2215/CJN.05030610. Epub 2010/10/23. PubMed PMID: 20966119; PubMed Central PMCID: PMC3022241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baxmann AC, Ahmed MS, Marques NC, Menon VB, Pereira AB, Kirsztajn GM, et al. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol. 2008;3(2):348–354. doi: 10.2215/CJN.02870707. Epub 2008/02/01. PubMed PMID: 18235143; PubMed Central PMCID: PMC2390952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The hypertension detection and follow-up program: Hypertension detection and follow-up program cooperative group. Prev Med. 1976;5(2):207–215. doi: 10.1016/0091-7435(76)90039-6. PubMed PMID: 935073. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Annals of internal medicine. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. Epub 1999/03/13. PubMed PMID: 10075613. [DOI] [PubMed] [Google Scholar]
- 21.Svendsen OL, Haarbo J, Hassager C, Christiansen C. Accuracy of measurements of body composition by dual-energy x-ray absorptiometry in vivo. Am J Clin Nutr. 1993;57(5):605–608. doi: 10.1093/ajcn/57.5.605. Epub 1993/05/01. PubMed PMID: 8480673. [DOI] [PubMed] [Google Scholar]
- 22.Rogan WJ, Gladen B. Estimating prevalence from the results of a screening test. Am J Epidemiol. 1978;107(1):71–76. doi: 10.1093/oxfordjournals.aje.a112510. Epub 1978/01/01. PubMed PMID: 623091. [DOI] [PubMed] [Google Scholar]
- 23.Frontera WR, Meredith CN, O'Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol. 1988;64(3):1038–1044. doi: 10.1152/jappl.1988.64.3.1038. Epub 1988/03/01. PubMed PMID: 3366726. [DOI] [PubMed] [Google Scholar]
- 24.Corsonello A, Pedone C, Corica F, Mussi C, Carbonin P, Antonelli Incalzi R. Concealed renal insufficiency and adverse drug reactions in elderly hospitalized patients. Arch Intern Med. 2005;165(7):790–795. doi: 10.1001/archinte.165.7.790. Epub 2005/04/13. PubMed PMID: 15824299. [DOI] [PubMed] [Google Scholar]
- 25.Doupe MB, Martin AD, Searle MS, Kriellaars DJ, Giesbrecht GG. A new formula for population-based estimation of whole body muscle mass in males. Can J Appl Physiol. 1997;22(6):598–608. doi: 10.1139/h97-039. Epub 1998/01/07. PubMed PMID: 9415832. [DOI] [PubMed] [Google Scholar]
- 26.Noori N, Kovesdy CP, Bross R, Lee M, Oreopoulos A, Benner D, et al. Novel equations to estimate lean body mass in maintenance hemodialysis patients. Am J Kidney Dis. 2011;57(1):130–139. doi: 10.1053/j.ajkd.2010.10.003. Epub 2010/12/28. PubMed PMID: 21184920; PubMed Central PMCID: PMC3026443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yavari R, McEntee E, McEntee M, Brines M. Anthropometric variables accurately predict dual energy x-ray absorptiometric-derived body composition and can be used to screen for diabetes. PLoS One. 2011;6(9):e24017. doi: 10.1371/journal.pone.0024017. Epub 2011/09/15. PubMed PMID: 21915276; PubMed Central PMCID: PMC3167829. [DOI] [PMC free article] [PubMed] [Google Scholar]