Abstract
Background
Based on pre-clinical and clinical activity in adult refractory tumors, and absence of significant neuro-, nephro-, or oto-toxicity, we conducted a pediatric phase 1 trial to determine the toxicities, maximum tolerated dose (MTD), and pharmacokinetics of satraplatin, an oral platinum analogue, in children and young adults with refractory solid tumors.
Procedure
Satraplatin was administered orally once daily on days 1–5 of a 28-day cycle at dose level (DL) 1 (60 mg/m2/dose), and DL2 (80 mg/m2/dose). Toxicities, responses, satraplatin pharmacokinetics, and pharmacogenomic expression of specific DNA repair genes were evaluated.
Results
Nine patients received 1–15 cycles (median=2). The MTD was exceeded at DL2 with delayed prolonged myelosuppression as dose-limiting toxicity (DLT) in 2/4 patients. At DL1, 0/5 patients had DLTs. Common non-DLTs included myelosuppression, gastrointestinal toxicities, fatigue, headache, liver enzyme elevation, and electrolyte abnormalities. No significant neuro-, nephro-, or oto-toxicity was observed. No objective responses were observed but 2 patients experienced prolonged disease stabilization (6–15 cycles). Satraplatin exposure (day 1 plasma ultrafiltrate area under the curve) was similar at DL1 and DL2. A strong correlation between estimated creatinine clearance and satraplatin pharmacokinetic parameters (clearance, area under the curve, and peak concentration) was observed.
Conclusions
The MTD of oral satraplatin in children with solid tumors was 60 mg/m2/dose daily × 5 days every 28 days, which is lower than the adult recommended dose of 80–120 mg/m2/dose. The toxicity profile was similar to adults and delayed myelosuppression was the DLT. No significant neuro-, nephro- or oto-toxicity was observed.
Keywords: Satraplatin, pediatric, phase 1 trial, solid tumors
Introduction
The platinum compounds cisplatin and carboplatin are standard agents in the treatment of several childhood cancers and oxaliplatin has been studied in single agent and combination trials in children. All these platinum compounds require intravenous administration, and long-term renal and otologic toxicity related to cisplatin are a concern, particularly in young children [1]. Satraplatin (JM216) is an orally administered platinum analogue, which, like other platinum-based drugs, exerts its cytotoxic effect via reactive bio-transformation products that form intra- and inter-strand DNA crosslinks, causing inhibition of DNA replication, cell cycle arrest, and apoptosis [2,3].
Satraplatin and its metabolite JM-118 have in vitro activity against several tumor cell lines (IC50 0.02–8.0 mcg/ml), including cisplatin-resistant cell lines. In vivo activity comparable to parenterally administered cisplatin or carboplatin was observed in murine and human xenograft models [2,4–9].
In adult clinical trials, once-daily dosing for 5 days every 21–35 days determined a maximum tolerated dose (MTD) ranging from 100–140 mg/m2/day, and doses of 80–120 mg/m2/day for 5 days were used in phase II and phase III studies [2,3,10–13]. Dose-limiting toxicities (DLTs) of satraplatin were myelosuppression (neutropenia and thrombocytopenia) and diarrhea. Nausea, vomiting, and asthenia were other common adverse effects. Nephrotoxicity, neurotoxicity and ototoxicity were infrequently reported [2,11,12]. Preliminary activity in early clinical trials was observed in patients with small cell lung cancer, and relapsed ovarian cancer; and in a phase III randomized controlled trial of patients with metastatic castration resistant prostate cancer who had progressed after prior chemotherapy, satraplatin significantly delayed time to pain progression and showed a small increase in progression free survival (PFS) compared to placebo, however, no overall survival benefit was observed [2,14–16].
Based on preclinical and preliminary clinical activity in adult refractory tumors, lack of significant cumulative neuro-, nephro-, or oto-toxicity, oral administration, and convenient schedule, we developed the first phase 1 clinical trial for pediatric patients. The objectives were to determine the MTD, toxicity profile, and pharmacokinetics of satraplatin in children with relapsed or refractory solid tumors including brain tumors. Pharmacogenomic expression of DNA repair genes that may predict treatment responses to platinum therapies were also evaluated.
Patients and Methods
Patient eligibility
Patients ≥ 3 and ≤ 21 years of age with treatment-refractory measurable or evaluable/nonmeasurable solid tumors, including brain tumors, with no other available standard curative treatment options were eligible. Other eligibility criteria included: recovery from the toxic effects of prior therapy; Karnofsky (patients >10 years) or Lansky (patients ≤ 10 years) performance score ≥ 50; interval from prior therapy ≥ 21 days for myelosuppressive chemotherapy (≥ 6 weeks for nitrosoureas, ≥ 4 weeks for temozolomide), ≥ 3 months for extensive radiation, ≥ 2 weeks for local radiation, ≥ 6 weeks for immunotherapy, ≥ 7 days for non-myelosuppressive biologic agents or growth factors, ≥ 30 days from any investigational drugs, ≥ 3 months for autologous transplant, ≥ 6 months for allogeneic transplant; adequate bone marrow function (absolute neutrophil count ≥ 1,000/mcl, transfusion independent platelet count ≥ 75,000/mcl); adequate liver function (bilirubin ≤ 1.5× upper limit of normal); and adequate renal function (creatinine clearance ≥ 60ml/min/1.73m2 or normal serum creatinine for age). Patients with brain tumors must have been on a stable or tapering dose of corticosteroids for 7 days prior to baseline scan.
Patients were excluded if they were receiving any concurrent tumor directed therapy including other investigational therapy, active pregnancy or breast-feeding, had prior treatment with satraplatin, or were unable to swallow capsules. This single institution trial was approved by the National Cancer Institute (NCI) Institutional Review Board, and all patients or their legal guardians provided written informed consent indicating their understanding of the investigational nature and risks of the study. Assent was obtained according to NCI guidelines.
Drug administration and study design
Satraplatin capsules (10 mg and 50 mg) were supplied by Agennix AG, and administered on an outpatient basis as a single daily oral dose from day 1 through day 5 of a 28 day cycle. As food may affect satraplatin pharmacokinetics, satraplatin was administered at least 1 hour before and 2 hours after a meal [17]. Additional cycles of satraplatin were provided if no DLTs were observed, toxicities from previous cycles were resolved, and there was no evidence of disease progression. All patients received an anti-emetic prior to each satraplatin dose and as clinically indicated.
Cohorts of 3–6 patients were enrolled at each dose level. The starting dose level was 60 mg/m2/dose (approximately 75% of adult recommended dose using the same dosing schedule), with escalations to 80, 110, 140 mg/m2/dose planned if 0 out of 3 or less than 2 out of 6 patients had a DLT. The MTD was determined from DLTs observed during the first treatment cycle and was defined as the dose level immediately below the dose at which 2 or more patients in a cohort of up to 6 patients experience a DLT attributable to satraplatin. Dose de-escalation to 40 mg/m2/dose (dose level −1) was also planned in case MTD was exceeded at dose level 1. Up to 6 additional patients could be enrolled at the MTD to further characterize satraplatin pharmacokinetics and toxicities in different age groups.
Toxicity assessment and disease status evaluation
Weekly history, physical, and neurologic exam, and twice weekly complete blood counts and serum chemistries were performed to monitor for satraplatin toxicities. Audiologic evaluation consisting of pure tone thresholds, speech audiometry, tympanometry, and distortion product otoacoustic emissions were performed prior to and after cycle 1, and then prior to every odd cycle. NCI Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v.4.0, http://ctep.info.nih.gov) were used for grading toxicity.
Disease status was evaluated prior to enrollment and then after every other cycle. Response Evaluation Criteria in Solid Tumors (RECIST) were used for solid tumors and world health organization (WHO) criteria for brain tumors for response evaluation in patients with measurable or with evaluable/nonmeasurable disease [18,19].
Definition of DLT
Hematologic DLT was defined as grade 4 neutropenia or thrombocytopenia on 2 consecutive blood counts drawn at least 72 hours apart, transfusion for a platelet count less than 50,000/mcl that occurred more than once in a cycle, or failure to recover neutrophil count to ≥ 1,000/mcl or platelet count to ≥ 75,000/mcl by day 42 of treatment cycle. Non-hematological DLT was defined as any grade 3 or 4 non-hematological toxicity related to satraplatin except grade 3 nausea, vomiting, or diarrhea that was controlled by symptomatic treatment within 72 hours, asymptomatic grade 3 elevation of serum transaminases that returned to ≤ grade 1 prior to next cycle, and asymptomatic grade 3 electrolyte abnormalities that were correctable to grade 2 or less within 48 hours.
Pharmacokinetic analyses
Pharmacokinetics and pharmacogenomics were assessed during the first cycle in consenting patients. For pharmacokinetic evaluation, serial plasma samples (prior to the first dose, 0.5, 1, 2, 3, 5, 8, 12, and 24 hours after first dose, and trough levels prior to day 3 and day 5) were obtained. Plasma was separated by centrifugation, and plasma ultrafiltrate (UF) was prepared using Microcon YM-10 filters. Plasma UF platinum was measured using a validated atomic absorption spectroscopy assay [20]. The lower limit of quantitation was 0.03 mcM (0.015 mcg/ml), and linear range 0.03–2.5 mcM (0.015–1.251 mcg/ml). Plasma UF satraplatin concentration-time data were analyzed using non-compartmental methods and day 1 satraplatin exposure (AUC0–24) was calculated using linear trapezoidal method.
Pharmacokinetic parameters were compared to baseline laboratory values that describe variability in organ functions that may influence the activity, metabolism, or elimination of satraplatin. As satraplatin pharmacokinetics have been shown to be influenced by renal function in adults, we compared satraplatin pharmacokinetic parameters to patients’ baseline serum creatinine, blood urea nitrogen, and estimated creatinine clearance (eCrCl) based on the Schwartz formula. Additionally, as platinum species are heavily protein bound, and because satraplatin undergoes reductive activation by heme and is metabolized by the liver, pharmacokinetic parameters were also compared to patients’ red blood cell count, hemoglobin concentration, serum total protein, serum albumin, and liver function tests (alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, and total and direct bilirubin). [21–24]
Pharmacogenomic analyses
Single nucleotide polymorphisms in DNA repair enzymes such as excision repair cross-complementing 1 (ERCC1) and x-ray cross-complementing group 1 (XRCC1) may influence response to, and toxicity from satraplatin therapy. ERCC1 rs11615 (N118N, 354T>C) and XRCC1 rs25487 (R399Q) polymorphisms were evaluated as the ERCC1 T-allele and the XRCC1 G-allele have been associated with improved outcomes in many adult cancers treated with platinum based therapies [25,26]. We additionally studied a polymorphism in ERCC2 (rs13181, K751Q) that was related to oxaliplatin-based chemotherapies in a large meta-analysis [27]. For pharmacogenomic evaluation, approximately 10 mL of blood was collected into an EDTA tube for DNA extraction. Genomic DNA was extracted from serum or white blood cell buffy coat layers of whole blood of patients and polymerase chain reaction (PCR) was performed with gene-specific primers as described previously [28].
Statistical considerations
Descriptive statistics were used to summarize demographic and toxicity data, and pharmacokinetic parameters. Regression analysis was used to correlate baseline demographic and laboratory values to pharmacokinetic parameters. Fisher’s Exact test or χ2 was used to test the relationship of hematological toxicity to age and genotype. Either the Wilcoxon rank-sum or the Kruskal-Wallis test was used to test the relationship of pharmacokinetic parameters to genotype and dose. Statistical analyses were conducted using Number Cruncher Statistical Software or GraphPad Prism.
Results
Patient characteristics
The patient characteristics are listed in Table I. All patients completed at least 1 cycle and were evaluable for toxicity. Most patients had tumors of the central nervous system. Nine patients received a total of 33 cycles (median=2, range 1–15).
Table I.
Baseline characteristics of all eligible patients
| Characteristic | Number of Patients (N=9) |
|---|---|
| Age: Median (range) | 17 (8–19) years |
| Sex: Female/ Male | 4/5 |
| Performance status (Lansky/Karnofsky): | |
| Median (range) | 90% (70–100%) |
| Prior therapy | |
| Any chemotherapy | 7 |
| Platinum containing chemotherapy | 3 |
| Any Radiation | 7 |
| Craniospinal radiation | 2 |
| Stem cell transplant | 0 |
| No. of prior chemotherapy regimens: | |
| Median (range) | 2 (0–4) |
| Tumor type | |
| Malignant glioma | 4 |
| Ependymoma | 2 |
| Medulloblastoma | 1 |
| Osteosarcoma | 1 |
| Hepatoblastoma | 1 |
Toxicity and MTD
There were no patient deaths attributed to satraplatin toxicity. MTD was exceeded at dose level 2 (80 mg/m2/dose) as 2 out of 4 patients developed dose-limiting myelosuppression during cycle 1. One patient developed grade 3 thrombocytopenia requiring platelet transfusions on days 22 and 28, and grade 4 neutropenia on day 39. The second patient developed grade 3 neutropenia without neutrophil count recovery by day 42. Dose level 1 was expanded and none of the 5 patients at dose level 1 experienced DLTs. The MTD of satraplatin was defined as 60 mg/m2/dose daily × 5 days every 28 days. The 2 patients who received craniospinal irradiation did not develop dose-limiting myelosuppression in cycle 1, and in our small sample of patients, we did not observe a clear relationship between degree of prior therapy and development of DLTs. Due to the decision by Agennix not to pursue the further development of this agent, we were unable to enroll additional patients at dose level 1 to further characterize the toxicities and pharmacokinetics of satraplatin.
The most frequent non-dose limiting toxicities in cycle 1 were myelosuppression, gastro-intestinal toxicities (anorexia, nausea, vomiting, diarrhea, abdominal pain), and laboratory abnormalities including abnormal serum electrolytes and elevated liver enzymes (Table II). The only renal toxicity attributed to satraplatin was transient grade 2 proteinuria in one patient, and no subjects had elevation in serum creatinine. No neurological deficits attributed to satraplatin were observed.
Table II.
Non dose-limiting toxicities possibly, probably, or definitely related to satraplatin (maximum toxicity grade per patient) observed in all patients
| Toxicity | N in cycle 1* | N in later cycles* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Dose level 1 | Dose level 2 | All dose levels | |||||||
| Grade | Grade | Grade | |||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |
| Hematologic | |||||||||
| Anemia | 2 | 1 | 3 | 1 | 1 | 1 | 1 | ||
| Lymphopenia | 1 | 2 | 1 | 1 | 1 | 1 | 2 | 3 | |
| Neutropenia | 1 | 2 | 1 | 1 | 2 | 3 | |||
| Thrombocytopenia | 2 | 1 | 1 | 1 | 1 | 1 | 3 | 2 | 1 |
| Cardiac | |||||||||
| Palpitations | 1 | ||||||||
| Constitutional | |||||||||
| Fatigue | 1 | 2 | 2 | 2 | |||||
| Generalized muscle weakness | 1 | ||||||||
| Myalgia | 1 | ||||||||
| Pain | 2 | 2 | |||||||
| Gastrointestinal | |||||||||
| Abdominal pain | 1 | 1 | 1 | ||||||
| Constipation | 1 | 1 | 1 | ||||||
| Diarrhea | 1 | 1 | 1 | 1 | |||||
| Nausea | 2 | 1 | 1 | 1 | 4 | ||||
| Vomiting | 1 | 1 | 1 | 2 | |||||
| Oral mucositis | 1 | 1 | |||||||
| Gum bleeding | 1 | ||||||||
| Metabolism/laboratory | |||||||||
| Anorexia | 2 | 3 | 1 | 1 | |||||
| Dehydration | 1 | ||||||||
| Weight loss | 1 | ||||||||
| Hypercalcemia | 1 | 2 | |||||||
| Hyperkalemia | 1 | ||||||||
| Hypermagnesemia | 1 | 1 | |||||||
| Hypernatremia | 1 | 1 | |||||||
| Hypoalbuminemia | 1 | ||||||||
| Hypokalemia | 1 | 1 | 2 | ||||||
| Hypomagnesemia | 1 | 1 | 2 | ||||||
| Hyponatremia | 1 | ||||||||
| Increased ALT | 2 | 1 | |||||||
| Increased AST | 1 | 1 | |||||||
| Increased alkaline phosphatase | 1 | 2 | 1 | 2 | |||||
| Increased bilirubin | 1 | ||||||||
| Neurologic/Psychiatric | |||||||||
| Dizziness | 1 | 1 | |||||||
| Headache | 2 | 1 | 2 | ||||||
| Anxiety | 1 | ||||||||
| Renal | |||||||||
| Proteinurea | 1 | 1 | |||||||
| Respiratory | |||||||||
| Dyspnea | 1 | ||||||||
| Dermatologic | |||||||||
| Rash | 1 | 1 | 1 | ||||||
| Bruising | 1 | ||||||||
| Hearing impairment | 1 | ||||||||
N=number of patients
Seven patients received more than 1 cycle of satraplatin. Toxicities in later cycles were similar to cycle 1 toxicities. New toxicities observed after cycle 1 included grade 2 hypoalbuminemia and grade 1 elevation in bilirubin in one patient. One patient with gliomatosis cerebri developed grade 1 unilateral mid-frequency hearing loss in cycle 2 which subsequently improved slightly, and was observed again in cycle 10 after which he had stable grade 1 hearing loss until he came off study. The hearing loss in this patient was possibly disease or satraplatin related. Dose reduction for myelosuppression was required in both patients who received >2 cycles. The first patient, enrolled on dose level 2, developed dose-limiting neutropenia in cycle 1, requiring 30% dose reduction. He needed a further 30% dose reduction in cycle 13 for dose limiting neutropenia in cycle 12. The second patient, who was enrolled at dose level 1, required dose reduction in cycle 3 for dose-limiting thrombocytopenia.
Response evaluation
No objective responses were observed. Two patients with evaluable/nonmeasurable only disease (patient #7 with gliomatosis cerebri, and patient #9 with spinal cord leptomeningeal involvement of medulloblastoma) had radiographic stable disease through cycle 15 and cycle 6 respectively, such that they had no clear increase in their existing lesions and did not develop new disease sites. Seven patients came off study for disease progression, and 2 with stable disease after the decision to discontinue the study by Agennix AG.
Pharmacokinetic analyses
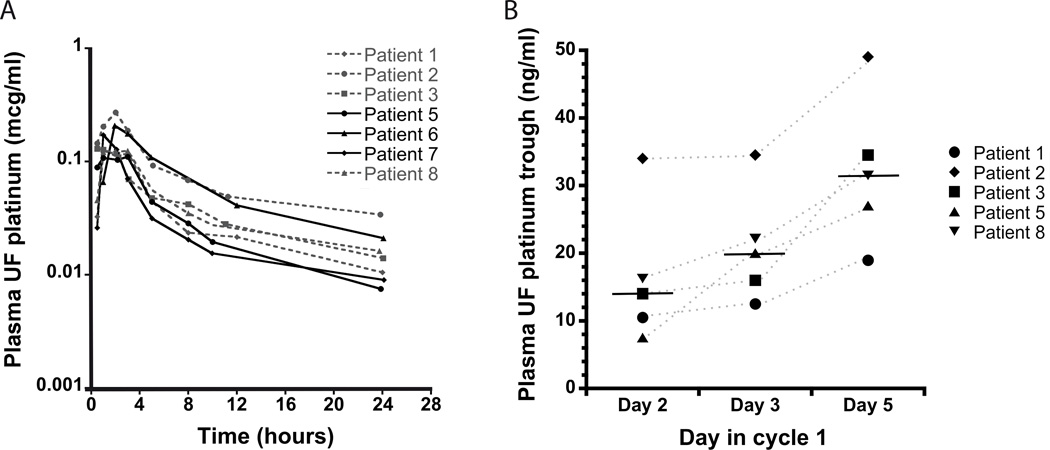
Pharmacokinetics were performed in 7/9 enrolled subjects (Figure 1A) during cycle 1, and the pharmacokinetic parameters are shown in Table III. After oral ingestion, platinum was detected in plasma within 30–60 min and peak levels were achieved at median of 2 hours (range 30 min-3 hours). Ultrafilterable platinum levels were detectable at 24 hours when the next dose was due (trough level median = 14 ng/ml, range 8–34 ng/ml). In five patients who had plasma UF platinum levels measured prior to the day 5 dose, day 5 trough levels were higher than trough levels prior to day 2 (Figure 1B), suggesting accumulation with repeated doses (median day 5 trough/day 2 trough ratio = 1.9, range 1.4–3.6). There was no significant increase in ultrafilterable platinum exposure with increasing dose from 60 to 80 mg/m2, and no correlation of dose to pharmacokinetic parameters was observed (P≥0.4).
Figure 1.
Satraplatin pharmacokinetics. Day 1 Plasma UF platinum concentration × time curves in 7 patients. Patients enrolled at DL1 are depicted with dashed lines and patients enrolled at DL2 are depicted with bold lines (A). Plasma UF platinum trough concentrations on days 2, 3 and 5 in five patients (B).
Table III.
Satraplatin day 1 pharmacokinetic parameters
| Dose level (mg/m2) |
Patient No. |
Age (years) |
BSA (m2) |
Actual dose (mg) |
Actual dose/m2 (mg/m2) |
Deviation (%)# |
Tmax (hr) |
Cmax (mcg/ml) |
AUC0–24h (mcg/ml*h) |
AUC0–inf (mcg/ml*h) |
T1/2 (h) |
Cl F (ml/min/m2) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 60 | 1 | 17 | 1.47 | 90 | 61.22 | 2.04 | 2 | 0.12 | 0.83 | 1.03 | 13.2 | 990.0 |
| 60 | 2 | 13 | 1.22 | 70 | 57.38 | −4.37 | 2 | 0.27 | 1.85 | 3.02 | 23.9 | 317.0 |
| 60 | 3 | 19 | 1.73 | 100 | 57.80 | −3.66 | 0.5 | 0.13 | 0.97 | 1.18 | 10.7 | 819.0 |
| 60 | 8 | 18 | 1.55 | 90 | 58.06 | −3.23 | 1 | 0.12 | 0.98 | 1.34 | 15.4 | 720.0 |
| Mean | 1.4 | 0.16 | 1.16 | 1.64 | 15.8 | 711.5 | ||||||
| SD | 0.8 | 0.07 | 0.47 | 0.93 | 5.7 | 285.7 | ||||||
| Median | 1.5 | 0.13 | 0.98 | 1.26 | 14.3 | 769.5 | ||||||
| 80 | 5 | 8 | 0.94 | 80 | 85.11 | 6.38 | 3 | 0.11 | 0.81 | 0.92 | 10.0 | 1,549.0 |
| 80 | 6* | 19 | 1.67 | 130 | 77.84 | −2.69 | 2 | 0.21 | 1.53 | 1.91 | 12.5 | 680.0 |
| 80 | 7* | 12 | 1.23 | 100 | 81.30 | 1.63 | 1 | 0.17 | 0.71 | 0.93 | 17.1 | 1,461.0 |
| Mean | 2.0 | 0.16 | 1.02 | 1.25 | 13.2 | 1,230.0 | ||||||
| SD | 1.0 | 0.05 | 0.45 | 0.57 | 3.6 | 478.3 | ||||||
| Median | 2.0 | 0.17 | 0.81 | 0.93 | 12.5 | 1,461.0 | ||||||
Abbreviations: BSA=body surface area, Tmax=time to peak concentration, Cmax=peak concentration, AUC0–24h= area under the concentration × times curve 0–24 hours, AUC0–inf = area under the concentration × times curve extrapolated to infinity, T1/2= terminal half life, Cl F= apparent clearance, SD=standard deviation.
Deviation of actual dose/m2 from dose level.
Patients with DLTs
Demographics and baseline laboratory values vs. pharmacokinetics and toxicities
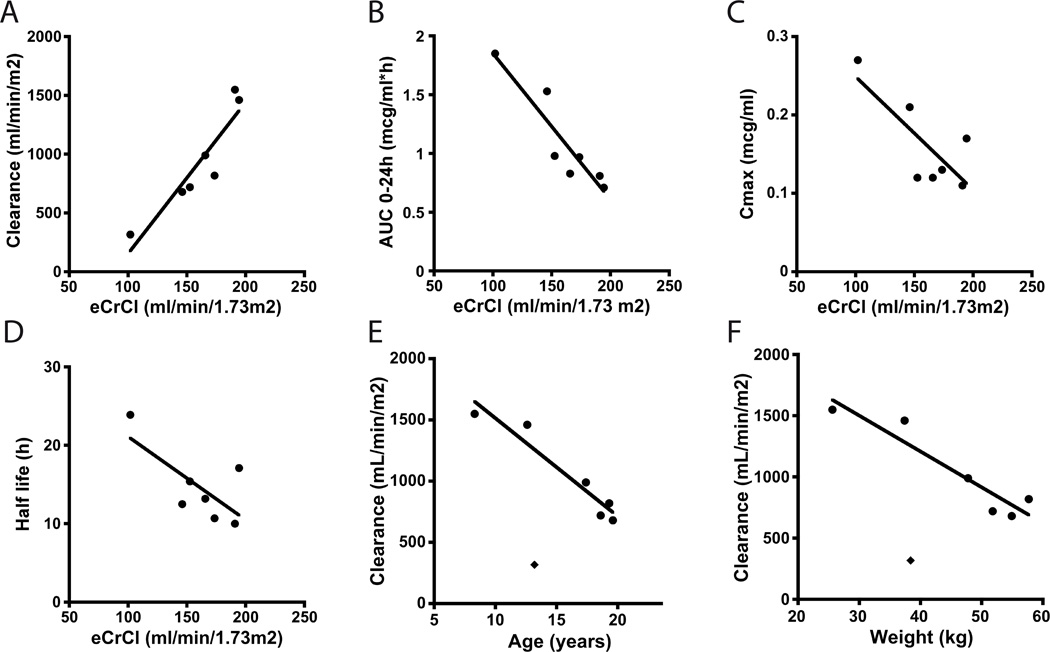
In children, eCrCl correlated well with several pharmacokinetic parameters. A strong correlation was observed between eCrCl and apparent satraplatin clearance (R2 =0.85, P=0.0031), AUC (R2 =0.85, P=0.0031), and Cmax (R2 =0.59, P=0.045) regardless of dose (Figure 2A–C). eCrCl also appeared to negatively correlate with half life, although this was not statistically significant. (R2 =0.49, P=0.078; Figure 2D).
Figure 2.
Relationship between eCrCl and satraplatin pharmacokinetic parameters: apparent clearance (A), exposure (AUC0–24h) (B), peak concentration (C), half life (D). Relationship between patient age (E) and patient weight (F) and satraplatin apparent clearance. Patient with the lowest eCrCl of 102 ml/min/1.73 m2 and lowest satraplatin apparent clearance of 317 ml/min/m2 (depicted here with a ◆) appeared to be an outlier and was excluded from analysis.
When the patient with the lowest eCrCl of 102ml/min/1.73m2 who appeared to be an outlier (as depicted by ◆ in Figure 2E and 2F) was excluded, patient age and body weight were also strongly correlated with apparent satraplatin clearance (R2 =0.92, P=0.0024 and R2 =0.89, P=0.0049 respectively).
All children younger than the median age of 17 years (all were ≤38.4 Kg) experienced clinically significant neutropenia (grade 3 or 4) or thrombocytopenia (grade 2 or 3 or requiring platelet transfusion) whereas only one patient ≥17 years experienced such toxicity (Table IV). This 19-year-old patient (weight 54.9 Kg) who developed clinically significant neutropenia and thrombocytopenia also had the lowest eCrCl and the highest satraplatin AUC of those aged ≥17 years.
Table IV.
Relationships between age, genotype and cycle 1 hematological toxicity
| Hematological toxicity (Grade) |
Age <17 (N*) |
Age ≥17 (N) |
Odds ratio (95% Confidence Interval) |
P |
| Neutropenia | ||||
| 0–2 | 0 | 4 | 1.0 (Reference) | 0.048 |
| 3–4 | 4 | 1 | 27.0 (0.9–857.3) | |
| Thrombocytopenia | ||||
| 0 | 0 | 4 | 1.0 (Reference) | 0.048 |
| 2–3 | 4 | 1 | 27.0 (0.9–857.3) | |
| Hematological toxicity (Grade) |
354TT (N) |
354TC/CC (N) |
Odds ratio (95% Confidence Interval) |
P |
| Neutropenia | ||||
| 0–2 | 3 | 1 | 1.0 (Reference) | 0.21 |
| 3–4 | 1 | 4 | 12.0 (0.5–280.3) | |
| Thrombocytopenia | ||||
| 0 | 3 | 1 | 1.0 (Reference) | 0.21 |
| 2–3 | 1 | 4 | 12.0 (0.5–280.3) | |
N=number of patients
Genotype vs. pharmacokinetics and hematological toxicities
No statistically significant relationships between polymorphisms in DNA repair genes and pharmacokinetic parameters were observed, although the ERCC1 N118N polymorphism appeared to be related to the Cmax of satraplatin such that the three lowest values were observed in those carrying only T alleles, the next highest in two carrying both C and T, and the highest value was observed in a patient carrying CC (P=0.057, Supplemental Figure 1).
No significant relationships were observed between genotype and hematologic toxicities used to determine the MTD (thrombocytopenia and neutropenia) (Table IV). However, there was a significant relationship between SNPN118N polymorphism in ERCC1 and leukopenia such that all 4 patients carrying only the T alleles did not have leukopenia whereas 4/5 patients carrying either 354TC or 354CC genotypes experienced grade 3 or 4 leukopenia (P=0.048). Severe hematological toxicities were also observed more frequently in carriers of the ERCC1 C-allele in a phase II trial of satraplatin in prostate cancer; this finding was contrary to the expectation that those with T-alleles would have a reduced DNA repair phenotype compared to those carrying C-alleles [28].
Discussion
The goal of this study was to evaluate a novel oral platinum agent, satraplatin, in pediatric refractory cancers. Satraplatin was overall well tolerated. The pediatric MTD was 60 mg/m2/day for 5 days every 28 days, which is lower than the recommended adult dose of 80–120 mg/m2/day. Differences in pharmacokinetics between children and adults may explain the lower MTD observed in these pediatric patients.
The toxicity profile in our pediatric population was similar to that in adults, and myelosuppression was the DLT. Myelosuppression was delayed with a median neutrophil nadir in cycle 1 occurring on day 27 (range 5–42,) and platelet count nadir on day 27 (range 11–30). This was similar to adults where median neutrophil nadir occurred on day 21 (range 2–63) and median platelet count nadir occurred on day 21 (range 2–64). Dose reduction was required for myelosuppression in both patients who received > 2 cycles which has also been observed in adults [2]. This suggests that with chronic dosing, more patients may require dose reductions. Common non-hematologic toxicities included gastro-intestinal toxicities and electrolyte abnormalities. Nausea was usually mild and managed with ondansetron alone and electrolyte abnormalities were also mostly grade 1. Overall, the observed toxicities were similar to toxicities observed with carboplatin [1]. Unlike cisplatin, satraplatin caused only mild nausea and we did not observe significant neurological or renal toxicities. Additionally, comprehensive audiologic monitoring did not reveal significant ototoxicity. Only one patient with gliomatosis cerebri had grade 1 unilateral hearing loss, which could have been due to underlying disease or therapy with satraplatin. This hearing loss was worse in the mid-frequencies and returned to normal in the high frequencies, unlike the typical high-frequency loss generally observed with cisplatin and other ototoxic drugs [29]. Evaluation of long-term cumulative toxicities was limited by the small sample size, as only 2 patients were on the study for >2 cycles.
Platinum concentration in plasma UF was used to calculate satraplatin exposure as it is a measure of the non-protein-bound free platinum concentration and has been used as a measure of satraplatin exposure in pre-clinical studies and adult clinical trials [2,11,12,20,30]. For an oral drug, satraplatin pharmacokinetics showed relatively limited variability (coefficient of variation 0.37 and 0.39 for Cmax and AUC0–24 respectively). In our patient population, dose proportional increase in exposure was not seen possibly because only two dose levels were evaluated. Peak levels were achieved at a median of 2 hours and ultrafilterable platinum levels were detectable at 24 hours (trough level prior to day 2 dose), similar to what has been observed in adult phase I trials [12]. However, satraplatin exposure of 1.10 ± 0.43 mcg/ml*h and peak concentration of 0.16 ± 0.06 mcg/ml (mean ± standard deviation, n=7) appeared higher in our patients compared to patients enrolled on adult phase I trials who were predominantly older adults (median age 50–62 years). Pharmacokinetic analyses using atomic absorption spectroscopy performed in two adult phase I trials showed mean plasma UF AUC was 0.28 ± 0.1 and 0.249 ± 0.044 mcg/ml*h and mean Cmax was 0.047 ± 0.005 and 0.052 ± 0.016 mcg/ml at doses of 60–80 mg/m2/dose once daily for 5 days [11,12]. Similar results were also observed in adult studies that used inductively coupled plasma mass spectrometry to measure ultrafilterable platinum (AUC0–24 0.419–0.466 mcg/ml*h and Cmax 0.054–0.057 mcg/ml) [17,23]. Although two of the adult studies used a similar assay for measuring plasma UF platinum, the adult and pediatric pharmacokinetic evaluations were not performed in the same laboratory, and the possibility that the observed differences in pharmacokinetic parameters were due to differences in the analytical assay cannot be completely excluded. However, if the exposure in pediatric patients was truly higher than in adults, it may explain the lower MTD observed in our study.
A limitation of this study is the small sample size that restricts detailed pharmacokinetic and pharmacogenomics analyses. Despite this, a strong correlation between eCrCl and satraplatin pharmacokinetic parameters was observed. This was similar to pharmacokinetics of other platinum agents in children such as carboplatin and oxaliplatin where clearance of the drug is highly correlated to creatinine clearance [1,31]. Renal function has also been shown to influence satraplatin pharmacokinetics in adults [23].
We additionally observed that younger patients, despite having more rapid clearance of satraplatin, appeared to experience greater hematologic toxicity compared to older patients, suggesting that age may influence development of clinically significant myelosuppression. No clear relationship between exposure or genotype and clinically relevant hematologic toxicities (neutropenia, thrombocytopenia) was observed. This may be a result of the small number of patients enrolled in this study.
Satraplatin was rationally designed to be an oral platinum analogue and have a better toxicity profile compared to cisplatin, with evidence of activity in cisplatin resistant cell lines. In our study, satraplatin was convenient to administer on an outpatient basis, required no intravenous hydration support, and had manageable toxicities. The only significant toxicity was delayed myelosuppression, which may limit chronic dosing. Although the current study was stopped due to the decision by Agennix AG to not pursue further development of this agent, other platinum compounds with such favorable properties may be selected for clinical evaluation in the future. Given that cisplatin and carboplatin are established components of therapies in many pediatric cancers, a novel platinum agent with a favorable toxicity profile and similar therapeutic range would be of benefit. Drugs that are orally bioavailable have the added advantage of being able to be administered on an outpatient basis, which is more convenient for patients and families and has the potential to reduce treatment costs.
Supplementary Material
Relationship between genotype ERCC1 N118N (354T>C) and peak concentration (Cmax)
Acknowledgements
We thank the patients and their families who volunteered to participate in the trial for their commitment and adherence to required study evaluations for the duration of the study, Agennix AG for providing satraplatin for this study, and Janet Therrien and Keith O’Neill for data management support.
Funding
This research was supported (in part) by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, and the National Institute on Deafness and Other Communication Disorders
Footnotes
Conflict of Interest: None
References
- 1.Pizzo PA, Poplack DG, editors. Principles and practice of pediatric oncology. Philadelphia: Lippincott Williams and Wilkins; 2006. p. 1780. [Google Scholar]
- 2.Agennix Satraplatin Investigator's Brochure version 6. 2012 [Google Scholar]
- 3.Choy H. Satraplatin: an orally available platinum analog for the treatment of cancer. Expert Rev Anticancer Ther. 2006;6(7):973–982. doi: 10.1586/14737140.6.7.973. [DOI] [PubMed] [Google Scholar]
- 4.Kelland LR, Abel G, McKeage MJ, et al. Preclinical antitumor evaluation of bis-acetato-ammine-dichloro-cyclohexylamine platinum(IV): an orally active platinum drug. Cancer Res. 1993;53(11):2581–2586. [PubMed] [Google Scholar]
- 5.Martelli L, Di Mario F, Ragazzi E, et al. Different accumulation of cisplatin, oxaliplatin and JM216 in sensitive and cisplatin-resistant human cervical tumour cells. Biochem Pharmacol. 2006;72(6):693–700. doi: 10.1016/j.bcp.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Mellish KJ, Kelland LR, Harrap KR. In vitro platinum drug chemosensitivity of human cervical squamous cell carcinoma cell lines with intrinsic and acquired resistance to cisplatin. Br J Cancer. 1993;68(2):240–250. doi: 10.1038/bjc.1993.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orr RM, O'Neill CF, Nicolson MC, et al. Evaluation of novel ammine/amine platinum (IV) dicarboxylates in L1210 murine leukaemia cells sensitive and resistant to cisplatin, tetraplatin or carboplatin. Br J Cancer. 1994;70(3):415–420. doi: 10.1038/bjc.1994.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Twentyman PR, Wright KA, Mistry P, et al. Sensitivity to novel platinum compounds of panels of human lung cancer cell lines with acquired and inherent resistance to cisplatin. Cancer Res. 1992;52(20):5674–5680. [PubMed] [Google Scholar]
- 9.Wosikowski K, Lamphere L, Unteregger G, et al. Preclinical antitumor activity of the oral platinum analog satraplatin. Cancer Chemother Pharmacol. 2007;60(4):589–600. doi: 10.1007/s00280-007-0502-z. [DOI] [PubMed] [Google Scholar]
- 10.Beale P, Raynaud F, Hanwell J, et al. Phase I study of oral JM216 given twice daily. Cancer Chemother Pharmacol. 1998;42(2):142–148. doi: 10.1007/s002800050797. [DOI] [PubMed] [Google Scholar]
- 11.Kurata T, Tamura T, Sasaki Y, et al. Pharmacokinetic and pharmacodynamic analysis of bis-acetato-ammine-dichloro-cyclohexylamine-platinum(IV) (JM216) administered once a day for five consecutive days: a phase I study. Jpn J Clin Oncol. 2000;30(9):377–384. doi: 10.1093/jjco/hyd102. [DOI] [PubMed] [Google Scholar]
- 12.McKeage MJ, Raynaud F, Ward J, et al. Phase I and pharmacokinetic study of an oral platinum complex given daily for 5 days in patients with cancer. J Clin Oncol. 1997;15(7):2691–2700. doi: 10.1200/JCO.1997.15.7.2691. [DOI] [PubMed] [Google Scholar]
- 13.Sessa C, Minoia C, Ronchi A, et al. Phase I clinical and pharmacokinetic study of the oral platinum analogue JM216 given daily for 14 days. Ann Oncol. 1998;9(12):1315–1322. doi: 10.1023/a:1008441416790. [DOI] [PubMed] [Google Scholar]
- 14.Fokkema E, Groen HJ, Bauer J, et al. Phase II study of oral platinum drug JM216 as first-line treatment in patients with small-cell lung cancer. J Clin Oncol. 1999;17(12):3822–3827. doi: 10.1200/JCO.1999.17.12.3822. [DOI] [PubMed] [Google Scholar]
- 15.Latif T, Wood L, Connell C, et al. Phase II study of oral bis (aceto) ammine dichloro (cyclohexamine) platinum (IV) (JM-216, BMS-182751) given daily × 5 in hormone refractory prostate cancer (HRPC) Invest New Drugs. 2005;23(1):79–84. doi: 10.1023/B:DRUG.0000047109.76766.84. [DOI] [PubMed] [Google Scholar]
- 16.Sternberg CN, Petrylak DP, Sartor O, et al. Multinational, double-blind, phase III study of prednisone and either satraplatin or placebo in patients with castrate-refractory prostate cancer progressing after prior chemotherapy: the SPARC trial. J Clin Oncol. 2009;27(32):5431–5438. doi: 10.1200/JCO.2008.20.1228. [DOI] [PubMed] [Google Scholar]
- 17.Ricart AD, Sarantopoulos J, Calvo E, et al. Satraplatin, an oral platinum, administered on a five-day every-five-week schedule: a pharmacokinetic and food effect study. Clin Cancer Res. 2009;15(11):3866–3871. doi: 10.1158/1078-0432.CCR-08-2373. [DOI] [PubMed] [Google Scholar]
- 18.Gehan EA, Tefft MC. Will there be resistance to the RECIST (Response Evaluation Criteria in Solid Tumors)? J Natl Cancer Inst. 2000;92(3):179–181. doi: 10.1093/jnci/92.3.179. [DOI] [PubMed] [Google Scholar]
- 19.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 20.Marcus L, Murphy R, Fox E, et al. The plasma and cerebrospinal fluid pharmacokinetics of the platinum analog satraplatin after intravenous administration in non-human primates. Cancer Chemother Pharmacol. 2012;69(1):247–252. doi: 10.1007/s00280-011-1659-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ando Y, Shimizu T, Nakamura K, et al. Potent and non-specific inhibition of cytochrome P450 by JM216, a new oral platinum agent. Br J Cancer. 1998;78(9):1170–1174. doi: 10.1038/bjc.1998.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carr JL, Tingle MD, McKeage MJ. Satraplatin activation by haemoglobin, cytochrome C and liver microsomes in vitro. Cancer Chemother Pharmacol. 2006;57(4):483–490. doi: 10.1007/s00280-005-0069-5. [DOI] [PubMed] [Google Scholar]
- 23.Galsky MD, Camacho LH, Chiorean EG, et al. Phase I study of the effects of renal impairment on the pharmacokinetics and safety of satraplatin in patients with refractory solid tumors. Ann Oncol. 2012;23(4):1037–1044. doi: 10.1093/annonc/mdr358. [DOI] [PubMed] [Google Scholar]
- 24.Raynaud FI, Boxall FE, Goddard P, et al. Metabolism, protein binding and in vivo activity of the oral platinum drug JM216 and its biotransformation products. Anticancer Res. 1996;16(4A):1857–1862. [PubMed] [Google Scholar]
- 25.Bowden NA. Nucleotide excision repair: why is it not used to predict response to platinum-based chemotherapy? Cancer Lett. 2014;346(2):163–171. doi: 10.1016/j.canlet.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Peters GJ, Avan A, Ruiz MG, et al. Predictive role of repair enzymes in the efficacy of Cisplatin combinations in pancreatic and lung cancer. Anticancer Res. 2014;34(1):435–442. [PubMed] [Google Scholar]
- 27.Yin M, Yan J, Martinez-Balibrea E, et al. ERCC1 and ERCC2 polymorphisms predict clinical outcomes of oxaliplatin-based chemotherapies in gastric and colorectal cancer: a systemic review and meta-analysis. Clin Cancer Res. 2011;17(6):1632–1640. doi: 10.1158/1078-0432.CCR-10-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Figg WD, Chau CH, Madan RA, et al. Phase II study of satraplatin and prednisone in patients with metastatic castration-resistant prostate cancer: a pharmacogenetic assessment of outcome and toxicity. Clin Genitourin Cancer. 2013;11(3):229–237. doi: 10.1016/j.clgc.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brock PR, Knight KR, Freyer DR, et al. Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J Clin Oncol. 2012;30(19):2408–2417. doi: 10.1200/JCO.2011.39.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKeage MJ, Mistry P, Ward J, et al. A phase I and pharmacology study of an oral platinum complex, JM216: dose-dependent pharmacokinetics with single-dose administration. Cancer Chemother Pharmacol. 1995;36(6):451–458. doi: 10.1007/BF00685793. [DOI] [PubMed] [Google Scholar]
- 31.Geoerger B, Doz F, Gentet JC, et al. Phase I study of weekly oxaliplatin in relapsed or refractory pediatric solid malignancies. J Clin Oncol. 2008;26(27):4394–4400. doi: 10.1200/JCO.2008.16.7585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relationship between genotype ERCC1 N118N (354T>C) and peak concentration (Cmax)