Abstract
Inbred mouse strains provide significant opportunities to understand the genetic mechanisms controlling ethanol-directed behaviors and neurobiology. They have been specifically employed to understand cellular mechanisms contributing to ethanol consumption, acute intoxication, and sensitivities to chronic effects. However, limited ethanol consumption by some strains has restricted our understanding of clinically relevant endpoints such as dependence-related ethanol intake. Previous work with a novel tastant-substitution procedure using monosodium glutamate (MSG or umami flavor) has shown that the procedure greatly enhances ethanol consumption by mouse strains that express limited drinking phenotypes using other methods. In the current study, we employ this MSG-substitution procedure to examine how ethanol dependence, induced with passive vapor inhalation, modifies ethanol drinking in C57BL/6J and DBA/2J mice. These strains represent ‘high’ and ‘low’ drinking phenotypes, respectively. We found that the MSG substitution greatly facilitates ethanol drinking in both strains, and likewise, ethanol dependence increased ethanol consumption regardless of strain. However, DBA/2J mice exhibited greater sensitivity dependence-enhanced drinking, as represented by consumption behaviors directed at lower ethanol concentrations and relative to baseline intake levels. DBA/2J mice also exhibited significant withdrawal-associated anxiety-like behavior while C57BL/6J mice did not. These findings suggest that the MSG-substitution procedure can be employed to examine dependence-enhanced ethanol consumption across a range of drinking phenotypes, and that C57BL/6J and DBA/2J mice may represent unique neurobehavioral pathways for developing dependence-enhanced ethanol consumption.
Keywords: dependence, substitution, anxiety, preference
Introduction
Inbred mouse strains have been employed by alcohol researchers to understand the genetic basis for ethanol consumption, sensitivity, and reward. For example, C57BL/6J (B6) and DBA/2J (D2) represent extremes in both drinking phenotype (B6 > D2; see Belknap & Atkins, 2001; Rhodes et al., 2007; Yoneyama, Crabbe, Ford, Murillo, & Finn, 2008) and behavioral/physiological sensitivity to ethanol (generally D2 > B6; reviewed by Crabbe, Phillips, Buck, Cunningham, & Belknap, 1999; Crawley et al., 1997). Recombinant inbred lines (BXD lines) and more recently F(2) populations derived from crosses between B6 and D2 mice have been used to understand genetic mechanisms controlling ethanol consumption/preference, reward, and sensitivity to both acute and chronic withdrawal. While these complex behaviors are clearly controlled by many gene products, several gene candidates strongly influence many of these phenotypes. For example, synaptic trafficking/scaffolding molecules including Syntaxin 12 (Weng, Symons, & Singh, 2009) and Munc18-1 (Fehr et al., 2005) help control ethanol consumption/preference. Additionally, dopamine D2 receptors appear to regulate ethanol reward, as represented by conditioned place preference (Hitzemann et al., 2003). Notably, dependence-enhanced ethanol drinking has not been as extensively studied using recombinant inbred lines. This likely reflects the strong negative correlation between ethanol consumption and withdrawal sensitivity (Metten et al., 1998).
We have recently described a tastant-substitution procedure using monosodium glutamate that initiates considerable ethanol drinking by traditionally low-drinking/preferring strains such as DBA/2J (D2) mice (McCool & Chappell, 2012, 2014). Although sucrose/saccharine substitution procedures are well established (Samson, 1986), they have limited efficacy in initiating ethanol drinking by D2 mice despite robust ethanol-seeking behaviors expressed by this strain (Chester & Cunningham, 1999). Since ethanol taste contains both sweet and bitter components (Blizard, 2007), the drinking versus seeking dichotomy in D2 mice may reflect their diminished ability to sense both the sweet components in ethanol itself as well as the sucrose/saccharine used to mask the bitter (aversive) components. Monosodium glutamate, the principle chemical component of umami flavor, is not only rewarding (Jones, Chappell, & Weiner, 2007; Uematsu et al., 2011), but also interacts with several different potential taste receptors (Delay et al., 2000; Inoue et al., 2007; Nakashima, Eddy, Katsukawa, Delay, & Ninomiya, 2012), which would theoretically limit effects of single-gene polymorphisms. Further, monosodium glutamate also conditions taste preference via post-ingestion mechanisms (Ackroff & Sclafani, 2013; Niijima, 1991; Shibata, Kameishi, Kondoh, & Torii, 2009). These characteristics may favorably interact with ethanol during the substitution procedure for mice like the D2 strain.
Ethanol dependence consistently enhances voluntary ethanol drinking both in humans and in a number of animal models. In rodents, ethanol dependence models have included forced consumption of an ethanol-containing liquid diet and passive inhalation of ethanol vapor. As long as ethanol taste aversion is avoided in the experimental design, these dependence models enhance ethanol drinking, seeking, and preference. In rats, for example, ethanol dependence via vapor exposure enhances operant responding for ethanol across a range of chronic exposure and abstinence periods (Ciccocioppo, Lin, Martin-Fardon, & Weiss, 2003; Roberts, Heyser, Cole, Griffin, & Koob, 2000; Sidhpura, Weiss, & Martin-Fardon, 2010). Ethanol drinking in dependent rats is also characterized by a distinct sensitivity to various pharmacological manipulations (de Guglielmo, Martin-Fardon, Teshima, Ciccocioppo, & Weiss, 2014; Funk, O'Dell, Crawford, & Koob, 2006; Gilpin & Koob, 2010; Rimondini, Thorsell, & Heilig, 2005; Sidhpura et al., 2010), suggesting that dependence exerts a unique influence on the neurobiological mechanisms controlling ethanol self-administration. Ethanol dependence also increases ethanol consumption in mouse strains such as C57BL/6J (B6) (Griffin, Lopez, & Becker, 2009), and shares some of the same pharmacology as in dependent rats (Chu, Koob, Cole, Zorrilla, & Roberts, 2007). While most studies have focused on B6 mice due to robust ‘baseline’ drinking, recent work using intragastic delivery has shown that dependence can in fact increase D2 ethanol self-administration (Cunningham, Fidler, Murphy, Mulgrew, & Smitasin, 2013; Fidler et al., 2012). However, the question remains whether oral consumption would be impacted in this strain. Further, the genetic diversity of mouse models has not been brought significantly to bear on dependence-enhanced drinking because of limited oral consumption by mice such as the D2 strain. The purpose of the current study was to employ a recently described substitution procedure with monosodium glutamate to ascertain whether ethanol dependence will increase ethanol drinking in D2 mice.
Materials and methods
Animals
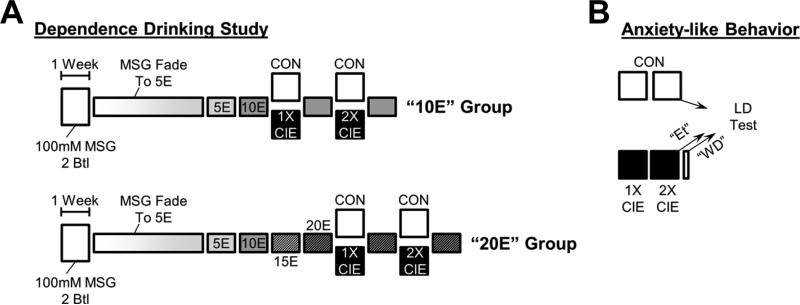
Adult male C57BL/6J (B6, n = 44) and DBA/2J (D2, n = 44) mice were obtained from The Jackson Laboratory (Bar Harbor, ME) at approximately 5 weeks of age. Mice were housed individually in a reversed 12-h light cycle (7:00 AM lights off), with water and standard mouse chow offered ad libitum. Four days after arrival at our facility, we divided mice into two experimental groups. We subjected one group of mice to the substitution procedures followed by chronic intermittent ethanol vapor inhalation (Study 1) to measure dependence-related ethanol drinking. To avoid confounds between the extensive handling required for the drinking study and the negative affective states potentially produced by the dependence procedure, a separate experimental group of mice received only the chronic intermittent ethanol vapor inhalation, followed by exposure to the light/dark box assay to measure anxiety-like behaviors (Study 2). The experimental design for both experimental groups is shown in Fig. 1. All animal procedures were approved by the Wake Forest School of Medicine IACUC in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Figure 1. Experimental Design.
(A) In the drinking study, animals were first subjected to a two-bottle preference test with 100-mM MSG during either 2-h or 24-h drinking sessions. We then used the MSG-substitution procedure as described previously (McCool & Chappell, 2012) to initiate drinking of 5% ethanol and subsequent increase in ethanol concentrations. Two cohorts, one drinking 10% (“10E” group) and another drinking 20% ethanol (“20E” group), were subjected to two separate exposures to either room air (CON) or chronic intermittent ethanol (CIE) inhalation to establish ethanol dependence (Becker & Hale, 1993). Ethanol drinking was measured 72 h after each dependence exposure. (B) In the anxiety study, animals were subjected to the two ethanol-dependence exposures. Anxiety-like behaviors were measured in the light/dark transition assay following the control exposure (CON), immediately after the last ethanol inhalation exposure while animals were still intoxicated (Et), or 72 h after the last ethanol inhalation (WD).
Drinking and substitution procedures
Mice were exposed to 100-mM monosodium glutamate (MSG in water; Sigma-Aldrich, St. Louis, MO) in the home cage using either a limited-access (2 h) or unlimited-access (24 h) two-bottle choice procedure (versus water alone) as described previously (McCool & Chappell, 2012, 2014). We used 5-mL serological pipettes (0.05-mL accuracy) to measure drinking during the 2-h limited-access, and 25-mL serological pipettes (0.2-mL accuracy) for the 24-h continuous-access drinking. Following this preference test, we employed an MSG-substitution procedure using a modified single-bottle, drinking-in-the-dark (DID) procedure (Rhodes, Best, Belknap, Finn, & Crabbe, 2005). Briefly, 5-mL ‘bottles’ were placed in cages for 2 h, beginning 30 min after lights-off, 5 days each week. Mice initially received 100-mM MSG for the first 2 days of DID drinking. Ethanol (from 95%; Warner-Graham Company, Cockeysville, MD) concentrations were increased from 2% to 5% in 1% increments adjusted every 2–3 days thereafter. Animals were never exposed to novel MSG/ethanol concentrations on a Monday. After consuming MSG + 5% ethanol, MSG concentrations were decreased from 100 mM to 0 mM in 25-mM increments adjusted every 2–3 days. The entire substitution procedure took 4 weeks (Fig. 1A). After 1 week of drinking 5% ethanol alone, the ethanol concentration was increased to 10% for 1 week. Mice were then randomly assigned to a “10E” group or a “20E” group. The “10E” group was subjected to the intermittent ethanol inhalation immediately following their first week of drinking 10% ethanol (baseline), and 10% ethanol drinking in the modified DID procedure was reassessed following the ethanol inhalation exposure (below). For the “20E” group, ethanol concentrations were increased in 5% increments every week over the next 2 weeks until the mice were consuming 20% ethanol in water during their ‘baseline’ week, and 20% ethanol drinking was reassessed following the ethanol inhalation.
Intermittent ethanol vapor exposure
To mimic ethanol physical dependence, we employed an intermittent ethanol vapor inhalation model based on previous work from Dr. Howard Becker (Becker & Hale, 1993), as defined by the standard operating procedure published on the website of the INIA-Stress research consortium (http://iniastress.org/dependence; 7/30/2014). Inhalation sessions were initiated by intra-peritoneal (i.p.) injection of 1.6 g/kg ethanol (8% v/v), and blood ethanol concentrations were stabilized by co-injection of 1 mmol/kg pyrazole (Sigma-Aldrich). Mice, housed in their home cages, were then placed into an airtight, custom-designed (Triad Plastics, Winston-Salem, NC) Plexiglas chamber. Ethanol vapor was introduced into the chambers using an aeration stone submerged in 95% ethanol; room air and ethanol vapor were mixed to produce blood ethanol concentrations sufficient to produce physical dependence across several repeated exposures (> 150mg/dL). Each day, the ethanol exposure consisted of a 16-h ethanol vapor inhalation (beginning 4 h before lights-off), followed by 8 h without ethanol vapor. These exposures were repeated for 4 consecutive days to complete a signal chronic intermittent exposure cycle (CIE, Fig. 1A). Ethanol drinking in the DID procedure was assessed 72 h after each CIE exposure. Control animals received i.p. pyrazole and were identically housed, but these mice were never exposed to any i.p. or inhaled ethanol.
Blood-ethanol determinations
Blood-ethanol concentrations (BECs) were measured using a commercially available enzyme/NADH-based assay (Carolina Liquid Chemistries Corp, Winston-Salem, NC) in samples collected from the facial vein (Golde, Gollobin, & Rodriguez, 2005). For the drinking study, samples were collected once from all mice during each of the ethanol vapor exposure periods (twice total on 2 separate weeks, once for each facial vein) and at the end of the drinking study in some animals as described in the results. For the anxiety-like behavior study, samples were collected from sentinel animals. Blood-ethanol concentrations were not significantly different between the B6 and D2 mice during the dependence exposure (184 ± 13 mg/dL and 205 ± 12 mg/dL, respectively; p > 0.05, unpaired t test).
Light/dark transition assay
Anxiety-like behaviors were measured in B6 and D2 mice using the light/dark transition assay. Briefly, animals were placed in the ‘light’ side of a light/dark box (Coulbourn Instruments, Whitehall, PA), and their position was monitored via infrared beam breaks for 5 min as described previously (DuBois, Perlegas, Floyd, Weiner, & McCool, 2006). Mice from each strain were divided into three treatment groups (Fig. 1B). ‘Ethanol’ (Et) animals received two consecutive CIE exposures (above) and were placed into the light/dark apparatus immediately after the last exposure while still intoxicated. ‘Withdrawal’ (WD) animals were placed into the assay 72 h after their last CIE exposure to mimic the withdrawal time frame in the drinking study. ‘Control’ (CON) mice were treated with pyrazole during the CIE process and were identically housed to the Et and WD groups, but never received any ethanol.
Statistics
For experiment 1 (drinking experiment), we employed repeated-measures two-way ANOVAs for each dependent variable across the main factors defined in the Results section. For experiment 2 (anxiety-like behaviors), separate experimental groups were required for each treatment condition, and we employed standard one-way ANOVAs across conditions. In both cases, we used a Bonferroni multiple-comparison post-test to examine relationships between groups. Significant differences are denoted by p values less than or equal to 0.05.
Results
MSG substitution
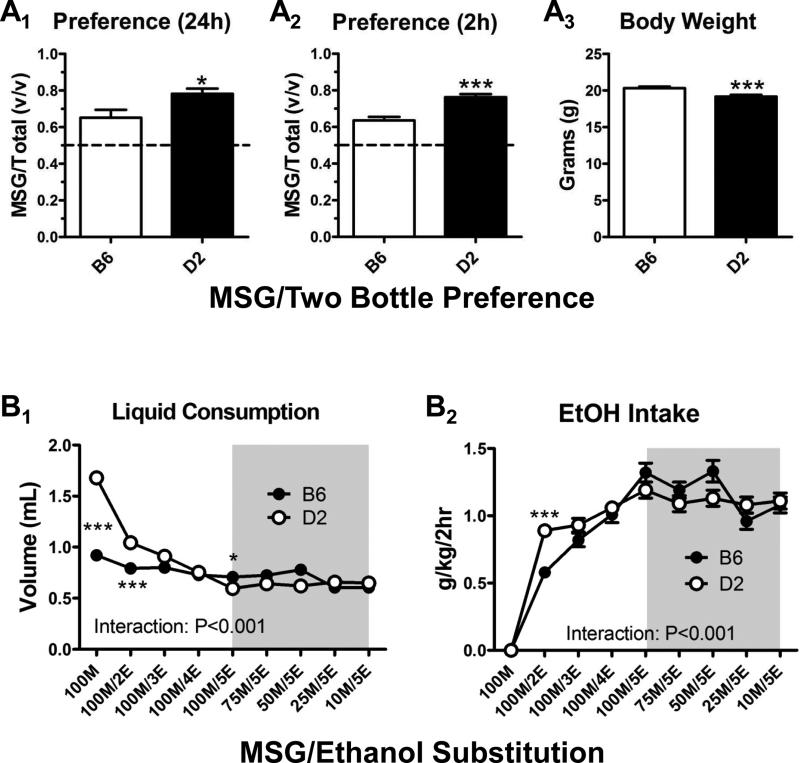
Mice from both strains were initially exposed to MSG in a 4-day, two-bottle preference test. In one experimental group (n = 12 from each strain), 100-mM MSG was placed in the home cage for 24 h each day. D2 mice exhibited significantly greater preference for MSG compared to B6 mice during this continuous access (Fig. 2A1; p < 0.05, t = 2.5, df = 22, t test). This difference in preference primarily resulted from significantly greater volumes of MSG consumed by D2 compared to B6 mice (5.7 ± 0.3 mL versus 4.0 ± 0.3 mL, p < 0.01, t = 3.6, t test), while water consumption tended to be less (2.1 ± 0.2 mL in D2, 1.6 ± 0.2 mL in B6), but was not significantly different between strains (p > 0.05, t = 1.8, t test). In a separate group of animals (n = 32 in each strain), we used a 2-h limited-access preference test (see Materials and methods) and found that the differences in MSG preference between strains was similar to the 24-h group (Fig. 2A2; p < 0.001, t = 4.9, df = 62, t test). Under these conditions, we found that the strain differences for volumes consumed mirrored the results from the 24-h test for both MSG (0.97 ± .04 mL in B6 and 1.43 ± 0.06 mL in D2; p < 0.001, t = 6.0, t test) and water (0.55 ± 0.03 mL in B6 and 0.40 ± 0.02 mL in D2, p < 0.001, t = 3.9, t test). The volume of 100-mM MSG consumed by D2 mice during the initial single-bottle DID session just 3 days after these preference tests was 1.63 ± 0.1 mL in the 24-h group and 1.7 ± 0.05 mL in the 2-h group (p > 0.05, t = 0.6, df = 42, t test). Similar outcomes were apparent in the B6 mice with no significant differences in volume consumed between the 2-h and 24-h groups (not shown, p > 0.05, t = 1.0, df = 42, t test). Importantly, we found that the type of initial experience with MSG, either limited or unlimited access, did not significantly alter the consumption of 5% ethanol (5E) measured 4 weeks later (see below). This strongly suggests that the different contexts (2 h versus 24 h) of the initial MSG exposures in these groups did not affect the DID drinking. We therefore combined the 2-h and 24-h MSG preference groups for the rest of our analysis.
Figure 2. The MSG-substitution procedure initiates similar levels of 5% ethanol consumption in both B6 and D2 mice.
(A) 100-mM MSG preference was significantly greater in D2 mic compared to B6 mice in both 24-h (A1) and 2-h (A2) drinking sessions. *p < 0.05 with t test. ***p < 0.001 with t test. Body weights at the end of the preference tests (A3) were also significantly lower (p < 0.001) in D2 mice (t test). (B) Despite dissimilar preference for MSG, B6 and D2 mice consume similar amounts of ethanol/MSG as MSG concentrations are lowered (gray boxes). There was a significant interaction (two-way ANOVA) between mouse strain and both the volume consumed (B1) and ethanol intake (g/kg/2 h; B2). *p < 0.05 and ***p < 0.001 with Bonferroni's multiple-comparison post-test across the strain factor.
During the MSG-substitution procedure, strain-specific differences in the consumption of MSG-containing solutions diminished as the ethanol concentrations increased. Repeated-measures, two-way analysis of variance using mouse strain and solution content as the main factors found significant interactions for both the volume consumed (mL; Fig. 2B1, p < 0.001, F = 25.2, df = 8) and the total ethanol intake (g/kg/2 h; Fig. 2B2, p < 0.001, F = 4.1, df = 8). Within the volume of solutions consumed, Bonferroni's multiple-comparison post-tests found significant differences between B6 and D2 mice for the 100-mM MSG (‘100M’, p < 0.001, t = 13.7), 100-mM MSG + 2% ethanol (‘100M/2E’, p < 0.001, t = 4.6), and 100-mM MSG + 5% ethanol (‘100M/5E’, p < 0.05, t = 2.9) solutions. For the total ethanol intake, these differences were only evident for the 100M/2E solution (p < 0.001, t = 4.1).
Baseline ethanol drinking
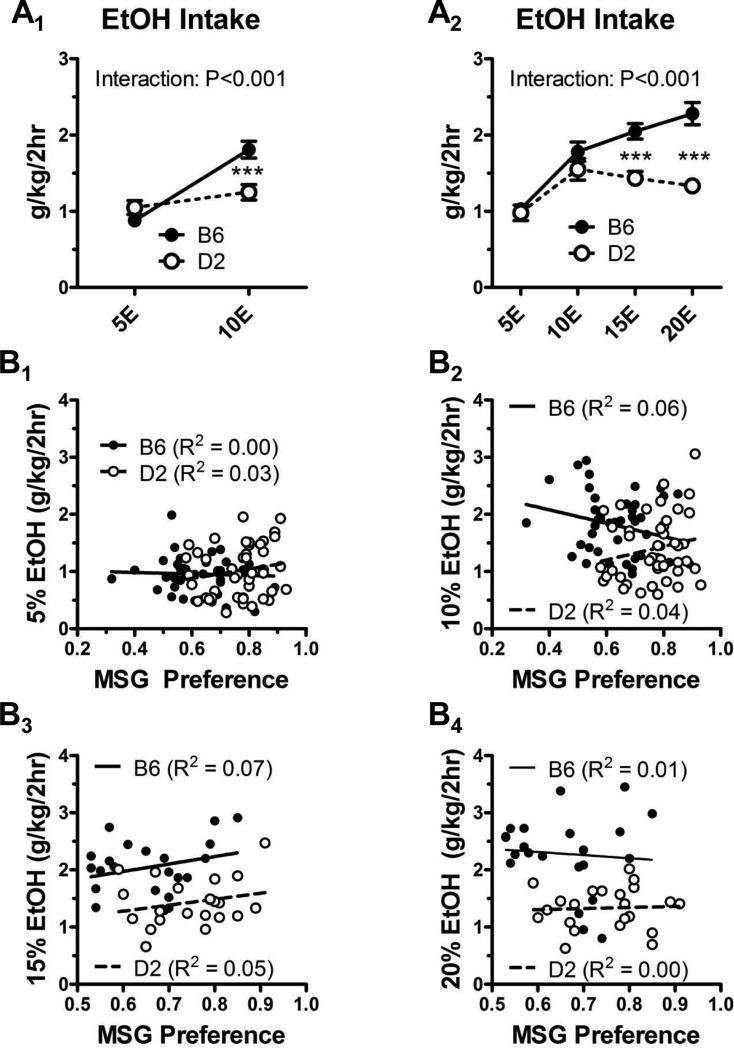
Following the MSG-substitution procedure, mice were randomly assigned to either the ‘10E’ or the ‘20E’ experimental group (n = 22 from each strain in each group). Intake (g/kg/2 h) of the 5% ethanol was not significantly different across any mouse strain within these two experimental groups (p > 0.05, F = 0.82, df = 87, one-way ANOVA). Within the ‘10E’ and ‘20E’ groups, however, there were significant interactions between mouse strain and the different ethanol concentrations (p < 0.001 for each experiment, F = 16.3 and df = 84 for the ‘10E’ group, F = 6.6 and df = 168 for the ‘20E’ group, two-way ANOVA; Fig. 3A1 & A2). For example, significant differences between strains were detected for 10E drinking in the ‘10E’ group and for 15E and 20E drinking in the ‘20E’ group, with B6 mice consuming more ethanol than D2 mice at ethanol concentrations greater than 10%. Given the power of the studies and the efficacy of MSG substitution to induce ethanol drinking in strains such as the D2 mice, we performed correlations between ethanol intake with the MSG preference values from the two-bottle choice experiment. There were no significant relationships between MSG preference ratios and the total amount of ethanol consumed across any of the ethanol concentrations (Fig. 3B1–3B4).
Figure 3. B6 mice consume more ethanol than D2 mice at concentrations greater than or equal to 10%.
(A) In the “10E” group during escalation of the ethanol concentration (A1), there was a significant interaction between mouse strain and ethanol concentration (two-way ANOVA) with B6 mice drinking significantly more 10% ethanol, but not 5% ethanol, than D2 mice (***p < 0.001, Bonferroni multiple-comparison post-test across the strain factor). Similarly, in the “20E” group (A2), there was also a significant interaction with B6 mice consuming more 15% and 20% ethanol (***p < 0.001, Bonferroni post-test across the strain factor). (B) There was no correlation (p > 0.05 in all cases) between the amount of ethanol consumed during the concentration escalation phase of the substitution procedure and the MSG preference exhibited during the initial two-bottle choice test. R2 values are indicated. Note that all animals are represented in the 5% ethanol (B1) and 10% ethanol (B2) graphs, while only the “20E” group is plotted in the 15% (B3) and 20% ethanol (B4) graphs. Lines derived from least-squares fits are shown for B6 (solid lines) and D2 (dashed lines) mice.
Ethanol dependence enhances ethanol drinking in both B6 and D2 mice
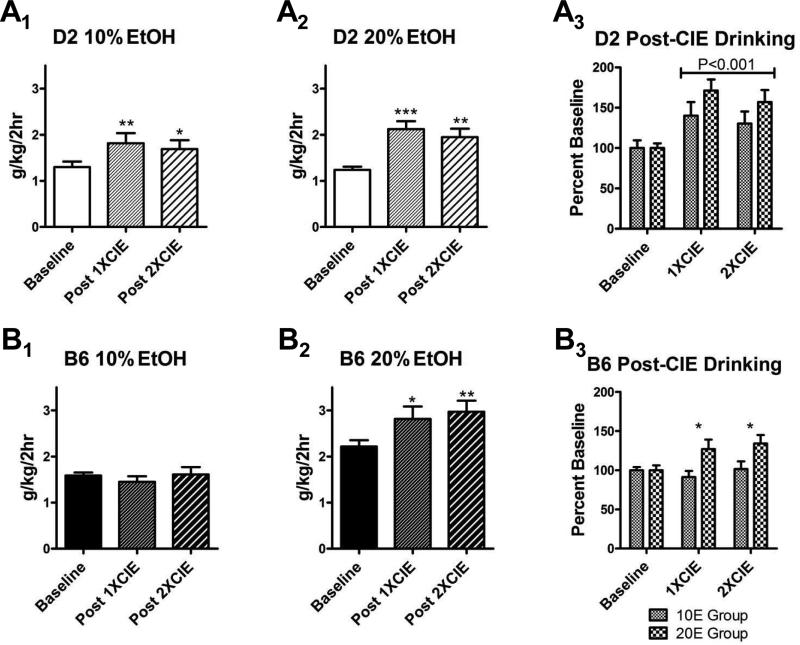
Since the MSG-substitution procedure produces measureable and persistent ethanol drinking by D2 mice, we examined whether ethanol dependence might increase drinking in these mice. Indeed, we found that ethanol drinking following both the first and second dependence ‘cycle’ (see Fig. 1 and Materials and methods) significantly increased consumption of both 10% ethanol as well as 20% ethanol relative to baseline (Fig. 4A1 & A2; p < 0.01, F = 7.9, df = 38 for ‘10E’ group; p < 0.001, F = 13.5, df = 41 for ‘20E’ group). In fact, when expressed as a percent of the baseline consumption within each animal, ethanol dependence increased ethanol drinking in D2 mice by 30–40% in the 10E group and 60–70% in the 20E group (Fig. 4A3), with a significant main effect of treatment (p < 0.001, F = 10.0, df = 75) and no significant interaction or group effect. In a subset of mice from the 10E and 20E groups (n = 8 per group), we measured blood ethanol concentrations after an extended (4-h) drinking session at the end of the study. The 10E-D2 mice drank 1.8 ± 0.3 g/kg after the 4-h session that produced BECs of 15 ± 4 mg/dL. In the 20E-D2 mice, ethanol consumption was 2.2 ± 0.3 g/kg, and the resulting BECs were 37 ± 7 mg/dL, with one animal having a BEC of 62 mg/dL. There was no significant correlation between ethanol consumption and BEC in the 10E-D2 mice, but BEC was significantly correlated with consumption (R2 = 0.59, p < 0.05) in the subset of 20E-D2 mice.
Figure 4. Ethanol consumption by B6 and D2 mice is differentially sensitive to ethanol dependence.
(A) Exposure to the CIE-dependence procedure significantly increased ethanol drinking in D2 mice relative to baseline. For the groups drinking 10% (A1, p < 0.01) and 20% (A2, p < 0.001) ethanol, there were significant differences in ethanol intake across the groups (repeated-measures ANOVA), with intakes being significantly greater than baseline following both exposures (*p < 0.05, **p < 0.01, ***p < 0.001, Bonferroni multiple-comparison post-test versus baseline). There was no significant difference between the 1XCIE and 2XCIE groups (p > 0.05, Bonferroni). When drinking data were expressed as percent baseline and compared across the 10E- and 20E-drinking groups (A3), there was a significant effect of treatment relative to baseline but no difference between either the drinking groups or the number of exposures (two-way ANOVA). (B) Enhanced ethanol drinking by B6 following exposure to the CIE dependence procedure is dependent upon the ethanol concentrations being consumed. In B6 mice drinking 10% ethanol (B1), CIE treatment did not significantly alter ethanol intake (p > 0.05, repeated-measures ANOVA). In the 20% ethanol group (B2), ethanol intake was significantly increased by CIE treatment (p < 0.01, repeated-measures ANOVA). Intakes here were significantly greater in both the 1XCIE (*p < 0.05, Bonferroni) and 2XCIE (**p < 0.01, Bonferroni) groups relative to controls, but these groups were not significantly different from one another (p > 0.05, Bonferroni). When normalized to baseline intake (B3), there was a significant main effect of the ethanol concentration being consumed (10E versus 20E; p < 0.01, two-way ANOVA across concentration and CIE treatments) with intakes being significantly greater in the 1XCIE and 2XCIE treatments in the 20% drinking group only (*p < 0.05, Bonferroni multiple-comparison post-test).
For the B6 mice, we found that neither of the vapor exposures increased ethanol drinking in animals consuming 10% ethanol (Fig. 4B1). In contrast, both vapor exposures significantly increased consumption of 20% ethanol in this strain (Fig. 4B2; p < 0.01, F = 7.1, df = 38). When these CIE-drinking data were normalized to baseline intakes for each animal, the two vapor exposures increased ethanol drinking in the 20E group by approximately 30% relative to baseline (Fig. 4B3), with a significant difference between the 10E- and 20E-B6 mice (p < 0.01, F = 9.8, df = 75), with only a trend for an interaction between the drinking group and treatment main factors. These findings with 20% ethanol are consistent with previous reports showing dependence-related increases in B6 mice drinking 15% ethanol (Griffin et al., 2009; Lopez & Becker, 2005). Again, in a subset of animals from the 10E- and 20E-drinking B6 mice (n = 8 per group), we tested BECs after a 4-h drinking session at the end of the experiment (post-CIE). Although the 10E-B6 mice did not increase ethanol consumption in response to ethanol vapor exposure, their consumption was 1.4 ± 0.3 g/kg, and their BEC was 48.6 ± 19.4 mg/dL at the end of the extended drinking session. The 20E-B6 subset drank 3.0 ± 0.4 g/kg ethanol and had BECs of 78 ± 17 mg/dL. There were significant correlations between ethanol intake (g/kg) and BEC for both the 10E- (R2 = 0.74, p < 0.01, F = 42.3, df = 14) and 20E- (R2 = 0.69, p < 0.05, F = 8.7, df = 6) B6 mice that were subjected to the extended drinking session.
To compare across the inbred strains, we performed an additional analysis by normalizing CIE-induced drinking to baseline consumption within each animal. We then employed repeated-measures two-way ANOVAs of the drinking data within each of the experimental groups (‘10E’ or ‘20E’) using inbred strain (B6 and D2) and treatment (CON, 1XCIE, 2XCIE) as the main factors. In the ‘10E’ experimental group, there was a significant interaction between the main factors (p < 0.01, F = 5.912, df = 50); a Bonferroni multiple- comparison post-test found significantly greater ethanol intake (g/kg/2 h) after both the 1XCIE (p < 0.01, t = 3.8) and 2XCIE (p < 0.05, t = 2.9) treatments in D2 mice, but not B6 mice. For the ‘20E’ group, dependence produced a significant effect across both the inbred strains (p < 0.001, F = 17.6, df = 50) and treatment groups (p < 0.001, F = 18.9), but no interaction. B6 and D2 mice from both the 1XCIE (p < 0.05 and t = 3.0 for B6; p < 0.001 and t = 4.6 for D2; Bonferroni multiple-comparison post-test) and 2XCIE (p < 0.01 for both, t = 3.8 for B6 and t = 3.7 for D2) treatment groups drank significantly more than in the CON condition. Because B6 mice drank more than D2 mice prior to dependence, we compared the effects of the dependence treatments on these strains by normalizing the drinking data to baseline consumption. For the 10E group, drinking by the D2 mice was significantly different from the B6 mice (p < 0.05, F = 2.8, df = 28, two-way repeated-measures ANOVA), but there was no interaction between strain and treatment group and no significant difference between the two CIE exposures. The main effect of mouse strain appeared to be primarily due to significant differences between B6 and D2 mice in the 1XCIE treatment group (p < 0.05, t = 2.9, Bonferroni multiple-comparison post-test). For the ‘20E’ group, there was again significantly greater drinking as a percent baseline in the D2 mice compared to B6 mice (p < 0.05, F = 5.7, df = 25), but there was neither an interaction nor a main effect of the different CIE treatments. The strain-specific differences in dependence-induced drinking was again greatest in the 1XCIE treatment groups (p < 0.05, t = 2.6, Bonferroni post-test).
As controls for the ethanol vapor-induced drinking increases in B6 and D2 mice, we tested the effects of the procedure on control animals that were identically housed and handled but never received ethanol (i.p. or inhaled). Ethanol drinking did not increase in either strain (Table 1). In fact, we found that this handling significantly decreased ethanol drinking in B6 mice, compared to both the 10E and 20E control groups. There was no effect in the D2 mice from either group.
Table 1.
Ethanol Drinking by Control Animals (g/kg/2 h)1
| Inbred Line2 | Baseline | Post-1XCON3 | Post-2XCON | Statistic4 |
|---|---|---|---|---|
| 10% Ethanol | ||||
| B6 | 1.20 ± 0.07 | 1.11 ± 0.07 (–6.5%) | 1.02 ± 0.04** (–13.4%) |
p < 0.01 F=8.0, df=23 |
| D2 | 1.08 ± 0.17 | 0.99 ± 0.08 (–2.6%) | 0.92 ± 0.08 (–8.0%) | n.s. |
| 20% Ethanol | ||||
| B6 | 2.31± 0.10 | 1.91 ± 0.16 (–17.1%) | 1.56 ± 0.07*** (–31.8%) |
p < 0.001 F=11.9, df=23 |
| D2 | 1.47 ± 0.1 | 1.31 ± 0.06 (–7.8%) | 1.28 ± 0.13 (–11.9%) | n.s. |
Animals were housed in the same conditions as CIE animals but received pyrazole only during room-air exposures.
n=8 in each line and experimental condition
mean ± SEM (percent baseline)
Repeated-measures ANOVA (“n.s.” denotes not significant).
p < 0.01
p < 0.001 from Bonferoni's multiple-comparison post-test versus baseline
B6 and D2 mice differentially express ethanol dependence-enhanced anxiety-like behavior
To establish the efficacy of the ethanol dependence exposure using vapor inhalation, we examined anxiety-like behaviors expressed in the light/dark transition test using B6 and D2 mice exposed to two consecutive ethanol vapor inhalation cycles (see Materials and methods). Mice were randomly assigned to three experimental groups: ‘CON’ animals received identical housing/handling but no ethanol vapor, ‘Et’ animals were placed in the light/dark box immediately after their final vapor exposure while still intoxicated, and ‘WD’ animals were placed in the light/dark box 72 h after their final ethanol vapor exposure. In the D2 mice, we found significant differences between the experimental groups for light-side time (p < 0.001, F = 9.4, df = 30, one-way ANOVA; Fig. 5A1), the number of light-dark transitions (p < 0.01, F = 7.9; Fig. 5A2), and light-side re-entry latency (p < 0.001, F = 11.1; Fig. 5A3). These differences were driven by significantly less light-side time (p < 0.05) and fewer light/dark transitions (p < 0.01) as well as significantly greater re-entry latencies (p < 0.01) in the D2 WD mice (Bonferroni's multiple-comparison test). There was also a trend (p < 0.1, F = 3.2) for dependence to alter egress latencies (CON – 63.0 ± 19.8 sec, Et – 86.4 ± 22.4 sec, WD – 16.6 ± 4.7 sec). There were no significant between-group differences in locomotor behavior reflected by the total number of moves, total move distance (cm), total move time (sec), or in the number of vertical plane entries (Table 2). In B6 mice, there were no significant differences in any dependent variable related to anxiety-like behavior (Fig. 5B), including egress latency (CON – 32.1 ± 10.6 sec, Et – 33.0 ± 10.6 sec, WD – 41.5 ± 10.4 sec). Of the variables describing locomotor behaviors, only the number of vertical plane entries was significantly diminished in B6 Et mice (Table 2).
Figure 5. CIE exposure differentially modulates anxiety-like behaviors expressed in the light/dark transition assay by D2 and B6 mice.
(A) Two consecutive CIE exposures increased the expression of anxiety-like behaviors in D2 mice. Light-side time (A1; p < 0.001, one-way ANOVA), light-dark transitions (A2, p < 0.01), and re-entry latency (A3, p < 0.001) were all significantly altered. Light-side times (A1) in the intoxicated D2 mice placed in the assay immediately after their last ethanol exposure (2XCIE) was significantly greater than control (CON), while 72 h after the last ethanol exposure (72WD), this measure was significantly less than both CON and 2XCIE. There were significantly fewer light-dark transitions (A2) in the 72WD group relative to both CON and 2XCIE. 72WD D2 mice (A3) had significantly longer light-side re-entry latencies compared to both CON and 2XCIE. *, **, ***p < 0.05, 0.01, and 0.001, respectively, relative to CON; ##, ###p < 0.01 and 0.001 relative to 2XCIE, respectively; Newman-Keuls multiple-comparison post-test.
Table 2.
Light/Dark Box Locomotor Behavior
| Control | CIE | 72-h Withdrawal | Statistic1 | |
|---|---|---|---|---|
| B6 | ||||
| Moves (#) | 27.4 ± 2.6 | 26.0 ± 2.7 | 30.5 ± 1.6 | n.s. |
| Move Distance (cm) | 1074 ± 54 | 947 ± 59 | 922 ± 33 | n.s. |
| Move Time (sec) | 264 ± 3 | 260 ± 3 | 258 ± 3 | n.s. |
| Vertical Plane Entries (#) | 42.2 ± 4.5 | 27.8 ± 2.4* | 37.1 ± 3.7 |
p < 0.05 F=3.7, df=23 |
| D2 | ||||
| Moves (#) | 29.9 ± 1.3 | 25.5 ± 2.0 | 27.6 ± 2.9 | n.s. |
| Move Distance (cm) | 861 ±54 | 1100 ± 84 | 881 ± 92 | n.s. |
| Move Time (sec) | 250 ± 5 | 264 ± 3 | 261 ± 4 | n.s. |
| Vertical Plane Entries | 31.2 ± 3.9 | 24.3 ± 3.5 | 29.5 ± 6.9 | n.s. |
Treatment groups were compared using standard one-way ANOVA (significance level noted). “n.s.” denotes no significant treatment effect. Post hoc comparisons between groups were made with Bonferroni's multiple-comparison post-test.
p < 0.05 relative to control group.
Discussion
In the current study we show that an MSG-substitution procedure initiates substantial ethanol drinking by both the ‘high-drinking’ B6 mouse strain and the ‘low-drinking’ D2 mouse strain (McCool & Chappell, 2012, 2014). This ethanol drinking was strain-dependent, with higher consumption in the B6 mice when ethanol concentrations were greater than or equal to 10%. Since the rank order of MSG consumption (D2 > B6) and consumption of ethanol concentrations ≥ 10% (B6 > D2) were dissimilar, it is unlikely that the consumption of different ethanol solutions was directly related to any long-term impact of MSG on ethanol preference/intake. Indeed, there was no correlation between ethanol consumption and MSG preference regardless of strain or ethanol concentration. This is consistent with previous suggestions (McCool & Chappell, 2014) that MSG may more effectively mask ethanol taste-related aversion than a sweetened solution, particularly in mouse strains that exhibit low preference for sweet flavors. Subsequently, we found that exposure to high levels of ethanol via passive inhalation enhanced ethanol consumption in both B6 and D2 mice. Surprisingly, relative to baseline consumption, there was a greater percent increase in D2 mice. Further, this dependence-enhanced ethanol drinking by D2 mice occurred at lower ethanol concentrations than for B6 mice. Finally, we showed that the passive ethanol inhalation exposure produces significant withdrawal-related increases in anxiety-like behavior in the D2 mice but not in the B6 strain.
Previous studies making direct comparisons between MSG- and sucrose-substitution procedures have demonstrated the superior efficacy of MSG (McCool & Chappell, 2012, 2014). Although the mechanism responsible for this is not entirely clear, it is likely that the taste of the MSG-ethanol mixture is a significant factor. The D2 genome carries a sac locus polymorphism encoding a taste receptor subunit with reduced sensitivity to sweet compounds such as sucrose and saccharine (Bachmanov et al., 2001; Max et al., 2001; Montmayeur, Liberles, Matsunami, & Buck, 2001; Nelson et al., 2001; Sainz, Korley, Battey, & Sullivan, 2001). Previous work on ethanol taste aversion shows ethanol possesses both ‘sweet’ and ‘bitter’ character, with B6 mice generalizing ethanol taste to both components while D2 mice generalize only to the bitter (Blizard, 2007). Knockout of the sweet taste receptor also dramatically reduces both ethanol consumption and the neurophysiological responses to oral ethanol in B6 mice (Blednov et al., 2008; Brasser, Norman, & Lemon, 2010). Finally, D2 mice will readily self-administer ethanol when it is delivered intragastrically, bypassing the oral cavity (Fidler et al., 2011). However, we cannot rule out possible direct interactions between MSG and the pharmacological effects of ethanol. Indeed, procedural differences between MSG-substitution procedures (McCool & Chappell, 2012, 2014) suggest that prolonged drinking experience with lower, less aversive ethanol concentrations might offer a better opportunity for D2 mice to establish the saliency of orally delivered ethanol. It is certainly possible that this type of ‘learning’ during the baseline drinking period could make critical contributions to enhanced post-dependence drinking.
It is furthermore noteworthy that the MSG-substitution procedure allowed us to measure dependence-related increases in oral ethanol consumption in D2 mice. We specifically observed a greater percent effect of dependence in D2 relative to B6 mice, and their dependence-enhanced drinking occurred at lower ethanol concentrations. It is possible that the robust drinking by B6 mice during the baseline period imposed a ‘ceiling’ on the levels of post-dependence drinking we could observe. While this might explain the strain differences with respect to the effect of dependence on intake itself, it is not clear how a potential ‘ceiling’ might insulate B6 mice drinking 10% ethanol from the effects of ethanol dependence. Regardless, our interpretation of greater sensitivity in the D2 strain again parallels previous work by Fidler and colleagues who used intragastric ethanol self-administration to show a greater enhancement of ethanol self-administration following dependence in D2 mice relative to B6 (Fidler et al., 2012). It is notable that the dependence induced in this study did not cause a persistent increase in ethanol self-administration in D2 mice when they had no experience with the self-administration prior to that dependence (Cunningham et al., 2013). Our finding and the findings from Fidler et al. can be contrasted with a study by Carmarini and Hodge (2004), who showed that intermittent i.p. administration of intoxicating ethanol doses insufficient to produce dependence caused similar increases in ethanol self-administration by B6 and D2 mice following a sucrose-substitution procedure. This suggests that neurobiological adaptations associated with dependence, ethanol pharmacology, and orosensory/interoceptive cues associated with self-administration must all interact to produce strain-specific drinking phenotypes. In the context of the current study, B6 mice drinking 10% ethanol may simply not have found this ethanol concentration salient enough to produce these interactions and subsequently did not increase drinking post-dependence.
The mechanisms driving dependence-related drinking in D2 mice are not well defined. Fidler et al. (2011) recently proposed several possible mechanisms related to dependence-enhanced intragastric ethanol self-administration in D2 mice: enhanced ethanol positive reward, reduced aversive effects, or relief of negative consequences associated with dependence/withdrawal. Greater reward valuation by D2 mice following dependence would be consistent with both their greater sensitivity in conditioned place-preference paradigms (Cunningham, Niehus, Malott, & Prather, 1992), as well as the finding that pairing acute withdrawal with acute ethanol enhances conditioned place preference for ethanol in this strain (Dreumont & Cunningham, 2014). Because we used a single-bottle self-administration procedure, we could not directly measure ethanol reward as it is represented by preference between ethanol and a non-rewarding solution. Regardless, reduced aversion to post-ingestive effects of ethanol is also a potential mechanism that is likely related to either the blood levels of ethanol itself or of one of its immediate metabolites, acetaldehyde. While B6 and D2 mice exhibit similar rates of ethanol metabolism, D2 mice reach higher blood and brain levels of acetaldehyde (Schneider, 1973; Tabakoff, Anderson, & Ritzmann, 1976). Additionally, acetaldehyde administration mimics many of the post-ingestive aversive effects associated with ethanol exposure (Dudek & Fuller, 1978). It is noteworthy that, despite remarkably similar consumption levels during a 4-h drinking session in the post-dependent B6 and D2 mice in the 10E groups (e.g., ~1.4 g/kg in B6 versus ~1.8 g/kg in D2), BECs were much higher in B6 mice (~50 mg/dL) than in D2 mice (< 20 mg/dL). Given their similar metabolic rates for ethanol, these BEC data suggest that D2 mice consumed most of the ethanol much earlier during the drinking session. Although this hypothesis needs to be directly tested, D2 mice consume sucrose with greater lick rates and have a tendency for both a greater numbers of licks during a drinking ‘bout’ and a greater volume per lick (Boughter, Baird, Bryant, St. John, & Heck, 2007). This ‘rapid consumption’ phenotype for D2 mice would tend to amplify post-ingestive positive effects of acute ethanol as well as any aversive effects of acetaldehyde for this strain in particular.
The well-established role of negative reinforcement in dependence-induced ethanol drinking in human populations and other rodent models suggests that the alleviation of withdrawal anxiety by drinking might also play a significant role in our study. However, the differential sensitivity of B6 and D2 mice to the anxiety-provoking environment in the light/dark transition test suggests to us that these strains represent distinct behavioral/neurobiological models for dependence-enhanced drinking. While B6 mice do not express robust withdrawal anxiety in our studies, chronic ethanol exposures strongly modulate learning-related behaviors in this strain across multiple experimental settings (Badanich, Becker, & Woodward, 2011; DePoy et al., 2013, 2014; Holmes et al., 2012; Kroener et al., 2012; Lopez, Griffin, Melendez, & Becker, 2012). Ethanol withdrawal in B6 mice also enhances compulsive-like behaviors (Umathe, Bhutada, Dixit, & Shende, 2008). In general, these studies suggest that dependence-enhanced ethanol drinking in B6 mice may rely especially upon cortical/striatal mechanisms associated with cognitive control and/or habit formation. Conversely, D2 mice express pronounced physical and emotional vulnerability to ethanol dependence. This vulnerability is manifested as increased incidence of withdrawal-related seizures (Crabbe, Kosobud, Young, & Janowsky, 1983), enhanced conditioned-taste aversion (Risinger & Cunningham, 1998) and ethanol reward (Dreumont & Cunningham, 2014), and relatively greater c-fos expression (compared to B6) within ‘emotional centers’ such as the lateral/basolateral and central amygdala, bed nucleus of the stria terminalis, hippocampus, and prelimbic cortex (Chen, Reilly, Kozell, Hitzemann, & Buck, 2009). Our finding that D2 mice express enhanced anxiety-like behavior within the light/dark transition assay is entirely consistent with the hypothesis that dependence drives drinking more through mechanisms related to negative affect in this strain. Thus, B6 and D2 mice may represent distinct psychopathological models for the development of dependence-related ethanol drinking.
In conclusion, we have shown that MSG substitution offers a unique opportunity to understand dependence-enhanced ethanol drinking across mouse strains that may not readily ingest significant quantities of ethanol. Further, we show that MSG substitution could be employed to specifically study dependence-enhanced drinking as an independent phenotype within the context of inbred mouse strains. This suggests a potential for identifying novel genetic mechanisms or novel models for distinct neurobehavioral/neurobiological pathways for ethanol addiction.
MSG substitution initiates ethanol intakes independent of MSG preference
DBA/2J ethanol drinking is more sensitive to dependence than in C57BL/6J mice
DBA/2J, but not C57BL/6J, mice express withdrawal-related anxiety
Acknowledgments
This work was supported by NIH/NIAAA grants U01 AA020942 and R01 AA014445.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackroff K, Sclafani A. Flavor preferences conditioned by intragastric monosodium glutamate in mice. Chemical Senses. 2013;38:759–767. doi: 10.1093/chemse/bjt042. [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Li X, Reed DR, Ohmen JD, Li S, Chen Z, et al. Positional cloning of the mouse saccharin preference (Sac) locus. Chemical Senses. 2001;26:925–933. doi: 10.1093/chemse/26.7.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badanich KA, Becker HC, Woodward JJ. Effects of chronic intermittent ethanol exposure on orbitofrontal and medial prefrontal cortex-dependent behaviors in mice. Behavioral Neuroscience. 2011;125:879–891. doi: 10.1037/a0025922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Hale RL. Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal “kindling”. Alcoholism: Clinical and Experimental Research. 1993;17:94–98. doi: 10.1111/j.1530-0277.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Atkins AL. The replicability of QTLs for murine alcohol preference drinking behavior across eight independent studies. Mammalian Genome. 2001;12:893–899. doi: 10.1007/s00335-001-2074-2. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Martinez M, Levine M, Damak S, Margolskee RF. Perception of sweet taste is important for voluntary alcohol consumption in mice. Genes, Brains, and Behavior. 2008;7:1–13. doi: 10.1111/j.1601-183X.2007.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blizard DA. Sweet and bitter taste of ethanol in C57BL/6J and DBA2/J mouse strains. Behavior Genetics. 2007;37:146–159. doi: 10.1007/s10519-006-9121-4. [DOI] [PubMed] [Google Scholar]
- Boughter JD, Jr., Baird JP, Bryant J, St. John SJ, Heck D. C57BL/6J and DBA/2J mice vary in lick rate and ingestive microstructure. Genes, Brain, and Behavior. 2007;6:619–627. doi: 10.1111/j.1601-183X.2006.00293.x. [DOI] [PubMed] [Google Scholar]
- Brasser SM, Norman MB, Lemon CH. T1r3 taste receptor involvement in gustatory neural responses to ethanol and oral ethanol preference. Physiological Genomics. 2010;41:232–243. doi: 10.1152/physiolgenomics.00113.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarini R, Hodge CW. Ethanol preexposure increases ethanol self-administration in C57BL/6J and DBA/2J mice. Pharmacology, Biochemistry, and Behavior. 2004;79:623–632. doi: 10.1016/j.pbb.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Chen G, Reilly MT, Kozell LB, Hitzemann R, Buck KJ. Differential activation of limbic circuitry associated with chronic ethanol withdrawal in DBA/2J and C57BL/6J mice. Alcohol. 2009;43:411–420. doi: 10.1016/j.alcohol.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, Cunningham CL. GABA(A) receptors modulate ethanol-induced conditioned place preference and taste aversion in mice. Psychopharmacology (Berl) 1999;144:363–372. doi: 10.1007/s002130051019. [DOI] [PubMed] [Google Scholar]
- Chu K, Koob GF, Cole M, Zorrilla EP, Roberts AJ. Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout. Pharmacology, Biochemistry, and Behavior. 2007;86:813–821. doi: 10.1016/j.pbb.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Lin D, Martin-Fardon R, Weiss F. Reinstatement of ethanol-seeking behavior by drug cues following single versus multiple ethanol intoxication in the rat: effects of naltrexone. Psychopharmacology (Berl) 2003;168:208–215. doi: 10.1007/s00213-002-1380-z. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Kosobud A, Young ER, Janowsky JS. Polygenic and single-gene determination of responses to ethanol in BXD/Ty recombinant inbred mouse strains. Neurobehavioral Toxicology and Teratology. 1983;5:181–187. [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Buck KJ, Cunningham CL, Belknap JK. Identifying genes for alcohol and drug sensitivity: recent progress and future directions. Trends in Neurosciences. 1999;22:173–179. doi: 10.1016/s0166-2236(99)01393-4. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, et al. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Fidler TL, Murphy KV, Mulgrew JA, Smitasin PJ. Time-dependent negative reinforcement of ethanol intake by alleviation of acute withdrawal. Biological Psychiatry. 2013;73:249–255. doi: 10.1016/j.biopsych.2012.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Niehus DR, Malott DH, Prather LK. Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharmacology (Berl) 1992;107:385–393. doi: 10.1007/BF02245166. [DOI] [PubMed] [Google Scholar]
- de Guglielmo G, Martin-Fardon R, Teshima K, Ciccocioppo R, Weiss F. MT-7716, a potent NOP receptor agonist, preferentially reduces ethanol seeking and reinforcement in post-dependent rats. Addiction Biology. 2014 doi: 10.1111/adb.12157. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delay ER, Beaver AJ, Wagner KA, Stapleton JR, Harbaugh JO, Catron KD, et al. Taste preference synergy between glutamate receptor agonists and inosine monophosphate in rats. Chemical Senses. 2000;25:507–515. doi: 10.1093/chemse/25.5.507. [DOI] [PubMed] [Google Scholar]
- DePoy L, Daut R, Brigman JL, MacPherson K, Crowley N, Gunduz-Cinar O, et al. Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:14783–14788. doi: 10.1073/pnas.1308198110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depoy L, Daut R, Wright T, Camp M, Crowley N, Noronha B, et al. Chronic alcohol alters rewarded behaviors and striatal plasticity. Addiction Biology. 2014 doi: 10.1111/adb.12131. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreumont SE, Cunningham CL. Effects of acute withdrawal on ethanol-induced conditioned place preference in DBA/2J mice. Psychopharmacology (Berl) 2014;231:777–785. doi: 10.1007/s00213-013-3291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois DW, Perlegas A, Floyd DW, Weiner JL, McCool BA. Distinct functional characteristics of the lateral/basolateral amygdala GABAergic system in C57BL/6J and DBA/2J mice. The Journal of Pharmacology and Experimental Therapeutics. 2006;318:629–640. doi: 10.1124/jpet.105.100552. [DOI] [PubMed] [Google Scholar]
- Dudek BC, Fuller JL. Task-dependent genetic influences on behavioral response of mice (Mus musculus) to acetaldehyde. Journal of Comparative and Physiological Psychology. 1978;92:749–758. doi: 10.1037/h0077506. [DOI] [PubMed] [Google Scholar]
- Fehr C, Shirley RL, Crabbe JC, Belknap JK, Buck KJ, Phillips TJ. The syntaxin binding protein 1 gene (Stxbp1) is a candidate for an ethanol preference drinking locus on mouse chromosome 2. Alcoholism: Clinical and Experimental Research. 2005;29:708–720. doi: 10.1097/01.alc.0000164366.18376.ef. [DOI] [PubMed] [Google Scholar]
- Fidler TL, Dion AM, Powers MS, Ramirez JJ, Mulgrew JA, Smitasin PJ, et al. Intragastric self-infusion of ethanol in high- and low-drinking mouse genotypes after passive ethanol exposure. Genes, Brain, and Behavior. 2011;10:264–275. doi: 10.1111/j.1601-183X.2010.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler TL, Powers MS, Ramirez JJ, Crane A, Mulgrew J, Smitasin P, et al. Dependence induced increases in intragastric alcohol consumption in mice. Addiction Biology. 2012;17:13–32. doi: 10.1111/j.1369-1600.2011.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, O'Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. The Journal of Neuroscience. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Koob GF. Effects of β-adrenoceptor antagonists on alcohol drinking by alcohol-dependent rats. Psychopharmacology (Berl) 2010;212:431–439. doi: 10.1007/s00213-010-1967-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde WT, Gollobin P, Rodriguez LL. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Animal (NY) 2005;34:39–43. doi: 10.1038/laban1005-39. [DOI] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Becker HC. Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcoholism: Clinical and Experimental Research. 2009;33:1893–1900. doi: 10.1111/j.1530-0277.2009.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitzemann R, Hitzemann B, Rivera S, Gatley J, Thanos P, Shou LL, et al. Dopamine D2 receptor binding, Drd2 expression and the number of dopamine neurons in the BXD recombinant inbred series: genetic relationships to alcohol and other drug associated phenotypes. Alcoholism: Clinical and Experimental Research. 2003;27:1–11. doi: 10.1097/01.ALC.0000047862.40562.27. [DOI] [PubMed] [Google Scholar]
- Holmes A, Fitzgerald PJ, MacPherson KP, DeBrouse L, Colacicco G, Flynn SM, et al. Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nature Neuroscience. 2012;15:1359–1361. doi: 10.1038/nn.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Glendinning JI, Theodorides ML, Harkness S, Li X, Bosak N, et al. Allelic variation of the Tas1r3 taste receptor gene selectively affects taste responses to sweeteners: evidence from 129.B6-Tas1r3 congenic mice. Physiological Genomics. 2007;32:82–94. doi: 10.1152/physiolgenomics.00161.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KM, Chappell AM, Weiner JL. A comparison of umami (MSG) vs. sweet (sucrose) fading in the initiation of operant ethanol self-administration. Alcoholism: Clinical & Experimental Research. 2007;31(6 Suppl):91A. [Google Scholar]
- Kroener S, Mulholland PJ, New NN, Gass JT, Becker HC, Chandler LJ. Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS One. 2012;7:e37541. doi: 10.1371/journal.pone.0037541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl) 2005;181:688–696. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Griffin WC, 3rd, Melendez RI, Becker HC. Repeated cycles of chronic intermittent ethanol exposure leads to the development of tolerance to aversive effects of ethanol in C57BL/6J mice. Alcoholism: Clinical and Experimental Research. 2012;36:1180–1187. doi: 10.1111/j.1530-0277.2011.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, et al. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nature Genetics. 2001;28:58–63. doi: 10.1038/ng0501-58. [DOI] [PubMed] [Google Scholar]
- McCool BA, Chappell AM. Using monosodium glutamate to initiate ethanol self-administration in inbred mouse strains. Addiction Biology. 2012;17:121–131. doi: 10.1111/j.1369-1600.2010.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Chappell AM. Persistent enhancement of ethanol drinking following a monosodium glutamate-substitution procedure in C57BL6/J and DBA/2J mice. Alcohol. 2014;48:55–61. doi: 10.1016/j.alcohol.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metten P, Phillips TJ, Crabbe JC, Tarantino LM, McClearn GE, Plomin R, et al. High genetic susceptibility to ethanol withdrawal predicts low ethanol consumption. Mammalian Genome. 1998;9:983–990. doi: 10.1007/s003359900911. [DOI] [PubMed] [Google Scholar]
- Montmayeur JP, Liberles SD, Matsunami H, Buck LB. A candidate taste receptor gene near a sweet taste locus. Nature Neuroscience. 2001;4:492–498. doi: 10.1038/87440. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Eddy MC, Katsukawa H, Delay ER, Ninomiya Y. Behavioral responses to glutamate receptor agonists and antagonists implicate the involvement of brain-expressed mGluR4 and mGluR1 in taste transduction for umami in mice. Physiology & Behavior. 2012;105:709–719. doi: 10.1016/j.physbeh.2011.09.028. [DOI] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- Niijima A. Effect of umami taste stimulations on vagal efferent activity in the rat. Brain Research Bulletin. 1991;27:393–396. doi: 10.1016/0361-9230(91)90131-3. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiology & Behavior. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr., et al. Mouse inbred strain differences in ethanol drinking to intoxication. Genes, Brain, and Behavior. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Thorsell A, Heilig M. Suppression of ethanol self-administration by the neuropeptide Y (NPY) Y2 receptor antagonist BIIE0246: evidence for sensitization in rats with a history of dependence. Neuroscience Letters. 2005;375:129–133. doi: 10.1016/j.neulet.2004.10.084. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Cunningham CL. Ethanol-induced conditioned taste aversion in BXD recombinant inbred mice. Alcoholism: Clinical and Experimental Research. 1998;22:1234–1244. [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Sainz E, Korley JN, Battey JF, Sullivan SL. Identification of a novel member of the T1R family of putative taste receptors. Journal of Neurochemistry. 2001;77:896–903. doi: 10.1046/j.1471-4159.2001.00292.x. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcoholism: Clinical and Experimental Research. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Schneider CW. Ethanol preference and behavioral tolerance in mice: biochemical and neurophysiological mechanisms. Journal of Comparative and Physiological Psychology. 1973;82:466–474. doi: 10.1037/h0034116. [DOI] [PubMed] [Google Scholar]
- Shibata R, Kameishi M, Kondoh T, Torii K. Bilateral dopaminergic lesions in the ventral tegmental area of rats influence sucrose intake, but not umami and amino acid intake. Physiology & Behavior. 2009;96:667–674. doi: 10.1016/j.physbeh.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Sidhpura N, Weiss F, Martin-Fardon R. Effects of the mGlu2/3 agonist LY379268 and the mGlu5 antagonist MTEP on ethanol seeking and reinforcement are differentially altered in rats with a history of ethanol dependence. Biological Psychiatry. 2010;67:804–811. doi: 10.1016/j.biopsych.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabakoff B, Anderson RA, Ritzmann RF. Brain acetaldehyde after ethanol administration. Biochemical Pharmacology. 1976;25:1305–1309. doi: 10.1016/0006-2952(76)90094-0. [DOI] [PubMed] [Google Scholar]
- Uematsu A, Tsurugizawa T, Kitamura A, Ichikawa R, Iwatsuki K, Uneyama H, et al. Evaluation of the ‘liking’ and ‘wanting’ properties of umami compound in rats. Physiological & Behavior. 2011;102:553–558. doi: 10.1016/j.physbeh.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Umathe S, Bhutada P, Dixit P, Shende V. Increased marble-burying behavior in ethanol-withdrawal state: modulation by gonadotropin-releasing hormone agonist. European Journal of Pharmacology. 2008;587:175–180. doi: 10.1016/j.ejphar.2008.03.035. [DOI] [PubMed] [Google Scholar]
- Weng J, Symons MN, Singh SM. Studies on Syntaxin 12 and alcohol preference involving C57BL/6J and DBA/2J strains of mice. Behavior Genetics. 2009;39:183–191. doi: 10.1007/s10519-008-9249-5. [DOI] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]