Abstract
Monoclonal antibodies (mAbs) are major reagents for research and clinical diagnosis. For their inherently high specificities to intended antigen targets and thus low toxicity in general, they are pursued as one of the major classes of new drugs. Yet binding properties of most monoclonal antibodies are not well characterized in terms of affinity constants and how they vary with presentations and/or conformational isomers of antigens, buffer compositions, and temperature. We here report a microarray-based label-free assay platform for high-throughput measurements of monoclonal antibody affinity constants to antigens immobilized on solid surfaces. Using this platform we measured affinity constants of over 1,410 rabbit monoclonal antibodies and 46 mouse monoclonal antibodies to peptide targets that are immobilized through a terminal cysteine residue to a glass surface. The experimentally measured affinity constants vary from 10 pM to 200 pM with the median value at 66 pM. We compare results of the microarray-based platform with those of a benchmarking surface-plasmon-resonance-based (SPR) sensor (Biacore 3000).
INTRODUCTION
Highly adaptive structures in paratope regions of antibodies afford their specific recognition capabilities and thus enable them the primary defense against foreign pathogens in a living organism. This remarkable molecular attribute also makes antibodies the leading choice of reagents for diagnosis and extraction of biomarkers from samples in clinical laboratories and in laboratories of life/medical sciences. In recent years, monoclonal antibodies are actively and in some cases successfully explored as one of the major forms of biologic drugs, for inherently high target-specificity and in turn low required dosage to achieve same therapeutic efficacy.1-3 Recent Ebola outbreaks in Africa and other parts of the world and the remarkable promise of combinations of monoclonal antibodies as an effective cure of infected patients highlight the importance of and urgent need for antibody-based drugs and antibody research in general.4, 5
Despite the aforementioned, most monoclonal antibodies from commercial vendors and in academic laboratories are not well characterized, in terms of quantitative binding properties against specific and non-specific targets. It is a common and often costly experience that one finds monoclonal antibodies against same antigen target but from different vendors or from the same vendor but of different lots to yield significantly different outcomes in “identically” executed assays. There are extensive studies revealing that on average 50% of commercial antibodies do not produce expected binding results as advertised and the success rate varies from 0% to 100% for different vendors.6 Even from the same lot, qualitative outcomes of antibody-antigen binding assays may vary from one type of assay to another; and from one laboratory to another. Some variations originate from changes in the paratope of the antibody that are often inadequately characterized. Others have to do with assay conditions, protocol details, and conformational presentations (denatured vs. natural form, free form vs. constrained form as a conjugate to a large carrier or as an integral part of a large protein) of antigen targets that can be understood and anticipated only if kinetic and thermodynamic information on antibody-antigen binding reactions are known even in limited circumstances, instead of merely IHC and Western Blot data or even less. The main reason that most antibodies are so insufficiently characterized and validated is the cost, in terms of materials, instrumentation, and skilled labor. We compared the results obtained from the microarray platform with those obtained from a benchmarking surface-plasmon-resonance-based (SPR) sensor (Biacore 3000).
We report a microarray-based label-free assay platform that affords high-throughput cost-effective measurement of binding curves of antibodies to antigen targets.7-10 We applied this platform to determine binding constants of 1,410 rabbit monoclonal antibodies and 46 mouse monoclonal antibodies to synthetic peptide targets that are immobilized through a terminal cysteine residue on a functionalized glass slide surface. The results compare well with measurements using a benchmark (but low throughput) SPR-based label-free sensor (Biacore 3000). Furthermore we find that the measured binding constants do not change when the target density changes by more than a factor of 4 (comparable to the target density in the SPR measurement) so that the average target separation is twice the dimension of a captured antibody, indicating that the measured binding constants are affinity constants instead of avidity constants that would involve both paratopes of bivalent antibody molecules.
METHODS AND MATERIALS
The essence of the present assay platform is as follows. Antigen targets are immobilized on a functionalized glass slide in form of a microarray in such a way that epitopes on the targets are available to subsequent solution-phase antibodies. The antigen microarray is incubated in solutions of specific antibodies raised against the targets at a series of concentrations. Afterward the microarray is kept in a constant flow of the buffer to allow antibody-antigen complexes formed during incubation to dissociate. Surface mass densities of antibody-antigen complexes on the microarray during incubation and subsequent dissociation are recorded in real time with a scanning ellipsometry sensor.9 The sensor measures the phase change of an illuminating optical beam as a result of antibody-antigen complex formation. The phase change has been shown to be proportional to the surface mass density of antibody-antigen complexes. The optical data yield binding curves that are subsequently used to extract binding kinetic constants.9
Peptide antigen microarray
1,410 antigens are synthetic peptides (15-aa with average molecular weight of 2 kDa) supplied by Epitomics, Inc (Burlingame, CA). They originate from a large collection of source proteins (See Supplemental Information) that are mostly targeted in drug discovery. At either N- or C-terminus, a cysteine residue is added intentionally. The peptides are lyophilized as received. To make printing solutions, each peptide is dissolved in 2 μL DMSO and diluted further in 38 μL 1× PBS to a final concentration of 0.25 mg/mL (~ 125 μM). The solutions are deposited in a 384-well plate for microarray fabrication. Peptide microarrays are printed on epoxy-functionalized glass slides (ArrayIt, Sunnyvale, CA) using an OmniGrid 100 contact-printing robot (Digilab, Holliston, MA) with 100 μm diameter stainless steel pins (Majer Precision Engineering, Tempe, AZ). Primary amine residues and thiol residues on the peptides react covalently with epoxide groups on the glass surface and anchor the peptides. A significant fraction of the peptides is immobilized through the terminal cysteine, making functional regions of these immobilized peptides intact and available in subsequent binding assays.
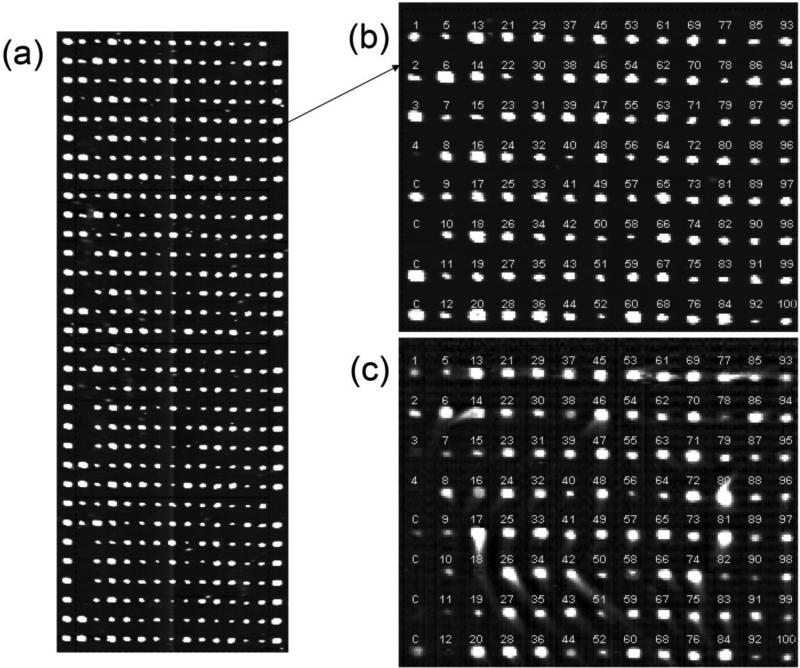
As shown in Figure 1, on one functionalized glass slide, we print 6 identical peptide microarrays so that after assembled in a fluidic cartridge each microarray is housed in a separate reaction chamber (12 mm L × 6 mm W × 0.4 mm D). In the present study, each peptide microarray (Figure 2(a)) consists of 4 identical subarrays as replicates, each having 100 distinct peptides and 20 control features in form of 8 rows × 15 columns. The left-most and the right-most columns consist of bovine serum albumin (BSA) and one blank. 100 peptides, 3 BSA and 1 blank form the remaining 13 columns (Fig. 2(b)). The diameters of printed peptide spots vary from 80 μm to 160 μm due to variations in wetting property of the peptide solutions. For the present study, 1,456 peptides are printed on 16 slides. We use 4 out of 6 microarrays on a glass slide to acquire a set of binding curves. The remaining two microarrays are for back-up. Before binding curve measurements, the peptide microarray is washed and blocked with a solution of BSA at 2 mg/mL in 1× PBS for 30 minutes and then washed and maintained in 1× PBS.8
Figure 1.
Sketch of a microarray-based label-free assay platform, consisting of a 1”×3” glass slide printed with 6 peptide target microarrays (the slide being an integral part of a 6-chamber fluidic assembly) and a scanning ellipsometry microscope (a.k.a. oblique-incidence reflectivity difference or OI-RD microscope). The microscope detects surface mass densities of target microarrays before, during and after reactions with unlabeled probes by measuring the extra phase change Δδ in a reflected optical beam due to targets or target-probe complexes on the glass surface. An OI-RD image of 6 peptide microarrays is shown. Each microarray is composed of 4 subarrays (for data statistics), and each subarray consists of 100 distinct 15-aa peptides and 20 control features including blanks.
Figure 2.
(a) OI-RD image of one peptide microarray (with 4 subarrays from top to bottom) after printing and before further processing. The left and right edges of each subarray are bovine serum albumin (BSA) and a blank. Each pixel of the image is 20 μm × 20 μm. (b) OI-RD image of one subarray without the left-most and right-most edges. It consists of 100 15-aa peptides and 4 control features (BSA and blank). The identities of the peptides are listed in Table 1. (c) Change in OI-RD image of the subarray after incubation with a monoclonal antibody mixture, at the individual probe concentration of 33 nM. The change measures the surface mass density of captured antibodies.
Monoclonal antibodies (MAbs)
To produce monoclonal antibodies, these peptides are conjugated to carrier protein KLH through the terminal cysteine residue using the standard maleimide conjugation scheme and Sulfo-SMCC cross-linkers (for example, see http://www.piercenet.com/product/maleimide-activated-klh-kit.) The conjugates are then introduced into rabbit or mouse for production of monoclonal antibodies. Harvested monoclonal antibodies are screened using direct peptide coating ELISA and western blot at Epitomics, Inc (Burlingame, CA) for specific binding. For direct peptide coating ELISA, we coated the ELISA plates using same peptides with terminal cysteine residues, either directly or having the peptides first conjugated to BSA and then using BSA-conjugated peptides for coating. We then added rabbit hybridoma supernatants to the plates and the binding of rabbit antibodies to the peptides were detected by HRP conjugated goat anti-rabbit secondary antibody (Jackson Immunoresearch). 1,410 rabbit mAbs and 46 mouse mAbs were selected. The selected antibodies are further purified and concentrated from supernatants by Nvigen, Inc (Santa Clara, CA) following a high-yield antibody enrichment protocol involving protein A/G coated magnetic beads (http://www.nvigen.com/docs/MagVigen_proteinAG_nanoparticles.pdf). Purified antibodies were quantified at Epitomics using SpectraDrop Micro-Volume Microplate/SpectraMax Microplate Reader (Molecular Devices) as follows. The antibodies were diluted with PBS (1:10 dilution) and 5 ul of the diluted antibodies were loaded onto SpectraDrop Micro-Volume Microplate (Molecular Device). The same buffer without the antibodies was also loaded as blank. SpectraMax Microplate Reader was used to read absorbance at 280 nm and in turn concentrations of the purified antibodies.
Scanning ellipsometry for label-free microarray detection
In this study the label-free sensor for real-time microarray detection is a scanning optical microscope that detects oblique- incidence reflectivity difference (OI-RD) due to an antigen microarray on a glass surface.8, 9 The optical signals are proportional to the surface mass density of antigen-antibody complexes. The OI-RD sensor does not require structured surfaces such as gold films or dielectric waveguides for detection and has a large “field of view” that easily expands from a few mm2 to 100 cm2. It is particularly suitable for detection of large microarrays on low-cost glass slides.
The arrangement of the scanning OI-RD microscope is shown in Figure 1.8, 9 A scan lens focuses a polarization-modulated laser beam (λ = 633 nm) to a 20-μm diameter spot on the back surface of a glass slide printed with antigen microarrays. The printed surface is in contact with either a buffer or an antibody solution. Images of a microarray are obtained by scanning the focused beam across the surface at 20 μm step size along the y-axis and by moving the microarray along the x-axis at the same step size with an encoded translation stage. Image “contrast” is the polarization change of the optical beam upon reflection from the surface. The ratio of reflectivities for p- and s-polarized components of the optical beam is given by rp /rs = tanφ · exp(iδ). The scanning OI-RD microscope measures the change in δ, namely, Δδ. Δδ varies linearly with the surface mass density Γ of antigen-antibody complexes as follows,8, 9
| (1) |
εs, ε0, and εd are optical dielectric constants of the glass slide, the aqueous ambient, and the antigen-antibody complex layer, respectively. ρ = 1.35 g/cm3 is the volume mass density of globular proteins. θ is the incidence angle in the glass slide. Given εs = 2.31, ε0 = 1.77, εd = 2.03 and θ = 65°, a layer of antigen-antibody complexes with Γ = 1 ng mm2 yields Δδ = +6.4 × 10−4. The current limit of our OI-RD microscope is |Δδ| ~ 9 × 10−6 , corresponding to a surface mass density of ~ 15 pg mm2.
High-throughput Binding Curve Acquisition
To exploit the high-throughput capability of the microarray-based platform, we take advantage of the fact that each monoclonal antibody is raised and ELISA-selected specifically against one peptide target and the cross-reactivity can be neglected. As a result we incubate a peptide microarray of 100 immobilized targets in an equally concentrated mixture of 100 corresponding specific monoclonal antibodies. We make four mixtures with individual antibody concentration C at 100 nM, 33 nM, 11 nM, and 3.7 nM, respectively. Identical peptide microarrays in four reaction chambers are used to acquire binding curves at four antibody concentrations.
For binding curve measurement, we first flow 1× PBS through a reaction chamber at 0.01 mL/min for 30 min to acquire the baseline. We next replace 1× PBS with an antibody mixture at 5 mL/min in 5 s and then reduce the flow rate to 0.01 mL/min to allow the microarray to incubate in the mixture under the flow condition for 30 minutes (association phase of the reaction). Afterward, we replace the mixture with 1× PBS at 5 mL/min in 7 s and then reduce the flow rate to 0.01 mL/min to allow antibody-antigen complexes to dissociate for 120 minutes (dissociation phase of the reaction).
To detect the amount of antigen-antibody complexes formed during association and dissociation phases of the reaction, we measure optical signals from a pixel in a target spot and two pixels in its neighboring unprinted region as the reference. The average of the signals read off the reference pixels is subtracted from the signal read off the target pixel to yield a background-corrected OI-RD signal Δδ.9 It takes 3 s for the scanning microscope to acquire one time-point from all 480 peptide targets in the microarray (120 targets in a subarray and 4 subarrays in one microarray). Each binding curve has 3600 time-points.
Langmuir Binding Model Analysis
We use the 1-to-1 Langmuir reaction model and the global curve fitting to analyze the binding curves. During the association phase, the optical signal Δδ(t), proportional to the surface mass density of antibody-antigen complexes Γ(t), is given by9
| (2a) |
Let t0 be the time when the antibody mixture is replaced with 1× PBS to mark the start of the dissociation phase. During dissociation,
| (2b) |
Δδ0 is proportional to the local peptide density where the target pixel is picked and thus varies from one replicate (in one subarray) to another (in another subarray) and from one binding curve (obtained in one chamber) to another (obtained in another chamber). As a result we fit the binding curve set for each antibody-antigen pair acquired at four antibody concentrations using kon and koff as global parameters valid for all four curves and Δδ0 as a local parameter adjustable for each curve. We compute the affinity constant (the equilibrium dissociation constant) as Kd = koff / kon9. In our present assay format, a set of four measurements in four chambers on one glass slide enable us to extract affinity constants for 100 antibody-antigen reactions.
Comparison with a benchmarking SPR-based label-free sensor
For comparison with a benchmark surface-plasmon-resonance (SPR) optical sensor for label-free binding curve acquisition,11, 12 we performed binding curve measurements on 6 antibody-antigen pairs using Biacore 3000 (https://www.biacore.com/lifesciences/products/systems_overview/3000) under similar incubation conditions. C1 sensor chips originally functionalized with carboxylic acids are converted to being NHS-functionalized after incubation in a mixture of EDC and NHS for 10 min. An NHS-functionalized surface is electrophilic, similar to an epoxy-functionalized surface that we use to immobilize cysteine-terminated peptides. Both NHS and epoxide residues react strongly with nucleophillic residues such as amine and sulf-hydryl groups on cysteine-terminated peptides. As a result we do not expect significant differences in immobilization efficiency and presentation of cysteine-terminated peptides on these two surfaces, except for the final peptide surface density determined by that of NHS groups on a C1 sensor chip or epoxide groups on a commercial epoxy-functionalized glass slide. For target immobilization, we incubate amine-reactive sensor surfaces of Chamber 2, 3 and 4 on the CM1 chip in 45 μM solutions of three different peptides for 20 min, according to the Biacore protocol. We then quench the surface of Chamber 1 and the unreacted surfaces of Chamber 2 through 4 by incubation in ethanolamine for 10 min followed by incubation in 0.2 mg/mL BSA for 10 min. Three rabbit monoclonal antibodies specifically raised against the 3 peptide targets in Chamber 2 through 4 are evenly mixed to make antibody mixtures at individual antibody concentration of C = 135 nM, 45 nM, and 15 nM. For binding reaction, a mixture flows throughall four chambers on the C1 sensor chip simultaneously for same incubation times and at comparable flow rates as in the OI-RD/microarray-based experiments. The signal from Chamber 1 is used as the reference to remove the background in the signals from the other three chambers. Different C1 sensor chips are used for different antibody mixtures as we do not regenerate the chip surface, - a procedure that is not reliable from our own experience.
RESULTS AND ANALYSIS
Figure 2(a) displays the optical image in Δδ (OI-RD image) of one printed peptide microarray before further processing. It consists of 4 identical subarrays. Each subarray contains 100 peptide targets (Figure 2(b)) and 20 control targets (bovine serum albumin and blank). The identities of these peptides (source proteins from which the peptides are derived) and their numeric assignments are listed in Table 1. Having 4 subarrays allows us to perform statistical analysis of binding kinetic parameters.9
Table 1.
Numeric ID and source proteins of 100 peptides along with association rates kon, dissociation rates koff, and equilibrium dissociation constants Kd = koff / kon with specific monoclonal antibodies. Peptide#4 yielded no binding curve data.
| Peptide ID | Original proteins from wich peptides are derived | kon 105 s−1M−1 | koff 10−5 s−1 | Kd pM |
|---|---|---|---|---|
| 1 | EEA1 | 2.3 | 2.8 | 120 |
| 2 | CD3 epsilon | 2.0 | 1.3 | 64 |
| 3 | MCSF | 18.0 | 3.2 | 18 |
| 4 | MLH1 | - | - | - |
| 5 | M110614 (1-6) | 1.0 | 1.3 | 129 |
| 6 | PK071 (22-20) | 0.7 | 10.1 | 1.370 |
| 7 | Histone H3 (tri methyl K27) - ChIP Grade | 7.5 | 4.6 | 61 |
| 8 | Histone H3 - ChIP Grade | 3.2 | 6.1 | 190 |
| 9 | Aurora B | 9.6 | 2.5 | 27 |
| 10 | Histone H3 (phospho S10) - ChIP Grade | 12.8 | 3.9 | 31 |
| 11 | APOBEC3G | 21.3 | 6.1 | 29 |
| 12 | HP1 alpha - Heterochromatin marker | 2.8 | 20.9 | 750 |
| 13 | PK036 (E7-B7) | 0.0 | 2.4 | 4,960 |
| 14 | PK011 (48-89) | 0.0 | 1.9 | 5,400 |
| 15 | PK022 (19-76) | 5.3 | 1.8 | 33 |
| 16 | PK040 (D0-G11) | 2.2 | 4.9 | 227 |
| 17 | PK023 (D11-7) | 1.0 | 1.2 | 118 |
| 18 | PK066 (20-119) | 4.8 | 4.9 | 103 |
| 19 | M120404 (A2-F6) | 9.9 | 1.5 | 15 |
| 20 | M120505-1 (1-B11) | 9.5 | 2.9 | 31 |
| 21 | DLGAP4 | 3.2 | 0.2 | 5.3 |
| 22 | Kallikrein 5 | 4.1 | 0.2 | 5.4 |
| 23 | PPP2CB | 2.4 | 2.2 | 92 |
| 24 | GLA | 3.8 | 0.9 | 24 |
| 25 | ACAT1 | 2.4 | 0.0 | 1.1 |
| 25 | Septin-10 | 1.8 | 0.3 | 15 |
| 27 | DOK5 | 4.0 | 4.2 | 106 |
| 28 | WDR5 | 4.2 | 0.6 | 15 |
| 29 | Lumican | 2.8 | 0.4 | 16 |
| 30 | NEDD4L Phospho (pS448) | 3.1 | 0.4 | 12 |
| 31 | Growth Arrest Soecific Protein 7 | 3.3 | 0.9 | 26 |
| 32 | PPP2CB | 1.7 | 0.1 | 7.6 |
| 33 | Glutamate dehydrogenase 1 | 1.7 | 1.5 | 91 |
| 34 | CDK5RAP3 | 2.0 | 0.3 | 15 |
| 35 | CLPX | 2.4 | 2.1 | 85 |
| 35 | Fbx32 | 2.6 | 0.6 | 22 |
| 37 | Pyruvate Dehydrogenase E1-alpha | 2.1 | 0.6 | 30 |
| 38 | NR5A1 | 0.2 | 2.8 | 1,730 |
| 39 | Na+/K+ ATPase a2 | 3.5 | 1.9 | 55 |
| 40 | BXDC1 | 0.7 | 1.8 | 235 |
| 41 | J1A | 1.6 | 0.8 | 48 |
| 42 | TCP1 eta | 2.8 | 0.4 | 13 |
| 43 | TAF1 | 2.4 | 0.3 | 13 |
| 44 | VMAT1 | 0.4 | 0.9 | 204 |
| 45 | COG7 | 1.8 | 0.7 | 41 |
| 46 | Glypican 4 | 1.5 | 0.1 | 7.4 |
| 47 | DNAJA2 | 0.8 | 0.6 | 78 |
| 48 | HoxC13 | 2.6 | 4.7 | 178 |
| 49 | PARM-1 | 1.8 | 0.2 | 10 |
| 50 | Alpha-1B Adrenergic Receptor | 8.1 | 0.6 | 7.7 |
| 51 | hnRNP E1 | 1.7 | 3.6 | 208 |
| 52 | hnRNP E1 | 2.3 | 0.5 | 20 |
| 53 | SHIP2 | 3.3 | 0.0 | 1.5 |
| 54 | CHMP1a | 2.4 | 1.1 | 48 |
| 55 | BASP1 | 1.8 | 2.0 | 109 |
| 56 | CUL2 | 1.7 | 1.2 | 70 |
| 57 | REEP5 | 1.2 | 0.5 | 41 |
| 56 | MRPS31 | 1.6 | 1.0 | 60 |
| 59 | H-Cadherin | 2.0 | 1.2 | 63 |
| 50 | Ribosomal Protein S15 | 1.1 | 0.2 | 20 |
| 61 | TTC14 | 4.6 | 0.3 | 7.1 |
| 62 | Histone Deaoetylase 11 | 2.9 | 1.9 | 65 |
| 63 | TPMT | 1.9 | 1.8 | 93 |
| 64 | PSAP | 5.2 | 4.8 | 92 |
| 65 | DDX20 | 1.6 | 1.2 | 74 |
| 66 | CDH29 | 1.6 | 0.2 | 13 |
| 67 | AARS | 2.0 | 0.2 | 12 |
| 68 | TXNRD1 | 1.8 | 0.1 | 6.6 |
| 69 | C1D | 2.1 | 0.2 | 11 |
| 70 | PRSS2 | 3.5 | 0.0 | 1.1 |
| 71 | TMEM45A | 1.8 | 0.0 | 1.2 |
| 72 | AKR1C1 | 2.3 | 0.0 | 0.88 |
| 73 | AK2 | 2.8 | 1.4 | 50 |
| 74 | CDCA3 | 3.0 | 0.2 | 5.5 |
| 75 | EDG2 | 5.1 | 0.7 | 13 |
| 76 | Beta-Glucuronidase | 2.7 | 1.2 | 46 |
| 77 | DEK | 1.0 | 0.3 | 24 |
| 78 | GPI | 1.7 | 0.9 | 55 |
| 79 | SPSB2 | 2.1 | 1.4 | 67 |
| 80 | PSMG1 | 1.4 | 0.0 | 1.1 |
| 81 | SPESP1 | 2.7 | 0.8 | 28 |
| 82 | IRF-6 | 2.0 | 5.7 | 283 |
| 83 | Doublecortin | 2.5 | 1.6 | 61 |
| 84 | POGZ | 4.9 | 0.6 | 12 |
| 85 | UGP2 | 3.3 | 1.6 | 49 |
| 86 | ACOT7 | 2.3 | 0.3 | 15 |
| 87 | ZAK | 9.2 | 4.2 | 45 |
| 88 | ACP1 | 6.4 | 1.7 | 26 |
| 89 | Glutamate dehydrogenase 1/2 | 20.6 | 5.1 | 25 |
| 90 | Napsin A | 3.7 | 1.8 | 48 |
| 91 | ERH | 2.0 | 4.7 | 239 |
| 92 | STOM | 4.4 | 1.9 | 43 |
| 93 | ISG20 | 1.8 | 4.4 | 242 |
| 94 | eIF-2A | 3.7 | 1.8 | 49 |
| 95 | HBXIP | 2.8 | 0.8 | 28 |
| 96 | Cathepsin L2 | 2.7 | 3.5 | 131 |
| 97 | UGT1A | 2.6 | 0.3 | 10 |
| 98 | Galectin-10 | 1.2 | 0.9 | 71 |
| 99 | ENO3 | 3.4 | 4.9 | 144 |
| 100 | TST | 2.0 | 1.5 | 78 |
Figure 2(c) shows the change in OI-RD image, obtained by subtracting the image taken before incubation from the image taken after incubation in an antibody mixture at the individual concentration of 33 nM. The contrast is proportional to the surface mass density of captured antibodies by immobilized peptide targets.
In Figure 3(a) we display binding curves (from one replicate for clarity) of 100 monoclonal antibodies to respective peptide targets simultaneously acquired during incubation in the antibody mixture at individual probe concentration C = 100 nM.9 Figure 3(b) combines binding curves acquired at 100 nM, 33 nM, 11 nM, and 3.7 nM into binding curve sets. Each set for one antibody-antigen pair is fit to Equation 2(a) and 2(b) with reaction rate constants kon and koff treated as global parameters.9 The results are listed in Table 1. The standard errors include contributions from the curve-fitting and variations from replicate to replicate in a microarray. For majority of the 100 antibody-peptide pairs, affinity constants Kd (equilibrium dissociation constants) are below 1 nM (10−9 M). The detection limit of Kd in the present study is 1 pM (10−12 M).
Figure 3.
(a) Binding curves of 100 monoclonal antibodies to respective peptide antigen targets simultaneously acquired during incubation in the antibody mixture with individual probe concentration at C = 100 nM. The vertical axis is the surface mass density change that has a full scale of 10 ng/mm2. The measurement on peptide#4 did not yield binding curves. (b) Binding curve sets for the same 100 antibodies acquired in 4 separate chambers at individual probe concentrations of 100 nM, 33 nM, 11 nM, and 3.7 nM. The curve sets are fit to the 1-to-1 Langmuir reaction model with kon and koff as global parameters. The equilibrium dissociation constant for each antibody-antigen pair is computed as Kd = koff/kon. These parameters are listed in Table 1.
This procedure is repeated for the remaining 1,356 monoclonal antibodies. The binding curve sets are fit to Equation (2a) and (2b) to yield association rates, dissociation rates, and affinity constants. These kinetic constants (kon, koff, and Kd) for all 1,456 monoclonal antibodies against their respective peptide targets are list in Table-S1 in Supplemental Information. To examine whether the binding kinetic constants are indeed affinity constants (monovalent) instead of avidity constant (bivalent) as each full rabbit antibody has two paratopes (two Fab domains), we repeated the association-dissociation curve measurement for three rabbit monoclonal antibodies with immobilized peptide targets at reduced surface densities (by reducing printing concentrations) so that the mean separation of the immobilized targets is twice the size of an IgG molecule. Though the captured rabbit antibody molecules per unit area are reduced in number by a factor of 4, the equilibrium dissociation constants Kd remain unchanged within a factor of 5. This shows that the measured Kd in the present study are indeed affinity constants.
To find the overall characteristic of affinity constants of all 1,410 rabbit monoclonal antibodies and the performance of our present OI-RD/microarray-based assay platform, we show in Figure 4 a plot of dissociate rate constants koff as a function of dissociation rate constants kon. Each point corresponds to one antibody-antigen pair and the ratio of the two constants is the affinity constant Kd for the pair. Contours of constant Kd are oblique straight lines shown so that the distribution of Kd can be seen along these lines. Histograms of the two kinetic constants and Kd are also displayed in the figure. Except for a small peak near 10−7 s−1 (the limit of the present study), koff are centered at around 10−5 s−1 with a full-width-at-half-max (FWHM) spanning one order of magnitude. kon are centered at around 105 s−1M−1 with a FWHM also over one order of magnitude. This makes Kd for 1,410 rabbit monoclonal antibody-peptide pairs to range from 20 pM to 200 pM with a median of 66 pM. For the 46 monoclonal mouse antibodies, albeit a much smaller collection, we find Kd to their respective peptide antigens ranging from 30 pM to 300 pM with a median of 72 pM, close to the median for 1,410 rabbit monoclonal antibodies.
Figure 4.
Maps of association rates, dissociation rates, and equilibrium dissociation constants for 1,410 rabbit monoclonal antibodies with specific 15-aa peptide antigen targets, from binding curves obtained using the microarray-based label-free assay platform. Each point is from one antibody-peptide pair. Diagonal lines show contours of constant equilibrium dissociation constant Kd = koff / kon in unit of molar (M).
Surface-plasmon-resonance-based (SPR) sensors such as Biacore 3000 were the first commercial label-free platform for biomolecular binding curve assays.11 Although all optical-reflection-based sensors including SPR sensors measure same surface mass density changes on solid surface, subsequent label-free sensors are often required to benchmark against an SPR sensor.13 For this purpose, we separately measured binding curves of 6 rabbit monoclonal antibodies against their peptide targets using both the OI-RD platform and a Biacore 3000.
Figure 5 shows binding curve sets of 3 rabbit monoclonal antibodies to peptide antigens obtained with the OI-RD platform and a Biacore 3000 under same reaction conditions except for immobilized target density and flow channel depth. The peptide targets are derived from EGFR Phospho (pY1068), CD34, and Cytokeratin 15. The quality of binding curves is comparable. It is noteworthy that net surface mass density changes in the SPR measurements are a factor of 4 smaller than those found in the OI-RD/microarray-based measurements, indicating that peptide target densities on the gold-coated surface of a C1 sensor chip (following the Biacore 3000 protocol) are smaller at least by a factor of 4 than the densities achieved on the epoxy-coated glass surface. It means that the mean separation between the immobilized peptide on the gold-coated surface is at least twice the size of an IgG molecule and the binding curves obtained with Biacore 3000 should yield affinity constants instead of avidity constants. We fit binding curve sets to Equation (2a) and (2b) with kon and koff as global parameters. The results are listed in Table 2. Kd's measured using both label-free sensors are comparable within a factor of 4. This once again confirms that our OI-RD/microarray-based assay platform truly measures affinity constants of antibodies against peptide antigens.
Figure 5.
Binding curve sets of 3 rabbit monoclonal antibodies with peptide targets, from CD34, Cytokeratin 15, and EGFR respectively, obtained using both Biacore 3000 and the microarray-based platform for comparison. The curve sets were globally fit to yield equilibrium dissociation constants (Kd). The results are listed in Table 2.
Table 2.
Comparison of the OI-RD/microarray-based assay platform with Biacore 3000 in characterization of specific affinity constants and rate constants of monoclonal rabbit antibodies with 6 immobilized peptide targets.
| Biacore 3000 | OI-RD scanning sensor | |||||
|---|---|---|---|---|---|---|
| Original proteins from which 6 peptides are derived | kon 105s−1M−1 | koff 10−5s−1 | Kd pM | kon 105s−1M−1 | koff 10−5s−1 | Kd pM |
| CD34 | 3.5 | 1.4 | 41 | 0.37 | 0.43 | 115 |
| Cytokeratin 15 | 7.3 | 1.8 | 247 | 0.16 | 0.89 | 564 |
| EGFR Phospho | 0.69 | 0.058 | 3.5 | 0.42 | 0.08 | 19 |
| Glucagon | 3.7 | 2.6 | 70 | 0.35 | 1.2 | 331 |
| Glut-1 | 4.6 | 1.1 | 24 | 0.36 | 0.028 | 7.7 |
| Vimentin | 2.3 | 7.1 | 306 | 0.25 | 4.3 | 1700 |
A closer examination of binding curves and fits to the 1-to-1 Langmuir reaction model (i.e., Equation (2a) and (2b)) reveals that there are more than one presentation of a peptide target upon immobilization so that the specific antibody molecules in the solution form more than one type of complexes with the immobilized peptides, each characterized with a distinct affinity constant.9, 14 This is particularly obvious for binding curves obtained with the Biacore 3000. More weakly bound complexes have significantly shorter dissociation times and may not be detected by endpoint measurements such as most fluorescence-based assays where measurements are performed after washing. When the assay time (incubation plus dissociation phase) is short, the presence of more weakly bound complexes makes “apparent” affinity constants determined from the fit to the 1-to-1 Langmuir reaction model larger than the constants for more tightly bound complexes. The latter are more closely resemble the complexes formed in solution.
DISCUSSION AND CONCLUSIONOWN
This study is the first where specific affinity constants of over 1,400 monoclonal antibodies raised with the same technology against synthetic peptide targets are determined on a microarray-based label-free assay platform. Qualities of binding curves and affinity constants obtained from this platform are comparable to those of a benchmark SPR-based platform (Biacore 3000).
Affinity constants for rabbit monoclonal antibodies to specific targets
Because 1,410 peptide targets come from a wide range of proteins or protein fragments, Figure 4 represents the typical distribution of affinity constants (20 ~ 200 pM) for rabbit monoclonal antibodies raised with a hybridoma technology of Epitomics, Inc against 15-aa peptides, measured using solid-surface-based assays. A small fraction of the 1,410 rabbit monoclonal antibodies have affinity constants in the order of 1 ~ 2 pM with dissociation rates close to koff= 10−7 s−1 (the limit of the present study). We should note here that by immobilizing peptide targets on a solid surface, available conformational isomers for such a peptide are significantly reduced in number from those for a same but free peptide in a solution so that association rates kon of corresponding antibodies to immobilized peptides are generally larger. Consequently affinity constants obtained from solid-surface-supported binding curve assays including the present OIRD/microarray-based assays and the SPR-based assays are expected to be smaller that the affinity constants obtained when both the antibody and the peptide antigen are in solution. A more detailed study of this topic will be separately reported.
Affinity constants of mouse monoclonal antibodies vs. affinity constants of rabbit monoclonal antibodies
For a pool of 46 monoclonal antibodies, their affinity constants to peptide targets cover a similar range (30 pM - 300 pM) as that for 1,410 rabbit monoclonal antibodies (20 ~ 200 pM). Within the limit of detection and the scope of this study (i.e., the pool size of investigated mouse monoclonal antibodies), it is inconclusive whether rabbit monoclonal antibodies have much higher affinity than mouse monoclonal antibodies.
Performance characteristics of the OI-RD/microarray-based assay platform
Using the OI-RD/microarray assay platform, we have measured Kd of protein-ligand binding reactions over a range from mM to 1 pM. Figure 4 highlights some of performance features of the platform such as limits of detectable kinetic constants and the extent of the mass transport effect on association rate measurement. The lower bound of detectable dissociation rates koff is 10−7 s−1. It is determined by the duration of the dissociation phase and the overall noise level of the assay platform.9 It may be further reduced by another order of magnitude with the improvement of the noise reduction. Though increasing the dissociation phase duration seems an equal option as well, it is often not practical. The upper bound of koff and konC is the larger of the sampling rate (0.33 s−1 in the present study) and the rate set by the effect of mass transport. As shown in Fig. 4 this platform yields kon as large as 106 M−1 s−1 at antibody probe concentrations at as high as 100 nM, thus the upper bound for konC and koff is mostly determined by the sampling rate of 0.33 s−1. It is worth emphasizing that key characteristics of antibody-antigen binding reaction or antibody-antigen complexes are dissociation rate constants koff. Since the latter are usually small for typical antibody-antigen pairs, their measurements over a long dissociation phase are not subject to the mass transport effect.
Binding curves in Figure 3 exhibit the sensitivity of the microarray-based platform for antibody characterization. A full layer of antibody molecules has a surface mass density of 5 ~ 16 ng/mm2, depending upon the average orientation of the aspheric molecules. It is a factor of 1000 larger than the background noise (15 pg/mm2) in our present assay system. Though it is not yet as sensitive as a Biacore 3000 (~ 1 pg/mm2), it is more than sufficient for assaying antibody-antigen binding reaction. Low cost per binding curve set and high-throughput make the microarray-based assay platform advantageous over Biacore 3000 or other similar platforms. As reported by Fei and coworkers,7 this platform has the capability to measure the temperature dependence of affinity constants and thus thermodynamic parameters of binding reactions that reveal details of antibody-antigen complex formation, such as entropic contributions to the stability of the complexes from available antigen isomers and effects of solvation/de-solvation.
Application of binding kinetic constants to understanding of various endpoint assays
Kinetic constants of antibody-antigen binding reactions including kon and koff as well as Kd obtained from microarray-based measurements can be qualitatively and even quantitatively applied to understanding of antibody binding to peptides/proteins in various endpoint assays such as ELISA; immuno-blotting; immuno-precipitation; immuno-histochemistry, etc. under different conditions or protocols. For a specific antibody-antigen reaction to reach saturation in any of these endpoint assays the association rate constant kon determines sufficient and but not excessive incubation times for a fixed antibody concentration or alternatively required antibody concentrations for a prescribed incubation time, while the dissociation rate constant determines the amount of the antibody-antigen complexes that survive repeated washing cycles. Furthermore dissociation rate constants for non-specific reactions of the same antibody with the backgrounds determine the effectiveness of washing cycles as the product of the total washing time and the non-specific dissociation rate constant should be much larger than unity. Poor performances of antibodies in these assays can be analyzed and understood in terms of (1) whether adequate incubation times are allocated for formation of intended antibody-antigen complexes, (2) whether washing cycles are excessive so that most intended antibody-antigen complexes may not survive; (3) whether washing cycles are sufficient for unintended complexes formed by same antibodies and background epitopes to become dissociated and removed; (4) whether the buffer condition during incubation and/or washing cycles in a specific protocol have modified binding kinetic constants and in turn the endpoints of the assay.
In conclusion, we measured affinity constants of 1,410 rabbit monoclonal antibodies and 46 mouse monoclonal antibodies to specific peptide targets on a microarray-based label-free binding curve assay platform. Affinity constants of rabbit monoclonal antibodies to immobilized peptide targets range from 20 to 200 pM with a median of 66 pM. The number of investigated mouse monoclonal antibodies is not large enough for such a general conclusion. We demonstrated that the ellipsometry/microarray-based assay platform is a high-throughput, cost-effective means of characterizing antibody affinity constants. In addition to the application as described in this study, this platform is useful for ranking/binning antibodies and evaluating cross-reactivity against a wide range of non-specific targets. Beyond antibodies, this platform is also suited for profiling a protein probe by affinity constants against a large collection of peptides that differ from each other by single amino-acids.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the University of California under U C Discovery Grant #bio09-156225 and in part by National Institutes of Health under R01-HD065122.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beck A, Wurch T, Bailly C, Corvaia N. Strategies and challenges for the next generation of therapeutic antibodies. Nat Rev Immunol. 2010 May;10(5):345–352. doi: 10.1038/nri2747. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen M, Marcellus RC, Roulston A, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A. 2007 Dec 4;104(49):19512–19517. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010 May;10(5):317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu X, Wong G, Audet J, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014 Oct 2;514(7520):47–53. doi: 10.1038/nature13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weingartl HM, Embury-Hyatt C, Nfon C, Leung A, Smith G, Kobinger G. Transmission of Ebola virus from pigs to non-human primates. Sci Rep. 2012;2:811. doi: 10.1038/srep00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perkel JM. The Antibody Challenge. Biotechniques. 2013;56:4. doi: 10.2144/000114143. [DOI] [PubMed] [Google Scholar]
- 7.Fei Y, Landry JP, Li Y, et al. An optics-based variable-temperature assay system for characterizing thermodynamics of biomolecular reactions on solid support. Review of Scientific Instruments. 2013 Nov;84(11):114102. doi: 10.1063/1.4826352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fei YY, Landry JP, Sun YS, et al. A novel high-throughput scanning microscope for label-free detection of protein and small-molecule chemical microarrays. Rev Sci Instrum. 2008 Jan;79(1):013708. doi: 10.1063/1.2830286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landry JP, Fei Y, Zhu X. Simultaneous Measurement of 10,000 Protein-Ligand Affinity Constants Using Microarray-Based Kinetic Constant Assays. Assay Drug Dev Technol. 2011 Dec 22; doi: 10.1089/adt.2011.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu XD, Landry JP, Sun YS, Gregg JP, Lam KS, Guo XW. Oblique-incidence reflectivity difference microscope for label-free high-throughput detection of biochemical reactions in a microarray format. Applied Optics. 2007 Apr 1;46(10):1890–1895. doi: 10.1364/ao.46.001890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loefas S, Malmqvist M, Roennberg I, Stenberg E, Liedberg B, Lundstroem I. Bioanalysis with surface plasmon resonance. Sensors and Actuators B. 1991;5(1-4):6. [Google Scholar]
- 12.Ritzefeld M, Sewald N. Real-Time Analysis of Specific Protein-DNA Interactions with Surface Plasmon Resonance. Journal of Amino Acids. 2012;2012:19. doi: 10.1155/2012/816032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu XD. Comparison of two optical techniques for label-free detection of biomolecular microarrays on solids. Optics Communications. 2006 Mar 15;259(2):751–753. [Google Scholar]
- 14.Sun YS, Landry JP, Fei YY, et al. Effect of fluorescently labeling protein probes on kinetics of protein-ligand reactions. Langmuir. 2008 Dec 2;24(23):13399–13405. doi: 10.1021/la802097z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.