Abstract
Introduction
Hair loss or alopecia affects the majority of the population at some time in their life, and increasingly, sufferers are demanding treatment. Three main types of alopecia (androgenic [AGA], areata [AA] and chemotherapy-induced [CIA]) are very different, and have their own laboratory models and separate drug-discovery efforts.
Areas covered
In this article, the authors review the biology of hair, hair follicle (HF) cycling, stem cells and signaling pathways. AGA, due to dihydrotesterone, is treated by 5-α reductase inhibitors, androgen receptor blockers and ATP-sensitive potassium channel-openers. AA, which involves attack by CD8+NK group 2D-positive (NKG2D+) T cells, is treated with immunosuppressives, biologics and JAK inhibitors. Meanwhile, CIA is treated by apoptosis inhibitors, cytokines and topical immunotherapy.
Expert opinion
The desire to treat alopecia with an easy topical preparation is expected to grow with time, particularly with an increasing aging population. The discovery of epidermal stem cells in the HF has given new life to the search for a cure for baldness. Drug discovery efforts are being increasingly centered on these stem cells, boosting the hair cycle and reversing miniaturization of HF. Better understanding of the molecular mechanisms underlying the immune attack in AA will yield new drugs. New discoveries in HF neogenesis and low-level light therapy will undoubtedly have a role to play.
Keywords: 5-α-reductase inhibitor, alopecia areata, androgenic alopecia, anti-androgen, baldness, chemotherapy-induced alopecia, hair follicle, low-level laser (light) therapy, stem cells, topical immunotherapy
1. Introduction
Hair follicles (HFs) are an integral part of the mammalian skin where they produce filaments largely composed of the protein, keratin [1]. Hair can be either thick, long terminal hair or and fine, short vellus hair. Hair helps the epidermis to maintain the body’s protective barrier against its external environment. In humans, however, the body hair has lost most of its importance as a physical protective barrier.
Several hypotheses have been put forward to explain how humans evolved to have radically different patterns of hair distribution compared to most other mammals [2]. These include the process of adaptation to provide a better thermoregulatory mechanism in the hot and dry climate conditions that arose about 3 million years ago when forests gave way to hot savanna grasslands. Another hypothesis is sexual selection based upon the dimorphism seen in the hair patterns of men and women. On average, men have more terminal body hair than women who have more vellus hair, which is less visible. A third hypothesis is adaptation to the advent of ectoparasites such as fleas and lice that could in theory have carried lethal diseases. The last hypothesis is that when humans began to walk upright, the need for hair by which young children could hold on to their mothers was lessened.
Nevertheless, hair still has great social significance for human beings. Healthy hair indicates health, youth and vigor. Male pattern baldness is taken to be a sign of age and loss of vigor, which may be concealed with a toupee, hats or simply by shaving the head. There is enormous demand for drugs and other treatments that can slow down or reverse hair loss; this has led to the creation of a multibillion-dollar industry [3]. In USA > 3.5 billion dollars is spent every year on treating hair loss. The American Hair Loss Association recognizes that hair loss is an extremely emotionally distressing disease that makes afflicted patients psychologically vulnerable [4]. This review aims to cover the biology of the HF, the different types of alopecia, laboratory models of hair loss and drug discovery efforts that are being undertaken for hair loss.
2. HFs, hair cycle, stem cells
Each strand of hair is made up of concentric regions, the medulla, cortex and cuticle (Figure 1). The innermost medulla is a variable space (sometimes present and sometimes not) and its function is still debated. The highly structured cortex is the primary source of the mechanical strength of the hair. The cortex contains melanin, which colors the fiber based on the number, distribution and types of melanin granules. The shape of the HF determines the shape of the cortex, so that hairs with circular cross-section are straight, and those with oval cross-section are curly. The cuticle is the outer covering of protein coated with a single molecular layer of lipid that makes the hair repel water. The diameter of the human hair varies from 17 to 180 µm [5].
Figure 1.
Cross-section of a normal hair showing central medulla, intermediate cortex, and outer cuticle.
The HF is a complex mini-organ [6] embedded in the skin and composed of the papilla, matrix, root sheath and bulge [7]. Structures closely associated with the HF are sebaceous glands, apocrine glands, the arrector pili muscle and mechanoreceptors that respond to touch [8]. Figure 2 shows the anatomy of the HF. There are between 250,000 and 500,000 HF on the human scalp and as many as 5,000,000 on the whole body.
Figure 2.
Schematic organization of the telogen-phase adult HF showing location of the stem cells. The stem cell populations are represented by their well-marked gene/protein-expression or promoter-activity: Lgr5 (hair germ and bulge), CD34 (bulge), LRC (bulge), Lgr6 (lower isthmus), Lrig1/MTS24 (isthmus), Blimp1 (sebaceous gland) and K15* (K15 promoter, bulge area).
HF: Hair follicle.
Hair grows in cycles during which it moves sequentially from one phase to another (Figures 3 and 4) [9]. Anagen is the growth phase; catagen is the involuting or regressing phase; and telogen, the resting or quiescent phase. There is also a shedding phase, or exogen, that is independent of anagen and telogen, in which one out of several hairs in a single follicle is physically shed. Normally, up to 90% of the HF are in anagen phase, while 10 – 14% are in telogen and 1 – 2% in catagen [10]. The length of the hair cycle varies between HF in different parts of the body. For eyebrows, the cycle is completed in around 4 months, while it takes the scalp 3 – 4 years to complete the cycle. The physical length of the hair depends on the duration of the hair cycle, which is why eyebrows are relatively short and scalp hair is often long.
Figure 3.
Hair follicle IRS structure. This features three distinct layers of epithelial cells, which are known as Henle’s layer, Huxley’s layer, and the IRS cuticle.
IRS: Inner root sheath.
Figure 4.
Hair cycle and its transitions. There are three main phases of the hair growth cycle; anagen, catagen and telogen with anagen further subdivided into proanagen, mesanagen and metanagen.
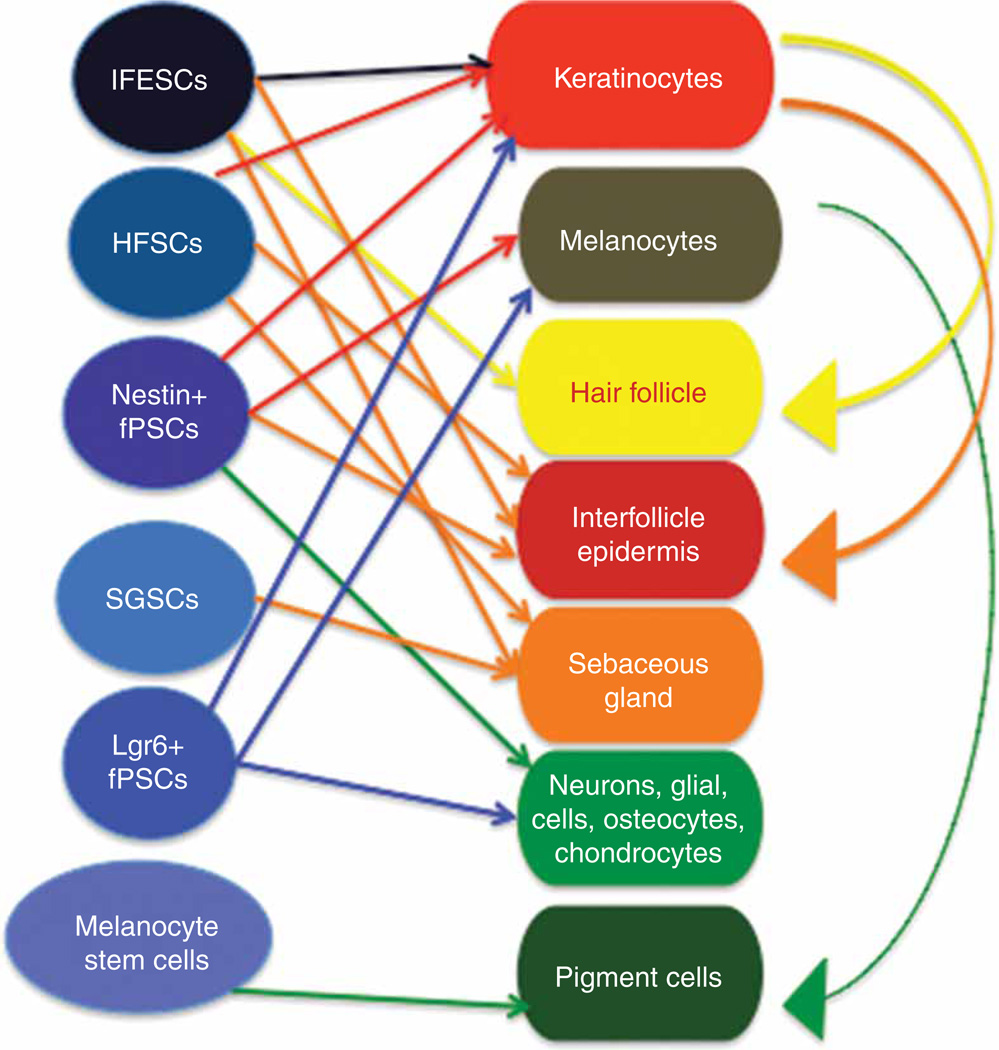
The signaling involved in the well-orchestrated process of hair growth and HF cycling is complex and incompletely understood [11]. The basic driving force is interaction between the mesenchymal and epithelial cell populations within the HF unit [12]. Figure 5 shows a schematic illustrating some of the different types of stem cells and the particular differentiated structures in the skin to which they contribute. The most important mesenchymal cells in the HF reside within the dermal papilla (DP). These cells produce signals to control sequential cycling of the follicular epithelium [13]. It is thought that epithelial stem cells, which reside in the bulge area of the HF, can respond to the signals from the DP [14]. This activation leads to production of progenitor cells from the stem cells in the bulge area, and then these progenitor cells become transiently amplifying cells that expand downward into the deep dermis, followed by differentiation into matrix cells that have the ability to produce the hair shaft, and its sheath. However, in both humans and especially in animals, the male or female genders have very different hair phenotypes, which are governed by the influence of sex hormones [15]. Several growth factor families are involved in HF cycling [13], namely fibroblast growth factor, EGF, hepatocyte growth factor, IGF-I, TGF-β families, among others. Signal transducer and activator of transcription 3 (stat3) is a latent cytoplasmic protein that conveys signals to the nucleus upon stimulation with cytokines/growth factors, leading to transcriptional activation of downstream genes that have the stat3 response element in their promoter region. Stat3 plays a critical role in HF cycling [16]. There is another, stat3 independent, pathway involving PKC, but both pathways eventually lead to activation of PI3K. It is thought the stat3-dependent pathway is involved in spontaneous HF cycling, while the stat3-independent pathway is involved in HF cycling after plucking for instance [16].
Figure 5.
Interactions between stem cells, progenitor cells, and cells in and related to the skin. IFESCs: Interfollicle epidermal stem cells; HFSCs: Hair follicle stem cells; SGSCs: Sebaceous gland stem cells; fPSCs: follicle nestin + pluripotent stem cells; Lgr6 + fPSCs, could be identical to fPSCs.
A series of signaling molecules is involved in each step of primary hair development and differentiation that have been elucidated in studies of embryogenesis [17]. Wingless type (Wnt) signaling is crucial for the initiation of HF development [18]. Wnt-protein is a ligand that binds to a cell-surface receptor ‘Frizzled’ family member, which then passes the biological signal to the intracellular protein ‘Dishevelled’ (Dsh). Dsh causes the accumulation of β-catenin in the cytoplasm (by protection from degradation) and its eventual translocation into the nucleus to act as a transcriptional co-activator of transcription factors that belong to the TCF/LEF family.
‘Sonic hedgehog’ (Shh) signaling plays an important role in both embryonic and adult HF development. Shh binds to and inhibits the extracellular domain ‘Patched,’ allowing the intracellular domain ‘Smoothened’ to accumulate and inhibit the proteolytic cleavage of the Gli family of zinc-finger transcription factors [19]. Shh signaling (and in particular activation of Gli2 [20]) is obligatory for development of the epithelial hair germ, comprising epidermal placodes and associated dermal condensates [21]. Noggin [22], bone morphogenetic protein (BMP) [23] and ectodysplasin [24] signaling also play important roles at early stages of HF placode development.
Dlx homeobox transcription factors regulate epidermal, neural and osteogenic cellular differentiation [25]. It was found Dlx3 played a central role as a transcriptional regulator of hair formation and regeneration. DLX3 co-localized with phosphorylated Smad1/5/8 complex, and regulated BMP signaling during hair morphogenesis in animal models [26].
An interesting study by Hamanaka et al. [27] showed that mitochondrial-generated reactive oxygen species (ROS) were critical mediators of cellular differentiation and HF morphogenesis. They generated mice with a keratinocyte-specific deficiency in mitochondrial transcription factor A (TFAM), which is required for the transcription of mitochondrial genes encoding electron transport chain subunits. Ablation of TFAM in keratinocytes impaired epidermal differentiation and HF growth and resulted in death 2 weeks after birth. TFAM-deficient keratinocytes failed to generate mitochondria-derived ROS, a deficiency that prevented the transmission of Notch and -catenin signals essential for epidermal differentiation and HF development [28]. In vitro keratinocyte differentiation was inhibited in the presence of antioxidants, and the decreased differentiation marker abundance in TFAM-deficient keratinocytes was partly rescued by application of exogenous H2O2.
The DP remains associated with the overlying epithelial matrix cells, which differentiate to give rise to the different HF lineages such as the cells that make the medulla, cortex and cuticle of the hair shaft and the inner root sheath (IRS) [29]. The matrix is derived from epithelial stem cells located in the bulge region of the HF [30]. Several important pathways and transcription factors that initiate and promote differentiation of the matrix cells have been determined, including Gata3 and Cutl (which regulate IRS differentiation and BMP signaling [31]), and transcription factors such as Gdsma3 [32], Msx2 [33], Foxn1 [34] and Hoxc13 [35] that are required for complete HF development and optimal hair shaft structure.
Cells with stem cell properties have recently been described in many integumental appendages including feathers [36] and teeth [37] but the HF stands out as one of the best model systems for studying adult stem cells [7]. The identification, characterization and transplantation of adult stem cells is currently one of the most intensively investigated areas of biological and biomedical research. HF are accessible, well defined in terms of their developmental biology, and their stem cell populations are located in discrete compartments or niches. Since the landmark paper almost 25 years ago by Cotsarelis et al. [14] highlighted the bulge as an important anatomical niche for HF epithelial stem cells in mice, much work directed at refining the location of stem cell compartments has been ongoing.
Aside from the location of these stem cells, the focus has been on three different aspects of their biology. Stem cells have the ability to self-renew and to generate progeny capable of differentiating into one or more cell types. The conventional view is that when stem cells divide, they give rise to one stem cell that remains in the niche and one transit-amplifying (TA) cell that can rapidly proliferate for a limited number of divisions, giving rise to the various differentiated cells of the tissue. It is thought that stem cells and TA cells are not interchangeable, and once the TA cell has left the niche, its progress toward differentiation is irreversible.
The role of tissue maintenance throughout the lifespan of an organism requires stem cells to preserve their genomic integrity. They do this by dividing infrequently, so as to reduce DNA replication errors, and are also commonly protected from environmental and chemical assault by the niches they inhabit. HF stem cells are suggested to be more quiescent than stem cells of other tissues such as the intestine. Using the accumulation of epigenetic errors as a measure of mitotic age, human scalp hairs from individuals of various ages were analyzed. Unexpectedly, no increase in epigenetic error frequency was observed relative to the age of individuals, suggesting a very slow cycling stem cell population that did not accumulate as many errors over time as stem cells seen in the colonic crypts [38].
3. Alopecia
Hair loss can be caused by any number of conditions, and can be classified as one out of a set of specific diagnoses, according to the ‘American Society of Hair Loss Association.’ Some diagnoses have alopecia in their title, such as androgenic alopecia (AGA), alopecia areata (AA) or chemotherapy-induced alopecia (CIA), while others do not. AGA is by far the commonest type of alopecia characterized by progressive hair loss caused by androgenic miniaturization of the HF, and has a gradual increase in incidence by age. Although AGA is often called ‘male pattern baldness’ it can affect up to 70% of men and also 40% of women at some point in their lifetime.
Probably the most common non-AGA alopecia a dermatologist will see are telogen effluvium (TE), AA and hair loss due to cosmetic over-processing. Other, more rare forms of hair loss may be difficult to diagnose, and some patients may wait months, even years for a correct diagnosis and undergo consultation with numerous dermatologists until they find one with knowledge of their condition. Plus, with rare diseases, there is little motivation for research to be conducted and for treatments to be developed. Often, even when a correct diagnosis is made, a dermatologist can offer no known treatment for the condition.
TE is a particularly alarming form of alopecia [39]. The typical patient is a woman claiming to have always had a ‘full head of hair’ and reporting her hair to come out suddenly ‘by the handful’. Patients are usually in good health, with no anorexia or nutrient deficiencies, possibly anxious, and have occasionally felt a painful or burning sensation at the scalp (trichodynia). Usually, the course of the disorder is chronic but intermittent, with apparent remissions followed by relapses. TE can be classified into three main categories: the premature teloptosis, the collective teloptosis and the premature entry into telogen: (drug-induced TE, TE due to dietary deficiencies and ‘autoimmune’ TE). The majority of TE patients are the autoimmune type, with some analogies with AA, including the triggering role of emotional stress.
4. Androgenic alopecia
4.1 Pathogenesis of AGA
AGA is often called ‘male pattern baldness,’ but the condition can affect large numbers of both men and women. Men typically present with hairline recession at the temples and balding at the vertex, while women normally suffer from diffuse hair thinning over the top of their scalp. Both genetic and environmental factors play a role, and the etiology remain incompletely understood.
Classic androgenic hair loss in males begins above the temples and vertex, or crown, of the scalp. As it progresses, only a thin rim of hair at the sides and rear of the head may remain (sometimes referred to as a ’Hippocratic wreath’), but AGA rarely progresses to complete baldness. The Hamilton–Norwood scale has been developed to grade AGA in males [40].
Female AGA has been colloquially referred to as ‘female pattern baldness,’ although this pattern can occur in males as well. It more often causes diffuse thinning without hairline recession, and like its male counterpart rarely leads to total hair loss [41]. The Ludwig scale grades severity of AA in females [42].
It is accepted that AGA is caused by changes in the male steroid hormones known as androgens [43], and variants in the gene for the androgen receptor has been implicated in AGA [44]. Androgens are important in male sexual development around birth and at puberty. They regulate sebaceous glands, apocrine hair growth and libido. With increasing age, androgens stimulate hair growth on the face, but suppress it at the temples and scalp vertex, a condition that has been referred to as the ‘androgen paradox.’ Men with AGA typically have lower total testosterone, higher unbound/free testosterone and higher free androgens, including dihydrotestosterone (DHT) [45]. The enzyme that transforms testosterone into DHT is known as 5-α-reductase (5-α-R) (or 3-oxo-5α-steroid 4-dehydrogenase). There are three isoenzymes of 5-α-R: known as 5α-R1, 5α-R2 and 5α-R3. 5α-R1 is expressed about 50 times more in the scalp of adult males than in the scalp of male fetus. Males with AGA have more 5α-R1 in scalp HF than males without AGA [45]. Therefore, the prevailing hypothesis is that DHT is responsible for miniaturization of HF [46].
Adrenal steroids such as dehydroepiandrosterone can be converted to 5α-DHT by isolated HFs, which may provide an additional source of intrafollicular DHT. Elevated urinary dehydroepiandrosterone and serum dehydroepiandrosterone sulfate have been reported to be present in balding young men. These reports suggest that dehydroepiandrosterone sulfate may act as an important endocrine factor in the development of AGA [47].
A difference between AGA and CIA can be summarized as follows. Chemotherapy targets transiently amplifying progenitor cells but spares the mostly static stem cells. Therefore, the hairs regrow after the chemotherapy treatment is finished. In contrast, androgens inhibit Wnt signaling [48], which is required for the ability of the DP to induce hair cycling and regeneration [49]. This mechanism implies that targeting androgens as a therapy for AGA gives only a transient effect, while CIA can completely recover and hair growth becomes normal again.
The growth and dormancy of HF have been related to the activity of IGF at the DP, which is affected by DHT [43]. Studies looking at serum levels of IGF-1 have shown it to be increased with vertex balding [50]. Earlier work looking at in vitro administration of IGF had no effect on HF when insulin was present, but when insulin was absent, IGF-1 caused follicle growth [51]. The effects on hair of IGF-I were found greater than IGF-II [52]. IGF-1 signaling controls the hair growth cycle and differentiation of hair shafts, possibly having an antiapoptotic effect during the catagen phase. Mutations of the gene encoding IGF-1 result in Laron syndrome shortened and morphologically bizarre hair growth and alopecia [53]. IGF-1 is modulated by IGF binding protein, which is produced in the DP [54].
DHT inhibits hair growth in mice by inhibiting IGF-1 at the DP [55]. The involvement of IGF signaling in the ongoing functioning of HF stem cells adds another angle to the effects of DHT on hair growth [56].
Extracellular histones inhibit hair shaft elongation and promote regression of HFs by decreasing IGF and alkaline phosphatase in transgenic mice [57]. Silencing P-cadherin, a HF protein at adherens junctions, decreases IGF-1, and increases TGF-β 2, although neutralizing TGF decreased catagenesis caused by loss of cadherin, suggesting additional molecular targets for therapy. P-cadherin mutants have short, sparse hair [58]. In addition, androgens enhance inducible nitric oxide synthase from occipital DP cells and stem cell factor for positive regulation of hair growth in beard and negative regulation of balding DP cells [59]. Other research suggests that the enzyme prostaglandin D2 (PGD2) synthase and its product, PGD2 in HF could contribute to hair loss in AGA [60].
Recently, a newly discovered class of tiny noncoding RNAs named microRNAs have been discovered to play a role in regulating gene expression, and have led to the new field of ‘regulomics’ [61]. These tiny bits of transcriptome fine-tune the expression of nearly one-third of the genes in mammals [62]. MicroRNAs affect skin development [63], and play a role in HF morphogenesis, maintenance and cycling (controlling proliferation, growth arrest and apoptosis) [64]. Micro- RNAs have also been shown to play a role in the pathogenesis of AGA, with higher expression in the DP of HF from AGA patients versus normal HF [65].
Reduced blood flow and oxygen pressure in the balding areas are also involved in male pattern baldness. It has been shown that decreased blood flow and lower partial pressure of oxygen occurs in the balding scalp compared with nonbalding areas and with controls [66]. The decreases found in partial pressure of oxygen and blood flow are ~ 40 and 62%, respectively. However, it is unlikely that these decreases in partial pressure of oxygen or blood flow are the root cause of hair loss. Only a fraction of the blood flow to the skin is used for metabolic needs and the rest is for temperature regulation. Furthermore, even for metabolically very active cells, the critical partial pressure of oxygen for impairment of mitochondrial function is < 5 mmHg and about 0.1 mmHg for hypoxic cell death [67].
4.2 Models for androgenic alopecia
The organ culture of entire HF isolated from the human scalp was first reported by Philpott et al. [68]. The HF organ culture model is commonly used to determine whether small molecules or biologics may have a hair growth-promoting effect [69]. The advantage is that the effect of a compound can be assessed simultaneously on the epithelial and mesenchymal compartment of the HF. However, obtaining sufficient human samples can be challenging and in such cases mouse or rat vibrissae follicles may serve as alternatives to human HF [70,71]. Another drawback of human HF is that, although they can be switched from anagen to catagen phase in vitro, via insulin/IGF-I starvation or culture with TGF-β, modeling of the human hair growth cycle in vitro is still not possible. Murine follicles may serve as excellent models for such cases [70]. A red deer mane follicle model described by Thornton et al. served a useful in vitro androgen-responsive model owing to their ability to produce a mane during the breeding season when plasma testosterone levels are high, which is replaced by the short neck hairs of the summer coat when testosterone levels are low [72].
A major challenge in developing new therapies for AGA is the lack of small animal models to support drug discovery research. Stump-tailed macaque (Macaca arctoides) that possesses hereditary balding characteristics similar in many respects to that of AGA remains the model of choice despite its high cost, difficulty of manipulation and low availability [73,74]. In an attempt to overcome this challenge, recently, Crabtree et al. developed the first transgenic mouse model of AGA overexpressing human androgen receptor (AR) in the skin under control of the keratin 5 promoter [45]. When the keratin 5-human AR transgenic mice was exposed to high levels of 5-αDHT, delayed hair regeneration was observed and upon administration of AR antagonist hydroxyflutamide (active metabolite of flutamide) DHT-induced baldness was prevented. This model enables investigation of both topically or systemically administered drugs for DHT-induced delay in hair regrowth. Furthermore, many studies of prostate cancer demonstrate that by binding to β-catenin, androgen-bound AR can inhibit Wnt signaling through sequesterization of β-catenin [75]. The same group has developed a double transgenic mouse model expressing both K5-hAR and tamoxifen-inducible β-catenin (S33Y-ER-ligand- binding domain) to directly assess the involvement of Wnt signaling in this mouse model. These models will also enable identification of specific genes and pathways regulated by AR and β-catenin within the context of AGA.
The fuzzy rat model, a genetic mutant between hairless and hairy albino rat, shows androgen-dependent hypersecretion of sebum and hyperplastic sebaceous glands [76,77]. Another testosterone-inducible model of alopecia, called androchronogenetic alopecia, has been reported in B6CBAF1/j mice that were a hybrid cross between a C57BL/6 female and a CBA male [73,78]. Xenografts of human skin on immunodeficient mice, nude (Hfh11nu) or severe combined immunodeficiency (Prkdcscid) as well as xenografts to double mutant mice for severe combined immunodeficiency (Prkdcscid/Prkdcscid) and a number of hormone receptor null mutations offer new alternatives to testosterone-inducible models [73,79]. Nude mice are especially suitable to study AGA due to their lack of readily observable hair, making xenografts easy to identify and follow [79].
4.3 Drug discovery for androgenic alopecia
There are only two FDA-approved current therapies for AGA, namely topical minoxidil (Rogaine) and oral finasteride (Propecia). In 1988, the FDA approved 2% minoxidil topical solution (Rogaine®) for use in treating AGA in men. A 2% solution marketed to women became available in 1991, and a 5% solution became available over the counter for use in men in 1997. In 1997, finasteride (Propecia) was approved by the FDA for the treatment of male AGA at a dose of 1 mg/day. This medication is a competitive inhibitor of type II 5-α-R that inhibits the conversion of testosterone to DHT, which is involved in miniaturizing the HF in AGA [80].
Table 1 lists the approved and investigational drugs that have been used, tested or considered for AGA. Drug candidates largely fall into the classes of 5-α-R inhibitors, androgen receptor antagonists, ATP-sensitive potassium channel openers, topically growth factors, and a diverse selection of antioxidants and botanical extracts suggested by studies in ethnophamacology. It should be noted that the duration of the therapeutic effect does vary between different treatments. For instance hair loss can rapidly resume after cessation of minoxidil therapy. In fact, since AGA is an ongoing process, it is likely that even the most successful treatment will not lead to a permanent cure.
Table 1.
Approved and Investigational drug therapies for androgenetic alopecia
| Drug (Trade name) | Structure | M, F, Both, animal | Class/route | Mechanism | Ref. |
|---|---|---|---|---|---|
| Minoxidil (Rogaine) |  |
Both; approved | Vasodilator, ATP-sensitive potassium channel opener; topical | Increases blood flow to HF, stimulates VEGF and PGE2 | [131] |
| PInacidil (Pindac) |  |
Both; clinical trial | Vasodilator, ATP-sensitive potassium channel opener; topical | Increases blood flow to HF, stimulates VEGF and PGE2 | [132] |
| Tretinoin (Retin-A) | 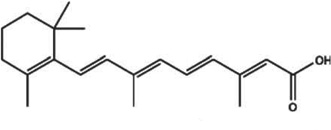 |
Both; clinical trial | Retinoid; topical | Promotes proliferation and differentiation, may promote vascular proliferation. | [133] |
| Finasteride (Propecia) |  |
Both, approved M | 5-α-reductase (5-α-R) inhibitor; oral, topical, mesotherapy | Inhibits 5-α-R2 | [134,135] |
| Dutasteride (Avodart) | 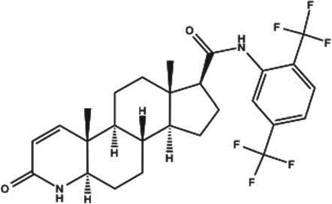 |
Both, approved M | 5-α-R inhibitor oral, topical, mesotherapy | Inhibits 5-α-R1 and 5-α-R2 | [136,137] |
| Bicalutamide (Casodex) |  |
F; clinical trial | Nonsteroidal antiandrogen; oral | Androgen receptor antagonist | [138] |
| Flutamide (Eulexin, Flutamin, Cytomid) |  |
F; clinical trial | Nonsteroidal antiandrogen; Oral | Androgen receptor antagonist | [139] |
| Fluridil (Eucapil) |  |
M; clinical trial | Nonsteroidal antiandrogen; topical | Androgen receptor antagonist | [140] |
| Cyproterone acetate (Androcur, Cyprostat) | 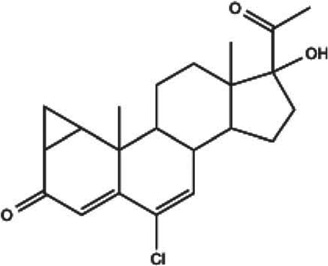 |
W; clinical trial | Steroidal antiandrogen | Androgen receptor antagonist; progesterone receptor agonist | [141] |
| Spironolactone (Aldactone) |  |
F; clinical trial | Steroidal potassium-sparing diuretic | Reduces adrenal androgen production and competitively blocks androgen receptors | [142] |
| Melatonin; N-acetyl-5-methoxytryptamine |  |
F; clinical trial | Hormone produced to regulate photo-periods, topical | Responsible for seasonal hair growth in animals | [143] |
| Bimatoprost (Lumigan, Latisse) |  |
Both; approved for eyebrows | Prostaglandin analog prodrug; topical | Activates prostamide α F2 receptors | [144] |
| RU-58841 myristate; |  |
Hamsters, macaques | Pro-drug for nonsteroidal antiandrogen; topical | Androgen receptor antagonist | [138] |
| SGF57 growth factors: bFGF, VEGF, IGF, SCF, KCF, SOD1, noggin | F; clinical trial | Growth factor cocktail; topical + microneedling | Stimulates HF | [145] | |
| Liposomal IGF-1 | Hamster | Growth factor, Topical | Stimulates HF | [146] | |
| Thymosin β 4 | Rats, mice | 43-amino acid protein; topical | Stimulates HF stem cells | [147] | |
| KF19418 |  |
Mice | Condensed naphthyridine derivative; topical | Anti-inflammatory, bronchodilator | [148] |
| Epigallocatechin-3-gallate (EGCG) |  |
Mice | Polyphenolic antioxidant; topical | Downregulated androgen receptor, reduced apoptosis | [149] |
| Polygonum multiflorum extract |  |
Mice | Botanical extract in water containing 2,3,5,4-tetrahydroxystilbene-2-O-β-dglucoside; topical with added lactobacilli | Increases anagen-phase HF; traditional folk-medicine for baldness | [150] |
| Eclipta alba extract | Mice | Botanical extract in methanol containing coumestans, triterpenes, saponins, flavonoids; topical | Increases melanogenesis, anagen-phase HF; traditional folk-medicine for baldness | [151] | |
| Thuja orientalis extract | Mice | Botanical extract in water containing flavonoids and diterpene; topical | Contains natural 5– reductase inhibitors; Traditional folk-medicine for baldness | [152] | |
| Steroid sulfatase inhibitors | Investigational | Topical | Reduce additional source of DHT | [153] | |
| Antisense oligonucleotides against AR | Investigational | Topical | Reduce expression of AR | [154] |
bFGF: Basic fibroblast growth factor: DHT: Dihydrotestosterone; HF: Hair follicle.
It is widely considered that topical application of active agents is superior to oral or parenteral administration of drugs for AGA [81]. The ideal therapeutic would be a cream or lotion that one could rub on one’s head in the morning after one’s shower. However this laudable desire comes up against the old problem of it being very difficult to get active drug molecules to cross the skin barrier. The problem may indeed be somewhat less daunting when the actual target of the drug is the HF itself [82] Lademann et al. at the Center for Experimental and Applied Cutaneous Physiology, Charité – Universitätsmedizin Berlin, Germany, suggested that HF are a highly relevant and efficient penetration pathway and reservoir for topically applied substances [83]. There have been efforts using specially designed nanoparticles to improve drug delivery via HF [84].
5. AA, alopecia totalis and alopecia universalis
5.1 Pathogenesis of AA
AA is a condition in which hair is lost from the scalp in patches sometimes called ‘spot baldness’. The number of people with AA, who go on to develop alopecia totalis (loss of hair from the entire scalp) or alopecia universalis (loss of hair from the entire body) is not known, but estimates range from 7 to 30% [85]. AA is typically multifocal and the bald areas are commonly oval or circular in shape and smooth to the touch. Hair shaped like an exclamation mark can be present around the margins of the patch. Vitiligo and autoimmune thyroid disorders are sometimes associated with AA [86], and patchy AA often spares gray hairs [87].
AA is a common autoimmune disease resulting from damage caused to the HFs by T cells. Evidence of autoantibodies to anagen stage HF structures is found in affected humans and experimental mouse models [88]. Research currently points to a cell-mediated autoimmune mechanism as the underlying etiology of this disorder, although autoantibodies are presumed to play an integral role in the mechanism of disease [89]. Biopsy specimens from affected individuals demonstrate a characteristic peri- and intrafollicular inflammatory infiltrate around anagen-stage HFs consisting of activated CD4 and CD8 T lymphocytes [90]. T lymphocytes cultivated from areas of affected scalp have also been shown to transfer AA to areas of nonaffected scalp in a severe combined immunodeficiency mouse model [91]. Recent studies found that transplantation of AA tissue to normal mice does not induce AA if the MoAb, anti-CD44v10, was injected into the normal mice shortly after transplant surgery [92]. CD44v10 is presumed to be involved in the activation mechanism of CD4 and CD8 lymphocyte and the migration into tissue and the subsequent initiation of the immune attack on HF. Similar investigations show that in vivo depletion of CD4+ cells with the CD4+ cell-depleting OX-35/OX-38 MoAb partially restores hair growth in AA-affected rats [93].
AA occurs more frequently in people who have affected family members, suggesting heredity may be a factor [94]. Strong evidence of genetic association with increased risk for AA was found by studying families with two or more affected members. This study identified at least four regions in the genome that are likely to contain these genes [95]. In addition, it is slightly more likely to occur in people who have relatives with autoimmune diseases.
Endogenous retinoids play a key role in the pathogenesis of AA [96]. Genes involved in retinoic acid (RA) synthesis were increased, whereas RA degradation genes were decreased both in AA mice, and in biopsies from AA patients. RA levels were also increased in C3H/HeJ mice with AA (see description of the model in next section). C3H/HeJ mice that were fed a purified diet containing high vitamin A showed accelerated development of AA.
AA has been linked with certain human leukocyte antigen (HLA) class II alleles, as have many autoimmune diseases. The HLA antigens DQB1*03 (DQ3) and DRB1*1104 (DR11) were strongly associated with a general susceptibility for AA [97]. Patients with alopecia totalis and alopecia universalis were found to express a significantly increased frequency of HLA alleles DQB1*0301 (DQ7), DRB1*0401 (DR4) and DRB1*1104 (DR11) [98].
In 2010, a genome-wide association study was completed that identified 129 single nucleotide polymorphisms that were associated with AA. The genes that were identified include those connected with regulatory T cells, cytotoxic T lymphocyte-associated antigen 4, IL-2, IL-2 receptor A, Eos, cytomegalovirus UL16-binding protein and the HLA region [89]. The study also identified two genes, PRDX5 and STX17 that were expressed in HF [99].
HF enjoy a relative degree of immune privilege that is characterized by downregulation of MHC class I, and local expression of immunosuppressants. Normally, NK cells attack cells with absent/low MHC class I expression, so healthy human anagen HF must somehow escape from NK cell attack. Ito et al. [100] found that immune avoidance happens via an active NK cell suppression. AA HF showed striking defects in NK cell inhibition/containment, with the NK cell inhibitor, macrophage migration inhibitory factor being strongly expressed by the HF epithelium, and very few CD56(+)/NK group 2D-positive (NKG2D+) NK cells were observed in and around normal anagen HF. By flow cytometry, many fewer NK function-activating receptors (NKG2D, NKG2C) and significantly more killer cell Ig-like receptors- 2D2/2D3 were found to be expressed on peripheral blood CD56(+) NK cells of healthy controls than on those of AA patients.
Xing et al. [101] showed that cytotoxic CD8(+)NKG2D(+) T cells were both necessary and sufficient for the induction of AA in mouse models of disease. Global transcriptional profiling of mouse and human AA skin revealed gene expression signatures indicative of cytotoxic T-cell infiltration, an IFN-γ response and upregulation of several γ-chain cytokines known to promote the activation and survival of IFN-γ-producing CD8(+)NKG2D(+) effector T cells.
As AA is accepted to be an autoimmune disease, it can be concluded that certain host proteins can act as autoantigens. Leung et al. [102] isolated AA-reactive HF-specific antigens from normal human scalp anagen HF extracts by immunoprecipitation using serum antibodies from 10 AA patients. Samples were analyzed by LCMALDI-TOF/TOF mass spectrometry, which indicated strong reactivity to the hair growth phase-specific structural protein trichohyalin in all AA sera and to keratin 16 (K16) in some sera. A MoAb to trichohyalin revealed that AA sera contained immunoreactivity that co-localized with trichohyalin in the growth phase-specific IRS of HF, and AA serum reactivity with anti-K16 antibody was observed in the outer root sheath of the HF.
5.2 Models for AA
Since the pathology of AA involves the interplay between the immune cells of the host and the cells of the HF, it is less convenient to use in vitro or ex vivo models to study AA, than it is for AGA which more involves the biology of HF alone. Therefore, animal models are needed to study this cell-mediated, organ-specific autoimmune disease.
AA-like hair loss has been observed in several species, including monkeys, dogs, cats, horses, cattle, poultry and nonhuman primates [88,103,104]. However, the use of these species in AA research is restricted due to their limited numbers, genetic variability, scattered geographical distribution [104] therefore inbred rodent strains are likely to be considered better research models. Several rodent models with spontaneous and induced AA have been identified and of these, C3H/HeJ mice and Dundee experimental bald rat (DEBR) are most commonly used. The DEBR develops spontaneous AA at a higher frequency than mice, they are more expensive to use in drug studies owing to their larger size [105]. The low frequency of AA and not being able to predict the stage of AA as it evolves in the naturally occurring C3H/HeJ model of AA can be converted into a predictable system by grafting full thickness skin from AA-affected mice to normal haired mice of the same strain [105]. Human scalp explants grafted onto severe combined immunodeficient (SCID) mice is another experimental model reported by Kyoizumi et al. [106]. Recently, Gilhar et al. [107] developed a new humanized mouse model of AA, by transplantation of healthy human scalp skin obtained from normal volunteers on to SCID mice. This was followed by intradermal injection of either autologous or allogeneic peripheral blood mononuclear cells, which had been cultured with a high dose of IL-2 and were enriched for NK group 2D-positive (NKG2D+) and CD56+ cells. This protocol led to rapid and predictable development of focal hair loss, with all the features of AA the Gu et al. [108] created a mouse model through repeated backcrossing/intercrossing between C57BL/6 and congenic AA(tj)mice (named B6.KM-AA). B6.KM-AA mice grew slower than B6 control mice and AA skin lesions developed by 4 weeks of age. The number of HF was reduced, but hair structures were normal. Loss of hair during disease progression was associated with CD4(+) and CD8(+) T lymphocytes infiltration peri- and intra-HF.
5.3 Drug discovery for AA
For the patient with AA, there is currently no universally proven therapy that induces and sustains remission, nor is there an accepted cure for this disease. The unpredictable course of the disease with spontaneous remissions frequently occurring also makes it challenging to design and conduct trials. Ito [109] pointed out that since spontaneous remission occurs in 80% of patients within 1 year, and not all patients require intense therapy, that no therapy at all (watchful observation) could be an option. Indeed many different therapies are available for this disease, and treatment choices are frequently based on disease extent, activity, duration of disease, and age of the patient. The drugs that are clinically used, in clinical trials or under laboratory investigation for AA are listed in Table 2.
Table 2.
Approved/investigational drug therapies for alopecia areata or alopecia universalis.
| Drug (Trade name) | Structure | M, F, Both, animal | Class/route | Mechanism | Ref |
|---|---|---|---|---|---|
| Hydrocortisone (Cortizone) |  |
Both; approved | Corticosteroid; topical; intradermal injection, oral | Anti-inflammatory, immunosuppressive | [155] |
| Clobetasol propionate (Temovate) |  |
Both; clinical trial | ‘Strong’ corticosteroid; topical; intradermal injection | Anti-inflammatory, immunosuppressive | [155] |
| Dinitrochlorobenzene |  |
Both | Local irritant; topical | Triggering sensitization counteracting autoreactive T cells | [110] |
| Squaric acid dibutylester |  |
Both | Local irritant; topical | Triggering sensitization counteracting autoreactive T cells | [156] |
| Diphenylcyclopropenone (Diphencyprone) |  |
Both | Local irritant; topical | Triggering sensitization counteracting autoreactive T cells | [157] |
| 8-Methoxypsoralen and UVA (PUVA) |  |
Both | Photochemotherapy, drug-light combination; topical | Kills autoreactive T cells; induces tolerogenic dendritic cells | [158] |
| Sulfasalazine (Azulfidine) |  |
Both; clinical trial | Developed as antibiotic; oral | Anti-inflammatory immunomodulator | [159] |
| Adalimumab (Humira) | F; case report | Recombinant human IgG1 MoAb against TNFα; s.c. injection | Anti-inflammatory; inhibits T cells | [160] | |
| Alefacept (Amevive) | F; case report | 1-92-LFA-3 (human) fusion protein with Ig G1 | Inhibits T cells | [161] | |
| Ruxolitinib (Jakavi) |  |
Case reports, mice | JAK inhibitor; topical, systemic | Affects downstream signaling of IFN-γ; and GC cytokine receptors | [101] |
| Tofacitinib (Jakvinus) |  |
Mice | JAK inhibitor; topical, systemic | Affects downstream signaling of IFN-γ and GC cytokine receptors | [101] |
Because of its autoimmune character, topical corticosteroids remain the cornerstone of initial treatment, as they are for many other inflammatory skin disorders. There have been a number of topical ‘sensitizing’ agents such as diphenylcyclopropenone, employed that are known as ‘topical immunotherapy.’ These compounds have in common the ability to cause the induction and periodic elicitation of allergic contact dermatitis as they act as potent contact allergens [110]. There is a decrease in CD4 to CD8 T-cell ratio from 4:1 to 1:1 [111] together with a decrease in lymphocytes and Langerhans cells in the HF bulb. Happle proposed [112] the concept of ‘antigenic competition,’ where an allergic reaction generates suppressor T cells that nonspecifically inhibit the autoimmune reaction against an HF antigen.
6. Chemotherapy-induced alopecia
6.1 Pathogenesis of CIA
Cancer chemotherapy is associated with severe side effects due to the induction of apoptosis in rapidly dividing cells within sensitive tissues (such as the hematopoietic system, the epithelia of the digestive tract and other organs). This apoptosis largely depends on p53, known as ‘the guardian of the genome,’ which accumulates in sensitive cells after a variety of stresses, resulting in growth arrest or induction of programmed cell death [113]. Chemotherapy affects the proliferating matrix keratinocytes in the bulb in the anagen HF that are producing the hair shaft. Damage to proliferating cells causes the HF to enter a dystrophic catagen stage in which the integrity of the hair shaft is compromised and the hair falls out. A significant fraction of HF is in the anagen phase at any one time, and these hairs are rapidly lost during chemotherapy, with massive apoptosis seen in the proximal bulb region. Chemotherapy-induced DNA damage leads to the rapid accumulation of p53 protein in the affected cells, followed by upregulation of Fas, IGF-BP3 and Bax, all of which are encoded by the corresponding p53-responsive genes [114].
Moderate-to-severe CIA is produced by the anthracyclines (e.g., doxorubicin), taxanes (e.g., taxol), alkylating compounds (e.g., cyclophosphamide) and the topoisomerase inhibitor etoposide [115].
6.2 Models of CIA
The usual models of cultured HF have been used to study CIA, but one interesting addition is use of the feather follicle. Xie et al. [116] found that cyclophosphamide induced distinct defects in feather formation: feather branching was transiently and reversibly disrupted, whereas the rachis (feather axis) remained unperturbed. Similar defects were observed in feathers treated with 5-fluorouracil or taxol but not with doxorubicin or arabinofuranosyl cytidine. Selective blockade of cell proliferation was seen in the feather branching area, along with a downregulation of Shh transcription. Local delivery of the Shh inhibitor, cyclopamine, or Shh silencing both recapitulated this effect.
Mice, rats and rabbits have been studied as animal models of CIA. The main difference in hair growth on human versus rodent skin/scalp is the growth pattern, which can be either mosaic or wave pattern [117]. In wave pattern hair growth, the entry of HF into anagen begins from the head and moves toward the tail, which is a pattern seen in neonatal rats and humans with CIA [117] Animal models of CIA typically involve procedures that cause the HF to enter the anagen growth phase in order to be able to mimic the human scenario [117]. Two main approaches are used: i) neonatal rats that show spontaneous anagen hair growth; and ii) synchronizing the HF in adult mice by depilation [117].
Wikramanayake et al. [118] showed that neonatal pigmented Long-Evans rats developed CIA in response to etoposide and cyclophosphamide. The rat dorsal hair was clipped during the early telogen stage to synchronize the hair cycle, and starting 15 days later, the rats were treated with etoposide for 3 days. HF in the clipped areas had the typical CIA follicular dystrophy (dystrophic catagen). When the hair in the pigmented alopecic areas regrew, it had normal pigmentation.
6.3 Treatment of CIA
For CIA the overriding goal of therapy is to protect the HF from being killed by the chemotherapeutic drugs. This goal can be accomplished by two broad strategies; first, the toxic drugs can be prevented from reaching the HF, and second, the cells in the HF can be protected against dying, by intervening in the apoptotic signaling, or by inhibiting ROS, or by counteracting other mediators of cytotoxicity. Since most interventions are applied topically onto the scalp, the interference with the desired cytotoxic effects of the chemotherapy against the cancer cells is thought likely to be minimal.
Table 3 lists the clinically used and investigational drugs that have been used for CIA.
Table 3.
Investigational drug therapies for chemotherapy-induced alopecia.
| Drug (Trade name) | Structure | M, F, Both, animal | Class/route | Mechanism | Ref. |
|---|---|---|---|---|---|
| Cyclosporin A (Neoral) |  |
Rats | Inhibitor of T cells; topical | Binds to cyclophilin and inhibits calcineurin; possibly increases P-glycoporotein | [162] |
| Immuvert/N-acetyl cysteine |  |
Rats | Immunomodulator derived from Serratia marscescens/thiol-based antioxidant; topical | Activates NK cells, produces IL1β/inhibits ROS | [163] |
| IL-1 | Rats | Pro-inflammatory cytokine, i.p. injection | Prevents HF cells undergoing mitosis | [164] | |
| Calcitriol, 1,25-dihydroxyvitamin D-3 (Topitriol) |  |
Rats | Active metabolite of vitamin D; topical | Stimulates differentiation in HF | [165] |
| PTH(1–34), PTH(7–34) | Mice | Parathyroid hormone receptor agonist or antagonist; systemic | Shifted HF towards ‘mild dystrophic anagen’; reduced apoptosis | [166] | |
| AS101 | 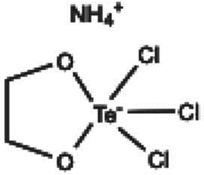 |
Both; Clinical Trial; Rats | Tellurium containing redox modulator; topical/ IV injection | Immunomodulator Anti-inflammatory Antioxidant | [167] |
| liposomal MAD11 | Rats | Topical liposomes containing mAb against doxorubicin | Neutralizes any chemotherapy that arrives at HF | [168] | |
| Adrenalin (Epinephrine) or norepinephrine |  |
Rats | Topical vasoconstrictors | Prevents chemotherapy accumulating in HF | [169] |
| EGF | Rats | Growth factor; topical | Prevents HF cells undergoing mitosis | [170] | |
| Acidic Fibroblast growth factor | Rats | Growth factor; s.c. injection | Prevents HF cells undergoing mitosis | [170] | |
| Cuscuta reflexa Roxb extract | Rats | Botanical extract in EtOH/PE containing coumestans, triterpenes, saponins, flavonoids; topical | [171] | ||
| α-Melanocyte-stimulating hormone |  |
ex vivo HF | Endogenous peptide hormone of the melanocortin family | Cytoprotective to HF, upregulates heme oxidase-1 | [172] |
| p53 inhibitors; Caspase-3 inhibitors | Investigational | Small molecule inhibitors; topical | Prevent apoptosis in HF | [173] | |
| Anti-death rFNK protein-TAT peptide | Rats | Site-directed mutagenesis of rat Bcl-x(L); topical | Anti-apoptotic and cytoprotective activity | [174] |
HF: Hair follicle; ROS: Reactive oxygen species.
7. Nondrug therapies
A number of nondrug therapies for alopecia are listed in Table 4. Hair transplantation or surgical hair restoration is widely employed by men with AGA. The introduction of robotic systems for HF harvesting has improved the technical function of this procedure [119,120]. Research efforts are underway to allow reconstituted HF to be transplanted as an alternative to harvested HF. There has been a report of GMP-compliant culture of human HF cells for encapsulation and transplantation [121]. Stem cell therapies are being increasingly discussed as possible alternatives for alopecia, but the exact manner of application has not yet been entirely worked out. Fractional laser (Fraxel) treatment for alopecia works on the basic premise that initiation of the wound healing response in the scalp will cause new HF to be formed in the skin [122,123]. Fraxel is widely used for photorejuvenation of the facial skin, but when it is used on the scalp instead, new hair can be seen to grow [124,125]. Perhaps the most exciting nondrug therapy is the use of low levels of red or near-infrared light known as low-level laser (light) therapy (LLLT) or photobiomodulation. The reason that LLLT is so promising is that it can be applied to AGA, AA or CIA with an approximately equal chance of success [126–128], it works equally well for men and women [129], it is relatively inexpensive and it has no known side effects or adverse effects.
Table 4.
Nondrug therapies for alopecia.
| Treatment | Mechanism | Application | Ref. |
|---|---|---|---|
| Hair transplantation | Replaces old with new HF | AGA | [175] |
| LLLT | Stimulates HF and stem cells, protects HF from dying | AGA, AA, CIA | [176] |
| Platelet rich plasma | Mixed growth factors in dalteparin and protamine microparticles | AGA | [177] |
| Follicular neogenesis | Dissociated epidermal and dermal cells in suspension reconstitute on a matrix | AGA | [178] |
| Fractional laser | Induction of thermal micro-wounds stimulates HF | AGA | [124] |
| Inductive cell therapy | Use spheroids of dermal papilla cells | AGA | [179] |
| Stem cells | Skin derived progenitor cells | AGA | [180] |
AA: Alopecia areata; AGA: Androgenic alopecia; CIA: Chemotherapy-induced alopecia; HF: Hair follicle; LLLT: Low-level laser (light) therapy.
8. Conclusion
The three main forms of alopecia are AGA caused by miniaturization of HF due to male hormones, AA caused by immune system attack on HF, and CIA caused by toxicity to fast growing cells in HF. The laboratory models and drug discovery efforts are very different for all of these forms because of the diverse etiologies and pathways involved. The one common factor is that topical delivery routes are preferred over systemic routes. AGA is being mainly investigated using 5-α-R inhibitors, androgen receptor antagonists and drugs that affect stem cells. AA is being investigated using immunosuppressive drugs, topical immunotherapy with contact sensitizers and JAK inhibitors. CIA is being investigated using cytokines, antioxidants and inhibitors of apoptosis. Some nondrug therapies such as hair transplantation, follicular neogenesis and LLLT may be applied to all forms of alopecia.
9. Expert opinion
In recent years, there has been an explosion of interest in discovering new drugs and nondrug treatments for hair loss [130]. The commonly used topical minoxidil preparation (Rogaine) was originally discovered by accident as a side effect of an oral blood-pressure medication. The other commonly used medication, oral finasteride (Propecia) was originally an antiandrogen drug used for benign enlarged prostate. However, many men are conflicted when they have to choose between accepting progressive hair loss, and taking antiandrogen drugs with their associated possible side effects of sexual dysfunction and feminization. Therefore (in common with most biomedical disease areas), there is now a trend toward trying to discover more molecularly targeted therapeutics. This search is connected with the development of the concept of personalized medicine, that aims to understand the patterns of gene expression that occur in the particular patient and in the particular disease state the patient is suffering from, so that the drugs can be tailored for each set of circumstances. Gene expression analysis is expected to reveal difference between individuals relating to present and future risk of hair loss, and possible at-risk persons may be able to take preventive measures. These new developments have arisen in parallel with groundbreaking discoveries about stem cells, the biology of the HF and elucidation of the signaling pathways involved in the hair cycle. This explosion of stem cell science and cell biology is taking place hand-in-hand with another explosion of interest in tissue engineering. Drug discovery efforts are now involved with finding compounds that may affect known signaling pathways involved in hair growth, HF cycling and stem cell mobilization, differentiation and proliferation.
The difference in the etiologic factors and pathophysiology that are involved with the three different types of alopecia, means that drug discovery efforts will likely take three divergent paths. Drugs that reverse the miniaturization of the HF seen in AGA and caused by 5-DHT will likely be ineffective against AA caused by attack against the HF by aberrantly activated NK2GD T cells. Likewise, CIA caused by cytotoxic chemotherapy killing rapidly proliferating cells in the HF is unlikely to respond well to either antiandrogens or to immunosuppressive drugs.
In the future, it is expected that completely new HF can be constructed in the laboratory using stem cell and tissue engineering technology to replace those that have been lost by age, disease or trauma. Since hair transplantation is already becoming popular using HF harvested from unaffected parts of the scalp, HF neogenesis could dramatically increase the supply of new HF. The topical delivery route is preferred for administering drugs to treat alopecia as the target tissue is well localized, application is easy for the patient to carry out and the risk of side- effects is much lower. However transdermal and trans-follicular drug delivery is another upcoming area of research, where further advances are expected to be made. LLLT (or photobiomodulation) mediated by red or near-infrared light that can be either LED or laser, is another rapidly growing treatment for hair loss. It is almost unique among alopecia therapies in that it can treat all three forms of alopecia (AGA, AA and CIA). Moreover, LLLT is also particularly effective in mobilizing stem cells, out of their hypoxic niches, and inducing them to differentiate and proliferate. The wide spectrum of activity, the noninvasive nature, lack of side effects, ease of application and relative affordability, suggests that LLLT will also play an increasing role in hair loss therapy.
Despite the otherwise rosy outlook for drug discovery for alopecia, it behooves us to sound a note of caution. The progressive loss of hair typical of AGA can most likely only be delayed and not completely reversed by drug treatment. Even supposedly long-lasting approaches, such as hair transplantation, may be defeated by the inevitable onslaught of DHT, and continuous androgen signaling. The newly transplanted HF may still go the way of their predecessors. The immunological mechanisms underlying AA might be thought to not suffer from the same long-term problems of recurrence as the hormonal mechanisms underlying AGA. However, autoimmune disease can often be progressive and chronic in nature (see, for example, multiple sclerosis and rheumatoid arthritis). Moreover, treatments that tackle autoimmune diseases by various immunosuppressive approaches are notoriously plagued by side effects. The form of alopecia with the most favorable prognosis is CIA. However in that case, for the eventual regrowth of hair to be meaningful for the patient, the chemotherapy for cancer has actually to be successful, which only applies to a minority of chemotherapy treatments. So for these reasons, treatments for alopecia, although highly desired by the general population, may not be as dramatically effective as hoped. ‘Gone today, hair tomorrow’ may only be a far-off goal to be striven after.
Article highlights.
There is an increasing demand for alopecia treatments due to the aging population, more awareness of body image and higher disposable incomes.
Hair follicle biology, role of stem cells and in vitro/in vivo models for drug discovery are active areas of research.
Androgenic alopecia (AGA), alopecia areata (AA) and chemotherapy-induced alopecia (CIA) have very different pathophysiology and treatments.
5-α-reductase inhibitors, androgen receptor antagonists and modulators of stem cells are being studied for AGA.
Immunosuppressive drugs, topical immunotherapy with contact sensitizers and JAK inhibitors are being investigated for AA.
Cytokines, antioxidants and inhibitors of apoptosis are being investigated for CIA.
Topical drug application is the preferred route.
This box summarizes key points contained in the article.
Acknowledgments
Z Santos was supported by CAPES grant 99999.002158/2014-00. MR Hamblin was supported by US NIH grant R01AI050875. MR Hamblin is also a present member of the Transdermal Cap, Inc. scientific advisory board. He has also been a member of the scientific advisory board and received research support from Lexington International, Inc. Both companies manufacture LLLT devices for hair regrowth.
Footnotes
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Harel S, Christiano AM. Genetics of structural hair disorders. J Invest Dermatol. 2012;132(E1):E22–E26. doi: 10.1038/skinbio.2012.7. [DOI] [PubMed] [Google Scholar]
- 2.Pagel M. What is the latest theory of why humans lost their body hair? Why are we the only hairless primate? Scientific American. 2007 [Google Scholar]
- 3.Semalty M, Semalty A, Joshi GP, et al. Hair growth and rejuvenation: an overview. J Dermatolog Treat. 2011;22(3):123–132. doi: 10.3109/09546630903578574. [DOI] [PubMed] [Google Scholar]
- 4.The American Hair Loss Association. [Cited 9 January 2015]; Available from: http://www.americanhairloss.org/ [Google Scholar]
- 5.Draelos ZK. Hair cosmetics. Dermatol Clin. 1991;9(1):19–27. [PubMed] [Google Scholar]
- 6. Schneider MR, Schmidt-Ullrich R, Paus R. The hair follicle as a dynamic miniorgan. Curr Biol. 2009;19(3):R132–R142. doi: 10.1016/j.cub.2008.12.005. •• Good review of hair follicle (HF) biology and its involvement in hair loss and regeneration.
- 7. Mokos ZB, Mosler EL. Advances in a rapidly emerging field of hair follicle stem cell research. Coll Antropol. 2014;38(1):373–378. • Up-to-date summary of the role of stem cells im HF biology and hair growth.
- 8. Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341(7):491–497. doi: 10.1056/NEJM199908123410706. •• Authoritative review on HF biology.
- 9.Paus R, Muller-Rover S, Van Der Veen C, et al. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol. 1999;113(4):523–532. doi: 10.1046/j.1523-1747.1999.00740.x. [DOI] [PubMed] [Google Scholar]
- 10.Sennett R, Rendl M. Mesenchymal-epithelial interactions during hair follicle morphogenesis and cycling. Semin Cell Dev Biol. 2012;23(8):917–927. doi: 10.1016/j.semcdb.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oro AE, Scott MP. Splitting hairs: dissecting roles of signaling systems in epidermal development. Cell. 1998;95(5):575–578. doi: 10.1016/s0092-8674(00)81624-4. • Good review of signaling pathways in HF cycling and hair growth.
- 12.Jahoda CA, Reynolds AJ. Dermal-epidermal interactions. Adult follicle-derived cell populations and hair growth. Dermatol Clin. 1996;14(4):573–583. doi: 10.1016/s0733-8635(05)70385-5. [DOI] [PubMed] [Google Scholar]
- 13.Peus D, Pittelkow MR. Growth factors in hair organ development and the hair growth cycle. Dermatol Clin. 1996;14(4):559–572. doi: 10.1016/s0733-8635(05)70384-3. [DOI] [PubMed] [Google Scholar]
- 14. Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61(7):1329–1337. doi: 10.1016/0092-8674(90)90696-c. •• Important paper describing the original discovery of stem cells in the HF bulge region.
- 15.Mayer JA, Chuong CM, Widelitz R. Rooster feathering, androgenic alopecia, and hormone-dependent tumor growth: what is in common? Differentiation. 2004;72(9–10):474–488. doi: 10.1111/j.1432-0436.2004.07209003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sano S, Kira M, Takagi S, et al. Two distinct signaling pathways in hair cycle induction: stat3-dependent and - independent pathways. Proc Natl Acad Sci USA. 2000;97(25):13824–13829. doi: 10.1073/pnas.240303097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rishikaysh P, Dev K, Diaz D, et al. Signaling involved in hair follicle morphogenesis and development. Int J Mol Sci. 2014;15(1):1647–1670. doi: 10.3390/ijms15011647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Andl T, Reddy ST, Gaddapara T, et al. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2(5):643–653. doi: 10.1016/s1534-5807(02)00167-3. • First description of the importance of wingless type signaling in HF development.
- 19.Harris PJ, Takebe N, Ivy SP. Molecular conversations and the development of the hair follicle and basal cell carcinoma. Cancer Prev Res (Phila) 2010;3(10):1217–1221. doi: 10.1158/1940-6207.CAPR-10-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mill P, Mo R, Fu H, et al. Sonic hedgehog-dependent activation of Gli2 is essential for embryonic hair follicle development. Genes Dev. 2003;17(2):282–294. doi: 10.1101/gad.1038103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiang C, Swan RZ, Grachtchouk M, et al. Essential role for Sonic hedgehog during hair follicle morphogenesis. Dev Biol. 1999;205(1):1–9. doi: 10.1006/dbio.1998.9103. [DOI] [PubMed] [Google Scholar]
- 22.Botchkarev VA, Botchkareva NV, Roth W, et al. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat Cell Biol. 1999;1(3):158–164. doi: 10.1038/11078. [DOI] [PubMed] [Google Scholar]
- 23.Mou C, Jackson B, Schneider P, et al. Generation of the primary hair follicle pattern. Proc Natl Acad Sci USA. 2006;103(24):9075–9080. doi: 10.1073/pnas.0600825103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pummila M, Fliniaux I, Jaatinen R, et al. Ectodysplasin has a dual role in ectodermal organogenesis: inhibition of Bmp activity and induction of Shh expression. Development. 2007;134(1):117–125. doi: 10.1242/dev.02708. [DOI] [PubMed] [Google Scholar]
- 25.Takechi M, Adachi N, Hirai T, et al. The Dlx genes as clues to vertebrate genomics and craniofacial evolution. Semin Cell Dev Biol. 2013;24(2):110–118. doi: 10.1016/j.semcdb.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Hwang J, Mehrani T, Millar SE, et al. Dlx3 is a crucial regulator of hair follicle differentiation and cycling. Development. 2008;135(18):3149–359. doi: 10.1242/dev.022202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hamanaka RB, Glasauer A, Hoover P, et al. Mitochondrial reactive oxygen species promote epidermal differentiation and hair follicle development. Sci Signal. 2013;6(261):ra8. doi: 10.1126/scisignal.2003638. • Interesting study showing that reactive oxygen species promote beneficial signaling in the HF.
- 28.Aubin-Houzelstein G. Notch signaling and the developing hair follicle. Adv Exp Med Biol. 2012;727:142–160. doi: 10.1007/978-1-4614-0899-4_11. [DOI] [PubMed] [Google Scholar]
- 29.Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002;118(2):216–225. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- 30.Taylor G, Lehrer MS, Jensen PJ, et al. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102(4):451–461. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- 31.Ellis T, Gambardella L, Horcher M, et al. The transcriptional repressor CDP (Cutl1) is essential for epithelial cell differentiation of the lung and the hair follicle. Genes Dev. 2001;15(17):2307–2319. doi: 10.1101/gad.200101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Zhou Y, Yang T, et al. Gsdma3 is required for hair follicle differentiation in mice. Biochem Biophys Res Commun. 2010;403(1):18–23. doi: 10.1016/j.bbrc.2010.10.094. [DOI] [PubMed] [Google Scholar]
- 33.Kim BK, Yoon SK. Hairless downregulates expression of Msx2 and its related target genes in hair follicles. J Dermatol Sci. 2013;71(3):203–209. doi: 10.1016/j.jdermsci.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 34.Potter CS, Pruett ND, Kern MJ, et al. The nude mutant gene Foxn1 is a HOXC13 regulatory target during hair follicle and nail differentiation. J Invest Dermatol. 2011;131(4):828–837. doi: 10.1038/jid.2010.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jave-Suarez LF, Schweizer J. The HOXC13-controlled expression of early hair keratin genes in the human hair follicle does not involve TALE proteins MEIS and PREP as cofactors. Arch Dermatol Res. 2006;297(8):372–376. doi: 10.1007/s00403-005-0623-3. [DOI] [PubMed] [Google Scholar]
- 36.Lin SJ, Wideliz RB, Yue Z, et al. Feather regeneration as a model for organogenesis. Dev Growth Differ. 2013;55(1):139–148. doi: 10.1111/dgd.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ponnaiyan D. Do dental stem cells depict distinct characteristics? - Establishing their "phenotypic fingerprint". Dent Res J (Isfahan) 2014;11(2):163–172. [PMC free article] [PubMed] [Google Scholar]
- 38.Shibata D. Inferring human stem cell behaviour from epigenetic drift. J Pathol. 2009;217(2):199–205. doi: 10.1002/path.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rebora A. Telogen effluvium revisited. G Ital Dermatol Venereol. 2014;149(1):47–54. [PubMed] [Google Scholar]
- 40.Guarrera M, Cardo P, Arrigo P, et al. Reliability of hamilton-norwood classification. Int J Trichology. 2009;1(2):120–122. doi: 10.4103/0974-7753.58554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11(4):e9860. doi: 10.5812/ijem.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guarrera M, Semino MT, Rebora A. Quantitating hair loss in women: a critical approach. Dermatology. 1997;194(1):12–16. doi: 10.1159/000246049. [DOI] [PubMed] [Google Scholar]
- 43. Kaufman KD. Androgens and alopecia. Mol Cell Endocrinol. 2002;198(1–2):89–95. doi: 10.1016/s0303-7207(02)00372-6. • Discusses the role of androgens in androgenetic alopecia (AGA).
- 44.Levy-Nissenbaum E, Bar-Natan M, Frydman M, et al. Confirmation of the association between male pattern baldness and the androgen receptor gene. Eur J Dermatol. 2005;15(5):339–340. [PubMed] [Google Scholar]
- 45. Crabtree JS, Kilbourne EJ, Peano BJ, et al. A mouse model of androgenetic alopecia. Endocrinology. 2010;151(5):2373–2380. doi: 10.1210/en.2009-1474. • Describes an animal model of AGA.
- 46.Kaliyadan F, Nambiar A, Vijayaraghavan S. Androgenetic alopecia: an update. Indian J Dermatol Venereol Leprol. 2013;79(5):613–625. doi: 10.4103/0378-6323.116730. [DOI] [PubMed] [Google Scholar]
- 47.Poor V, Juricskay S, Telegdy E. Urinary steroids in men with male-pattern alopecia. J Biochem Biophys Methods. 2002;53(1–3):123–130. doi: 10.1016/s0165-022x(02)00100-8. [DOI] [PubMed] [Google Scholar]
- 48.Leiros GJ, Attorresi AI, Balana ME. Hair follicle stem cell differentiation is inhibited through cross-talk between Wnt/beta-catenin and androgen signalling in dermal papilla cells from patients with androgenetic alopecia. Br J Dermatol. 2012;166(5):1035–1042. doi: 10.1111/j.1365-2133.2012.10856.x. [DOI] [PubMed] [Google Scholar]
- 49.Dong L, Hao H, Xia L, et al. Treatment of MSCs with Wnt1a-conditioned medium activates DP cells and promotes hair follicle regrowth. Sci Rep. 2014;4:5432. doi: 10.1038/srep05432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Platz EA, Pollak MN, Willett WC, et al. Vertex balding, plasma insulin-like growth factor 1, and insulin-like growth factor binding protein 3. J Am Acad Dermatol. 2000;42(6):1003–1007. [PubMed] [Google Scholar]
- 51. Kamiya T, Shirai A, Kawashima S, et al. Hair follicle elongation in organ culture of skin from newborn and adult mice. J Dermatol Sci. 1998;17(1):54–60. doi: 10.1016/s0923-1811(97)00068-6. • One of the first papers to describe in vitro growth of HFs.
- 52.Philpott MP, Sanders DA, Kealey T. Effects of insulin and insulin-like growth factors on cultured human hair follicles: IGF-I at physiologic concentrations is an important regulator of hair follicle growth in vitro. J Invest Dermatol. 1994;102(6):857–861. doi: 10.1111/1523-1747.ep12382494. [DOI] [PubMed] [Google Scholar]
- 53.Lurie R, Ben-Amitai D, Laron Z. Laron syndrome (primary growth hormone insensitivity): a unique model to explore the effect of insulin-like growth factor 1 deficiency on human hair. Dermatology. 2004;208(4):314–318. doi: 10.1159/000077839. [DOI] [PubMed] [Google Scholar]
- 54.Batch JA, Mercuri FA, Werther GA. Identification and localization of insulinlike growth factor-binding protein (IGFBP) messenger RNAs in human hair follicle dermal papilla. J Invest Dermatol. 1996;106(3):471–475. doi: 10.1111/1523-1747.ep12343649. [DOI] [PubMed] [Google Scholar]
- 55.Zhao J, Harada N, Okajima K. Dihydrotestosterone inhibits hair growth in mice by inhibiting insulin-like growth factor-I production in dermal papillae. Growth Horm IGF Res. 2011;21(5):260–267. doi: 10.1016/j.ghir.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 56.Li J, Yang Z, Li Z, et al. Exogenous IGF-1 promotes hair growth by stimulating cell proliferation and down regulating TGF-beta1 in C57BL/6 mice in vivo. Growth Horm IGF Res. 2014;24(2–3):89–94. doi: 10.1016/j.ghir.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 57.Shin SH, Joo HW, Kim MK, et al. Extracellular histones inhibit hair shaft elongation in cultured human hair follicles and promote regression of hair follicles in mice. Exp Dermatol. 2012;21(12):956–958. doi: 10.1111/exd.12033. [DOI] [PubMed] [Google Scholar]
- 58.Samuelov L, Sprecher E, Tsuruta D, et al. P-cadherin regulates human hair growth and cycling via canonical Wnt signaling and transforming growth factor-beta2. J Invest Dermatol. 2012;132(10):2332–2341. doi: 10.1038/jid.2012.171. [DOI] [PubMed] [Google Scholar]
- 59.Inui S, Itami S. Androgen actions on the human hair follicle: perspectives. Exp Dermatol. 2013;22(3):168–171. doi: 10.1111/exd.12024. [DOI] [PubMed] [Google Scholar]
- 60.Garza LA, Liu Y, Yang Z, et al. Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia. Sci Transl Med. 2012;4(126):126ra34. doi: 10.1126/scitranslmed.3003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 62.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 63.Sand M, Gambichler T, Sand D, et al. MicroRNAs and the skin: tiny players in the body’s largest organ. J Dermatol Sci. 2009;53(3):169–175. doi: 10.1016/j.jdermsci.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 64.Andl T, Murchison EP, Liu F, et al. The miRNA-processing enzyme dicer is essential for the morphogenesis and maintenance of hair follicles. Curr Biol. 2006;16(10):1041–1049. doi: 10.1016/j.cub.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Goodarzi HR, Abbasi A, Saffari M, et al. MicroRNAs take part in pathophysiology and pathogenesis of Male Pattern Baldness. Mol Biol Rep. 2010;37(6):2959–2965. doi: 10.1007/s11033-009-9862-2. • Highlights the role of microRNAs in AGA.
- 66.Goldman BE, Fisher DM, Ringler SL. Transcutaneous PO2 of the scalp in male pattern baldness: a new piece to the puzzle. Plast Reconstr Surg. 1996;97(6):1109–1116. doi: 10.1097/00006534-199605000-00003. discussion 17. [DOI] [PubMed] [Google Scholar]
- 67.Klemp P, Peters K, Hansted B. Subcutaneous blood flow in early male pattern baldness. J Invest Dermatol. 1989;92(5):725–726. doi: 10.1111/1523-1747.ep12721603. [DOI] [PubMed] [Google Scholar]
- 68.Philpott MP, Green MR, Kealey T. Human hair growth in vitro. J Cell Sci. 1990;97(Pt 3):463–471. doi: 10.1242/jcs.97.3.463. [DOI] [PubMed] [Google Scholar]
- 69.Higgins CA, Christiano AM. Regenerative medicine and hair loss: how hair follicle culture has advanced our understanding of treatment options for androgenetic alopecia. Regen Med. 2014;9(1):101–111. doi: 10.2217/rme.13.87. [DOI] [PubMed] [Google Scholar]
- 70.Philpott MP, Kealey T. Cyclical changes in rat vibrissa follicles maintained In vitro. J Invest Dermatol. 2000;115(6):1152–1155. doi: 10.1046/j.1523-1747.2000.00183.x. [DOI] [PubMed] [Google Scholar]
- 71.Robinson M, Reynolds AJ, Jahoda CA. Hair cycle stage of the mouse vibrissa follicle determines subsequent fiber growth and follicle behavior in vitro. J Invest Dermatol. 1997;108(4):495–500. doi: 10.1111/1523-1747.ep12289730. [DOI] [PubMed] [Google Scholar]
- 72.Thornton MJ, Kato S, Hibberts NA, et al. Ability to culture dermal papilla cells from red deer (Cervus elaphus) hair follicles with differing hormonal responses in vivo offers a new model for studying the control of hair follicle biology. J Exp Zool. 1996;275(6):452–458. doi: 10.1002/(SICI)1097-010X(19960815)275:6<452::AID-JEZ7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 73.Sundberg JP, King LE, Bascom C. Animal models for male pattern (androgenetic) alopecia. Eur J Dermatol. 2001;11(4):321–325. [PubMed] [Google Scholar]
- 74.Diani AR, Mills CJ. Immunocytochemical localization of androgen receptors in the scalp of the stumptail macaque monkey, a model of androgenetic alopecia. J Invest Dermatol. 1994;102(4):511–514. doi: 10.1111/1523-1747.ep12373176. [DOI] [PubMed] [Google Scholar]
- 75.Chesire DR, Isaacs WB. Ligand-dependent inhibition of beta-catenin/TCF signaling by androgen receptor. Oncogene. 2002;21(55):8453–8469. doi: 10.1038/sj.onc.1206049. [DOI] [PubMed] [Google Scholar]
- 76.Ye F, Imamura K, Imanishi N, et al. Effects of topical antiandrogen and 5-alpha-reductase inhibitors on sebaceous glands in male fuzzy rats. Skin Pharmacol. 1997;10(5–6):288–297. doi: 10.1159/000211517. [DOI] [PubMed] [Google Scholar]
- 77.Park WS, Lee CH, Lee BG, et al. The extract of Thujae occidentalis semen inhibited 5alpha-reductase and androchronogenetic alopecia of B6CBAF1/j hybrid mouse. J Dermatol Sci. 2003;31(2):91–98. doi: 10.1016/s0923-1811(02)00146-9. [DOI] [PubMed] [Google Scholar]
- 78.Matias JR, Malloy V, Orentreich N. Animal models of androgen-dependent disorders of the pilosebaceous apparatus 1 The androchronogenetic alopecia (AGA) mouse as a model for malepattern baldness. Arch Dermatol Res. 1989;281(4):247–253. doi: 10.1007/BF00431058. [DOI] [PubMed] [Google Scholar]
- 79.Sundberg JP, Beamer WG, Uno H, et al. Androgenetic alopecia: in vivo models. Exp Mol Pathol. 1999;67(2):118–130. doi: 10.1006/exmp.1999.2276. [DOI] [PubMed] [Google Scholar]
- 80. Rittmaster RS. Finasteride. N Engl J Med. 1994;330(2):120–125. doi: 10.1056/NEJM199401133300208. •• Important paper describing the introduction of finasteride for AGA.
- 81.Semalty A, Semalty M, Joshi GP, et al. Techniques for the discovery and evaluation of drugs against alopecia. Expert Opin Drug Discov. 2011;6(3):309–321. doi: 10.1517/17460441.2011.553831. [DOI] [PubMed] [Google Scholar]
- 82. Patzelt A, Lademann J. Drug delivery to hair follicles. Expert Opin Drug Deliv. 2013;10(6):787–797. doi: 10.1517/17425247.2013.776038. • Drug delivery may be failitated through HFs.
- 83.Lademann J, Knorr F, Richter H, et al. Hair follicles – an efficient storage and penetration pathway for topically applied substances. Summary of recent results obtained at the Center of Experimental and Applied Cutaneous Physiology, Charite -Universitatsmedizin Berlin, Germany. Skin Pharmacol Physiol. 2008;21(3):150–155. doi: 10.1159/000131079. [DOI] [PubMed] [Google Scholar]
- 84.Gupta M, Agrawal U, Vyas SP. Nanocarrier-based topical drug delivery for the treatment of skin diseases. Expert Opin Drug Deliv. 2012;9(7):783–804. doi: 10.1517/17425247.2012.686490. [DOI] [PubMed] [Google Scholar]
- 85.Islam N, Leung PS, Huntley A, et al. The autoimmune basis of alopecia areata: a comprehensive review. Autoimmun Rev. 2015;14(2):81–89. doi: 10.1016/j.autrev.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 86.Walker A, Mesinkovska NA, Boncher J, et al. Colocalization of vitiligo and alopecia areata presenting as poliosis. J Cutan Pathol. 2014 doi: 10.1111/cup.12437. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 87.Jia WX, Mao QX, Xiao XM, et al. Patchy alopecia areata sparing gray hairs: a case series. Postepy Dermatol Alergol. 2014;31(2):113–116. doi: 10.5114/pdia.2014.40956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McElwee KJ, Boggess D, Olivry T, et al. Comparison of alopecia areata in human and nonhuman mammalian species. Pathobiology. 1998;66(2):90–107. doi: 10.1159/000028002. [DOI] [PubMed] [Google Scholar]
- 89. Petukhova L, Duvic M, Hordinsky M, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466(7302):113–117. doi: 10.1038/nature09114. •• Important paper describing results of a genome-wide investigation into the immune mechamisms behind alopecia areata (AA).
- 90.Gregoriou S, Papafragkaki D, Kontochristopoulos G, et al. Cytokines and other mediators in alopecia areata. Mediators Inflamm. 2010;2010:928030. doi: 10.1155/2010/928030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Deeths MJ, Endrizzi BT, Irvin ML, et al. Phenotypic analysis of T-cells in extensive alopecia areata scalp suggests partial tolerance. J Invest Dermatol. 2006;126(2):366–373. doi: 10.1038/sj.jid.5700054. [DOI] [PubMed] [Google Scholar]
- 92.Freyschmidt-Paul P, Seiter S, Zoller M, et al. Treatment with an anti-CD44v10-specific antibody inhibits the onset of alopecia areata in C3H/HeJ mice. J Invest Dermatol. 2000;115(4):653–657. doi: 10.1046/j.1523-1747.2000.00113.x. [DOI] [PubMed] [Google Scholar]
- 93.McElwee KJ, Spiers EM, Oliver RF. Partial restoration of hair growth in the DEBR model for Alopecia areata after in vivo depletion of CD4+ T cells. Br J Dermatol. 1999;140(3):432–437. doi: 10.1046/j.1365-2133.1999.02705.x. [DOI] [PubMed] [Google Scholar]
- 94.Martinez-Mir A, Zlotogorski A, Ott J, et al. Genetic linkage studies in alopecia areata. J Investig Dermatol Symp Proc. 2003;8(2):199–203. doi: 10.1046/j.1087-0024.2003.00809.x. [DOI] [PubMed] [Google Scholar]
- 95.Martinez-Mir A, Zlotogorski A, Gordon D, et al. Genomewide scan for linkage reveals evidence of several susceptibility loci for alopecia areata. Am J Hum Genet. 2007;80(2):316–328. doi: 10.1086/511442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Duncan FJ, Silva KA, Johnson CJ, et al. Endogenous retinoids in the pathogenesis of alopecia areata. J Invest Dermatol. 2013;133(2):334–343. doi: 10.1038/jid.2012.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Colombe BW, Price VH, Khoury EL, et al. HLA class II antigen associations help to define two types of alopecia areata. J Am Acad Dermatol. 1995;33(5 Pt 1):757–764. [PubMed] [Google Scholar]
- 98.Colombe BW, Lou CD, Price VH. The genetic basis of alopecia areata: HLA associations with patchy alopecia areata versus alopecia totalis and alopecia universalis. J Investig Dermatol Symp Proc. 1999;4(3):216–219. doi: 10.1038/sj.jidsp.5640214. [DOI] [PubMed] [Google Scholar]
- 99.Jagielska D, Redler S, Brockschmidt FF, et al. Follow-up study of the first genome-wide association scan in alopecia areata: IL13 and KIAA0350 as susceptibility loci supported with genome-wide significance. J Invest Dermatol. 2012;132(9):2192–2197. doi: 10.1038/jid.2012.129. [DOI] [PubMed] [Google Scholar]
- 100.Ito T, Ito N, Saatoff M, et al. Maintenance of hair follicle immune privilege is linked to prevention of NK cell attack. J Invest Dermatol. 2008;128(5):1196–1206. doi: 10.1038/sj.jid.5701183. [DOI] [PubMed] [Google Scholar]
- 101. Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20(9):1043–1049. doi: 10.1038/nm.3645. •• Paper highlighting the role of T-cell attack on HFs in AA, and showing that small molecule JAK inhibitors can treat the condition.
- 102.Leung MC, Sutton CW, Fenton DA, et al. Trichohyalin is a potential major autoantigen in human alopecia areata. J Proteome Res. 2010;9(10):5153–5163. doi: 10.1021/pr100422u. [DOI] [PubMed] [Google Scholar]
- 103.McElwee KJ, Boggess D, Miller J, et al. Spontaneous alopecia areata-like hair loss in one congenic and seven inbred laboratory mouse strains. J Investig Dermatol Symp Proc. 1999;4(3):202–206. doi: 10.1038/sj.jidsp.5640211. [DOI] [PubMed] [Google Scholar]
- 104.McElwee KJ, Hoffmann R. Alopecia areata - animal models. Clin Exp Dermatol. 2002;27(5):410–417. doi: 10.1046/j.1365-2230.2002.01075.x. [DOI] [PubMed] [Google Scholar]
- 105.Sun J, Silva KA, McElwee KJ, et al. The C3H/HeJ mouse and DEBR rat models for alopecia areata: review of preclinical drug screening approaches and results. Exp Dermatol. 2008;17(10):793–805. doi: 10.1111/j.1600-0625.2008.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kyoizumi S, Suzuki T, Teraoka S, et al. Radiation sensitivity of human hair follicles in SCID-hu mice. Radiat Res. 1998;149(1):11–18. [PubMed] [Google Scholar]
- 107.Gilhar A, Keren A, Paus R. A new humanized mouse model for alopecia areata. J Investig Dermatol Symp Proc. 2013;16(1):S37–S38. doi: 10.1038/jidsymp.2013.11. [DOI] [PubMed] [Google Scholar]
- 108.Gu ME, Song XM, Zhu CF, et al. Breeding and preliminarily phenotyping of a congenic mouse model with alopecia areata. Dongwuxue Yanjiu. 2014;35(4):249–255. doi: 10.13918/j.issn.2095-8137.2014.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ito T. Advances in the management of alopecia areata. J Dermatol. 2012;39(1):11–17. doi: 10.1111/j.1346-8138.2011.01476.x. [DOI] [PubMed] [Google Scholar]
- 110.Singh G, Lavanya M. Topical immunotherapy in alopecia areata. Int J Trichology. 2010;2(1):36–39. doi: 10.4103/0974-7753.66911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Buckley DA, Du Vivier AW. The therapeutic use of topical contact sensitizers in benign dermatoses. Br J Dermatol. 2001;145(3):385–405. doi: 10.1046/j.1365-2133.2001.04399.x. [DOI] [PubMed] [Google Scholar]
- 112.Happle R. Antigenic competition as a therapeutic concept for alopecia areata. Arch Dermatol Res. 1980;267(1):109–114. doi: 10.1007/BF00416931. [DOI] [PubMed] [Google Scholar]
- 113.Giaccia AJ, Kastan MB. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 1998;12(19):2973–2983. doi: 10.1101/gad.12.19.2973. [DOI] [PubMed] [Google Scholar]
- 114.Paus R, Haslam IS, Sharov AA, et al. Pathobiology of chemotherapy-induced hair loss. Lancet Oncol. 2013;14(2):e50–e59. doi: 10.1016/S1470-2045(12)70553-3. [DOI] [PubMed] [Google Scholar]
- 115.Jimenez JJ, Roberts SM, Mejia J, et al. Prevention of chemotherapy-induced alopecia in rodent models. Cell Stress Chaperones. 2008;13(1):31–38. doi: 10.1007/s12192-007-0005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xie G, Wang H, Yan Z, et al. Testing Chemotherapeutic Agents in the Feather Follicle Identifies a Selective Blockade of Cell Proliferation and a Key Role for Sonic Hedgehog Signaling in Chemotherapy-Induced Tissue Damage. J Invest Dermatol. 2014 doi: 10.1038/jid.2014.409. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 117.Wang J, Lu Z, Au JL. Protection against chemotherapy-induced alopecia. Pharm Res. 2006;23(11):2505–2514. doi: 10.1007/s11095-006-9105-3. [DOI] [PubMed] [Google Scholar]
- 118.Wikramanayake TC, Amini S, Simon J, et al. A novel rat model for chemotherapy-induced alopecia. Clin Exp Dermatol. 2012;37(3):284–289. doi: 10.1111/j.1365-2230.2011.04239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rose PT, Nusbaum B. Robotic hair restoration. Dermatol Clin. 2014;32(1):97–107. doi: 10.1016/j.det.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 120.Marshall BT, Ingraham CA, Wu X, et al. Future horizons in hair restoration. Facial Plast Surg Clin North Am. 2013;21(3):521–528. doi: 10.1016/j.fsc.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 121.Marazzi M, Crovato F, Bucco M, et al. GMP-compliant culture of human hair follicle cells for encapsulation and transplantation. Cell Transplant. 2012;21(1):373–378. doi: 10.3727/096368911X565010. [DOI] [PubMed] [Google Scholar]
- 122. Chueh SC, Lin SJ, Chen CC, et al. Therapeutic strategy for hair regeneration: hair cycle activation, niche environment modulation, wound-induced follicle neogenesis, and stem cell engineering. Expert Opin Biol Ther. 2013;13(3):377–391. doi: 10.1517/14712598.2013.739601. • Poses the question whether stem cell technologies will really be able to treat aloipecia.
- 123. Ito M, Yang Z, Andl T, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447(7142):316–320. doi: 10.1038/nature05766. •• Shows that the wound healing response can encourage regeneration of HFs.
- 124.Kim WS, Lee HI, Lee JW, et al. Fractional photothermolysis laser treatment of male pattern hair loss. Dermatol Surg. 2011;37(1):41–51. doi: 10.1111/j.1524-4725.2010.01833.x. [DOI] [PubMed] [Google Scholar]
- 125.Lee GY, Lee SJ, Kim WS. The effect of a 1550 nm fractional erbium-glass laser in female pattern hair loss. J Eur Acad Dermatol Venereol. 2011;25(12):1450–1454. doi: 10.1111/j.1468-3083.2011.04183.x. [DOI] [PubMed] [Google Scholar]
- 126. Lanzafame RJ, Blanche RR, Bodian AB, et al. The growth of human scalp hair mediated by visible red light laser and LED sources in males. Lasers Surg Med. 2013;45(8):487–495. doi: 10.1002/lsm.22173. • Clinical trial showing that low-level laser (light) therapy (LLLT) was beneficial in AGA.
- 127.Wikramanayake TC, Villasante AC, Mauro LM, et al. Low-level laser treatment accelerated hair regrowth in a rat model of chemotherapy-induced alopecia (CIA) Lasers Med Sci. 2013;28(3):701–706. doi: 10.1007/s10103-012-1139-7. [DOI] [PubMed] [Google Scholar]
- 128.Wikramanayake TC, Rodriguez R, Choudhary S, et al. Effects of the Lexington LaserComb on hair regrowth in the C3H/HeJ mouse model of alopecia areata. Lasers Med Sci. 2012;27(2):431–436. doi: 10.1007/s10103-011-0953-7. [DOI] [PubMed] [Google Scholar]
- 129.Jimenez JJ, Wikramanayake TC, Bergfeld W, et al. Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: a multicenter, randomized, sham device-controlled, double-blind study. Am J Clin Dermatol. 2014;15(2):115–127. doi: 10.1007/s40257-013-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Paus R. Therapeutic strategies for treating hair. loss. Drug Discov Today Ther Strateg. 2006;3:101–110. [Google Scholar]
- 131.Rossi A, Cantisani C, Melis L, et al. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2012;6(2):130–136. doi: 10.2174/187221312800166859. [DOI] [PubMed] [Google Scholar]
- 132.Buhl AE, Waldon DJ, Conrad SJ, et al. Potassium channel conductance: a mechanism affecting hair growth both in vitro and in vivo. J Invest Dermatol. 1992;98(3):315–319. doi: 10.1111/1523-1747.ep12499788. [DOI] [PubMed] [Google Scholar]
- 133.Bazzano GS, Terezakis N, Galen W. Topical tretinoin for hair growth promotion. J Am Acad Dermatol. 1986;15(4 Pt 2):880–883. 90–93. doi: 10.1016/s0190-9622(86)80024-x. [DOI] [PubMed] [Google Scholar]
- 134.Caserini M, Radicioni M, Leuratti C, et al. A novel finasteride 0.25% topical solution for androgenetic alopecia: pharmacokinetics and effects on plasma androgen levels in healthy male volunteers. Int J Clin Pharmacol Ther. 2014;52(10):842–849. doi: 10.5414/CP202119. [DOI] [PubMed] [Google Scholar]
- 135.Kaufman KD. Finasteride, 1 mg (Propecia), is the optimal dose for the treatment of men with male pattern hair loss. Arch Dermatol. 1999;135(8):989–990. doi: 10.1001/archderm.135.8.989. [DOI] [PubMed] [Google Scholar]
- 136.Olszewska M, Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J Drugs Dermatol. 2005;4(5):637–640. [PubMed] [Google Scholar]
- 137.Jung JY, Yeon JH, Choi JW, et al. Effect of dutasteride 0.5 mg/d in men with androgenetic alopecia recalcitrant to finasteride. Int J Dermatol. 2014;53(11):1351–1357. doi: 10.1111/ijd.12060. [DOI] [PubMed] [Google Scholar]
- 138.Pan HJ, Wilding G, Uno H, et al. Evaluation of RU58841 as an antiandrogen in prostate PC3 cells and a topical anti-alopecia agent in the bald scalp of stumptailed macaques. Endocrine. 1998;9(1):39–43. doi: 10.1385/ENDO:9:1:39. [DOI] [PubMed] [Google Scholar]
- 139.Yazdabadi A, Sinclair R. Treatment of female pattern hair loss with the androgen receptor antagonist flutamide. Australas J Dermatol. 2011;52(2):132–134. doi: 10.1111/j.1440-0960.2010.00735.x. [DOI] [PubMed] [Google Scholar]
- 140.Sovak M, Seligson AL, Kucerova R, et al. Fluridil, a rationally designed topical agent for androgenetic alopecia: first clinical experience. Dermatol Surg. 2002;28(8):678–685. doi: 10.1046/j.1524-4725.2002.02017.x. [DOI] [PubMed] [Google Scholar]
- 141.Scheinfeld N. A review of hormonal therapy for female pattern (androgenic) alopecia. Dermatol Online J. 2008;14(3):1. [PubMed] [Google Scholar]
- 142.Rathnayake D, Sinclair R. Innovative use of spironolactone as an antiandrogen in the treatment of female pattern hair loss. Dermatol Clin. 2010;28(3):611–618. doi: 10.1016/j.det.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 143.Fischer TW, Burmeister G, Schmidt HW, et al. Melatonin increases anagen hair rate in women with androgenetic alopecia or diffuse alopecia: results of a pilot randomized controlled trial. Br J Dermatol. 2004;150(2):341–345. doi: 10.1111/j.1365-2133.2004.05685.x. [DOI] [PubMed] [Google Scholar]
- 144.Khidhir KG, Woodward DF, Farjo NP, et al. The prostamide-related glaucoma therapy, bimatoprost, offers a novel approach for treating scalp alopecias. FASEB J. 2013;27(2):557–567. doi: 10.1096/fj.12-218156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lee YB, Eun YS, Lee JH, et al. Effects of topical application of growth factors followed by microneedle therapy in women with female pattern hair loss: a pilot study. J Dermatol. 2013;40(1):81–83. doi: 10.1111/j.1346-8138.2012.01680.x. [DOI] [PubMed] [Google Scholar]
- 146.Castro RF, Azzalis LA, Feder D, et al. Safety and efficacy analysis of liposomal insulin-like growth factor-1 in a fluid gel formulation for hair-loss treatment in a hamster model. Clin Exp Dermatol. 2012;37(8):909–912. doi: 10.1111/j.1365-2230.2012.04441.x. [DOI] [PubMed] [Google Scholar]
- 147.Philp D, Nguyen M, Scheremeta B, et al. Thymosin beta4 increases hair growth by activation of hair follicle stem cells. FASEB J. 2004;18(2):385–387. doi: 10.1096/fj.03-0244fje. [DOI] [PubMed] [Google Scholar]
- 148.Shirai A, Ikeda J, Kawashima S, et al. KF19418, a new compound for hair growth promotion in vitro and in vivo mouse models. J Dermatol Sci. 2001;25(3):213–218. doi: 10.1016/s0923-1811(00)00147-x. [DOI] [PubMed] [Google Scholar]
- 149.Kim YY, Up No S, Kim MH, et al. Effects of topical application of EGCG on testosterone-induced hair loss in a mouse model. Exp Dermatol. 2011;20(12):1015–1017. doi: 10.1111/j.1600-0625.2011.01353.x. [DOI] [PubMed] [Google Scholar]
- 150.Park HJ, Zhang N, Park DK. Topical application of Polygonum multiflorum extract induces hair growth of resting hair follicles through upregulating Shh and beta-catenin expression in C57BL/6. mice. J Ethnopharmacol. 2011;135(2):369–375. doi: 10.1016/j.jep.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 151.Datta K, Singh AT, Mukherjee A, et al. Eclipta alba extract with potential for hair growth promoting activity. J Ethnopharmacol. 2009;124(3):450–456. doi: 10.1016/j.jep.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 152.Zhang NN, Park DK, Park HJ. Hair growth-promoting activity of hot water extract of Thuja orientalis. BMC Complement Altern Med. 2013;13:9. doi: 10.1186/1472-6882-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hoffmann R, Rot A, Niiyama S, et al. Steroid sulfatase in the human hair follicle concentrates in the dermal papilla. J Invest Dermatol. 2001;117(6):1342–1348. doi: 10.1046/j.0022-202x.2001.01547.x. [DOI] [PubMed] [Google Scholar]
- 154.Dugour A, Hagelin K, Smus C, et al. Silencing the androgen receptor: new skills for antiandrogen oligonucleotide skin and hair therapy. J Dermatol Sci. 2009;54(2):123–125. doi: 10.1016/j.jdermsci.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 155.Lenane P, Macarthur C, Parkin PC, et al. Clobetasol propionate1 0.05%, vs hydrocortisone, 1%, for alopecia areata in children: a randomized clinical trial. JAMA Dermatol. 2014;150(1):47–50. doi: 10.1001/jamadermatol.2013.5764. [DOI] [PubMed] [Google Scholar]
- 156.Micali G, Cicero RL, Nasca MR, et al. Treatment of alopecia areata with squaric acid dibutylester. Int J Dermatol. 1996;35(1):52–56. doi: 10.1111/j.1365-4362.1996.tb01618.x. [DOI] [PubMed] [Google Scholar]
- 157.Chiang K, Atanaskova Mesinkovska N, Amoretti A, et al. Clinical efficacy of diphenylcyclopropenone in alopecia areata: retrospective data analysis of 50 patients. J Am Acad Dermatol. 2014;71(3):595–597. doi: 10.1016/j.jaad.2014.04.036. [DOI] [PubMed] [Google Scholar]
- 158.Mohamed Z, Bhouri A, Jallouli A, et al. Alopecia areata treatment with a phototoxic dose of UVA and topical 8-methoxypsoralen. J Eur Acad Dermatol Venereol. 2005;19(5):552–555. doi: 10.1111/j.1468-3083.2005.01226.x. [DOI] [PubMed] [Google Scholar]
- 159.Aghaei S. An uncontrolled, open label study of sulfasalazine in severe alopecia areata. Indian J Dermatol Venereol Leprol. 2008;74(6):611–613. doi: 10.4103/0378-6323.45103. [DOI] [PubMed] [Google Scholar]
- 160.Gorcey L, Spratt EA, Leger MC. Alopecia universalis successfully treated with adalimumab. JAMA Dermatol. 2014;150(12):1341–1344. doi: 10.1001/jamadermatol.2014.1544. [DOI] [PubMed] [Google Scholar]
- 161.Bui K, Polisetty S, Gilchrist H, et al. Successful treatment of alopecia universalis with alefacept: a case report and review of the literature. Cutis. 2008;81(5):431–434. [PubMed] [Google Scholar]
- 162.Hussein AM, Stuart A, Peters WP. Protection against chemotherapy-induced alopecia by cyclosporin A in the newborn rat animal model. Dermatology. 1995;190(3):192–196. doi: 10.1159/000246683. [DOI] [PubMed] [Google Scholar]
- 163.Jimenez JJ, Huang HS, Yunis AA. Treatment with ImuVert/N-acetylcysteine protects rats from cyclophosphamide/cytarabine-induced alopecia. Cancer Invest. 1992;10(4):271–276. doi: 10.3109/07357909209032751. [DOI] [PubMed] [Google Scholar]
- 164.Jimenez JJ, Wong GH, Yunis AA. Interleukin 1 protects from cytosine arabinoside-induced alopecia in the rat model. FASEB J. 1991;5(10):2456–2458. doi: 10.1096/fasebj.5.10.2065892. [DOI] [PubMed] [Google Scholar]
- 165.Jimenez JJ, Yunis AA. Protection from chemotherapy-induced alopecia by 1,25-dihydroxyvitamin D3. Cancer Res. 1992;52(18):5123–5125. [PubMed] [Google Scholar]
- 166.Peters EM, Foitzik K, Paus R, et al. A new strategy for modulating chemotherapy-induced alopecia, using PTH/PTHrP receptor agonist and antagonist. J Invest Dermatol. 2001;117(2):173–178. doi: 10.1046/j.0022-202x.2001.01410.x. [DOI] [PubMed] [Google Scholar]
- 167.Sredni B, Xu RH, Albeck M, et al. The protective role of the immunomodulator AS101 against chemotherapy-induced alopecia studies on human and animal models. Int J Cancer. 1996;65(1):97–103. doi: 10.1002/(SICI)1097-0215(19960103)65:1<97::AID-IJC17>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 168.Balsari AL, Morelli D, Menard S, et al. Protection against doxorubicin-induced alopecia in rats by liposome-entrapped monoclonal antibodies. FASEB J. 1994;8(2):226–230. doi: 10.1096/fasebj.8.2.8119493. [DOI] [PubMed] [Google Scholar]
- 169.Soref CM, Fahl WE. A new strategy to prevent chemotherapy and radiotherapyinduced alopecia using topically applied vasoconstrictor. Int J Cancer. 2015;136(1):195–203. doi: 10.1002/ijc.28961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jimenez JJ, Yunis AA. Protection from 1- beta-D-arabinofuranosylcytosine-induced alopecia by epidermal growth factor and fibroblast growth factor in the rat model. Cancer Res. 1992;52(2):413–415. [PubMed] [Google Scholar]
- 171.Patel S, Sharma V, Chauhan NS, et al. A study on the extracts of Cuscuta reflexa Roxb. in treatment of cyclophosphamide induced alopecia. Daru. 2014;22(1):7. doi: 10.1186/2008-2231-22-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bohm M, Bodo E, Funk W, et al. alpha-Melanocyte-stimulating hormone: a protective peptide against chemotherapy-induced hair follicle damage? Br J Dermatol. 2014;170(4):956–960. doi: 10.1111/bjd.12759. [DOI] [PubMed] [Google Scholar]
- 173.Botchkarev VA. Molecular mechanisms of chemotherapy-induced hair loss. J Investig Dermatol Symp Proc. 2003;8(1):72–75. doi: 10.1046/j.1523-1747.2003.12175.x. [DOI] [PubMed] [Google Scholar]
- 174.Nakashima-Kamimura N, Nishimaki K, Mori T, et al. Prevention of chemotherapy-induced alopecia by the anti-death FNK protein. Life Sci. 2008;82(3–4):218–225. doi: 10.1016/j.lfs.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 175.Vogel JE, Jimenez F, Cole J, et al. Hair restoration surgery: the state of the art. Aesthet Surg J. 2013;33(1):128–151. doi: 10.1177/1090820X12468314. [DOI] [PubMed] [Google Scholar]
- 176. Avci P, Gupta GK, Clark J, et al. Low-level laser (light) therapy (LLLT) for treatment of hair loss. Lasers Surg Med. 2014;46(2):144–151. doi: 10.1002/lsm.22170. • Good review of LLLT for treatment of different forms of alopecia.
- 177.Takikawa M, Nakamura S, Nakamura S, et al. Enhanced effect of platelet-rich plasma containing a new carrier on hair growth. Dermatol Surg. 2011;37(12):1721–1729. doi: 10.1111/j.1524-4725.2011.02123.x. [DOI] [PubMed] [Google Scholar]
- 178.Asakawa K, Toyoshima KE, Ishibashi N, et al. Hair organ regeneration via the bioengineered hair follicular unit transplantation. Sci Rep. 2012;2:424. doi: 10.1038/srep00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Higgins CA, Richardson GD, Ferdinando D, et al. Modelling the hair follicle dermal papilla using spheroid cell cultures. Exp Dermatol. 2010;19(6):546–548. doi: 10.1111/j.1600-0625.2009.01007.x. [DOI] [PubMed] [Google Scholar]
- 180.Toyoshima KE, Asakawa K, Ishibashi N, et al. Fully functional hair follicle regeneration through the rearrangement of stem cells and their niches. Nat Commun. 2012;3:784. doi: 10.1038/ncomms1784. [DOI] [PMC free article] [PubMed] [Google Scholar]