Abstract
Objective
The high risk of nonunion represents a challenge in vertebral surgery, thus stimulating new strategies to improve fusion rates. We investigated the effect of 2 different bone grafts and amniotic fluid application on radiologically and histologically evaluated vertebral fusion in an experimental rat model.
Materials and methods
Forty-eight 24-week-old Sprague Dawley rats were included and assigned into 1 of 4 groups: allograft group, allograft plus human amniotic fluid group, demineralized bone matrix (DBM) group, or DBM plus human amniotic fluid group. After decortication and L4–L6 spinal fusion, study treatments were applied. Fusion in each rat was examined radiologically and histologically 8 weeks after the intervention.
Results
The group that received only allograft had better radiologic scores (median = 3.5; range = 3–4) when compared with the group that received only DBM (median = 2; range = 1–4) (P = 0.002); however, histologic scores did not differ. When amniotic fluid was added to the grafting, allograft-based treatments performed better than DBM-based treatments both on radiologic (median = 4; range = 3–4 vs median = 3; range = 3–4; P = 0.003) and histologic (median = 7; range = 6–7 vs median = 5; range = 3–6; P < 0.001) evaluation. Addition of amniotic fluid did not result in better outcomes in the rats that received DBM-based treatments but based on histologic evaluation, rats that received allograft-based treatments benefited from this application.
Conclusions
Amniotic fluid seems to have an enhancing effect on posterior spinal fusion, particularly when combined with allograft.
Key words: Allograft, amniotic fluid, demineralized bone matrix, experimental study, vertebral fusion
Introduction
Advances in the field of vertebral surgery have inevitably placed more significance on fusion surgery, which is still associated with a 10% to 15% risk of nonunion even in the presence of internal fixation.1 In addition, it is a well-known fact that nonunion often requires revision surgery due to patient dissatisfaction.
Vertebral fusion demands a concerted effect of certain biologic and mechanical factors.1 Although the former of these includes the extraction of joint cartilage, decortication, grafting, and immobilization of the segment to be fused, the latter involves the use of fixation equipment such as rods, plaques, wires, hooks, plasters, corsets, and a variety of apparatuses.2 Reinforcing the fusion with solid internal fixation does not exclude the possibility of nonunion, again placing increasing emphasis on biologic factors.
A number of studies have been undertaken to investigate alternative strategies, particularly looking at alternatives to bone grafts at a clinical level,2 due to the high rate of nonunion and donor site morbidity following the use of autografts in primary spinal fusion surgery.2 In this regard, demineralized bone matrix (DBM) represents a readily available graft alternative with osteoinductive potential that has shown promising results in several studies.3,4
Several mediator molecules with anabolic effects such as the transforming growth factor, fibroblast growth factor (FGF), platelet-derived growth factor, interleukin-1, and interleukin-6 can also provide additional benefit in these procedures.5 Potentially similar growth and trophic factors include insulin-like growth factor (IGF)-I and IGF-II and epidermal growth factor. Specifically, IGF-I and IGF-II are growth factors that are known to be associated with matrix synthesis during the bone recovery phase.6
In addition, amniotic fluid has been reported to be a rich source of certain extracellular macromolecules such as epidermal growth factor, IGF-I, IGF-II, FGF, fibronectin and laminin,7,8 hyaluronic acid (HA) (which is a high molecular weight polysaccharide that is abundant in body fluids and in connective tissue), chondroitin sulphate, and an HA activating factor.7,8 HA is particularly found in soft connective tissues, with some osteoblastic bone-forming effect.7 The role of amniotic fluid, which has a variety of biologic features in vertebral fusion, has been the subject of very few studies until now.
We investigated the effect of bone grafts (allograft or DBM) and amniotic fluid on vertebral fusion in an experimental rat vertebral fusion model.
Materials and Methods
Experimental animals and study groups
The study conformed to the Turkish national recommendations of the ethics committees for animal research, in line with the European Commission Directive 86/609/EEC for animal experiments. A total of 48 Sprague Dawley rats with a mean weight of 250 g (range = 200–300 g) and age of 24 weeks were included in this study. Animals were placed in cages of 2 and were kept at a stable temperature of 20°C to 24°C with 12 hours of dark and 12 hours of light cycles. Rats were assigned into 4 groups consisting of 12 rats in each: Group I had allograft only, Group II had allograft plus human amniotic fluid, Group III had DBM only, and Group IV had DBM plus human amniotic fluid application. Fusion in each rat was examined radiologically and histologically 8 weeks after the experimental application of the study treatments following experimental decortication and spinal fusion between the fourth and sixth vertebrae.
Preparation of allografts and DBM
To obtain allografts and DBM, 8 rats that were not included in the study were killed, after which both iliac wings, femur, and tibia were stripped off from the soft tissues. Iliac wings were used to obtain allografts.
To prepare DBM, femurs and tibias were frozen at –70°C after removal of the soft tissues. Sterilization was carried out by ethylene oxide. Fragments of 0.5 mm were dissected to obtain DBM. They then were ground to achieve fragments with an average dimension of 106 to 500 μm. The decalcification process was completed by storing the material for 16 hours at 4°C in 0.6 normal hydrochloric acid (N HCL) (100 g/2 L). Materials were then washed in sterile water and soaked in 70% ethanol. DBM was dried using a vacuum dryer overnight, sterilized with ethylene oxide, and kept at –70°C.
Preparation of amniotic fluid
Amniotic fluid was obtained from pregnant women attending the obstetrics outpatient unit in our hospital who completed 20 weeks of pregnancy and signed an informed consent. Centrifugation was performed using a Heraeus Sepatech Megafuge 1.0R (Langenselbold, Germany) device at 4300 revolutions/min for 15 minutes. About 0.1 cc precipitate was obtained from this procedure. The remaining 8 cc supernatant was taken and kept at –20°C. Amniotic fluid to be used in surgery was thawed by keeping at room temperature for 20 minutes.
Surgical methods and follow-up
In anesthetized rats in the prone position, a surgical midline incision was made on the lumbar region along the spinous processes. After skin, subcutaneous tissue, and fascia were incised, the longissimus lumbarum muscle, which is localized posteriorly, was stripped off and spinous processes and transverse processes were exposed. Spinal fusion was performed in L4 to L6. Spinous processes of the lumbar vertebrae were taken off by rongeur and bones were cleared of their soft tissues. Transverse processes were decorticated. The lumbar region in which the graft was placed was decorticated with rongeur, curette, and thin burr. In Groups I and III, grafts were applied without amniotic fluid (ie, allograft only in Group I and DBM only in Group III). In Group II and Group IV, in addition to allograft or DBM, 0.5 cc processed amniotic fluid was applied to the posterior spinal elements that were decorticated following fusion with grafts.
During the first 7 days of postsurgical follow-up, wound dressings and examination of the surgical site were performed. Immobilization was not implemented. Rats were killed at Week 8 using high-dose ether anesthesia. Cervical dislocation was not performed on rats because it could affect the fusion. The fusion line was accessed through a posterior midline incision involving the skin, subcutaneous tissue, and muscle layer. The fusion area was carefully dissected with bone scissors avoiding injury to the fusion site at its proximal and distal ends. The removed segments with fusion were placed in bottles containing 10% formaldehyde.
Radiologic evaluations
Each fusion segment extracted was assigned a number and placed on a 30 × 24 cm radiograph cassette so that tube distance was 90 cm. Then anteroposterior radiographs digitally shot were evaluated. Radiographic images were evaluated by a single radiologist who was blinded to the type of grafting implemented. Radiologic assessments were based on Lenke’s radiologic evaluation system9 using a 1- to 4-point scale, where 1 = bilateral graft resorption or fusion mass with obvious bilateral pseudarthrosis; 2 = bilateral small, thin fusion masses; 3 = unilateral large fusion mass with contralateral small fusion mass; and 4 = solid large trabeculated bilateral fusion masses.
Histologic examinations
Samples from which radiologic images were obtained after the rats were killed were decalcified in 10% formic acid at room temperature for 80 days. Decalcification solution was changed every 3 days during this period. Samples were dehydrated with ethanol, cleaned with xylene, and buried in paraffin. Longitudinal sections of 5 μm were done by microtome knife and hematoxylin-eosin stain was applied. All cross-sections were evaluated with a light microscope (Olympus BX-51, Postfach, Hamburg, Germany) at the histology laboratory and microphotographs were obtained. A 0- to 7-point scaling system described by Emery et al10 was used for histopathologic evaluations, where 0 = empty cleft, 1 = fibrous tissue only, 2 = more fibrous tissue than fibrocartilage, 3 = more fibrocartilage than fibrous tissue, 4 = fibrocartilage only, 5 = more fibrocartilage than bone, 6 = more bone than fibrocartilage, and 7 = bone only.
Statistical analyses
Statistical evaluation was done using SPSS 13.0 for Windows (IBM-SPSS Inc, Armonk, NY). Differences between the groups in terms of scores were analyzed using Kruskal-Wallis variance analysis and Mann-Whitney U test was used for pairwise comparisons. A P value < 0.05 was considered an indication of statistical significance for the variance analyses. For post-hoc analyses, Bonferroni correction was made and the level of significance was set at P < 0.0083 for Mann Whitney U test.
Results
None of the rats had an immunologic reaction after local administration of amniotic fluid. During the 8-week period after the intervention, none of the rats developed rash or purulent discharge at the site of incision.
Comparison of allograft-based treatments versus DBM-based treatments
Comparisons of the groups in terms of radiologic and histologic scores and images are shown in Figures 1 and 2 and Table I. The group that received only allograft (ie, no amniotic fluid application) had better radiologic scores (median = 3.5; range = 3–4) when compared with the group that received only DBM without amniotic fluid (median = 2; range = 1–4) (P = 0.002). However, these 2 groups did not differ with regard to histologic scores (median = 6; range = 5–6 vs median = 5; range = 4–6) (P > 0.0083). On the other hand, when amniotic fluid was added to the treatment, allograft-based treatments performed better than DBM-based treatments based both on radiologic (median = 4; range = 3–4 vs median = 3; range = 3–4; P = 0.003) and histologic (median = 7; range = 6–7 vs median = 5; range = 3–6; P < 0.001) scores.
Figure 1.
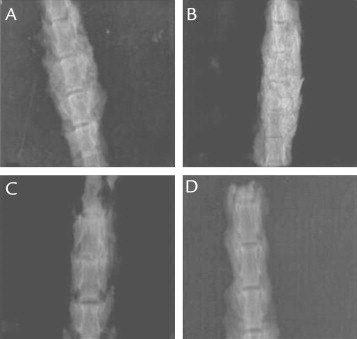
Anteroposterior radiograph images of (A) allograft group, (B) allograft plus amniotic fluid group, (C) demineralized bone matrix group, and (D) demineralized bone matrix plus amniotic fluid group.
Figure 2.
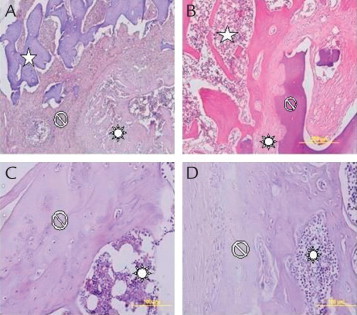
Histologic (hematoxylin-eosin staining [× 40]) images of (A) allograft group, (B) allograft plus amniotic fluid group, (C) demineralized bone matrix group, and (D) demineralized bone matrix plus amniotic fluid group.  = allograft;
= allograft;  = bone tissue;
= bone tissue;  = fibrocartilage tissue.
= fibrocartilage tissue.
Table I.
Comparison of the groups in terms of radiologic and histologic scores.
| Group | Median (min–max) | Group I | Group II | Group III | Group IV | |
|---|---|---|---|---|---|---|
| P value | ||||||
| Group I | Radiologic scores | 3 (3–4) | 0.001* | 0.002* | ||
| Histologic scores | 6 (5–6) | >0.0083 | >0.0083 | |||
| Group II | Radiologic scores | 4 (3–4) | 0.001* | 0.003* | ||
| Histologic scores | 7 (6–7) | >0.0083 | <0.001* | |||
| Group III | Radiologic scores | 2 (1–4) | 0.002* | >0.0083 | ||
| Histologic scores | 5 (4–6) | >0.0083 | >0.0083 | |||
| Group IV | Radiologic scores | 3 (3–4) | 0.003* | >0.0083 | ||
| Histologic scores | 5 (3–6) | <0.001* | >0.0083 | |||
P < 0.0083 for the pairwise comparison, indicating statistical significance.
Effect of amniotic fluid
Addition of amniotic fluid did not result in better outcomes in the rats that received DBM-based treatments. When the group that received DBM only and the group that received DBM plus amniotic fluid were compared, no significant differences was found in radiologic or histologic scores (P > 0.0083 for both comparisons). On the other hand, addition of amniotic fluid to the allograft resulted in better histologic scores (median = 7; range = 6–7 versus median = 6; range = 5–6; P = 0.001), but not better radiologic scores (median = 4; range = 3–4 versus median = 3.5; range = 3–4).
Discussion
Posterior fusion is frequently performed for the surgical management of a number of conditions, including vertebra fractures, spondylolisthesis, and vertebral instability. However, controversy still exists on the type of grafts to be used in these applications. Although autografts represent the most commonly preferred strategy, their disadvantages include their paucity and high morbidity rates in graft sites. The volume of bone that can be used for autografting may be limited.11 Presence of inflammatory and osteogenic cells in the microanatomic environment and a healthy blood flow constitute the key elements of a successful spinal arthrodesis. The few transplanted autologous cells that survive in the fusion site can be a source for osteogenic cells.
Allografts, used in combination with autografts or as a substitute for autografts, represent the most commonly used type of graft. Bridwell et al12 found it convenient to use allografts in patients with paralysis in whom obtaining autogenic bone is not possible. Several studies have also demonstrated successful posterior fusion using allografts in children. Although a high rate of compression is found with allografts in the lumbar vertebra, an acceptable rate of fusion is obtained in structural allografts supported with anterior autologous bone grafts. Also, it needs to be taken into consideration that there can be disadvantages to allografts—like the risk of bacterial-viral infections and immunologic reactions—because they need cold-chain during the time they are preserved and transferred. In a study where 29 patients treated with fresh frozen allograft were compared with those treated with autografts, Brown et al13 did not find any difference in terms of fusion and graft resorption. These abovementioned studies examined single-level fusion and the authors pointed out that higher rates of resorption occurs in multilevel fusions done with allograft. Young and Rossenwasser14 performed fusion using fibular allografts and did not find any difference in terms of postoperative fusion success rates between fibular allograft and iliac wing.
Although several studies have demonstrated that DBM is a convenient graft alternative,3,4 there are scarce data on its use for vertebral fusion. Several experimental animal models were developed to investigate the osteoinductive capacity of DBM or to improve the biologic activity of autografts.3,4 Morone and Boden15 demonstrated that a low volume of autograft revealed similar results with the fusion obtained by a mixture prepared with DBM gel. Many experimental study models have been published reporting on the effect of DBM. In a study conducted by Urist16 almost 40 years ago, osteoinductive capacity of DBM was established. It was shown in experiments and in a limited number of studies that DBM increased bone formation. Peterson et al17 reported satisfactory fusion rates in a study in which they investigated the fusion potential of commercial DBM derivatives compared with autografts. In our study, scores of DBM based on histologic and radiologic evaluation were inferior compared with those obtained from the allograft group.
Studies of amniotic fluid in this context showed positive effects on cell differentiation, migration, and invasion of various cell types.7 Ozgenel et al7 suggested that amniotic fluid accelerates new cartilage formation and this could be due to the rich HA and growth factor content of amniotic fluid.7 In another study in which the authors examined the effect of amniotic fluid on cartilage formation in pericondrial flaps, Ozgenel et al18 suggested that at the end of eighth week, amniotic factor had a positive effect on scar tissue and new cartilage formation, most likely due to the presence of growth factors and extracellular matrix precursors. In a study examining the osteoblastic bone production induced by amniotic fluid, Karacal et al19 reported that bone formation was improved histopathologically when amniotic fluid was applied to bone defects in rabbits at the end of a 6-week period. In an experimental study by Aydin et al,20 significantly better radiologic fusion rates at 6 weeks and significantly better histologic fusion quality at 3 and 6 weeks were observed in rats receiving amniotic fluid. These results are consistent with our observations, where histologic scores were statistically significantly better in the allograft plus amniotic fluid group compared with those in the allograft only group; however, such a superiority was not evident in terms of radiologic scores, probably due to low number of subjects. On the other hand, histologic and radiologic fusion scores were not better in the DBM plus amniotic fluid group compared with those in the DBM only group, thus not supporting beneficial effects of amniotic fluid on fusion when combined with DBM. These data show that amniotic fluid is effective in enhancing vertebral fusion, particularly when combined with allograft, probably due to its high content of several growth factors.
Both allografts and DBM are relatively more available for surgical use compared with autografts. Furthermore, the amniotic fluid used in our study seemed to have an additional osteoinductive effect. However, cost may be a limiting factor for its use and the use of allografts and DBM may be associated with higher treatment costs. Considering the continuous pressure on health care providers in terms of cost-containment strategies, the cost issue may eventually prove to be significant from the viewpoint of health care costs within specific countries. On the other hand, it is also obvious that higher success and lower nonunion rates would be expected to reduce treatment costs.
Conclusions
Amniotic fluid seems to have an enhancing effect on posterior spinal fusion, particularly when combined with allograft, as shown radiologically and histologically at the end of the eighth week. Use of amniotic fluid in this setting seems to be a promising approach in improving fusion rates and further studies are warranted to better define its clinical role and benefit.
Conflicts of Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Acknowledgments
Mithat Oner were responsible in study design and writing. Turan Cihan Dülgeroglu were responsible in study design and data collection. Ibrahim Karaman were responsible in writing and data collection. Ahmet Guney were responsible in writing. Ibrahim Halil Kafadar were responsible in figure creation. Sevki Erdem were responsible in study design.
References
- 1.Sama A.A., Khan S.N., Myers E.R. High-dose alendronate uncouples osteoclast and osteoblast function: a study in a rat spine pseudarthrosis model. Clin Orthop Relat Res. 2004:135–142. doi: 10.1097/00003086-200408000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Hsu W.K., Wang J.C. Biologic substitutes in spinal arthrodesis. COLUNA/COLUMNA. 2007;6:174–182. [Google Scholar]
- 3.Aghdasi B., Montgomery S.R., Daubs M.D. A review of demineralized bone matrices for spinal fusion: the evidence for efficacy. Surgeon. 2013;11:39–48. doi: 10.1016/j.surge.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Tilkeridis K., Touzopoulos P., Ververidis A. Use of demineralized bone matrix in spinal fusion. World J Orthop. 2014;5:30–37. doi: 10.5312/wjo.v5.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hannouche D., Petite H., Sedel L. Current trends in the enhancement of fracture healing. J Bone Joint Surg Br. 2001;83:157–164. doi: 10.1302/0301-620x.83b2.12106. [DOI] [PubMed] [Google Scholar]
- 6.Tuncel M., Halici M., Canoz O. Role of insulin like growth factor-I in repair response in immature cartilage. Knee. 2005;12:113–119. doi: 10.1016/j.knee.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Ozgenel G.Y., Filiz G., Ozcan M. Effects of human amniotic fluid on cartilage regeneration from free perichondrial grafts in rabbits. Br J Plast Surg. 2004;57:423–428. doi: 10.1016/j.bjps.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 8.Merimee T.J., Grant M., Tyson J.E. Insulin-like growth factors in amniotic fluid. J Clin Endocrinol Metab. 1984;59:752–755. doi: 10.1210/jcem-59-4-752. [DOI] [PubMed] [Google Scholar]
- 9.Lenke L.G., Bridwell K.H., Bullis D. Results of in situ fusion for isthmic spondylolisthesis. J Spinal Disord. 1992;5:433–442. doi: 10.1097/00002517-199212000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Emery S.E., Brazinski M.S., Koka A. The biological and biomechanical effects of irradiation on anterior spinal bone grafts in a canine model. J Bone Joint Surg Am. 1994;76:540–548. doi: 10.2106/00004623-199404000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Sonntag V.K., Marciano F.F. Is fusion indicated for lumbar spinal disorders? Spine. 1995;20:138S–142S. [PubMed] [Google Scholar]
- 12.Bridwell K.H., Lenke L.G., McEnery K.W. Anterior fresh frozen structural allografts in the thoracic and lumbar spine. Do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects? Spine. 1995;20:1410–1418. [PubMed] [Google Scholar]
- 13.Brown M.D., Malinin T.I., Davis P.B. A roentgenographic evaluation of frozen allografts versus autografts in anterior cervical spine fusions. Clin Orthop Relat Res. 1976:231–236. [PubMed] [Google Scholar]
- 14.Young W.F., Rosenwasser R.H. An early comparative analysis of the use of fibular allograft versus autologous iliac crest graft for interbody fusion after anterior cervical discectomy. Spine. 1993;18:1123–1124. doi: 10.1097/00007632-199307000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Morone M.A., Boden S.D. Experimental posterolateral lumbar spinal fusion with a demineralized bone matrix gel. Spine. 1998;23:159–167. doi: 10.1097/00007632-199801150-00003. [DOI] [PubMed] [Google Scholar]
- 16.Urist M.R., Silverman B.F., Buring K. The bone induction principle. Clin Orthop Relat Res. 1967;53:243–283. [PubMed] [Google Scholar]
- 17.Peterson B., Whang P.G., Iglesias R. Osteoinductivity of commercially available demineralized bone matrix. Preparations in a spine fusion model. J Bone Joint Surg Am. 2004;86-A:2243–2250. doi: 10.2106/00004623-200410000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Ozgenel G.Y. The influence of human amniotic fluid on the potential of rabbit ear perichondrial flaps to form cartilage tissue. Br J Plast Surg. 2002;55:246–250. doi: 10.1054/bjps.2002.3811. [DOI] [PubMed] [Google Scholar]
- 19.Karacal N., Kosucu P., Cobanglu U. Effect of human amniotic fluid on bone healing. J Surg Res. 2005;129:283–287. doi: 10.1016/j.jss.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 20.Aydin H., Saracoglu M., Kerimoglu G. [Effects of human amniotic fluid on posterolateral spinal fusion: an experimental preliminary study] Eklem Hastalik Cerrahisi. 2011;22:166–171. [PubMed] [Google Scholar]


