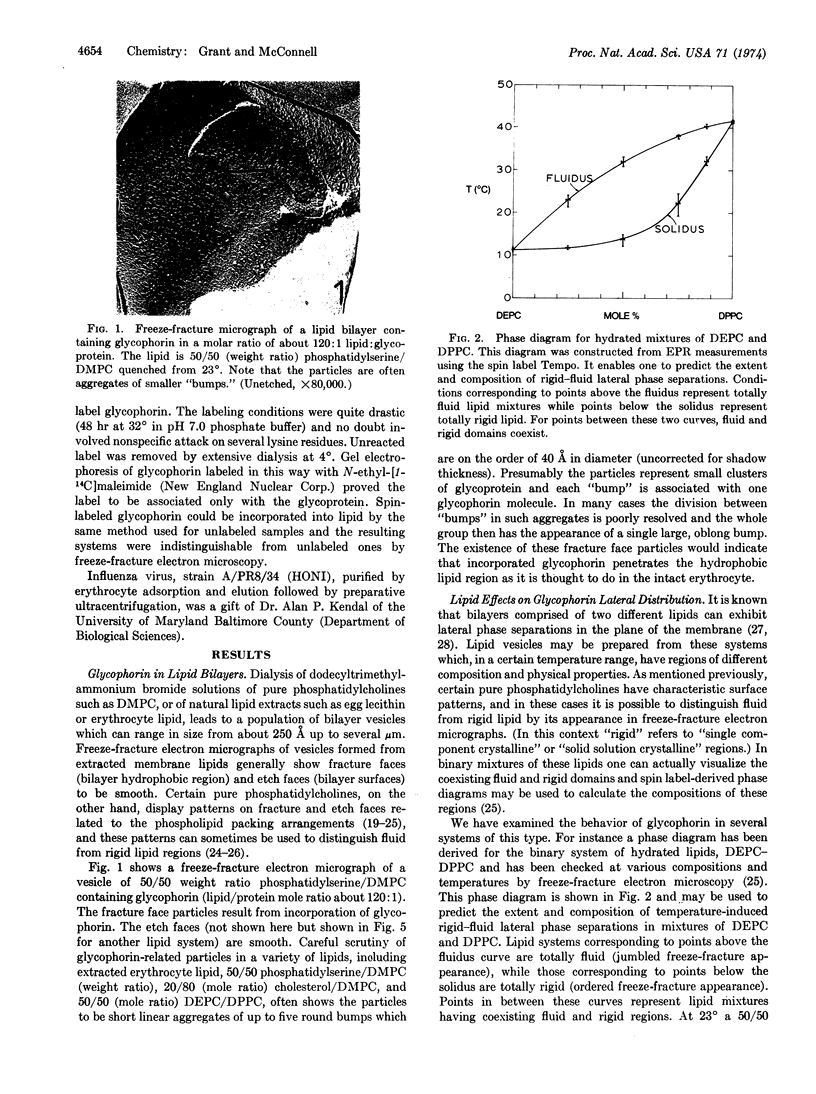
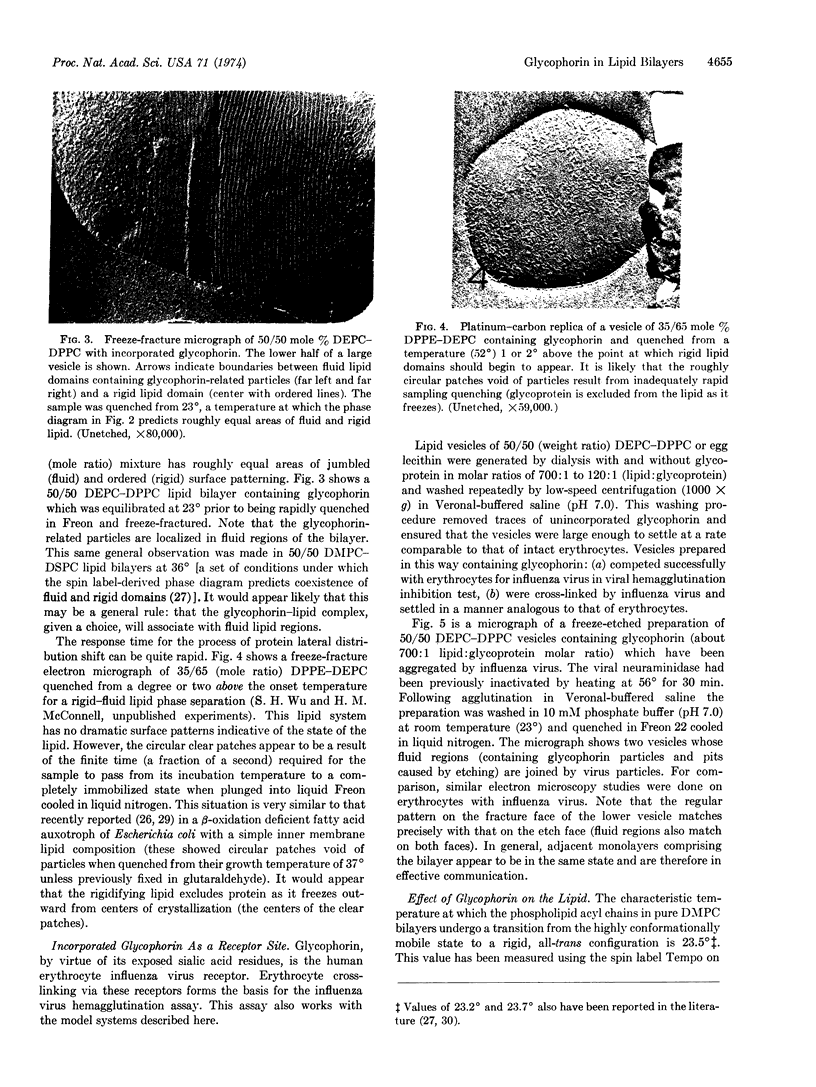
Abstract
Glycophorin, the major glycoprotein of human erythrocytes, has been isolated and reincorporated into lipid vesicles. Freeze-fracture electron microscopy shows the reincorporated glycophorin to occur as small particles in vesicle fracture faces while the etch faces are smooth. The glycoprotein has a tendency to cluster into groups of several particles. Evidence is presented that, although lipids in immediate contact with glycopherin are likely somewhat immobilized, the entire lipid-protein complex has a tendency to occupy fluid regions of the bilayer. Reincorporated glycophorin assumes its proposed conformation in the intact erythrocyte in so far as it penetrates the hydrophobic membrane interior while its N-terminal end with attached carbohydrate residues is exposed to the aqueous compartment and is available as a specific recognition site.
Keywords: lipid-protein interactions, freeze-fracture electron microscopy, spin labels, glycoproteins, lateral phase separations
Full text
PDF
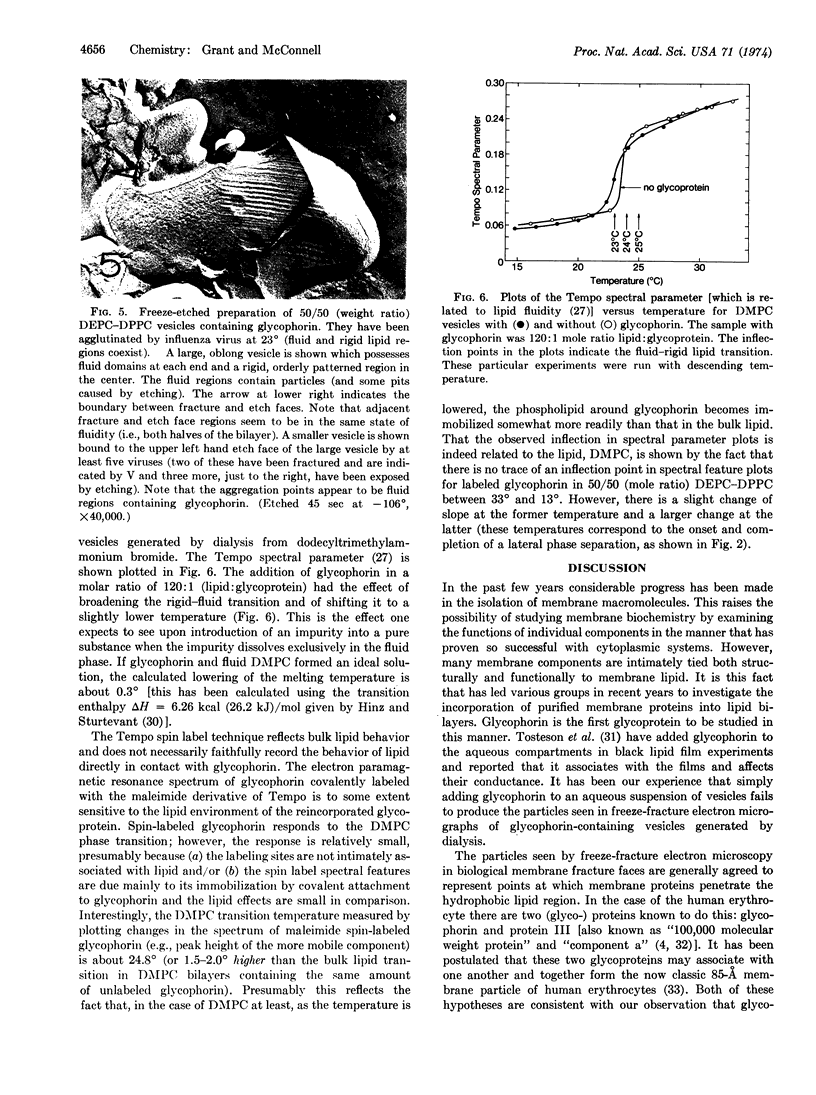

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bretscher M. S. A major protein which spans the human erythrocyte membrane. J Mol Biol. 1971 Jul 28;59(2):351–357. doi: 10.1016/0022-2836(71)90055-6. [DOI] [PubMed] [Google Scholar]
- Cubero Robles E., van den Berg D. Synthesis of lecithins by acylation of O-(sn-glycero-3-phosphoryl) choline with fatty acid anhydrides. Biochim Biophys Acta. 1969 Dec 17;187(4):520–526. doi: 10.1016/0005-2760(69)90049-6. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Dehlinger P. J., Jost P. C., Griffith O. H. Lipid binding to the amphipathic membrane protein cytochrome b5. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2280–2284. doi: 10.1073/pnas.71.6.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluck D. J., Henson A. F., Chapman D. The structure of dilute lecithin-water systems revealed by freeze-etching and electron microscopy. J Ultrastruct Res. 1969 Dec;29(5):416–429. doi: 10.1016/s0022-5320(69)90063-x. [DOI] [PubMed] [Google Scholar]
- Grant C. W., Wu S. H., McConnell H. M. Lateral phase separations in binary lipid mixtures: correlation between spin label and freeze-fracture electron microscopic studies. Biochim Biophys Acta. 1974 Sep 6;363(2):151–158. doi: 10.1016/0005-2736(74)90055-8. [DOI] [PubMed] [Google Scholar]
- Griffith O. H., McConnell H. M. A nitroxide-maleimide spin label. Proc Natl Acad Sci U S A. 1966 Jan;55(1):8–11. doi: 10.1073/pnas.55.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti G. Membrane proteins. Annu Rev Biochem. 1972;41:731–752. doi: 10.1146/annurev.bi.41.070172.003503. [DOI] [PubMed] [Google Scholar]
- Hinz H. J., Sturtevant J. M. Calorimetric studies of dilute aqueous suspensions of bilayers formed from synthetic L- -lecithins. J Biol Chem. 1972 Oct 10;247(19):6071–6075. [PubMed] [Google Scholar]
- Hong K., Hubbell W. L. Preparation and properties of phospholipid bilayers containing rhodopsin. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2617–2621. doi: 10.1073/pnas.69.9.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. L., Segrest J. P., Kahane I., Marchesi V. T. Studies on the major sialoglycoprotein of the human red cell membrane. Isolation and characterization of tryptic glycopeptides. Biochemistry. 1973 Jul 31;12(16):3131–3138. doi: 10.1021/bi00740a600. [DOI] [PubMed] [Google Scholar]
- Ji T. H., Nicolson G. L. Lectin binding and perturbation of the outer surface of the cell membrane induces a transmembrane organizational alteration at the inner surface. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2212–2216. doi: 10.1073/pnas.71.6.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost P. C., Capadil R. A., Vanderkooi G., Griffith O. H. Lipid-protein and lipid-lipid interactions in cytochrome oxidase model membranes. J Supramol Struct. 1973;1(4):269–280. doi: 10.1002/jss.400010404. [DOI] [PubMed] [Google Scholar]
- Juliano R. L. The proteins of the erythrocyte membrane. Biochim Biophys Acta. 1973 Dec 28;300(4):341–378. doi: 10.1016/0304-4157(73)90013-0. [DOI] [PubMed] [Google Scholar]
- Kleemann W., McConnell H. M. Lateral phase separations in Escherichia coli membranes. Biochim Biophys Acta. 1974 Apr 29;345(2):220–230. doi: 10.1016/0005-2736(74)90260-0. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Andrews E. P. Glycoproteins: isolation from cellmembranes with lithium diiodosalicylate. Science. 1971 Dec 17;174(4015):1247–1248. doi: 10.1126/science.174.4015.1247. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Tillack T. W., Jackson R. L., Segrest J. P., Scott R. E. Chemical characterization and surface orientation of the major glycoprotein of the human erythrocyte membrane. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1445–1449. doi: 10.1073/pnas.69.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L. Anionic sites of human erythrocyte membranes. I. Effects of trypsin, phospholipase C, and pH on the topography of bound positively charged colloidal particles. J Cell Biol. 1973 May;57(2):373–387. doi: 10.1083/jcb.57.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto da Silva P., Douglas S. D., Branton D. Localization of A antigen sites on human erythrocyte ghosts. Nature. 1971 Jul 16;232(5307):194–196. doi: 10.1038/232194a0. [DOI] [PubMed] [Google Scholar]
- SINGLETON W. S., GRAY M. S., BROWN M. L., WHITE J. L. CHROMATOGRAPHICALLY HOMOGENEOUS LECITHIN FROM EGG PHOSPHOLIPIDS. J Am Oil Chem Soc. 1965 Jan;42:53–56. doi: 10.1007/BF02558256. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Marchesi V. T., Guyer R. B., Terry W. Red cell membrane glycoprotein: amino acid sequence of an intramembranous region. Biochem Biophys Res Commun. 1972 Nov 15;49(4):964–969. doi: 10.1016/0006-291x(72)90306-3. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Kahane I., Jackson R. L., Marchesi V. T. Major glycoprotein of the human erythrocyte membrane: evidence for an amphipathic molecular structure. Arch Biochem Biophys. 1973 Mar;155(1):167–183. doi: 10.1016/s0003-9861(73)80019-0. [DOI] [PubMed] [Google Scholar]
- Shimshick E. J., Kleemann W., Hubbell W. L., McConnell H. M. Lateral phase separations in membranes. J Supramol Struct. 1973;1(4):285–294. doi: 10.1002/jss.400010406. [DOI] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separation in phospholipid membranes. Biochemistry. 1973 Jun 5;12(12):2351–2360. doi: 10.1021/bi00736a026. [DOI] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separations in binary mixtures of cholesterol and phospholipids. Biochem Biophys Res Commun. 1973 Jul 17;53(2):446–451. doi: 10.1016/0006-291x(73)90682-7. [DOI] [PubMed] [Google Scholar]
- Stier A., Sackmann E. Spin labels as enzyme substrates. Heterogeneous lipid distribution in liver microsomal membranes. Biochim Biophys Acta. 1973 Jul 6;311(3):400–408. doi: 10.1016/0005-2736(73)90320-9. [DOI] [PubMed] [Google Scholar]
- Tardieu A., Luzzati V., Reman F. C. Structure and polymorphism of the hydrocarbon chains of lipids: a study of lecithin-water phases. J Mol Biol. 1973 Apr 25;75(4):711–733. doi: 10.1016/0022-2836(73)90303-3. [DOI] [PubMed] [Google Scholar]
- Tillack T. W., Scott R. E., Marchesi V. T. The structure of erythrocyte membranes studied by freeze-etching. II. Localization of receptors for phytohemagglutinin and influenza virus to the intramembranous particles. J Exp Med. 1972 Jun 1;135(6):1209–1227. doi: 10.1084/jem.135.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosteson M. T., Lau F., Tosteson D. C. Incorporation of a functional membrane glycoprotein into lipid bilayer membranes. Nat New Biol. 1973 May 23;243(125):112–114. [PubMed] [Google Scholar]
- Ververgaert P. H., Verkleij A. J., Elbers P. F., van Deenen L. L. Analysis of the crystallization process in lecithin liposomes: a freeze-etch study. Biochim Biophys Acta. 1973 Jul 6;311(3):320–329. doi: 10.1016/0005-2736(73)90313-1. [DOI] [PubMed] [Google Scholar]
- Ververgaert P. H., Verkleij A. J., Verhoeven J. J., Elbers P. F. Spray-freezing of liposomes. Biochim Biophys Acta. 1973 Jul 18;311(4):651–654. doi: 10.1016/0005-2736(73)90140-5. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]