Abstract
Background
Ewing sarcoma (ES) is driven by fusion of the EWS gene with an ETS transcription factor, most often FLI1. Neuropeptide Y (NPY) is an EWS-FLI1 transcriptional target. NPY is highly expressed in ES and exerts opposing effects, ranging from ES cell death to angiogenesis and cancer stem cell propagation. The functions of NPY are regulated by dipeptidyl peptidase IV (DPPIV), a hypoxia-inducible enzyme that cleaves the peptide and activates its growth-promoting actions. The goal of this study was to determine clinically relevant functions of NPY by identifying the associations between its concentrations and DPP activity in patients and ES phenotype.
Methods
NPY concentrations and DPP activity were measured in serum samples from 223 patients with localized and 9 patients with metastatic ES provided by Children’s Oncology Group.
Results
Serum NPY levels were elevated in ES patients, as compared to healthy control and osteosarcoma populations, independently of the EWS-ETS translocation type. Significantly higher NPY concentrations were detected in ES patients with tumors of pelvic and bone origin. A similar trend was observed in patients with metastatic ES. There was no effect of NPY on survival in patients with localized ES. DPP activity in sera of ES patients was not significantly different from healthy control and osteosarcoma patients. However, high DPP levels were associated with improved survival.
Conclusion
Systemic NPY is elevated in ES patients and its high levels associate with unfavorable disease features. DPPIV in patients’ sera is derived from non-tumoral sources and its high activity correlates with improved survival.
Keywords: Ewing sarcoma, neuropeptide Y, dipeptidyl peptidase IV, survival, disease phenotype
Introduction
Ewing sarcoma (ES) is an aggressive malignancy of children and adolescents arising in bones or soft tissues. The presence of metastases is the most powerful adverse prognostic factor in ES, with a 5-year event-free survival (EFS) at 72% and 3-year EFS at 27% for patients with localized and metastatic disease, respectively.1,2 While pulmonary metastases are the most common, the prognosis is worse for patients with secondary bone tumors, particularly when both bone and lung metastases are present (8–14% EFS).1 Among patients with localized disease, pelvic tumors carry a worse prognosis.3
Malignant transformation of ES is driven by chromosomal translocations resulting in the fusion of the EWS gene with an ETS transcription factor.4 Most common fusion types include EWS-FLI1 transcripts varying in their fusion sites and EWS-ERG. However, recent studies identified other ETS proteins that form fusion with EWS, as well as novel translocations associated with ES and ES-related tumors that do not contain EWS gene.5
Microarray analyses have identified multiple transcriptional targets of the EWS-FLI1 protein that are up-regulated in ES, including neuropeptide Y (NPY) and its receptors – Y1R and Y5R.6 NPY is a 36 amino acid sympathetic neurotransmitter known to regulate cell proliferation and differentiation and act as an angiogenic factor.7–9 Moreover, the peptide controls bone homeostasis by blocking osteoblast differentiation.10 There is also growing evidence of NPY’s role in the regulation of tumor growth, both via its angiogenic activity and direct effects on tumor cells.8,11–13 Importantly, in tumors of sympathetic origin, such as neuroblastoma and pheochromocytoma, NPY release manifested by its elevated systemic levels in patients has been associated with an aggressive phenotype of the disease.14–17 No such correlations were found for intratumoral NPY mRNA.
The role of NPY in ES remains unclear. Initial studies from our laboratory indicated that NPY stimulates cell death via activation of both Y1R and Y5R.11,12 Consequently, exogenous NPY inhibits ES cell survival in vitro and growth of primary tumors in an ES xenograft model.11,12 However, we have also shown that in the hypoxic tumor microenvironment, the actions of the endogenous NPY shift to Y2R/Y5R-driven effects that are known to promote tumor dissemination, such as ES cancer stem cell proliferation and migration, as well as angiogenesis.13 This hypoxia-induced switch in NPY actions is mediated by increased Y2R and Y5R expression, but also by stimulation of dipeptidyl peptidase IV (DPPIV), a membrane protease that converts full length NPY1–36 to the selective Y2R/Y5R agonist, NPY3–36.12,13 Thus, DPPIV is a key regulator of NPY actions in ES, shifting its activity from Y1R/Y5R-mediated growth inhibition to Y2R/Y5R-mediated potentially pro-metastatic effects. However, the protease also modifies a variety of other peptides and augments the cellular immune response.18,19
High endogenous NPY expression in tumors often leads to its elevated systemic levels.14–17 We have also shown that high levels of DPPIV in ES xenografts result in its elevated activity in plasma.12 Therefore, the goal of the present study was to assess levels of NPY and DPPIV activity in sera of ES patients and determine if the pattern of their release correlates with specific disease phenotype, providing insight into clinically relevant functions of the peptide. We have shown for the first time that systemic NPY levels are highly elevated in ES patients with unfavorable disease features. Thereby, our data corroborate results of previous experimental studies demonstrating hypoxia-induced pro-metastatic effect of NPY.13 In contrast, DPPIV detectable in patients’ sera is derived from non-tumoral sources and its high activity correlates with better EFS.
Methods
Human samples
232 serum samples from ES patients and 21 serum samples from osteosarcoma patients were received from Children’s Oncology Group (COG). 31 serum samples from healthy volunteer children, ages 6–18 years of age, were collected at the Georgetown University Clinical Research Unit. Human tissue sections from 17 archival paraffin embedded ES samples were collected from multiple institutions in Poland by one of the co-authors (EIS) in compliance with institutional ethical regulations. Use of these samples was approved by Georgetown University Institutional Review Board.
Cell culture
Human ES cell lines were obtained and cultured as previously reported.12
ES xenografts
SK-ES1 or TC71 cells were injected orthotopically into gastrocnemius muscles of SCID/bg mice.13 Once tumors reached 1cm3 the primary tumors were excised and tissues collected for analyses.
Real time RT-PCR
RNA was isolated using High Pure RNA Isolation Kit (Roche Applied Science, Indianapollis, IN). cDNA was synthesized using iScript cDNA Synthesis Kit and amplified using ICycler iQ Detection System (Bio-Rad Laboratories, Hercules, CA), TaqMan Universal PCR Master Mix and pre-designed primers and fluorescein-labeled probes (Applied Biosystems, Foster City, CA). The results were calculated by the comparative CT method using β-actin as a reference gene.
Tumor translocations and gene expression data
Translocation and gene expression profiling data for primary ES tumor samples were provided by COG. Fusion type was known for 50 of the evaluated patients as determined from archived tumor specimens.4 Gene expression profiling of 56 archived COG tumor samples was performed using Affymetrix Human Exon arrays and normalization and transcript summarization of data achieved using Partek Genomics Suites (Partek, St. Louis, Mo).
NPY ELISA
Conditioned media were collected from ES cells upon 24h culture and cells were trypsinized, counted and lysed in ELISA assay buffer. NPY concentration in the cell extracts and culture media was determined using Neuropeptide Y Enzyme Immunoassay Kit (Bachem Peninsula Laboratories, San Carlos, CA) and normalized per mg of protein or cell number, respectively. Patient sera were extracted using C18 Sep-columns (Bachem Peninsula Laboratories, San Carlos, CA) and NPY measured by ELISA, as above. For samples that reached the upper limit of detection (1.267 ng/ml) in the initial ELISA test and had sufficient volume, a second assay with a higher dilution was performed. For volume-limited samples, the upper limit of detection from the first ELISA test was used. Subsequent statistical analyses relied on categorized NPY data.
Dipeptidyl peptidase (DPP) activity
DPP activity in human serum was measured colorimetrically at 405nm, using 1mM p-nitroanilide (pNA)-conjugated Gly-Pro dipeptide substrate (Sigma, St. Louis, MO), as previously described.12
Immunohistochemistry
Immunostaining of ES xenografts and human tumors was performed on formalin-fixed, paraffin-embedded tissue samples using rabbit polyclonal anti-NPY antibody (Sigma, St. Louis, MO).
Statistical analyses
Statistical analyses were performed using SigmaStat®, GraphPad and SPSS software. For systemic NPY levels and DPP activity, measurements were divided into 4 rank groups or analyzed as continuous variates. The comparisons were performed using Kruskal-Wallis test, Fisher’s exact test, log-rank test and the Cox proportional hazards model, when appropriate. For the Cox model, a stepwise variable selection procedure was utilized, starting with gender, age at enrollment, primary site, randomized treatment assignment, rank group for NPY, and DPP as a continuous variate. General NPY-immunoreactivity and DPP activity were compared via One-way ANOVA and post hoc Bonferroni correction. The comparison of gene expression levels was performed using t-test.
Results
Systemic NPY levels are elevated in ES patients
We have previously shown that ES cells express high levels of NPY. To determine if this high NPY expression in tumors results in secretion of the peptide into the circulation we measured NPY concentrations in sera from 232 ES patients, including 223 patients with localized and 9 patients with metastatic disease. Table 1 summarizes demographic characteristics of the ES patients. Patients with osteosarcoma (n = 21) or healthy children (6–18 years of age; n = 31) served as reference populations.
Table 1.
Demographic characteristics of ES patient population included in study on serum NPY levels.
| Clinical feature: | n (%) | |
|---|---|---|
| Gender | Female | 94 (42.2%) |
| Male | 129 (57.8%) | |
| Age at enrollment | <18 | 196 (87.9%) |
| 18+ | 27 (12.1%) | |
| Race | Black | 2 (0.9%) |
| White | 198 (88.8%) | |
| Other | 9 (4.0%) | |
| Unknown | 14 (6.3%) | |
| Ethnicity | Hispanic | 18 (8.1%) |
| Non-Hispanic | 198 (88.8%) | |
| Unknown | 7 (3.1%) | |
| Primary Tumor Site | Non-pelvic | 185 (83.0%) |
| Pelvic | 38 (17.0%) | |
| Randomized Treatment Assignment | Standard | 113 (50.7%) |
| Intensive | 110 (49.3%) | |
| First event in EFS analysis | No event | 156 (69.3%) |
| Relapse | 59 (26.5%) | |
| Second malignant neoplasm | 5 (2.2%) | |
| Death | 3 (1.4%) | |
| Follow-up time for “No event” patients | Median | 4.9 years |
| Minimum | 1.8 years | |
| Maximum | 7.7 years |
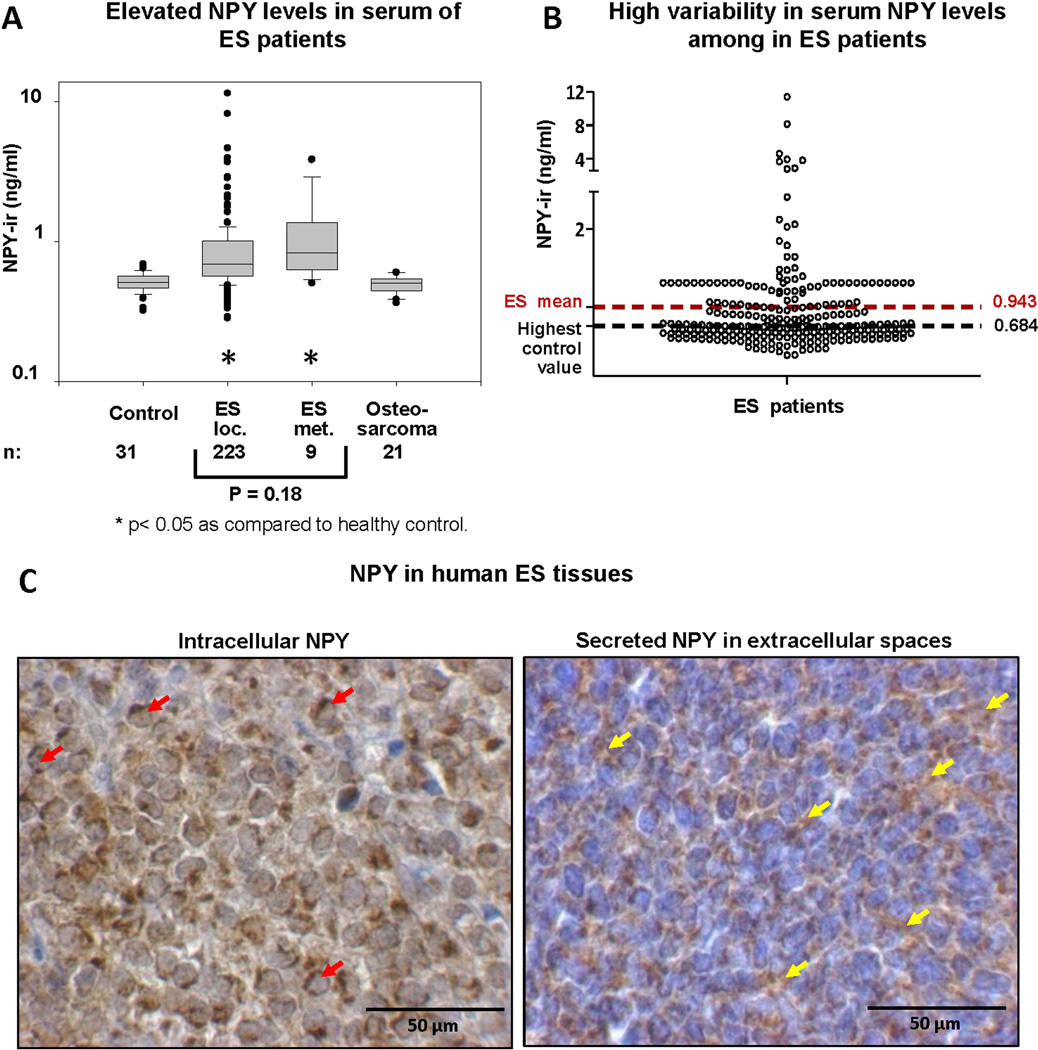
NPY concentrations in sera of ES patients with localized and metastatic disease (mean 0.940 and 1.212 ng/ml, respectively) were significantly higher, as compared to healthy control (mean 0.517 ng/ml), suggesting release of the peptide from ES tumors (Fig. 1A). Patients with metastatic disease tended to have higher NPY serum levels as compared to those with localized ES (p = 0.18). However, due to the limited sample size, no definitive conclusion could be made. No increased serum NPY was observed in osteosarcoma patients (mean 0.492 ng/ml).
Figure 1. ES tumors secrete variable levels of NPY to the circulation.
A. NPY-immunoreactivity (NPY-ir) was measured by ELISA in sera from healthy children (ages 6–18), ES patients with localized and metastatic disease, and osteosarcoma patients. B. Variability in serum NPY concentrations among patients with ES (localized and metastatic patients combined). C. Representative images of human ES tissues exhibiting different patterns of NPY immunostaining – accumulation in tumor cell cytoplasm or in extracellular spaces. Red arrows designate tumor cells with evident cytoplasmic NPY accumulation, while yellow arrows indicate NPY immunoreactivity in extracellular spaces.
Serum NPY levels in ES patients are highly variable
Despite the overall elevated NPY levels in ES patients, the peptide concentrations were highly variable within the ES population (Fig. 1B). While 50% of patients with localized ES had NPY concentrations greater than the highest control value, the remaining patients were at the level of healthy control. Variability in systemic NPY concentrations corresponded to a heterogeneous pattern of NPY immunostaining in human ES tissues (Fig. 1C). 11 out of 17 tested tissue samples (65%) exhibited prevalent intracellular staining, while in 6 samples (35%) the staining was observed in both cytoplasm and extracellular spaces, suggesting increased release of the peptide.
ES cells vary in NPY release
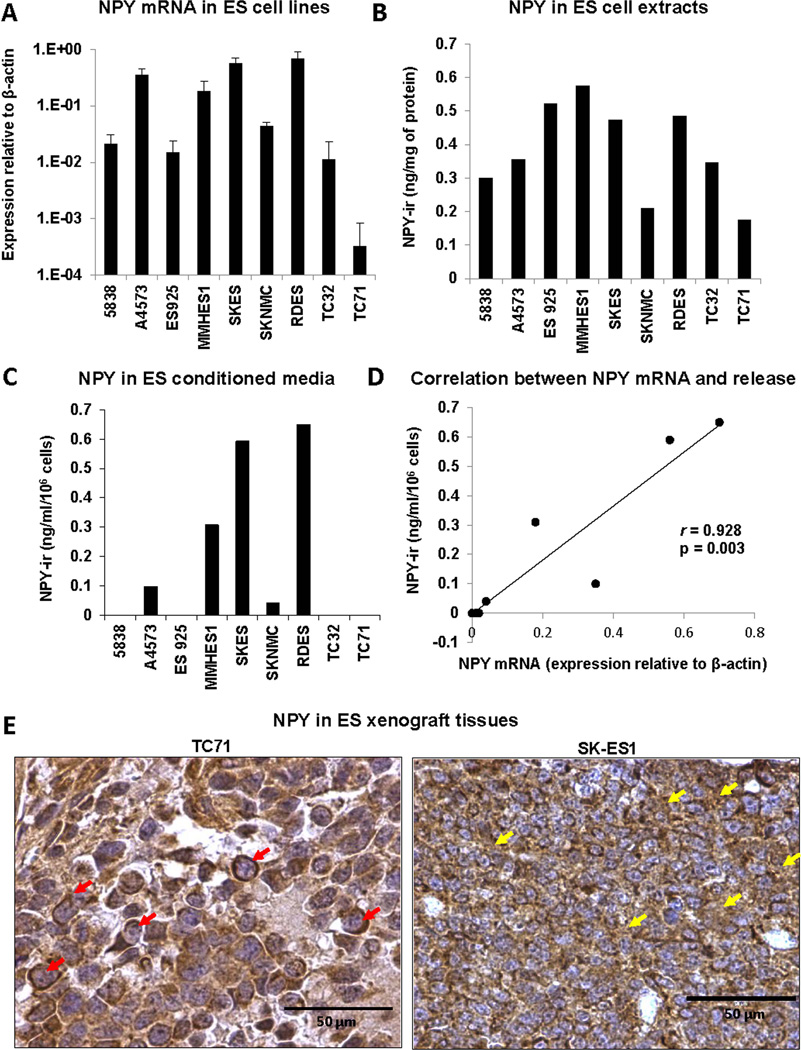
The variability in NPY systemic levels and in its immunostaining patterns suggested differential NPY secretion from ES tumors. To test this, we compared NPY expression and release in a panel of ES cell lines. All tested cells had detectable NPY mRNA and intracellular NPY protein (Fig. 2A, B), however they varied in their release of the peptide. NPY was detectable in conditioned media from 5 out of 9 cell lines (Fig. 2C) and its concentrations positively correlated with mRNA levels (Fig. 2D). No significant correlation between NPY mRNA and its intracellular levels was observed.
Figure 2. ES cells constitutively express NPY but vary in its release.
A. NPY mRNA levels measured in a panel of ES cell lines by real-time RT-PCR. B. NPY intracellular content determined in cell extracts from ES cells by ELISA. C. Concentration of NPY in conditioned media from ES cells measured by ELISA. D. Positive correlation between intracellular NPY mRNA and its concentrations in the corresponding conditioned media. E. NPY immunostaining performed in orthotopic xenograft tissues derived from two ES cell lines varying in NPY release – TC71 and SK-ES1. Red arrows specify tumor cells with cytoplasmic NPY accumulation, while yellow arrows indicate NPY immunoreactivity in extracellular spaces.
To determine if the differences in NPY secretion observed in ES cell lines are maintained in vivo, ES xenograft tissues derived from two cell lines varying in NPY release, TC71 and SK-ES1, were immunostained for NPY. In TC71 xenografts, NPY accumulated mainly in the cytoplasm of tumor cells, while in SK-ES1 xenografts NPY immunoreactivity was also detected in extracellular spaces, suggesting its secretion (Fig. 2E). This observation was in agreement with high concentration of the peptide in SK-ES1, but not TC71 conditioned media (Fig. 2C). These results corroborated our data in human ES tissues, confirming variability in NPY release from ES tumors.
NPY levels are increased in ES patients with various translocation types
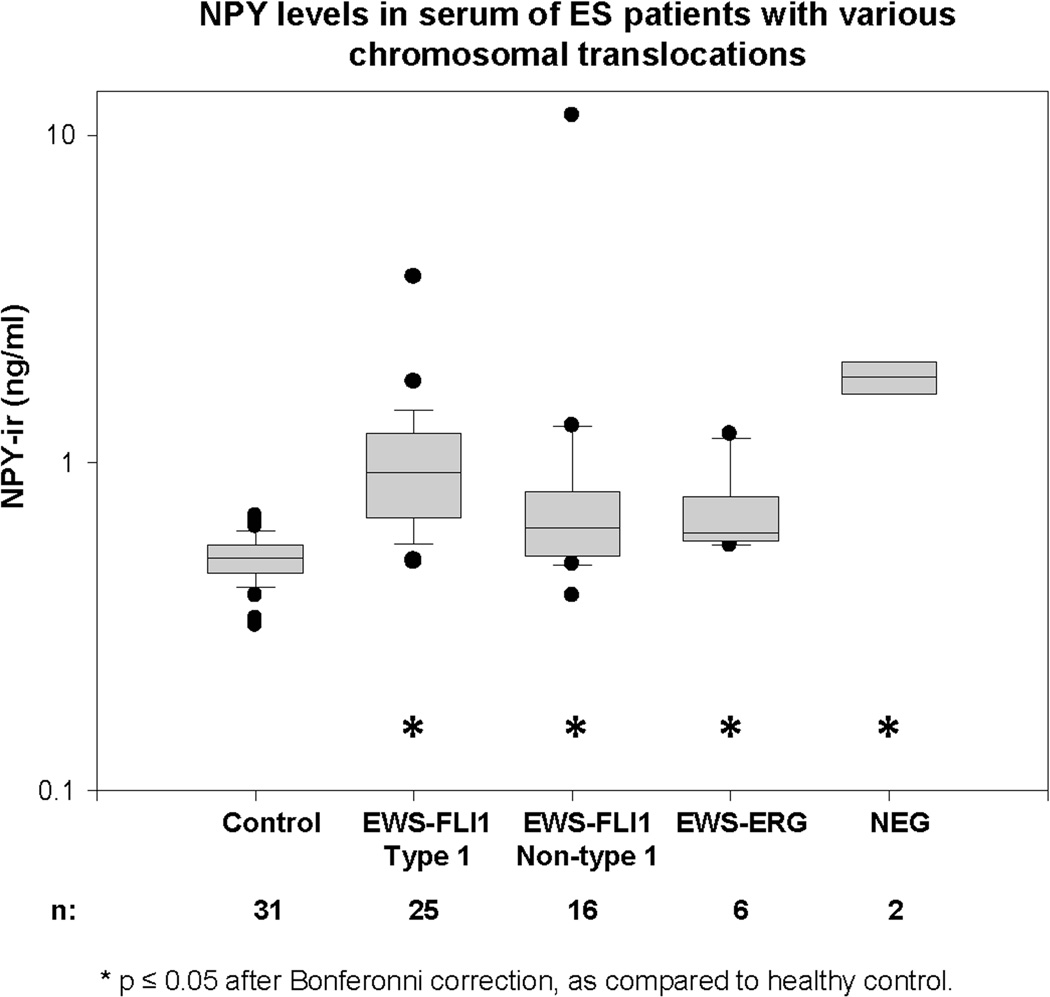
Having determined the variability in systemic NPY levels between ES patients, we sought to identify the phenotype of the disease that is associated with elevated peptide levels. Since NPY is a transcriptional target of EWS-FLI1, we tested whether its release depends on the fusion type. Serum NPY levels were compared in a cohort of 50 patients with known translocation status. All groups of patients with various EWS-FLI1 translocation types and EWS-ERG fusion had significantly elevated NPY levels, as compared to healthy control (Fig. 3). NPY concentrations were particularly high in patients negative for EWS-FLI1 and EWS-ERG, although the limited number of samples precluded reliable group comparison.
Figure 3. Serum NPY levels are elevated in ES patients independently of the EWS-ETS translocation type.
NPY serum concentrations were compared between healthy control (children 6–18 years of age) and ES patients with various types of EWS-ETS translocations. The study was performed in a cohort of 50 patients tested for the presence of EWS-FLI1 and EWS-ERG fusions. Two of the patients were negative for both types of translocations (NEG).
NPY release is elevated in pelvic ES
Since fusion type did not have an effect on NPY release, we correlated serum concentrations of the peptide with the patients’ clinical characteristics (Table 1). No significant associations of gender, age or randomized treatment assignment and NPY levels were observed. However, NPY was significantly elevated in patients with pelvic tumors, as compared to non-pelvic ES (Table 2). 55.3% of patients with pelvic primary tumor sites were above the 75th percentile for NPY level, with only 18.4% total below the median (p = 1.966 × 10−5), which indicated increased NPY release from these tumors.
Table 2.
NPY concentrations in sera of ES patients with pelvic and non-pelvic tumors
| Primary Tumor Site: |
NPY levels by percentile: | Total | p-value | |||
|---|---|---|---|---|---|---|
| < 25th | 25th–50th | 50th – 75th | > 75th | |||
| Non-pelvic n (%) | 51 (27.6%) | 53 (28.6%) | 46 (24.9%) | 35 (18.9%) | 185 (100%) | |
| Pelvic n (%) | 4 (10.5%) | 3 (7.9%) | 10 (26.3%) | 21 (55.3%) | 38 (100%) | 1.966 × 10–5 |
NPY system expression is increased in bone ES
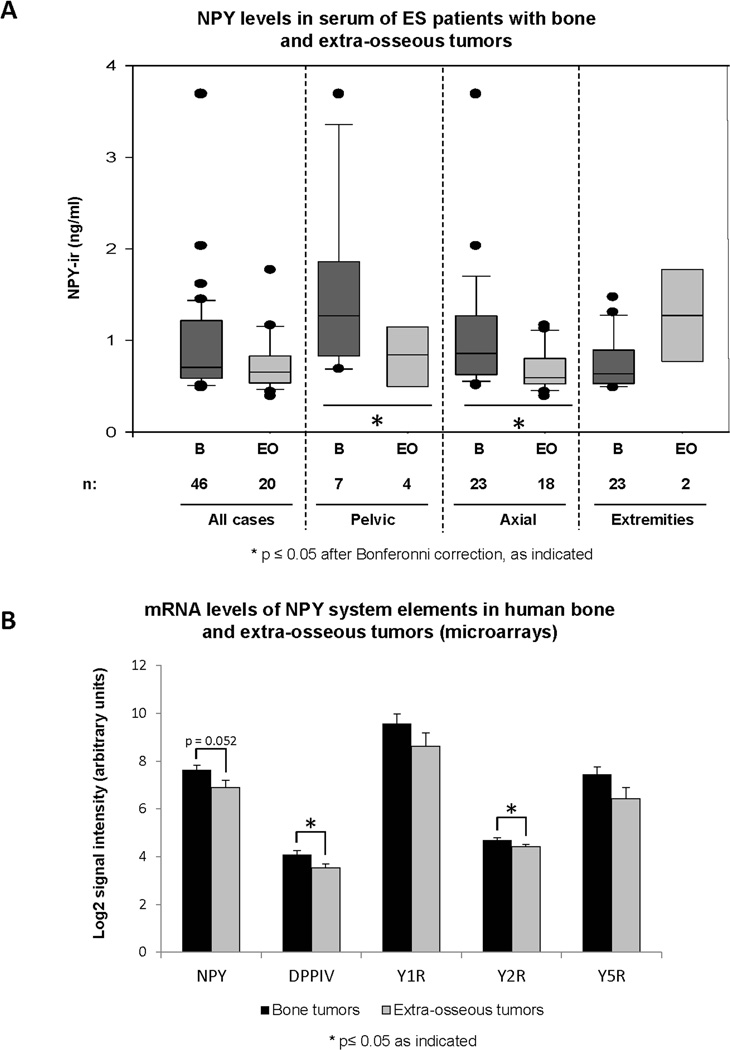
Aside from pelvic localization, bone origin of the primary tumor was also associated with increased NPY release (Fig. 4A). The comparison was performed in a subset of 66 patients with known tumor localization. The difference between bone and extraosseous tumors achieved statistical significance within subsets of pelvic and axial tumors, but not ES localized in extremities. To determine if these differences correlated with the levels of NPY transcription, we interrogated gene expression microarray data from 56 human ES tumors (Fig. 4B). mRNA of the NPY system was moderately, but consistently up-regulated in bone tumors, as compared to the extraosseous lesions. The differences in mRNA levels achieved statistical significance for DPPIV and Y2R (p = 0.003 and p = 0.046, respectively), while it was on the border of significance for NPY (p = 0.052).
Figure 4. NPY system expression is elevated in bone, as compared to soft tissue ES.
A. Serum NPY concentrations were compared between ES patients with bone (B) or extra-osseous (EO) primary tumors. These comparisons were made in the overall patient population and within sub-groups with particular tumor localization. B. mRNA levels of NPY, its receptors, Y1R, Y2R and Y5R, and DPPIV were measured by gene expression microarrays in tissues from 56 human ES primary tumors originating from bone or soft tissues (n = 32 and 23, respectively) and compared between the two groups.
Among ES patients with localized disease, NPY levels had no effect on survival
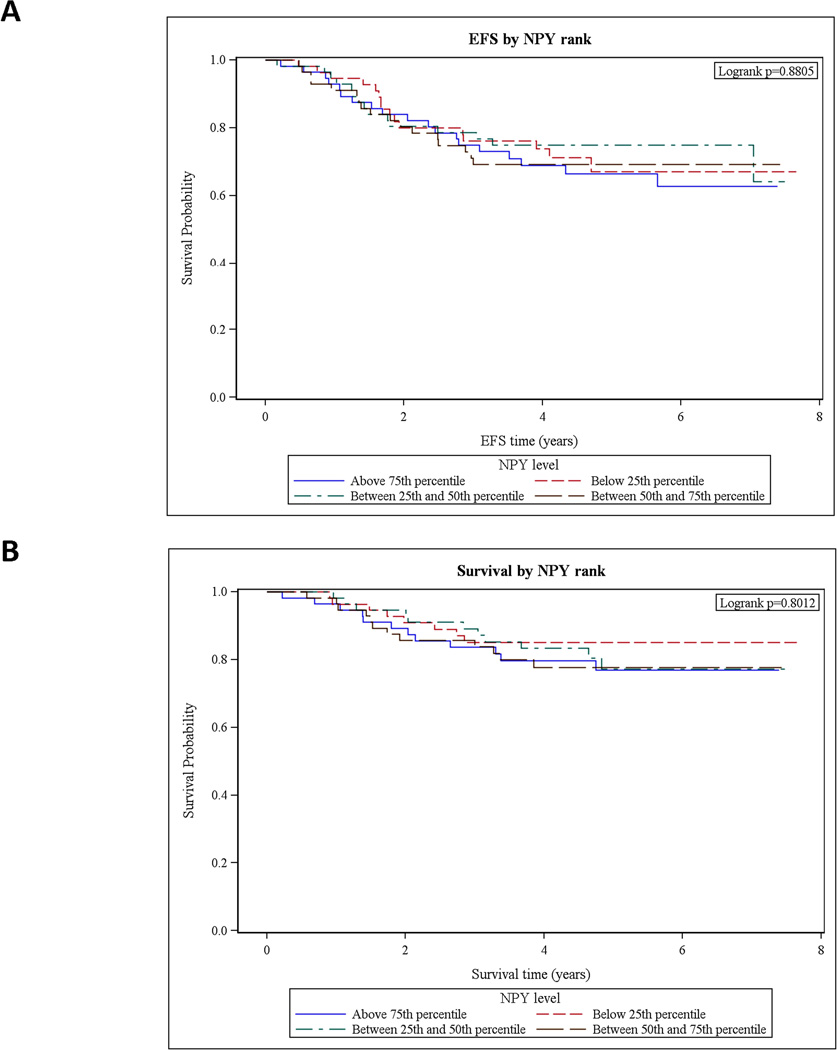
Patients with localized ES were grouped based on serum NPY levels into four approximately equally sized groups. Based on analyses performed with these quartiles, serum concentration of NPY did not have an effect on patients’ survival (Fig. 5). Due to the small sample size, patients with metastatic ES were not included in this analysis.
Figure 5. In ES patients with localized disease, serum NPY levels do not affect survival.
A. ES patients with localized disease were divided into four groups, based on serum NPY levels (above 75th percentile, 50th–75th percentile, 25th–50th percentile and below 25th percentile). Patients’ event-free survival (EFS) was compared between the groups using log-rank test. No significant differences between groups were detected. B. The same approach revealed no significant differences in overall survival between ES patients with various NPY levels.
Tumor DPPIV does not affect systemic DPP activity in sera of ES patients
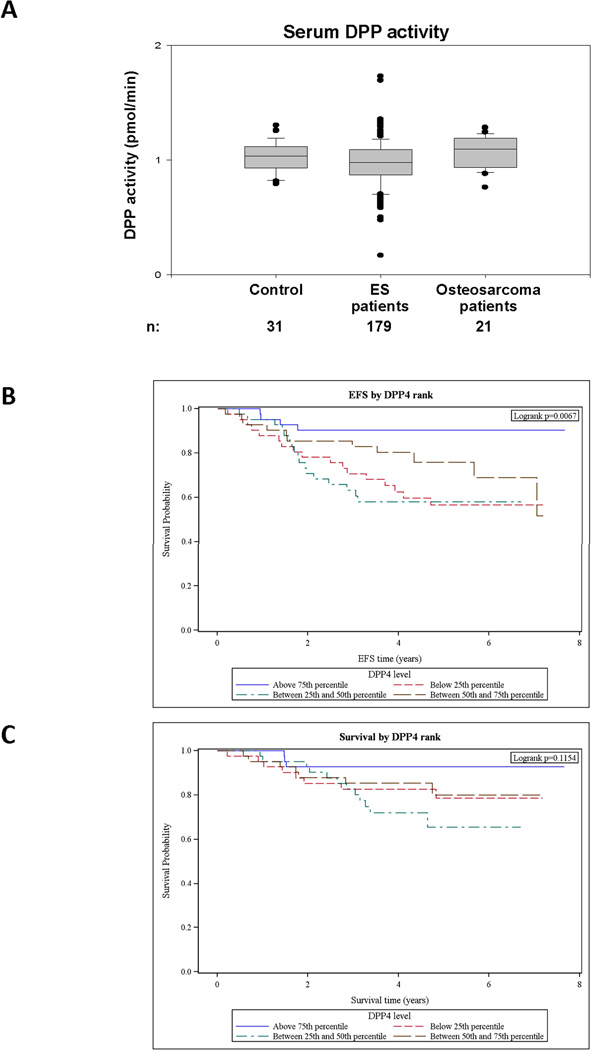
Since DPPIV regulates NPY actions and its plasma levels are elevated in mice bearing DPPIV-rich ES xenografts, we tested DPP activity in the sera of 179 ES patients; a part of the cohort used for measurement of NPY. DPP activity in ES patients was not significantly different as compared to healthy control and osteosarcoma patients (Fig. 6A). These data indicate that the enzyme activity measured in these sera is derived from non-tumoral sources.
Figure 6. DPP activity is not elevated in serum of ES patients, but its high levels associate with better event-free survival.
A. DPP activity was measured in sera of healthy children (ages 6–18), ES patients and osteosarcoma patients by colorimetric method. No significant differences between the experimental groups were observed. B. ES patients with localized disease were categorized in quartiles based on their serum DPP activity. High DPP activity associated with significantly better event-free survival (EFS) among ES patients, as determined by log-rank test (p = 0.0067). C. The effect of DPP levels on overall survival of ES patients with localized disease, tested as above, did not achieve statistical significance (p = 0.1154).
High systemic DPP activity is a strong predictor of better EFS in patients with localized ES
In contrast to NPY levels, DPP activity had a significant effect on EFS of patients with localized ES. The log-rank test for the effect on EFS of DPP activity level categorized in quartiles had a p-value of 0.0067, and the stepwise variable selection in the Cox proportional hazards model gave a model with only DPP level statistically significant, and an estimated hazard ratio of 0.7546 per 0.1 change in DPP activity (95% Wald CI: 0.6626–0.8595, p = 2.216 × 10−5) (Fig. 6B). The effect on overall survival was less pronounced; the log-rank test for the effect on survival of DPP activity level categorized in quartiles had a p-value of 0.1154, and the stepwise selection process in the Cox model similarly had only DPP level as statistically significant, with an estimated hazard ratio of 0.8227 per 0.1 change in DPP activity (95% Wald CI: 0.6921–0.9779, p = 0.0269) (Fig. 6C).
Discussion
As one of the EWS-FLI1 target genes, NPY is highly expressed in ES. In these tumors, the peptide exerts several opposing effects, such as Y1R/Y5R-mediated cells death and Y2R/Y5R-dependent angiogenesis and cancer stem cell proliferation and migration.11–13 NPY functions are regulated by DPPIV and a hypoxic tumor environment, both of which favor its Y2R/Y5R-driven actions.13 Such complex activities of NPY raise a question as to which of the functions of the peptide affect phenotype of the disease and survival of ES patients. To address this, we sought to identify clinical features of ES associated with elevated NPY release and DPP activity.
The average serum NPY concentration was significantly elevated in ES patients, as compared to healthy controls. No such increase in NPY levels was observed in patients with osteosarcoma, a tumor that affects the patient population comparable to ES, indicating that NPY release is characteristic for ES. This observation is consistent with EWS-FLI1-driven expression of NPY.6 The fact that NPY levels were elevated in ES patients with various types of EWS-FLI1 rearrangements and EWS-ERG fusion implicates NPY as a universal EWS-ETS target.
Despite the overall elevated NPY levels in ES patients, its serum concentrations were highly variable, with 50% of patients with localized disease exhibiting normal serum NPY levels. The study on ES cell lines revealed significant differences in peptide secretion, suggesting that heterogeneous NPY release can underlie the variability in its systemic concentrations among ES patients. This was further confirmed by extracellular NPY immunostaining observed in ES xenografts derived from cell lines releasing NPY and some human ES tissues. Since NPY acts through cell membrane receptors, the ability of ES cells to secrete the peptide is an important mechanism regulating its actions. In the cell lines we observed a strong correlation between NPY mRNA and its concentrations in conditioned media. However, no such trend was observed in the small population (n = 6) of ES patients with matching NPY mRNA and serum levels data (data not shown). Thus, NPY serum concentrations in patients may be further modified by tumor mass or factors promoting its release in the tumor microenvironment.
One of the stromal factors known to regulate NPY synthesis and release is brain-derived neurotrophic factor (BDNF).20 High levels of BDNF are present in the bone environment, which correlates with elevated expression and secretion of the peptide from bone ES, at least in the pelvic and axial locations.21 This observation suggests the potential involvement of NPY in ES bone invasion and is in agreement with the role of the peptide in regulating bone homeostasis. NPY has been shown to inhibit osteoblast differentiation, which impairs osteogenesis and decreases bone density.10 Although bone lesions in ES are mainly osteolytic, blocking osteogenesis in pediatric and adolescent patients with ongoing bone formation may shift bone homeostasis toward osteolysis and promote their degradation. A similar phenomenon has been observed in neuroblastoma, another pediatric tumor with frequent bone metastases.22
Aside from bone ES, elevated NPY serum concentrations were also observed in patients with pelvic tumors, known to carry worse prognosis.3 It is not clear, however, if this unfavorable prognosis results from a higher tumor mass at diagnosis, their proximity to the internal organs, frequent incomplete resection or different biology of these tumors. Similarly, increased NPY levels in these tumors may result from their larger size or more aggressive phenotype. The trend toward increased NPY serum concentrations in patients with metastatic ES suggest its elevated release associated with blood-borne disease dissemination. This notion is further supported by our previous experimental data indicating that hypoxic microenvironment, known to increase ES malignancy, stimulates endogenous NPY and promotes its Y2R/Y5R-mediated growth-promoting and pro-metastatic actions.13
Analysis performed within a population of patients with localized disease indicated no effect of serum NPY levels on ES patients’ survival. However, given a trend toward increased peptide levels in patients with metastatic ES, the potential role of NPY as a prognostic factor in the overall ES population cannot be excluded. Nevertheless, even if further studies show no prognostic value of NPY, systemic levels of the peptide can be used to monitor disease progression and response to treatment in the subset of patients with elevated NPY release. This has been previously proposed for neuroblastoma and pheochromocytoma patients.23,24 Moreover, high systemic levels of NPY may identify a subset of potential candidates for anti-NPY therapies among a population of ES patients, once the role of the endogenous peptide in these tumors is fully elucidated. Lastly, the universal, fusion type-independent tissue expression of NPY may serve as a marker in differential diagnosis between ES and other small blue round cell tumors.
NPY actions are modified by DPPIV, which cleaves NPY and facilitates its Y2R/Y5R-mediated growth-promoting and pro-metastatic effects.12,13 Since our previous studies indicated elevated DPP activity in plasma of mice bearing DPPIV-rich ES xenografts, we sought to determine if the same measure can apply to ES patients.12 The assay used in our study measured the overall DPP activity in patients’ sera and did not distinguish between different types of DPPs. However, DPPIV is the only known membrane DPP that can be converted to a soluble enzyme by its shedding from the cell surface, while others (DPP8 and DPP9) are intracellular proteases.12 Therefore, DPP activity detectable in sera can be attributed mainly to DPPIV. Nevertheless, serum DPP activity in ES patients was not significantly different than that observed in healthy control and osteosarcoma patients. This data suggests that enzyme shedding from ES tumors is not sufficient to affect its activity in blood. Instead, the DPP activity detectable in serum is dependent on non-tumoral sources, such as endothelium and immune cells.13,18,19 This discrepancy between the animal studies and clinical data may be associated with the relatively higher tumor burden in mice than in patients.
Despite the non-tumoral origin of DPPIV detected in the serum, its high activity significantly associated with better EFS in ES patients, and trended towards such an effect on overall survival. This surprising discovery may be associated with the known role of DPPIV in regulating the immune response. DPPIV is a crucial factor in activation and propagation of T lymphocytes and natural killers, two key elements of the cellular immunity responsible for a host’s anti-tumor response.18,19 Thus, improved overall survival of patients with high DPPIV activity may reflect a more efficient immune response, which inhibits the disease progression. This observation strongly suggests that in the event that pro-metastatic actions of NPY are confirmed in animal models, directly blocking this pathway will be a better therapeutic strategy than the previously proposed inhibition of multifaceted DPPIV activity.12 It also raises a question regarding the safety of long-term administration of DPPIV inhibitors recently introduced as routine treatment for diabetes.19
In summary, we provide the first evidence for elevated systemic levels of NPY in ES patients, comparable to those described for patients with tumors of sympathetic origin.14–17 Although we did not observe a direct effect of high NPY levels on survival of patients with localized ES, the trend toward elevated NPY release in patients with unfavorable disease features suggests a potential role of the peptide in ES dissemination, and warrants further survival analyses including patients at the metastatic stage. This notion is also supported by our previous data indicating that hypoxic tumor microenvironment activates pro-metastatic actions of NPY in ES.13 Moreover, increased NPY systemic levels and intratumoral expression of the entire NPY system in bone tumors suggest a potential role of this pathway in ES bone invasion. On the other hand, the association of high DPP activity with better survival implicates a potential role for DPPIV in anti-cancer immune response.
Acknowledgments
Funding sources:
This work was supported by National Institutes of Health grants: UL1TR000101 (previously UL1RR031975) through the Clinical and Translational Science Awards Program, 1RO1CA123211 and 1R03CA178809 to JK, COG Group Chair Grant U10CA098543, Statistics and Data Center Grant U10 CA98413-08, COG NCTN Network Group Operations Center Grant 1U10CA180886 and COG NCTN Statistics & Data Center 1U10CA180899, as well as funding from Children’s Cancer Foundation (Baltimore, MD) to JK and JT. Additional support came from AFLAC Foundation, Burroughs Wellcome Clinical Scientist Award in Translational Research and the NIH grants R01CA88004, R01CA133662, R01CA138212, and RC4CA156509 to JT. The experiments were performed with use of Lombardi Comprehensive Cancer Center Shared Resources (Histopathology & Tissue, The Tissue Culture and Biostatistics & Bioinformatics) supported by NIH/NCI grant P30-CA051008 and statistical analyses with support provided by a grant from the WWWW (QuadW) Foundation, Inc. to COG.
Footnotes
The authors declare no conflict of interest.
References
- 1.Ladenstein R, Potschger U, Le Deley MC, et al. Primary disseminated multifocal Ewing sarcoma: results of the Euro-EWING 99 trial. J Clin Oncol. 2010;28:3284–3291. doi: 10.1200/JCO.2009.22.9864. [DOI] [PubMed] [Google Scholar]
- 2.Womer RB, West DC, Krailo MD, et al. Randomized Controlled Trial of Interval-Compressed Chemotherapy for the Treatment of Localized Ewing Sarcoma: A Report From the Children's Oncology Group. J Clin Oncol. 2012;30:4148–4154. doi: 10.1200/JCO.2011.41.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernstein M, Kovar H, Paulussen M, et al. Ewing's sarcoma family of tumors: current management. Oncologist. 2006;11:503–519. doi: 10.1634/theoncologist.11-5-503. [DOI] [PubMed] [Google Scholar]
- 4.van Doorninck JA, Ji L, Schaub B, et al. Current treatment protocols have eliminated the prognostic advantage of type 1 fusions in Ewing sarcoma: a report from the Children's Oncology Group. J Clin Oncol. 2010;28:1989–1994. doi: 10.1200/JCO.2009.24.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonescu C. Round cell sarcomas beyond Ewing: emerging entities. Histopathology. 2014;64:26–37. doi: 10.1111/his.12281. [DOI] [PubMed] [Google Scholar]
- 6.Hancock JD, Lessnick SL. A transcriptional profiling meta-analysis reveals a core EWS-FLI gene expression signature. Cell Cycle. 2008;7:250–256. doi: 10.4161/cc.7.2.5229. [DOI] [PubMed] [Google Scholar]
- 7.Lee EW, Michalkiewicz M, Kitlinska J, et al. Neuropeptide Y induces ischemic angiogenesis and restores function of ischemic skeletal muscles. J Clin Invest. 2003;111:1853–1862. doi: 10.1172/JCI16929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu C, Everhart L, Tilan J, et al. Neuropeptide Y and its Y2 receptor: potential targets in neuroblastoma therapy. Oncogene. 2010 doi: 10.1038/onc.2010.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Son MY, Kim MJ, Yu K, Koo DB, Cho YS. Involvement of neuropeptide Y and its Y1 and Y5 receptors in maintaining self-renewal and proliferation of human embryonic stem cells. J Cell Mol Med. 2011;15:152–165. doi: 10.1111/j.1582-4934.2009.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee NJ, Herzog H. NPY regulation of bone remodelling. Neuropeptides. 2009;43:457–463. doi: 10.1016/j.npep.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Kitlinska J, Abe K, Kuo L, et al. Differential effects of neuropeptide Y on the growth and vascularization of neural crest-derived tumors. Cancer Res. 2005;65:1719–1728. doi: 10.1158/0008-5472.CAN-04-2192. [DOI] [PubMed] [Google Scholar]
- 12.Lu C, Tilan JU, Everhart L, et al. Dipeptidyl Peptidases as Survival Factors in Ewing Sarcoma Family of Tumors: IMPLICATIONS FOR TUMOR BIOLOGY AND THERAPY. J Biol Chem. 2011;286:27494–27505. doi: 10.1074/jbc.M111.224089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tilan JU, Lu C, Galli S, et al. Hypoxia shifts activity of neuropeptide Y in Ewing sarcoma from growth-inhibitory to growth-promoting effects. Oncotarget. 2013;4:2487–2501. doi: 10.18632/oncotarget.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen PS, Cooper MJ, Helman LJ, Thiele CJ, Seeger RC, Israel MA. Neuropeptide Y expression in the developing adrenal gland and in childhood neuroblastoma tumors. Cancer Res. 1990;50:6055–6061. [PubMed] [Google Scholar]
- 15.Dotsch J, Christiansen H, Hanze J, Lampert F, Rascher W. Plasma neuropeptide Y of children with neuroblastoma in relation to stage, age and prognosis, and tissue neuropeptide Y. Regul Pept. 1998;75–76:185–190. doi: 10.1016/s0167-0115(98)00067-6. [DOI] [PubMed] [Google Scholar]
- 16.Kogner P, Bjork O, Theodorsson E. Plasma neuropeptide Y in healthy children: influence of age, anaesthesia and the establishment of an age-adjusted reference interval. Acta Paediatr. 1994;83:423–427. doi: 10.1111/j.1651-2227.1994.tb18134.x. [DOI] [PubMed] [Google Scholar]
- 17.de SSP, Denker J, Bravo EL, Graham RM. Production, characterization, and expression of neuropeptide Y by human pheochromocytoma. J Clin Invest. 1995;96:2503–2509. doi: 10.1172/JCI118310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mentlein R. Dipeptidyl-peptidase IV (CD26)--role in the inactivation of regulatory peptides. Regul Pept. 1999;85:9–24. doi: 10.1016/s0167-0115(99)00089-0. [DOI] [PubMed] [Google Scholar]
- 19.Stulc T, Sedo A. Inhibition of multifunctional dipeptidyl peptidase-IV: is there a risk of oncological and immunological adverse effects? Diabetes Res Clin Pract. 2010;88:125–131. doi: 10.1016/j.diabres.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 20.Wirth MJ, Patz S, Wahle P. Transcellular induction of neuropeptide Y expression by NT4 and BDNF. Proc Natl Acad Sci U S A. 2005;102:3064–3069. doi: 10.1073/pnas.0404712102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamashiro T, Fukunaga T, Yamashita K, Kobashi N, Takano-Yamamoto T. Gene and protein expression of brain-derived neurotrophic factor and TrkB in bone and cartilage. Bone. 2001;28:404–409. doi: 10.1016/s8756-3282(01)00405-7. [DOI] [PubMed] [Google Scholar]
- 22.Granchi D, Baglio SR, Amato I, Giunti A, Baldini N. Paracrine inhibition of osteoblast differentiation induced by neuroblastoma cells. Int J Cancer. 2008;123:1526–1535. doi: 10.1002/ijc.23654. [DOI] [PubMed] [Google Scholar]
- 23.Grouzmann E, Fathi M, Gillet M, et al. Disappearance rate of catecholamines, total metanephrines, and neuropeptide Y from the plasma of patients after resection of pheochromocytoma. Clin Chem. 2001;47:1075–1082. [PubMed] [Google Scholar]
- 24.Kogner P, Bjork O, Theodorsson E. Neuropeptide Y in neuroblastoma: increased concentration in metastasis, release during surgery, and characterization of plasma and tumor extracts. Med Pediatr Oncol. 1993;21:317–322. doi: 10.1002/mpo.2950210502. [DOI] [PubMed] [Google Scholar]