Abstract
c-Kit (CD117) is a tyrosine kinase receptor found in various types of immune cells. It has been shown that c-Kit plays a role in the pathogenesis of multiple sclerosis, an inflammatory demyelinating disorder of the CNS. Recent data have suggested an immunoregulatory effect of c-Kit. We therefore examined the role of c-Kit in autoantigen-induced i.v. tolerance in experimental autoimmune encephalomyelitis (EAE), an animal model of MS. Our results show that induction of intravenous tolerance against EAE in B6 mice is characterized by increased numbers of CD117+ cells and altered mast-cell associated molecules in the periphery and in the CNS. W−sh (c-Kit deficient) mice were resistant to i.v autoantigen-induced tolerance, with increased proinflammatory cytokine production in the periphery. I.v. autoantigen in WT mice suppressed production of proinflammatory cytokines IFN-γ and IL-6 and up-regulated expression of FoxP3, a transcription factor of Tregs; however, in W−sh mice IFN-γ and IL-6 were increased with a failure of FoxP3 induction upon i.v. autoantigen injection, and is thus a mechanism for resistance to i.v. tolerance induction in these mice. We conclude that c-kit signaling has a regulatory role in i.v. tolerance and could be a target for potential immunotherapy in autoimmune disorders.
Keywords: tolerance, experimental autoimmune encephalomyelitis, c-kit, CD117, autoimmunity
INTRODUCTION
Auto-antigen tolerance is an important aspect of the pathogenesis of autoimmune diseases. Antigen tolerance can be achieved by administration of specific auto-antigens using different methods such as intravenous (i.v.) injection of soluble peptide or antigen-coupled splenocytes (1). Various mechanisms have been shown to induce i.v. tolerance, such as deletion of antigen-reactive T cells or production of regulatory cells and cytokines (2–4). Administration of soluble antigen after onset of experimental autoimmune encephalomyelitis (EAE) leads to apoptosis of antigen-specific T cells and amelioration of disease (5). However, i.v. injection of antigen before EAE induction activates regulatory mechanisms to induce tolerance (6).
c-Kit (CD117) is a tyrosine kinase receptor found in several hematopoietic and lymphoid cell types (7). Binding c-Kit receptor to its ligand plays a role in development of myeloid cells (8). It has also been shown that c-kit signaling is involved in antigen independent B cell (9) and early T cell development (10). Importantly, c-kit expression on some fully differentiated immune cells such as dendritic cells, natural killer cells and mast cells highlights its possible role in the pathogenesis of a wide variety of inflammatory diseases. We have previously shown that c-kit deficient mice demonstrate a greater inflammatory response in EAE disease and that c-kit signaling may act as an immunoregulatory factor; however, the role of c-kit in immune tolerance and autoimmunity has not been demonstrated.
In order to determine whether c-kit receptor plays a regulatory role in autoimmune diseases, we studied and compared EAE disease and i.v. tolerance in c-kit-deficient (W−sh) and WT mice. Our results showed that W−sh mice were resistant to MOG i.v. tolerance, with extensive inflammation and demyelination in the CNS. This may have been due to lack of IFN-γ suppression after MOG i.v. injection and a significantly lower level of FoxP3 expression in W−sh mice. Our data demonstrate that c-kit signaling has tolerogenic effects following i.v. administration of antigen. Based on these findings, we hypothesize that c-kit may play a role in induction of tolerance in autoimmune diseases and could be a good target for immunotherapy of multiple sclerosis and other autoimmune diseases.
RESULTS
I.v. tolerance increased the number of CD117+ cells in spleen and CNS
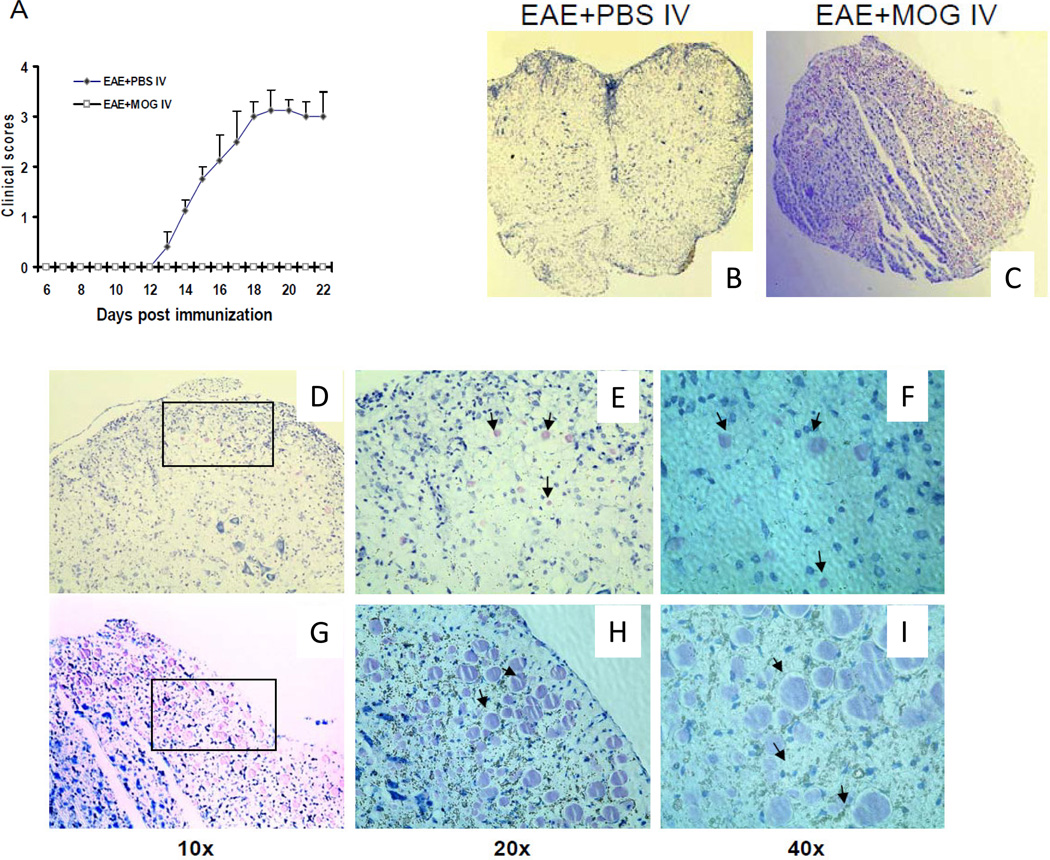
To induce tolerance, MOG35-55 peptide was administered i.v. when B6 mice were immunized for EAE. PBS-injected mice served as controls. MOG-injected mice showed protection from EAE (Fig. 1A, p<0.01).
Figure1. CD117(c-kit) after MOG iv tolerance in EAE A).
EAE clinical disease was significantly suppressed in WT mice after MOG iv injection compared to control (p <0.01). Histochemical staining of CD117 in spinal cords of WT mice after i.v. PBS (B) or MOG injection (C) is shown. Data are representative of 3 separate experiments and 4–5 WT mice were used. Sections were fixed and stained with CD117 antibody as described in Materials and Methods. A high power objective field (10×,20×,40×) is shown in spinal cords of either PBS- (D,E,F) or MOG-treated (G,H,J) mice.
In order to determine the involvement of c-kit in induction of i.v. tolerance, we examined the percentage of CD117, one of the major c-kit gene downstream proteins, in the spleens of i.v. tolerized and control mice after EAE induction by using flow cytometry. Toluidine Blue Staining showed an increased number of CD117+ cells in spinal cords of tolerized mice compared to control (Fig. 1 B,C). Morphologically, CD117+ cells in the CNS of tolerized mice were larger and appeared more activated than in non-tolerized mice (Fig. 1D–I).
I.v. tolerance enhanced mast cell-associated gene expression
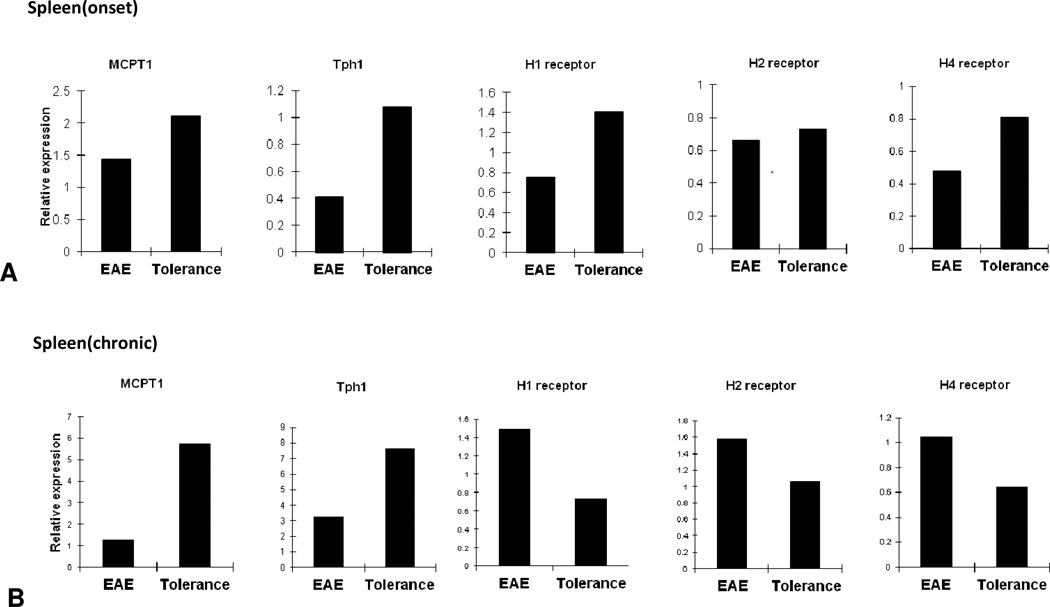
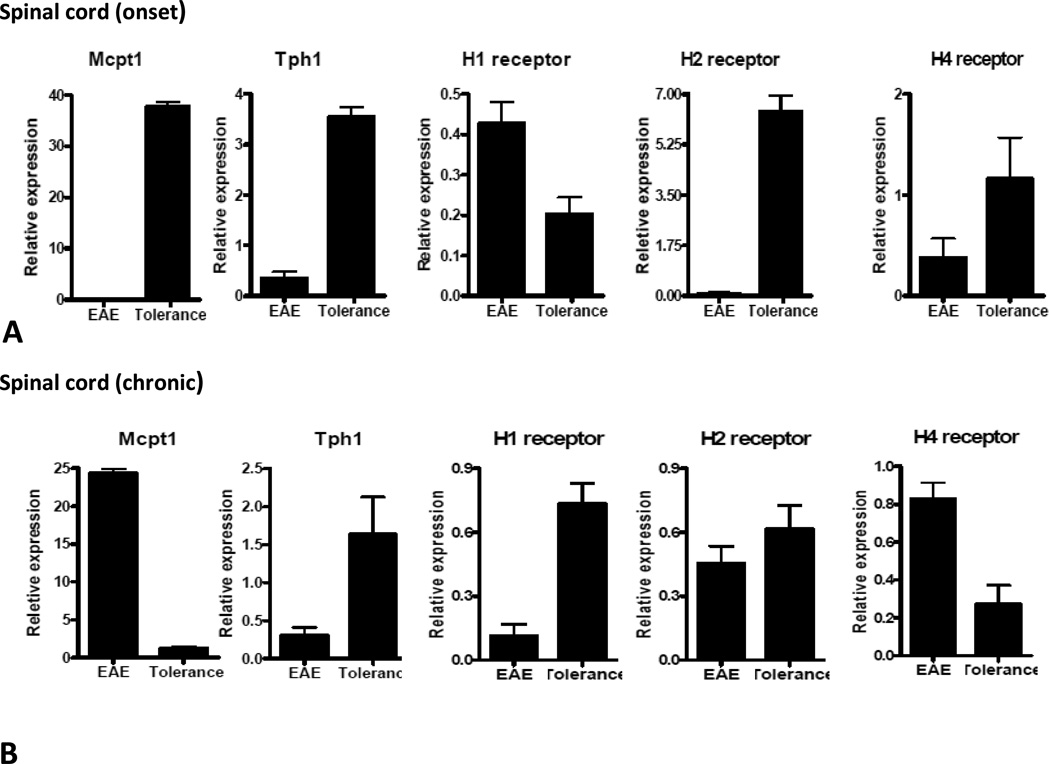
Although CD117 is expressed on different types of immune cells, its expression on mast cells led us to further examine the role of c-kit deficiency on mast cell-associated genes after induction of i.v. tolerance Although CD117 is expressed on different types of immune cells, its expression on mast cells led us to further examine the role of c-kit deficiency on mast cell-associated genes in induction of i.v. tolerance. We therefore determined gene expression of Tph1, MCPT1 and H1, H2 and H4 receptors at the onset of EAE (day 12 p.i.) and during the chronic phase of the disease (day 42 p.i.). At disease onset, all of the above-mentioned molecules were up-regulated in the spleen of tolerized mice; however, we detected increased expression of Tph1 and MCPT1 during the chronic phase of EAE (Fig. 2A, B). At disease onset all these molecules except for H1 receptor had significantly higher levels of expression in spinal cords of tolerized mice (Fig 3A). On day 42 p.i, spinal cords of the tolerized group expressed higher levels of Tph1 and H1, H2 and H3 receptors, while expression of mcpt1 and H4 receptor was reduced (Fig. 3B).
Figure2. Expression of Mast cell-related genes in spleen after i.v. tolerance.
Spleens from EAE control (PBS i.v.) and tolerized mice (MOG i.v.) were isolated at onset and chronic phase of disease. Quantitative real-time RT-PCR analysis was performed on pooled splenocytes from tolerized and control mice at onset and chronic phase of disease (A, B). Data are representative of two separate experiments.
Figure3. Expression of mast cell-related genes in the CNS after i.v. tolerance.
Spinal cords from EAE control (PBS i.v.) and tolerized mice (MOG i.v.) were isolated at onset and chronic phase of disease. Quantitative real-time RT-PCR analysis was performed on spinal cords of 4–5 mice in each group individually and data demonstrates an average of gene expression in 4–5 mice from each experimental group at onset and chronic phase of disease (A, B). Data are representative of two separate experiments.
C-Kit-deficient mice are not capable of establishing efficient i.v. tolerance
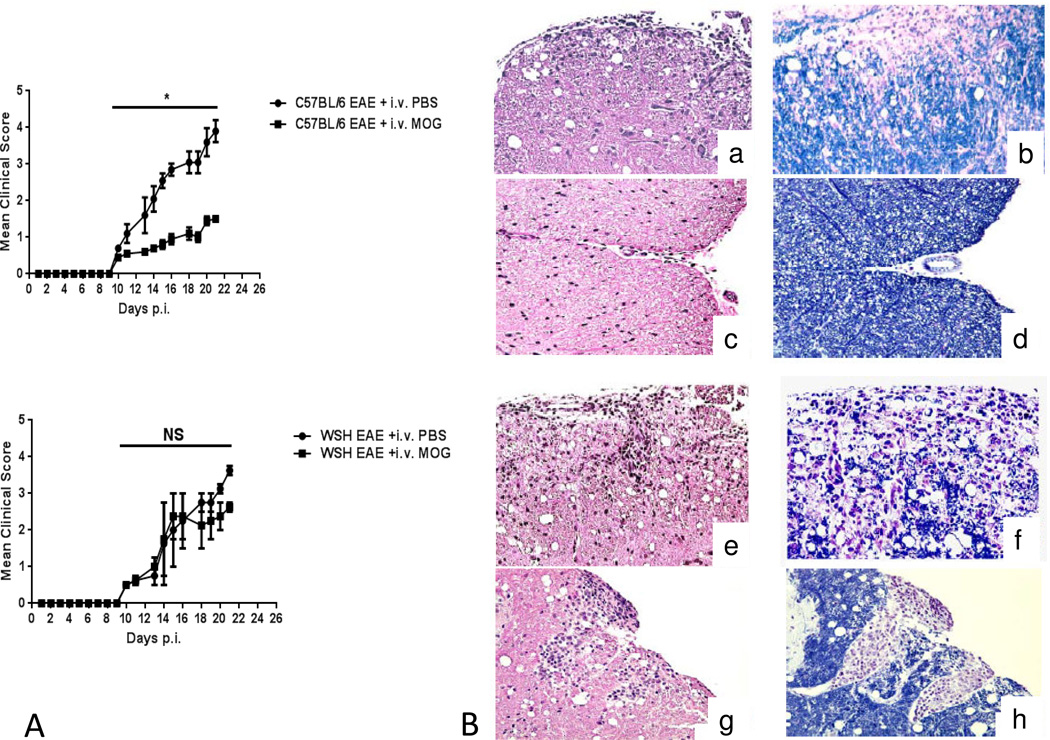
In order to investigate whether c-kit is required to induce i.v. tolerance, we immunized W−sh mice and WT controls with MOG to induce EAE. Animals were i.v injected with either MOG35-55 (200 μg/mouse) or PBS starting 3 days before immunization, three times, at three-day intervals. In agreement with our previous findings, clinical EAE was significantly suppressed in WT mice after MOG35-55 injection; however, W−sh mice, which had received i.v MOG, did not show significant disease suppression (Fig. 4A). Histology findings confirmed that WT mice manifested less inflammation and demyelination after i.v. tolerance (3B (c–d)) compared to WT control (4B (a–b)), but i.v. tolerance did not change CNS inflammation and demyelination in W−sh mice (Fig. 4B (g–h)) (4B (e–f)). These findings indicate that CD117 plays a role in the induction of i.v. tolerance in EAE.
Figure4. c-kit deficient mice are not capable of establishing efficient MOG i.v. tolerance.
Female WT and W−sh KO mice (n =5 in each group) were immunized with 200 μg of MOG35–55 peptide in CFA. MOG was i.v. injected on days -3, 0, 3 p.i. Clinical EAE was evaluated daily according to a 0–5 severity scale. (A) MOG i.v. injection suppressed EAE in WT but not W−sh mice. Data represent the mean clinical score + SEM (representative of 3 separate experiments). (B) Spinal cords were harvested after extensive perfusion, and 5-μm sections were stained with H&E or Luxol fast blue (myelin stain). WT mice manifested less inflammation and demyelination after i.v. tolerance (c–d) compared to WT control (a–b), but i.v. tolerance (g–h) did not change either CNS inflammation or demyelination in W−sh mice (e–f).
I.v. tolerance did not suppress all inflammatory cytokines in W−sh mice
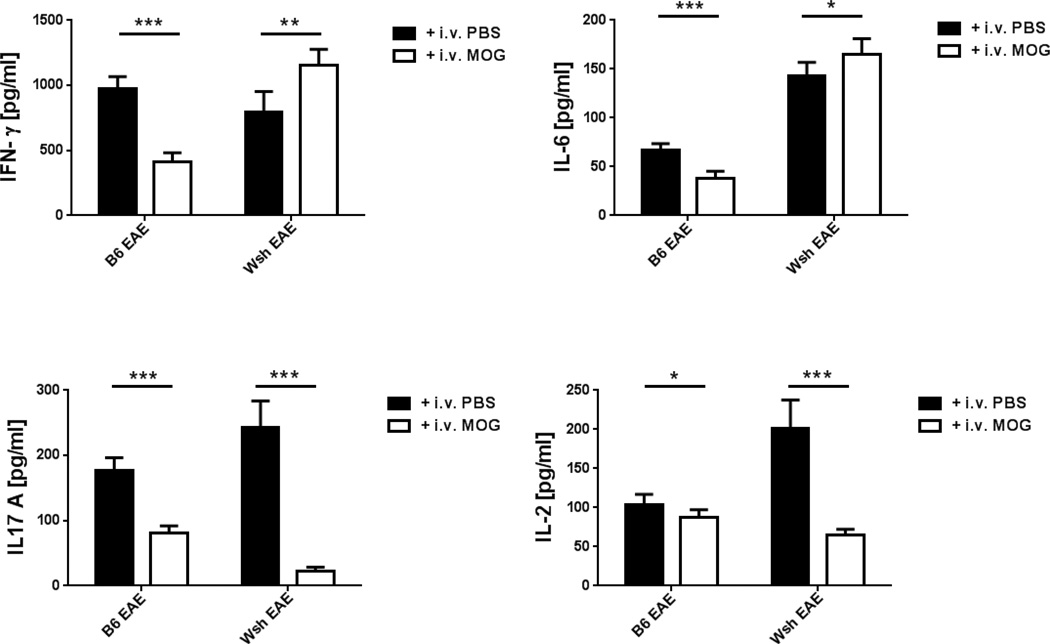
To determine the role of cytokines in i.v. tolerance induction in W−sh mice, we examined splenocytes from EAE and tolerized mice at day 21 p.i. Following stimulation with MOG35-55, supernatants were analyzed for anti- and pro-inflammatory cytokine production in both groups. Splenocytes from W−sh mice with EAE showed a higher baseline level of IL-17, IL-2 and IL-6 than those from WT mice after stimulation. After MOG i.v. injection, IL-17, IL-2, IL-6 and IFN-γ were suppressed in tolerized compared to control WT mice. MOG i.v injection decreased the level of IL-2 and IL-17 in W−sh mice, but higher levels of IFN-γ and IL-6 were detected in tolerized W−sh mice compared to W−sh PBS-injected control mice (Fig. 5).
Figure5. Effect of c-kit deficiency on cytokine production after i.v. tolerance.
Pooled splenocytes from 5 mice in i.v. tolerized WT and 4 mice in W−sh mice were cultured and stimulated with MOG35–55 (25 ug/ml) at a concentration of 1×106 cells/ml for 72h. IFN-γ, IL-17, IL-6 and IL-2 were measured by ELISA. Data represent mean values and SEM of one out of three separate experiments. Values refer to comparison between two groups. *, p < 0.05 , ** p <0.01
Lack of c-kit signaling promotes Th1 and eliminates Treg after i.v tolerance
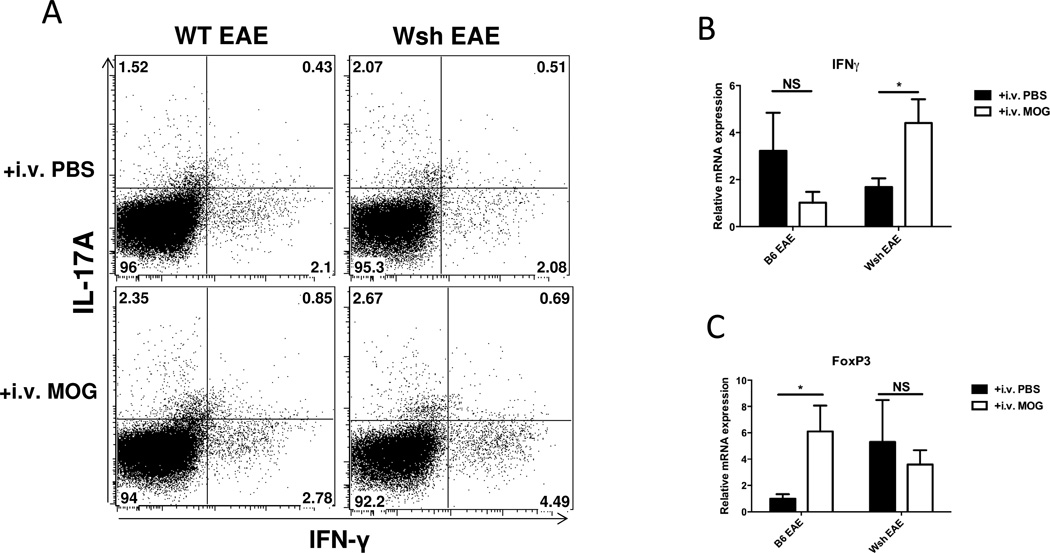
In order to determine the effect of c-kit-deficiency on Th1 and Th17 cells in i.v. tolerance, splenocytes from PBS- or MOG-i.v. EAE from either WT or W−sh mice were stimulated with PMA in the presence of GolgiPlug for 5 h. Gated CD4+ T cells were analyzed for intracellular expression of IL-17 and IFN-γ. We found a twofold increase in IFN-γ+ CD4+ T cells (Th1 cells) in MOG tolerized W−sh mice compared to i.v. PBS W−sh mice; however, there was no difference in the percentage of Th17 cells (Fig. 6A). This finding was confirmed by RT-PCR results showing that IFN-γ expression had significantly increased in W−sh tolerized mice compared to control; however, i.v. tolerance caused IFN-γ expression to decrease in WT mice (Fig 6B). While IFN-γ production after MOG stimulation in both WT and W−sh mice was not different at baseline, i.v. MOG injection significantly increased IFN-γ in W−sh mice compared to control (Fig 5). We also examined mRNA expression of FoxP3, IL-10, and IL-27 in WT and W−sh mice after i.v. PBS or MOG injection. WT mice expressed significantly higher FoxP3 after i.v. tolerance, while there was no difference in FoxP3 expression between tolerized and non-tolerized W−sh mice (Fig 6C). We also looked at IL-10 and IL-27 expression in both spleens and spinal cords. Although there was a higher level of IL-27 expressed in WT tolerized mice compared to control, at disease peak we did not find any statistically significant results in either the WT or W−sh group (data not shown). This suggests that c-kit signaling may contribute to suppression of IFN-γ and induction of FoxP3+ Tregs after i.v. tolerance.
Figure6. c-Kit gene deficiency leads to Th1 immune deviation and elimination of Tregs after i.v. tolerance.
After sacrificing EAE mice from WT and W-sh KO in both tolerized and nontolerized groups at disease peak, pooled splenocytes were stimulated with MOG for three days. After 72h, PMA and ionomycin plus GolgiPlug were added to culture for 5 hours. Supernatant and cells were also used for ELISA and RT-PCR. Cells were stained for CD4 surface marker and intracellular staining of IFN-γ and IL-17. (A) The percentage of IFN-γ positive CD4 cells was higher in W−sh tolerized mice compared to control. (B) ELISA and (C) RT-PCR confirmed higher expression of IFN-γ in W−sh tolerized mice compared to control. (D) RT-PCR of FoxP3 transcription factor is shown in spleens of WT and W−sh after i.v. PBS or i.v. MOG injection. Data are representative of two separate experiments, with 4–5 mice in each experimental group.
DISCUSSION
C-kit is a tyrosine kinase receptor which binds to its ligand, called stem cell factor (SCF), leading to different biological and immunological functions (11). The role of c-kit in mast cell development and differentiation is well described (12). c-kit receptor activation enhances degranulation and release of large numbers[??] of chemokines and cytokines from mast cells (13, 14). In addition, several findings demonstrate that c-kit plays a role in the function of various types of immune cells including eosinophil and dendritic cells (11). Increased expression of c-kit on dendritic cells promotes Th2 and Th17 responses (15). Moreover, adding c-kit antibody to dendritic cells enhanced natural killer cell activation, which might have been due to a decrease in IL-6 production (16) Furthermore, it has been recently shown that mice lacking CD117 molecule developed more severe inflammatory response after EAE induction, indicating the anti-inflammatory effect of this molecule (36).
In the present study, we found that induction of i.v. tolerance increased the number of CD117+ cells in the periphery and in the CNS. Increased numbers of CD117+ cells in the inflammatory foci suggest that c-kit may play a role in i.v. tolerance induction against EAE. We also provide evidence that c-kit expression is required for peripheral immune tolerance, which effectively suppresses EAE and other autoimmune diseases (17–18). In addition, several studies demonstrate that c-Kit play immunosuppressive role rather than immune activation (13–14). In an allograft transplant mouse model, enhanced rejection was found in c-kit deficient mice combined with up-regulated expression of MCP-1, CCR2, and IL-2 in these mice (13)
Given that a large proportion of c-Kit+ cells are mast cells (12), we have determined the regulation of mast cell-associated molecules including Tph1, MCPT1, H1, H2, and H4 receptors. We have shown that expression of Tph1, MCPT1, H1, H2, and H4 receptors increased after i.v. tolerance induction in B6 mice. It has also been shown that mast cells may contribute to tumor growth and metastasis (20–23); mast cells may thus also have an immunosuppressive role in dampening the immune response to tumors. It has already been described that c-kit deficient mice completely lack mast cells (24 In light of this, resistance to i.v. tolerance in c-kit deficient mice may be due to their lack of mast cells. Tph1 is an enzyme that can metabolize tryptophan and create a tryptophan-deficient environment (25); Tph1 may thus be utilized by mast cells to limit T-cell activation (13). Lack of Tryptophan hydroxylase-1(Tph-1) disrupts allograft tolerance (19). The decreased expression of Mcpt1 in arthritis-susceptible DA rats compared to arthritis-resistant E3 rats suggests an immunoregulatory role of this mast cell-associated molecule in this autoimmune disorder (26). It has been shown that that H4R plays a role in frequency of regulatory T cells in lymphoid organs (27)We found that i.v. tolerance increases H4R expression in spleen in the acute phase of EAE, which may be involved in i.v. tolerance induction. Although expression of H1-4R in the CNS correlated with the progression of EAE, its role in this disease is not known (28–29). We also observed that H4 receptor expression decreased in both spleen and CNS after i.v. tolerance in the chronic phase of EAE, which may indicate its immunoregulatory role In mice, deletion of H1R results in suppression of IFN-γ and increased secretion of Th2 cytokines (IL-4 and IL-13), indicating an important regulatory mechanism in the control of inflammatory functions through the H1 pathway with a different intracellular signaling pathway (30–31). On the other hand, cells transmigrating to the H4R agonist, but not to H1R, were skewed toward CD4 cells expressing CD25 and intracellular FoxP3. In addition, H4R agonist administration resulted in accumulation of FoxP3+ T cells, suggesting its direct effect on T regulatory cell recruitment (32). H4R-responsive cells suppressed proliferation of autologous T cells, an effect that was dependent on IL-10 production. These results indicate that H4R stimulation endows regulatory T cells with a potent capacity to suppress proliferation (32). It has also been shown that inhibition of H4 receptor exacerbates EAE through induction of Th1 and IFN-γ (33). Our observations of increased levels of H1-4R in the CNS of i.v. tolerized mice in the acute phase of EAE also suggest that these mast cell-associated molecules may play an important role in tolerance induction, thus suppressing EAE.
There are other possible underlying mechanisms. i.e., the effect of the c-kit gene on phenotype of DCs can play a role in i.v. tolerance in these mice. I.V. tolerance decreased IL-6 production in WT splenocytes, while in W-sh splenocytes the amount of IL-6 increased after MOG i.v. injection. C-kit expression is directly related to IL-6 production (34). Our current finding implies that the c-kit signaling pathway plays a role in IL-6 suppression after i.v. tolerance. In addition, we have shown that i.v. tolerance in WT mice suppresses IFN-γ, IL-17 and IL-2 (35). However, i.v. tolerance in c-kit deficient mice suppressed IL-17 and IL-2 production. The fact that c-kit-deficient mice, which were less susceptible to i.v. tolerance induction in EAE, showed increased Th1 and IFN-γ production emphasizes the immunosuppressive role of CD117+ cells in i.v. tolerance through Th1 and IFN-γ pathways. C-kit-deficient splenocytes produced increased amounts of IL-17, IL-6 and IL-2 after EAE induction, in accordance with a previous study showing more proinflammatory and fewer immunoregulatory cytokines in lymphocytes in EAE of W−sh mice (36). In a previous study, we found that MOG i.v. injection induces CD11c+CD11b+ DCs with a regulatory profile having IL-10 and TGF-β producing capacity, which leads to a dramatic enhancement of FoxP3 regulatory T cells (6). Upon i.v. tolerance induction, while WT mice expressed a high level of Treg transcription factor FoxP3, this expression was not changed in c-kit-deficient mice, suggesting that i.v. tolerance resistance in these mice may be due to lack of Treg induction.
In summary, our observation that, in c-kit-deficient mice after i.v. tolerance, T cell differentiation is skewed toward the Th1/Th17, but not the Treg pathway indicates a key role for c-Kit in auto-antigen induced tolerance. The information obtained from this study may thus not only define a novel immunomodulation pathway, but also have clinical implications for future immunotherapy of autoimmune diseases such as MS.
MATERIALS AND METHODS
Mice, EAE and i.v. tolerance induction
Female, 8–10 week old, W−sh (c-Kit–deficient) mice and wild type C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). To induce EAE, mice were injected subcutaneously with 200 µg MOG35-55 in CFA containing 4 mg/ml Mycobacterium tuberculosis H37Ra (Difco, Detroit, MI) over 2 sites at the back. Two hundred ng pertussis toxin was given i.v. on days 0 and 2 post immunization (p.i.). To induce i.v. tolerance, MOG35-55 at a dose of 200 μg/mouse was i.v. injected on days −3, 0, +3 p.i. Identical volumes (100 μl) of PBS were i.v. injected in parallel to serve as controls. EAE was scored according to a 0–5 scale, as follows (29): 1, limp tail or waddling gait with tail tonicity; 2, waddling gait with limp tail (ataxia); 2.5, ataxia with partial limb paralysis; 3, full paralysis of one limb; 3.5, full paralysis of one limb with partial paralysis of second limb; 4, full paralysis of 2 limbs; 4.5, moribund; and 5, death. All work was performed in accordance with the Thomas Jefferson University guidelines for animal use and care.
Histopathology
Mice were extensively perfused with PBS and spinal cords were harvested. Five-µm sections were stained with H&E or Luxol fast blue (myelin stain). Slides were assessed in a blinded fashion for inflammation and demyelination, as follows (37). For inflammation: 0, none; 1, a few inflammatory cells; 2, organization of perivascular infiltrates; and 3, increasing severity of perivascular cuffing with extension into the adjacent tissue. For demyelination: 0, none; 1, rare foci; 2, a few areas of demyelination; 3, large (confluent) areas of demyelination.
Flow-cytometric analysis of MNCs in the CNS
Mononuclear cells (MNCs) from pooled spinal cords were isolated as previously described (30). Cells were cultured overnight with MOG (10mg/ml) at 1×106 cells/ml concentration. Cells were washed in staining buffer (1% FCS + 0.1% NaN3 in PBS). After blocking with anti-CD16/CD32 antibodies, cells were stained with fluorescent antibodies to murine CD4, CD8, IFN-γ and IL-17. (BD PharMingen, San Jose, CA). Cells were fixed and data acquired on a FACSAria (Becton-Dickinson, Mountain View, CA). MNCs were gated and fluorescence was analyzed using CellQuest (Beckson-Dickson) software.
Cytokine production
MNCs from spleen of MOG-i.v. and PBS-i.v. EAE mice were isolated on day 21 p.i. Erythrocytes in the cell pellets from spleen were hemolyzed by adding NH4Cl-Tris Buffer for 5 min. at room temperature followed by washing. These cells were then suspended at a density of 2 ×106/ml in complete RPMI 1640 with 10% FCS, with or without MOG35-55, at a final concentration of 25μg/ml. Triplicate aliquots (1 ml) of MNCs suspensions were added to 48-well round-bottomed microtiter plates (Nunc). Supernatants were collected after 72 h. Quantitative ELISA for IFN-γ, IL-2, IL-6 and IL-17 was performed using paired mAbs according to the manufacturer's recommendations (R&D).
Flow cytometric analysis
Splenocytes and Mononuclear cells (MNCs) from pooled spinal cords were isolated as previously described (17). CNS Cells were cultured overnight with MOG (10ug/ml) at 1×106 cells/ml concentration. Splenocytes were cultured for 3 days with MOG (25ug/ml) at a density of 2×106/ml. Both cell suspensions were re-stimulated with PMA (50 ng/ml), ionomycin (500 ng/ml) (Sigma) and treated with GolgiStop (1 μg/106 cells) (BD PharMingen, San Jose, CA) for 4 hours before FACS staining. Cells were harvested, washed in staining buffer containing 1% FCS, 0.1% NaN3 in PBS and blocked with anti-CD16/CD32 antibodies. Following another wash step, cells were stained with fluorescently labeled antibodies to CD4 and CD8 for 30 mins in the dark at 4°C. Cells were washed, fixed and permeabilized using Fix and Perm® cell permeabilization reagents (Caltag Laboratories, Burlingame, CA). Cells were stained for intracellular cytokines with rat anti-mouse IFN-γ and IL-17 antibodies. All flow cytometric analyses were performed using appropriate isotype controls. All antibodies were purchased from BD PharMingen, San Jose, CA. Data were acquired on a FACSAria (BD Biosciences, San Jose, CA) and analyzed using FlowJo Software.
Real-time PCR
Total RNA isolated from spinal cords and spleens of all groups was purified using the RNeasy system (Qiagen). Complementary DNA was then prepared and analyzed using real-time PCR Taqman Assay (Applied Biosystems). Splenocytes of PBS-i.v. and MOG-i.v. mice were isolated and mRNA levels of mast-cell-related genes and immune molecules were quantified by real-time RT-PCR.
Immunohistochemistry
For immunohistology, spinal cords were snap frozen, cryocut, and acetone-fixed. Slides were blocked with normal mouse serum. Tissue sections stained for CD117 (clone 2B8) were used. The specificity of CD117 staining was confirmed by the absence of staining in CNS tissue from W-sh mice (data not shown). Histochemical analysis of c-kit+ cells was carried out as described before (30). Briefly, 5 μm thickness sections were de-waxed in xylene and rehydrated by decreasing concentrations of ethanol to distilled water. They were stained with toluidine blue for 2 min and washed with distilled water, after which they were stained using light green SF for 30 s, washed using distilled water and dehydrated in increasing concentrations through alcohol series, xylene and mounted using DPX.
Statistical analysis
Clinical scores were analyzed using the Mann-Whitney U test, and all other experiments were tested for statistical differences using unpaired, 2-tailed, Student’s t tests. Differences were considered significant if p<0.05.
Acknowledgment
This study was supported by the NIH/NINDS (AR). We thank Katherine Regan for editorial assistance.
Footnotes
None of co-authors has a conflict of interest to declare.
References
- 1.Kennedy MK, Dal Canto MC, Trotter JL, Miller SD. Specific immune regulation of chronic-relapsing experimental allergic encephalomyelitis in mice. Journal of immunology (Baltimore, Md : 1950) 1988;141(9):2986–2993. [PubMed] [Google Scholar]
- 2.Benson JM, Campbell KA, Guan Z, Gienapp IE, Stuckman SS, Forsthuber T, et al. T-cell activation and receptor downmodulation precede deletion induced by mucosally administered antigen. The Journal of clinical investigation. 2000;106(8):1031–1038. doi: 10.1172/JCI10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ilarregui JM, Croci DO, Bianco GA, Toscano MA, Salatino M, Vermeulen ME, et al. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nature immunology. 2009;10(9):981–991. doi: 10.1038/ni.1772. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson TA, Stuart PM, Herndon JM, Griffith TS. Apoptosis, tolerance, and regulatory T cells--old wine, new wineskins. Immunological reviews. 2003;193:111–123. doi: 10.1034/j.1600-065x.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhang GX, Liu TT, Ventura ES, Chen Y, Rostami A. Reversal of spontaneous progressive autoimmune encephalomyelitis by myelin basic protein-induced clonal deletion. Autoimmunity. 1999;31(4):219–227. doi: 10.3109/08916939908994067. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Zhang GX, Chen Y, Xu H, Fitzgerald DC, Zhao Z, et al. CD11c+CD11b+ dendritic cells play an important role in intravenous tolerance and the suppression of experimental autoimmune encephalomyelitis. Journal of immunology (Baltimore, Md : 1950) 2008;181(4):2483–2493. doi: 10.4049/jimmunol.181.4.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sperling C, Schwartz S, Buchner T, Thiel E, Ludwig WD. Expression of the stem cell factor receptor C-KIT (CD117) in acute leukemias. Haematologica. 1997;82(5):617–621. [PubMed] [Google Scholar]
- 8.Kinashi T, Springer TA. Steel factor and c-kit regulate cell-matrix adhesion. Blood. 1994;83(4):1033–1038. [PubMed] [Google Scholar]
- 9.Rolink A, Haasner D, Nishikawa S, Melchers F. Changes in frequencies of clonable pre B cells during life in different lymphoid organs of mice. Blood. 1993;81(9):2290–2300. [PubMed] [Google Scholar]
- 10.Wolf SS, Cohen A. Expression of cytokines and their receptors by human thymocytes and thymic stromal cells. Immunology. 1992;77(3):362–368. [PMC free article] [PubMed] [Google Scholar]
- 11.Ray P, Krishnamoorthy N, Oriss TB, Ray A. Signaling of c-kit in dendritic cells influences adaptive immunity. Annals of the New York Academy of Sciences. 2010;1183:104–122. doi: 10.1111/j.1749-6632.2009.05122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miettinen M, Lasota J. KIT (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Applied immunohistochemistry & molecular morphology: AIMM / official publication of the Society for Applied Immunohistochemistry. 2005;13(3):205–220. doi: 10.1097/01.pai.0000173054.83414.22. [DOI] [PubMed] [Google Scholar]
- 13.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442(7106):997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 14.St John AL, Abraham SN. Innate immunity and its regulation by mast cells. Journal of immunology (Baltimore, Md : 1950) 2013;190(9):4458–4463. doi: 10.4049/jimmunol.1203420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ray P, Krishnamoorthy N, Ray A. Emerging functions of c-kit and its ligand stem cell factor in dendritic cells: regulators of T cell differentiation. Cell cycle (Georgetown, Tex) 2008;7(18):2826–2832. doi: 10.4161/cc.7.18.6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vredevoe DL, Widawski M, Fonarow GC, Hamilton M, Martinez-Maza O, Gage JR. Interleukin-6 (IL-6) expression and natural killer (NK) cell dysfunction and anergy in heart failure. The American journal of cardiology. 2004;93(8):1007–1011. doi: 10.1016/j.amjcard.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 17.Tete S, Tripodi D, Rosati M, Conti F, Maccauro G, Saggini A, et al. Role of mast cells in innate and adaptive immunity. Journal of biological regulators and homeostatic agents. 2012;26(2):193–201. [PubMed] [Google Scholar]
- 18.Gilfillan AM, Beaven MA. Regulation of mast cell responses in health and disease. Critical reviews in immunology. 2011;31(6):475–529. doi: 10.1615/critrevimmunol.v31.i6.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nowak EC, de Vries VC, Wasiuk A, Ahonen C, Bennett KA, Le Mercier I, et al. Tryptophan hydroxylase-1 regulates immune tolerance and inflammation. The Journal of experimental medicine. 2012;209(11):2127–2135. doi: 10.1084/jem.20120408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Theoharides TC, Conti P. Mast cells: the Jekyll and Hyde of tumor growth. Trends in immunology. 2004;25(5):235–241. doi: 10.1016/j.it.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Wedemeyer J, Galli SJ. Decreased susceptibility of mast cell-deficient Kit(W)/Kit(W-v) mice to the development of 1, 2-dimethylhydrazine-induced intestinal tumors. Laboratory investigation; a journal of technical methods and pathology. 2005;85(3):388–396. doi: 10.1038/labinvest.3700232. [DOI] [PubMed] [Google Scholar]
- 22.Khazaie K, Blatner NR, Khan MW, Gounari F, Gounaris E, Dennis K, et al. The significant role of mast cells in cancer. Cancer metastasis reviews. 2011;30(1):45–60. doi: 10.1007/s10555-011-9286-z. [DOI] [PubMed] [Google Scholar]
- 23.Stockmann C, Schadendorf D, Klose R, Helfrich I. The impact of the immune system on tumor: angiogenesis and vascular remodeling. Frontiers in oncology. 2014;4:69. doi: 10.3389/fonc.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geissler EN, Ryan MA, Housman DE. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988;55(1):185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- 25.Lee GK, Park HJ, Macleod M, Chandler P, Munn DH, Mellor AL. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology. 2002;107(4):452–460. doi: 10.1046/j.1365-2567.2002.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wester L, Koczan D, Holmberg J, Olofsson P, Thiesen HJ, Holmdahl R, et al. Differential gene expression in pristane-induced arthritis susceptible DA versus resistant E3 rats. Arthritis research & therapy. 2003;5(6):R361–R372. doi: 10.1186/ar993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.del Rio R, Noubade R, Saligrama N, Wall EH, Krementsov DN, Poynter ME, et al. Histamine H4 receptor optimizes T regulatory cell frequency and facilitates anti-inflammatory responses within the central nervous system. Journal of immunology (Baltimore, Md : 1950) 2012;188(2):541–547. doi: 10.4049/jimmunol.1101498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dimitriadou V, Pang X, Theoharides TC. Hydroxyzine inhibits experimental allergic encephalomyelitis (EAE) and associated brain mast cell activation. International journal of immunopharmacology. 2000;22(9):673–684. doi: 10.1016/s0192-0561(00)00029-1. [DOI] [PubMed] [Google Scholar]
- 29.Rouleau A, Dimitriadou V, Trung Tuong MD, Newlands GF, Miller HR, Schwartz JC, et al. Mast cell specific proteases in rat brain: changes in rats with experimental allergic encephalomyelitis. Journal of neural transmission (Vienna, Austria : 1996) 1997;104(4–5):399–417. doi: 10.1007/BF01277659. [DOI] [PubMed] [Google Scholar]
- 30.Fujimoto S, Komine M, Karakawa M, Uratsuji H, Kagami S, Tada Y, et al. Histamine differentially regulates the production of Th1 and Th2 chemokines by keratinocytes through histamine H1 receptor. Cytokine. 2011;54(2):191–199. doi: 10.1016/j.cyto.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Jutel M, Watanabe T, Klunker S, Akdis M, Thomet OA, Malolepszy J, et al. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature. 2001;413(6854):420–425. doi: 10.1038/35096564. [DOI] [PubMed] [Google Scholar]
- 32.Morgan RK, McAllister B, Cross L, Green DS, Kornfeld H, Center DM, et al. Histamine 4 receptor activation induces recruitment of FoxP3+ T cells and inhibits allergic asthma in a murine model. Journal of immunology (Baltimore, Md : 1950) 2007;178(12):8081–8089. doi: 10.4049/jimmunol.178.12.8081. [DOI] [PubMed] [Google Scholar]
- 33.Ballerini C, Aldinucci A, Luccarini I, Galante A, Manuelli C, Blandina P, et al. Antagonism of histamine H4 receptors exacerbates clinical and pathological signs of experimental autoimmune encephalomyelitis. British journal of pharmacology. 2013;170(1):67–77. doi: 10.1111/bph.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krishnamoorthy N, Oriss TB, Paglia M, Fei M, Yarlagadda M, Vanhaesebroeck B, et al. Activation of c-Kit in dendritic cells regulates T helper cell differentiation and allergic asthma. Nature medicine. 2008;14(5):565–573. doi: 10.1038/nm1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fitzgerald DC, Zhang GX, Yu S, Cullimore ML, Zhao Z, Rostami A. Intravenous tolerance effectively overcomes enhanced pro-inflammatory responses and experimental autoimmune encephalomyelitis severity in the absence of IL-12 receptor signaling. Journal of neuroimmunology. 2012;247(1–2):32–37. doi: 10.1016/j.jneuroim.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li H, Gonnella P, Safavi F, Vessal G, Nourbakhsh B, Zhou F, et al. Low dose zymosan ameliorates both chronic and relapsing experimental autoimmune encephalomyelitis. Journal of neuroimmunology. 2013;254(1–2):28–38. doi: 10.1016/j.jneuroim.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H, Nourbakhsh B, Safavi F, Li K, Xu H, Cullimore M, et al. Kit (W-sh) mice develop earlier and more severe experimental autoimmune encephalomyelitis due to absence of immune suppression. Journal of immunology (Baltimore, Md : 1950) 2011;187(1):274–282. doi: 10.4049/jimmunol.1003603. [DOI] [PMC free article] [PubMed] [Google Scholar]