Highlight
Arabinogalactan proteins (AGPs) recognized by the LM2, LM14, and MAC207 antibodies are diversely deposited in rhizodermis of barley root hair-producing genotypes, but evenly deposited in rhizodermal cells of the root hairless mutant.
Key words: Arabinogalactan proteins (AGPs), barley (Hordeum vulgare), cell differentiation, monoclonal antibodies, root hairs, Yariv.
Abstract
The arabinogalactan proteins (AGPs) are involved in a range of plant processes, including cell differentiation and expansion. Here, barley root hair mutants and their wild-type parent cultivars were used, as a model system, to reveal the role of AGPs in root hair development. The treatment of roots with different concentrations of βGlcY (a reagent which binds to all classes of AGPs) inhibited or totally suppressed the development of root hairs in all of the cultivars. Three groups of AGP (recognized by the monoclonal antibodies LM2, LM14, and MAC207) were diversely localized in trichoblasts and atrichoblasts of root hair-producing plants. The relevant epitopes were present in wild-type trichoblast cell walls and cytoplasm, whereas in wild-type atrichoblasts and in all epidermal cells of a root hairless mutant, they were only present in the cytoplasm. In all of cultivars the higher expression of LM2, LM14, and MAC207 was observed in trichoblasts at an early stage of development. Additionally, the LM2 epitope was detected on the surface of primordia and root hair tubes in plants able to generate root hairs. The major conclusion was that the AGPs recognized by LM2, LM14, and MAC207 are involved in the differentiation of barley root epidermal cells, thereby implying a requirement for these AGPs for root hair development in barley.
Introduction
The arabinogalactan proteins (AGPs) are a large heterogeneous family of hydroxyproline-rich glycoproteins found both within and on the surface of plant cells (Fincher et al., 1983; Nguema-Ona et al., 2012), and in representatives of the entire plant kingdom, including mosses (Lee et al., 2005). They are involved in a large number of biological processes, including cell division (Langan and Nothnagel, 1997), programmed cell death (Gao and Showalter, 1999; Guan and Nothnagel, 2004), cell differentiation (Majewska-Sawka and Nothngel, 2000; dos Santos et al., 2006), cell expansion (Darley et al., 2001; Lu et al., 2001) and host/microbe interactions (van Buuren et al., 1999; Johnson et al., 2003; Nguema-Ona et al., 2013). Some AGPs are directed to the cytosol, and some others to the extracellular matrix (Youl et al., 1998); they typically attach to the plasma membrane by means of a glycosylphosphatidylinositol (GPI) anchor. Other AGPs are secreted into either the intercellular space (Samaj et al., 2000) or to the plant’s exterior in the form of mucilage (Moody et al., 1988). Although their molecular size can vary from 60 to 300kDa, they all consist of a short peptide core surrounded by carbohydrate moieties which comprise at least 90% of the molecule’s mass (Serpe and Nothnagel, 1999). The glycan part consists of sugars (arabinose, galactose, rhamnose, fucose, glucuronic acid, and xylose) (Nothnagel, 1997; Showalter, 2001) generating a carbohydrate moiety which varies greatly both between species, and even between organs of a given species (Tsumuraya et al., 1988; Pennell et al., 1991; Seifert and Roberts, 2007). The form of post-translational modification of the AGPs may influence their function more strongly than does their peptide sequence (Nguema-Ona et al., 2012).
An important tool for AGP investigation is a Yariv reagent. The reactive form, containing β-D-glucosyl residues (βGlcY), is capable of binding and/or precipitating AGPs (Yariv et al., 1962). Plants or organs treated with a Yariv reagent are deprived of functional AGPs naturally present on their surface, which allows their functional investigation in vivo. However, βGlcY binds to all AGPs, what prevents its application for analyses of individual classes of AGPs (Yariv et al., 1962; Paulsen et al., 2014). For more detailed investigations of AGPs, monoclonal antibodies (mAbs) recognizing specific epitopes associated with the carbohydrate moieties have been used (Nguema-Ona et al., 2012).
The role of AGPs during root development and morphogenesis was shown using the active form of Yariv reagent. Exposure of the Arabidopsis thaliana root to βGlcY suppresses the elongation of epidermal cells and hence reduces root growth (Willats and Knox, 1996). AGPs are known to influence the organization of cortical microtubules, which control the elongation of epidermal cells (Nguema-Ona et al., 2007). Periplasmic AGPs can also act as calcium capacitors, which is significant because calcium ion gradients are important for cell expansion (Lamport and Varnai, 2012). In the barley (Hordeum vulgare) root hairless mutant rhl.1a, an HvAGP gene was upregulated by four orders of magnitude compared to the wild-type level, but there was no such upregulation in a second mutant (rhp1.a) which developed root hairs unable to progress beyond the primordium stage (Kwasniewski et al., 2010). There is no more evidence about the role of AGPs in root hair development, although the pollen tube as another cell expressing tip growth was extensively studied in this context (Qin et al., 2007; Dardelle et al., 2010; Wang et al., 2010). Treatment of pollen tubes with βGlcY halts tip growth (Mollet et al., 2002) and some AGP epitopes have been localized on the tube surface (Jauh and Lord, 1996; Chen et al., 2007; Speranza et al., 2009). The AGP epitopes recognized by the mAbs LM2 and JIM13 are deposited on the pollen tube surface of the majority of mono- and dicotyledonous species analysed to date, so are likely to be of central importance for pollen tip growth (Nguema-Ona et al., 2012).
The literature indicates a possible function of AGPs in root hair development. To validate this hypothesis we analysed the role of AGPs in this process using barley rhizodermis of root hair mutants and their parent lines as a model system. We investigated the effect of βGlcY treatment on barley root hair development and the localization of 10 AGP epitopes in roots, with particular focus on the rhizodermis.
Materials and methods
Plant material and growing conditions
The analysis involved the following wild-type cultivars of barley (Hordeum vulgare L.): Dema, Diva, Karat, and Optic, along with the root hair mutants rhl1.b, rhp1.a rhs1.a, rhs2.a, rhs.3a, and rhs4.a (Table 2), all of which have been described by Chmielewska et al. (2014). Caryopses were surface sterilized by immersion in 20% household bleach and then germinated under aeroponic conditions in glass tubes sealed with Parafilm (Szarejko et al., 2005) maintained under a 16h photoperiod (180 µEm–2 s–1 light) at 20°C for 5 days.
Table 2.
Root hair mutants and their parent cultivar
| Gene symbol | Mutant name | Parent cultivar | Phenotype |
|---|---|---|---|
| rhl1.b | root hairless 1.b | Karat | Root hairless phenotype |
| rhp1.a | root hair primoria 1.a | Dema | Trichoblasts produce only bulges (primordia) unable to enter tip growth |
| rhs1.a | root hair short 1.a | Diva | Root hairs shorter (from 40 to 90%) in comparison to those in the parent cultivar |
| rhs2.a | root hair short 2.a | Dema | |
| rhs3.a | root hair short 3.a | Karat | |
| rhs4.a | root hair short 4.a | Optic |
βGlcY treatment
The Yariv reagent βGlcY [1,3,5-tris (4-β-D-glycopyranosyloxyphenylazo)-2,4,6-trihydroxy-benzene] (Biosupplies, Bundoora, Australia) stock solution (2mg ml–1) prepared in 0.15M NaCl was dissolved in de-mineralized water to obtain working solutions of 25 µM, 10 µM, and 1 µM. The seedlings were exposed to βGlcY in hydroponic culture for 5 days following Marzec et al. (2014b ), while control sets of seedlings were grown in either de-mineralized water or in 25 µM α-D-galactosyl Yariv reagent (αGalY) (Biosupplies), prepared as described above. Three biological replicates, each comprising at least five seedlings per treatment, were included. Mean root hair lengths were based on at least 1000 root hairs measured from 15 roots, and were compared with one another using the Student’s t-test (P < 0.05).
Immunolocalization of AGP epitopes
Root sections of length 2mm were fixed by immersion for 4h at room temperature in 50mM cacodylate buffer (pH 7.2) containing 0.5% (v/v) glutaraldehyde and 2.0% (v/v) formaldehyde. Following a 15min rinse in cacodylate buffer and two washes in distilled water, the materials were dehydrated by passage through an ethanol series (30–100%), then infiltrated with LR White resin (Sigma Aldrich, Munich, Germany), initially 33%, then 66%, and finally 100%. The samples were thereafter transferred into BEEM capsules (SPI Supplies, West Chester, USA) and polymerized at 60°C for 48h. Ultra-thin (70nm) sections and semi-thin (0.5 µm) ones were cut using an Ultracut UCT instrument (Leica, Wetzlar, Germany). The former were transferred onto copper grids for subsequent immunogold labelling while the latter were mounted on poly-L-lysine-covered slides. The anti-AGP mAbs JIM4, JIM8, JIM13-17, LM2, LM14, and MAC207 (PlantProbes, Leeds, UK) were diluted 1:20 for both the fluorescence- and immunogold-labelled detection of AGPs. The fluorescence-labelling procedure followed that of Srivastava et al. (2007), and was based on the use of goat anti-rat antibody conjugated with DyLight 488 fluorochrome (Thermo Scientific, Rockford, USA). Sections were analysed using a confocal laser scanning microscope (Zeiss LSM 510 META; Zeiss, Jena, Germany); cell wall autofluorescence was detected using a 364nm laser line equipped with a 385 long-pass filter, while the fluorescence of secondary antibodies was captured by an argon 488-laser equipped with a 560–615nm band pass filter. Immunogold labelling was based on the use of a goat anti-rat antibody conjugated with 10nm gold particles, as described by Teige et al. (1998); for ultrastructural analysis, an FEI Tecnai Sphera G2 (FEI, Eindhoven, The Netherlands) was used operating at 120kV.
Whole-mount immunolabelling of AGP epitopes
The same root sections described above were used for whole-mount immunolabelling, employing the same buffers and antibody dilutions. Goat anti-rat DyLight 488 was used as a secondary antibody for fluorescence labelling. For scanning electron microscopy (SEM), the secondary antibody was goat anti-rat conjugated with 1nm gold particles. A Silver Enhancing kit (BBI Solutions, Cardiff, UK) was included, following Talbot et al. (2002). The signal was detected using a FESEM S 4100 device (Hitachi High-Technologies Europe GmbH, Krefeld, Germany).
Results
βGlcY treatment inhibited root hair development in barley
There was no difference with respect to either the length or number of seminal roots formed by the parent cultivar plants in response to any of the three concentrations of βGlcY tested (Fig. 1A, B). In the presence of 25 µM βGlcY, the roots of cultivars Dema, Diva, Karat, and Optic all failed to form root hair tubes (Fig. 1C; Supplementary Table S1). Exposure to 10 µM βGlcY stopped root hair development at the primordium stage, while the 1 µM treatment had no effect on root hair length (Fig. 1C–J). In control plants treated with either demineralized water or αGalY (AGP-unreactive form of Yariv reagent), fully developed root hairs were formed, confirming the inhibitory effect of βGlcY on root hair tube elongation (Fig. 1C,J). Both light microscopy and SEM analysis showed that root hairs failed to develop on roots exposed to 25 µM βGlcY (Fig. 1D–F; Supplementay Figure S1), but the alternation of trichoblasts and atrichoblasts was maintained (Fig. 1D). A few epidermal cells bulged as a consequence of radial expansion (Fig. 1E, F).
Fig. 1.
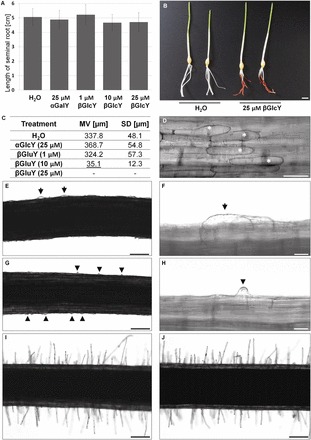
The effect of βGlcY treatment on root growth and root hair differentiation in barley cv. Karat. (A, B) Root length was not significantly influenced, while (C–J) root hair development was inhibited. (C) Root hair lengths estimated from at least 1000 root hairs sampled from 15 roots. (D) The response of root epidermal cells to 25 µM βGlcY. (E–J) Light microscopy analysis: (E,F) hairless mutant roots exposed to 25 µM βGlcY; (G–H) primordia only producing mutant roots exposed to 10 µM βGlcY; (I) treatment with 1 µM βGlcY had no effect on root hair elongation; (J) a control treatment with 25 µM αGalY. Asterisks, shorter epidermal cells; arrows, bulging cells; arrowheads, primordia; MV, mean value; SD, standard deviation. Scale bars in (B) 1cm, (D) 100 µm, (E, G, and I–J) 200 µm; (F, H) 20 µm. Underlined mean value in table indicate the statistical significance, in comparison to control conditions (Student’s t test (P < 0.05)).
The presence of AGP in the barley root
Of the set of mAbs used to detect AGP, JIM4, JIM15, and JIM17 all failed to detect any epitopes in either transverse or longitudinal sections of the meristematic and mature root hair zone of the parent cultivars Dema, Diva, Karat, and Optic. Otherwise, epitopes were detected as follows: JIM8, endodermis and metaphloem sieve elements (Fig. 2B); JIM13, throughout the root but especially in the rhizodermis, the external layer of the cortex, the endodermis, and the metaphloem sieve elements (Fig. 2C); JIM14, only in the metaphloem sieve elements (Fig. 2D); JIM16, in the endodermis (Fig. 2E); LM2, throughout the root, except for the external cell layer in the cortex and metaxylem, and most strongly in the root hair cells and endodermis (Fig. 2F) [a similar distribution was present in the root zone in which cell differentiation was initiated (Supplementary Figure S2)]; LM14 and MAC207, in the phloem companion cells and the root epidermis, again more abundantly in the root hair cells (Fig. 1G and Table 1), especially in the differentiation zone of the root, where the difference between trichoblast and atrichoblast cell size was most apparent (Supplementary Figure S3).
Fig. 2.
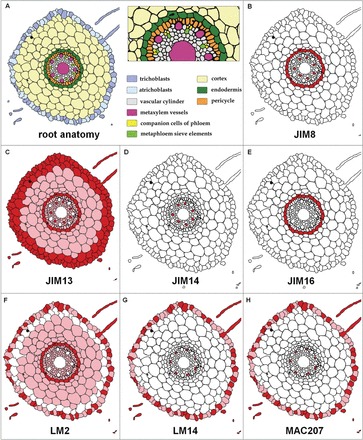
Schematic overview of AGP epitope distribution derived from transverse sections made from the mature root hair zone. (A) The organization of the root. (B–H) Abundance of the various AGP epitopes. (B) JIM8 in the endodermis and metaphloem sieve elements. (C) JIM13 was distributed throughout the root except in the metaxylem. (D) JIM14 was restricted to the metaphloem sieve elements, whereas (E) JIM16 was restricted to the endodermis. The three epitopes (F) LM2, (G) LM14, and (H) MAC207 were heterogeneously distributed in the epidermis. Signal strength indicated by colour: dark red (strong), light red (weak), and white (none).
Table 1.
AGP epitopes detected in the roots of wild-type cultivars
| mAb | Epidermis | Cortex | Endodermis | Pericycle | Xylem | Metaphloem sieve elements | Companion cells of phloem | Vascular cylinder | |
|---|---|---|---|---|---|---|---|---|---|
| Atrichoblasts | Trichoblasts | ||||||||
| JIM4 | – | – | – | – | – | – | – | – | – |
| JIM8 | – | – | – | + | – | – | + | – | – |
| JIM13 | ++ | ++ | + | ++ | + | – | + | – | + |
| JIM14 | – | – | – | – | – | – | + | – | – |
| JIM15 | – | – | – | – | – | – | – | – | – |
| JIM16 | – | – | – | + | – | – | – | – | – |
| JIM17 | – | – | – | – | – | – | – | – | – |
| LM2 | + | ++ | + | + | + | – | + | + | + |
| LM14 | + | ++ | – | – | – | – | – | + | – |
| MAC207 | + | ++ | – | – | – | – | – | + | – |
Distribution of LM2, LM14, and MAC207 epitopes in the Karat and root hairless mutant rhizodermis
The presence of LM2, LM14, and MAC207 epitopes within the root epidermis of cv. Karat, as demonstrated by transmission electron microscopy (TEM), was consistent with the patterns obtained using confocal laser scanning microscopy (CLSM). The LM2 epitope was present throughout the epidermis, but was especially abundant in the root hairs and trichoblasts (Fig. 3A, B). In the former cell type, it was concentrated in the cell wall and cytoplasm (Fig. 3J, M). There was little accumulation in the cytoplasm of non-root hair cells, and none in the atrichoblast cell wall (Fig. 3G). Both LM14 and MAC207 epitopes were present in the cytoplasm and cell walls of root hairs (Fig. 3C–F, N, and O) and trichoblasts (Fig. 3C–F, K, and L); and in the atrichoblast cytoplasm (Fig. 3H, I). A similar analysis of the rhl1.b root hairless mutant (derived from cv. Karat; Table 2) showed that all three epitopes were present in the rhizodermal layer, but that there was not diverse expression of epitopes analysed (Fig. 3P–U). TEM analysis revealed that LM2, LM14, and MAC207 epitopes were evenly distributed throughout the rhizodermis, but were restricted to the cytoplasm (Fig. 3V–X); this distribution resembled that seen in the atrichoblasts of cv. Karat (Fig. 3G–I).
Fig. 3.
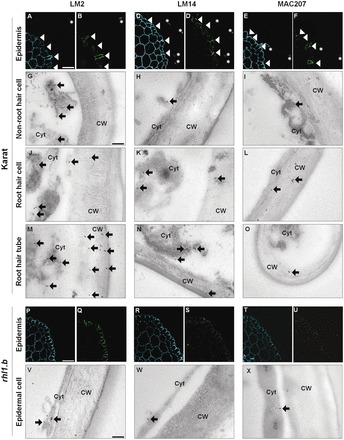
Immunolocalization of LM2, LM14, and MAC207 epitopes in the rhizodermis of barley cv. Karat and the rhl1.b mutant. (A, C, E, P, R, T) Autofluorescence illustrates cell patterning in the mature root hair zone. Fluorescence labelling of AGP epitopes in (B, D, F) cv. Karat and (Q, S, U) the rhl1.b mutant, with (B, D, F, Q, S, U, G–O, V–X) showing subcellular localization based on immunogold labelling. (A–F) Epitopes were more abundant in the trichoblasts and root hair tubes than in the atrichoblasts. (G–O) In the trichoblast cell wall, LM2, LM14, and MAC207 epitopes were only detected in the wild-type cultivars (H–X). In the root hairless mutant, the three epitopes were homogeneously distributed within the epidermis. Asterisks, root hair tubes; arrowheads, trichoblasts; arrows, gold particles; CW, cell wall; Cyt, cytoplasm. Scale bars: (A–F and P–U) 50 µm; (G–O and V–X), 100nm.
LM2 epitope was present on the wild-type root hair tube surface
Of the mAbs used to investigate the distribution of AGP epitopes in the meristematic and mature root hair zone, LM2 was the only one which detected epitopes exclusively on the root surface of root hair tubes of cultivars Dema, Diva, Karat, and Optic (Fig. 4 and Supplementary Figure S4). It was even detectable on the young primordia formed during the earliest stages of root hair development (Supplementary Figure S4). At the primordium stage, the epitopes were restricted to the tip of the outgrowth (Fig. 4D), but later they became homogeneously distributed along root hair tubes (Fig. 4B; Supplementary Figure S4). SEM analysis of preparations labelled with a gold-conjugated secondary antibody confirmed the deposition pattern of LM2 epitope AGPs. Even under light microscopy, the presence of LM2 epitope was observed in cv. Karat on the surface of primordia and root hair tubes (Fig. 5A, B, J), whereas in the negative control no signal was detected (Fig. 5C,D,M). SEM observations confirmed deposition of LM2 epitope on the surface of primordia (Fig. 5E, F) and young root hairs (Fig. 5G–I); moreover, LM2 was still present on the tip of the mature root hair (Fig. 5K, L), whereas in the negative control no LM2 was observed on the root surface (Fig. 5N, O).
Fig. 4.
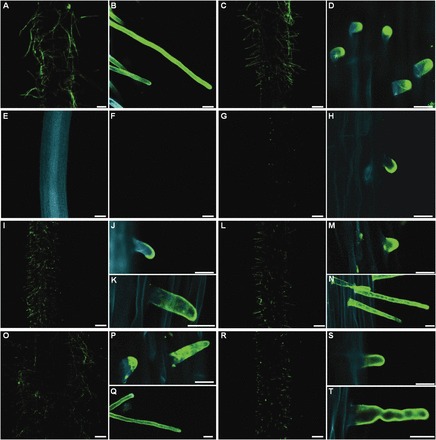
The localization of LM2 epitopes in whole-mount immunolabelled root sections of barley cultivars Karat and Dema, and the root hair mutants rhl1.b, rhp1.a, rhs1.a, 2.a, 3.a, and 4.a. Epitope was detected (A, B) on the surface of cv. Karat root-hair tubes and (C, D) in the zone harbouring root hairs in cv. Dema. (E) Autofluorescence in rhl1.b and (F) the lack of any epitope on the root surface. (G, H) Clear signal in the primordia formed by rhp1.a. (I) Epitope in rhs1.a, focusing on (J) young and (K) mature root hairs. Similar comparisons are shown for (L–N) rhs2.a, (O–Q) rhs3.a, (R–T) rhs4.a. Scale bar in (A, C, E–G, I, L, O, R) 200 µm, and in (B, D, H, J, K, M, N, P, Q, S, T) 20 µm.
Fig. 5.
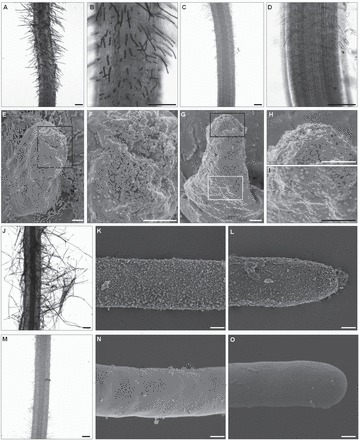
LM2 epitope distribution on the barley cv. Karat root hair surface as shown by immunogold labelling visualized by (A–D, J, M) light microscopy and (C–I, K, L, N, O) SEM. (A, B) Negative control (no primary antibody); (C, D) LM2 signal following the inclusion of gold-conjugated secondary antibody; (E) primordium; (F) detail of the primordium tip. Label strength decreased from (H) the tip to (I) the base of a growing root hair (G). (J) Root hairs displaying an even distribution of LM2 epitopes in (K) the central part and (L) the tip of fully developed root hairs. (M–O) No epitopes were detected in the negative control. Scale bar in (A–D, J, M) 200 µm, and in (E–L, N, O) 2 µm.
Localization of LM2 epitope on the root surface in root hair mutants
Neither SEM or CLSM was able to detect the presence of LM2 epitope on the roots of the rhl1.b root hairless mutant (Fig. 4F), while in the rhp1.a mutant (which produces only primordia; Table 2), epitopes were restricted to the tip of outgrowths (Fig. 4G, H). In the four non-allelic short root hair mutants rhs1.a, 2.a, 3.a, and 4.a (Table 2) the localization of LM2 epitopes was comparable to that observed in the wild-type root; they were distributed over the whole surface of the root hair tubes formed by fully developed root hairs (Fig. 4I–T).
Discussion
βGlcY inhibits root hair development
This research has shown that root hair development is quantitatively inhibited by the concentration of βGlcY present in the growing medium. A level of 25 µM suppressed primordium formation and therefore blocked root hair formation at an early stage, while a level of 10 µM was insufficient to prevent primordium formation, but sufficient to halt the elongation of the root hair tubes. In Arabidopsis roots, βGlcY treatment results in a significant degree of radial cell expansion in the rhizodermis, causing the tissue to bulge outwards (Willats and Knox, 1996; Ding and Zhu, 1997). At the subcellular level, this phenomenon reflects a disorganization of the cortical microtubules in the epidermis (Nguema-Ona et al., 2007). The inhibitory effect of βGlcY on pollen tube tip growth is well established (Mollet et al., 2002). In both lily (Lilium longiflorum) and Annona cherimola, pollen tube elongation is compromised (Jauh and Lord, 1996; Mollet et al., 2002); however, in Aquilegia eximia, Lycopersicon pimpinellifolium, and tobacco (Nicotiana tabacum), this is not the case (Mollet et al., 2002), and Arabidopsis pollen is completely prevented from germinating (Lennon and Lord, 2000). βGlcY treatment enhances the level of cytosolic Ca2+ in both lily pollen tubes (Roy et al., 1999) and cultured tobacco cells (Pickard and Fujiki, 2005). Both changes in cytosolic Ca2+ level and cytoskeleton organization are important parameters during the formation of primordia and the elongation of root hair tubes (Schiefelbein et al., 1992; Park and Nebenführ, 2011). With respect to the inhibitory effect of βGlcY on barley root hair development, therefore, it seems probable that AGPs regulate the organization of the cytoskeleton and the cytosolic Ca2+ level.
No βGlcY effect was observable during the early stage of rhizodermal cell differentiation, specifically when the alternation of trichoblasts and atrichoblasts is first apparent (Marzec et al., 2013). Since the highest concentration of βGlcY applied (sufficient to convert a wild-type into a hairless phenotype) did not modify this alternation, the implication was that the localization of AGPs on the root surface had no influence over rhizodermis patterning; rather, this must depend on asymmetric daughter cell elongation following symmetrical cell division (Marzec et al., 2013 2014a). Given that the inhibitory effect of βGlcY on the elongation of the Arabidopsis root (Willats and Knox, 1996) was not reproduced in barley, it would appear that species-distinct mechanisms must underlie the elongation of rhizodermal cells. However, in the presence of 25 µM βGlcY, some of the barley root cells did bulge outwards, implying some degree of cytoskeletal disorganization similar to that which occurs in the Arabidopsis rhizodermis (Nguema-Ona et al., 2007).
The distribution of AGP in the barley root
Variation in patterns of deposition of AGP epitopes between species has hindered the allocation of function to the individual classes of these proteins. Epitopes recognized by neither JIM4, JIM15, nor JIM17 were detectable in barley; similarly, in onion and pea, JIM4 epitopes are not present (Casero et al., 1998), while JIM15 and JIM17 epitopes are lacking in Benincasa hispida (wax gourd; Xie et al., 2011) (Table 3). In contast, JIM15 epitopes are ubiquitous in the carrot (Daucus carota) root (Knox et al., 1991), while those recognized by JIM4 are present in the protoxylem/pericycle of carrot and radish (Casero et al., 1998). The tissue distribution of JIM13 epitopes is particularly variable: they are seen throughout the barley root but are only detectable in the developing xylem in carrot (Dolan and Roberts, 1995) and in the pericycle and protophloem sieve elements in maize (Zea mays; Samaj et al., 1998); they are completely missing in B. hispida (Xie et al., 2011). Although JIM13 epitope was abundant in the rhizodermis, endodermis, and metaphloem sieve elements, no specific developmental process could be assigned to the class of AGP harbouring β-D-GlcA-(1,3)-α-D-GalA-(1,2)-α-L-Rha (Yates and Knox, 1994; Yates et al., 1996).
Table 3.
AGP epitopes detected in the roots of various plant species
| mAbs | Recognized epitope | Epitope expression in roots |
|---|---|---|
| JIM4 | β-D-GlcA-(1,3)-α-D-GalA-(1,2)-α-L-Rha (Yates et al., 1996) | Carrot: protoxylem, pericycle (Knox et al., 1989
1991; Casero et al., 1998) Radish: individual pericycle cells (Casero et al., 1998) Onion, pea: lack of expression in all root tissues (Casero et al., 1998) Barley: lack of expression in all root tissues (this work) |
| JIM8 | Unknown | Maize: protophloem sieve elements (Samaj et al., 1998) Wax gourd: protoxylem (Xie et al., 2011) Barley: endodermis and metaphloem sieve elements (this work) |
| JIM13 | β-D-GlcA-(1,3)-α-D-GalA-(1,2)-α-L-Rha (Yates and Knox, 1994; Yates et al., 1996) |
Arabidopsis: xylem (Dolan et al., 1995); root cap and all root apex cells (Vicre et al., 2005) Carrot: early stage of xylem development (Knox et al., 1991) Maize: pericycle, protophloem sieve elements, companion cells, root cap (Samaj et al., 1998) Wax gourd: lack of expression in all root tissues (Xie et al., 2011) Barley: all root cells, stronger in epidermis, endodermis, and metaphloem sieve elements (this work) |
| JIM14 | Unknown |
Arabidopsis: all root cells, stronger in metaphloem sieve elements (Dolan and Roberts, 1995) Carrot: all root cells (Knox et al., 1991) Wax gourd: lack of expression in all root tissues (Xie et al., 2011) Barley: metaphloem sieve elements (this work) |
| JIM15 | Unknown | Carrot: all root cells, except epidermis (Knox et al., 1991) Wax gourd: lack of expression in all root tissues (Xie et al., 2011) Barley: lack of expression in all root tissues (this work) |
| JIM16 | Unknown | Carrot: all cells in root meristem (Knox et al., 1989) Wax gourd: all root cells, weaker in cortex and parenchyma (Xie et al., 2011) Barley: endodermis (this work) |
| JIM17 | Unknown | Wax gourd: lack of expression in all root tissues (Xie et al., 2011) Barley: lack of expression in all root tissues (this work) |
| LM2 | β-Linked GlcA (Smallwood et al., 1994) |
Arabidopsis: epidermis, weaker in trichoblasts (Andeme-Onzighi et al., 2002) Maize: surface of root hair tubes (Samaj et al., 1999) Wax gourd: all root cells, except root epidermis (Xie et al., 2011) Barley: all cells except xylem, diverse expression in epidermal and parenchyma cells (this work) |
| LM14 | Arabinose- and galactose-enriched carbohydrate chains (Moller et al., 2008) | Wax gourd: all root cells, stronger in epidermis (Xie et al., 2011) Barley: companion cells of phloem and diverse expression in epidermis (this work) |
| MAC207 | β-GlcA-(1,3)-α-GalA-(1,2)-Rha (Bradley et al., 1988; van den Bosch et al.,1989) |
Arabidopsis: root cap and all root apex cells (Vicre et al., 2005) Carrot: all cells in root meristem (Knox et al., 1989) Wax gourd: lack of expression in all root tissues (Xie et al., 2011) Barley: companion cells of phloem and diverse expression in epidermis (this work) |
JIM8, JIM14, and JIM16 mAbs can each serve as a marker for particular root tissues in barley: the former two for metaphloem sieve elements, and the latter one for the endodermis. JIM8 epitopes are also detected in maize root immature and mature sieve elements (Samaj et al., 1998) and in the B. hispida protoxylem (Xie et al., 2011) (Table 3). Thus JIM8 epitope AGPs may be involved in the differentiation of two types of vascular tissue in both mono- and dicotyledonous species. JIM14 epitope localization is interspecifically rather variable (Knox et al., 1991; Dolan and Roberts, 1995; Xie et al., 2011). In barley, they were restricted to the metaphloem sieve elements, while in Arabidopsis they are abundant throughout the root (Dolan et al., 1995) (Table 3). JIM16 epitopes were observed solely in the root endodermis of barley, while in both carrot (Knox et al., 1989) and B. hispida (Xie et al., 2011) they are distributed throughout the root; the inference is that these epitopes are unlikely to be generally involved in cell differentiation.
The localization of LM2, LM14, and MAC207 epitopes in the barley rhizodermis
LM2 targets the β-linked GlcA molecule present in the AGP polysaccharide moiety (Smallwood et al., 1994), and was particularly abundant in trichoblasts/root hair tubes. In Arabidopsis, LM2 epitopes are present in the rhizodermis (especially in the trichoblasts), while in the reb1 mutant they are confined to atrichoblasts (Andeme-Onzighi et al., 2002). The implication is that AGPs containing GlcA are required for root hair differentiation in a wide range of species. In both maize and sundew (Drosera capensis), LM2 epitopes are deposited in the root epidermis, parenchyma, and cortex (Samaj et al., 2000). TEM analysis has revealed a preferential localization within the cytoplasm endomembrane system, in association with the endoplasmic reticulum, Golgi apparatus, and tonoplast in both species (Samaj et al., 2000). The present experiments have demonstrated the presence of LM2 epitopes in various tissues of the barley root, and of particular interest is their distribution between trichoblasts and atrichoblasts and among the various parenchyma layers. While they were largely restricted to the cell wall of root hairs and tubes, some were also present in the atrichoblast cytoplasm. In root hair tubes, they were associated with vesicles, just as they are in both maize and sundew (Samaj et al., 2000). At an early stage of root hair formation, when the cell alternation pattern is first visible (Marzec et al., 2013), more abundant LM2 epitope was present in the cytoplasm of nascent trichoblasts. The lack of LM2 epitope in the external layer of the cortex, in comparison to its low level presence in the cytoplasm of other layers, can be explained by a combination of differential expression of the gene encoding the protein recognized by LM2, variable post-transcriptional modification of proteins (Seifert et al., 2014), and the influence of symplasmic communication on the transport and location of AGPs among cells of the same tissue (Marzec and Kurczynska, 2014). AGPs are known to contribute to signalling and cell-to-cell communication, and the presence of the LM2 epitope in the layer of cells located below the root epidermis could probably interfere with communication between adjacent rhizodermal cells. As the greater abundance of the LM2 epitope coincided with an early stage of barley root hair development, it is possible that the relevant AGPs are transported to the cell wall only in root hair cells. In maize, the finding that LM2 epitope is deposited on the surface of root hair tubes has been suggested to imply that these AGPs have a function in root hair elongation (Samaj et al., 1999). The same epitopes were present on the barley root surface from the earliest stage of root hair development, although unlike in maize, they remained at the tip of the mature root hairs. In both the rhp1.a mutant and the four non-allelic rhs mutants, the epitopes remained detectable on the surface of primordia/root hair tubes. A consistent hypothesis is therefore that the presence of certain AGP epitopes on the root hair surface is required for the development of the root hair. Because there was no discernible effect of fixation or dehydration on the distribution of the LM2 epitope, the likelihood is that the relevant AGP remained anchored to GPI (and so to the plasma membrane), rather than being secreted into the extracellular matrix.
A similar distribution of epitopes applied to the AGPs recognized by LM14, a mAb which targets arabinose- and galactose-enriched carbohydrate chains (Moller et al., 2008), and MAC207, which targets β-GlcA-(1,3)-α-GalA-(1,2)-Rha (Bradley et al., 1988; van den Bosch et al., 1989). Although the abundance of these epitopes was lower than for those recognizing LM2, there remained a clear difference between their abundance on trichoblasts/root hair tubes and on atrichoblasts. LM14 epitopes are present throughout the B. hispida root and particularly in the rhizodermis, but no difference in abundance appears to exist between root hair and non-root hair cells (Xie et al., 2011) (Table 3). In barley, the presence of LM14 epitopes was restricted to the root epidermis and phloem sieve elements, a finding which allows this mAb to be informative as a marker for these tissues. More specifically, the epitope was detected in the trichoblast cell wall, all the way from the earliest stage of root hair formation to the final, mature stage. B. hispida roots lack any MAC207 epitopes (Xie et al., 2011), whereas the antigen is present in both Arabidopsis and carrot root cells (Knox et al., 1989; Vicre et al., 2005). In barley, the distribution of MAC207 epitope overlapped that of LM14 (Table 3). The pattern of LM2, LM14 and MAC207 epitope deposition in barley suggests a coincidence of epitope transport/localization and rhizodermal cell differentiation.
In contrast to the wild-type cultivars, in which the presence of all three epitopes was marginal in atrichoblasts but substantial in trichoblasts, in the root hairless mutant, LM2, LM14, and MAC207 epitopes were dispersed at a low level of abundance throughout the rhizodermis. This observation is fully consistent with the downregulation of a gene encoding AGP in the mutant root, whereas no such differential transcription could be observed between mutants producing primordia and the parent cultivar (Kwasniewski et al., 2010). The present immunolocalization experiments in mutants generating a distinct root hair phenotype have led to a suggested role for each of the three classes of AGP during the early stage of root hair development and have established correlations between their cellular localization and rhizodermal cell specialization.
Supplementary material
Supplementary data can be found at JXB online.
Supplementary Table S1. The effect of βGlcY treatment on root hair tube elongation in barley cultivars Dema, Diva, and Optic.
Supplementary Figure S1. SEM analysis of βGlcY-induced inhibition of root hair elongation in barley cv. Dema.
Supplementary Figure S2. CLSM analysis of LM2 epitope deposition in barley cv. Karat root.
Supplementary Figure S3. Localization of MAC207 epitopes in barley cv. Karat roots as visualized by CLSM.
Supplementary Figure S4. LM2 epitopes on the barley cv. Dema root surface as visualized by CLSM.
Funding
The research was carried out in the framework of the two Polish National Science Centre grants 2011/01/M/NZ2/02979 and 2013/08/T/NZ3/00811. M. Marzec was supported by a Foundation for Polish Science scholarship (START 071/2014).
Supplementary Material
Acknowledgements
We thank Kirsten Hoffie and Marion Benecke (IPK Structural Cell Biology Group) for their technical assistance and Aleksandra Muszynska for critical reading of the manuscript.
References
- Andeme-Onzighi C, Sivaguru M, Judy-March J, Baskin TI, Driouich A. 2002. The reb1-1 mutation of Arabidopsis alters the morphology of trichoblasts, the expression of arabinogalactan proteins and the organisation of cortical microtubules. Planta 215, 949–958. [DOI] [PubMed] [Google Scholar]
- Bradley DJ, Wood EA, Larkins AP, Galfre G, Butcher GW, Brewin NJ. 1988. Isolation of monoclonal antibodies reacting with peribacteroid membranes and other components of pea root nodules containing Rhizobium leguminosarum . Planta 173, 149–160. [DOI] [PubMed] [Google Scholar]
- Casero PJ, Casimiro I, Knox JP. 1998. Occurrence of cell surface arabinogalactan-protein and extensin epitopes in relation to pericycle and vascular tissue development in the root apex of four species. Planta 204, 252–259. [Google Scholar]
- Chen T, Teng N, Wu X, Wang Y, Tang W, Samaj J, Baluska F, Lin J. 2007. Disruption of actin filaments by latrunculin B affects cell wall construction in Picea meyeri pollen tube by disturbing vesicle trafficking. Plant and Cell Physiology 1, 19–30. [DOI] [PubMed] [Google Scholar]
- Chmielewska B, Janiak A, Karcz J, et al. 2014. Morphological, genetic and molecular characteristics of barley root hair mutants. Journal of Applied Genetics 10.1007/s13353-014-0225-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardelle F, Lehner A, Ramdani Y, Bardor M, Lerouge P, Driouich A, Mollet JC. 2010. Biochemical and immunocytological characterizations of Arabidopsis pollen tube cell wall. Plant Physiology 153, 1563–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darley CP, Forrester AM, McQueen-Mason SJ. 2001. The molecular basis of plant cell wall extension. Plant Molecular Biology 47, 179–195. [PubMed] [Google Scholar]
- Ding L, Zhu JK. 1997. A role for arabinogalactan-proteins in root epidermal cell expansion. Planta 203, 289–294. [DOI] [PubMed] [Google Scholar]
- Dolan L, Linstea P, Roberts K. 1995. An AGP epitope distinguishes a central metaxylem initial from other vascular initials in the Arabidopsis roots. Protoplasma 189, 145–155. [Google Scholar]
- Dolan L, Roberts K. 1995. Secondary thickening in roots of Arabidopsis thaliana: anatomy and cell surface changes. New Phytologist 131, 121–128. [DOI] [PubMed] [Google Scholar]
- dos Santos ALW, Wietholter N, Gueddari NE, Moerschbacher BM. 2006. Protein expression during seed development in Araucaria angustifolia: transient accumulation of class IV chitinases and arabinogalactan proteins. Physiologia Plantarum 127, 138–148. [Google Scholar]
- Fincher GB, Stone BA, Clarke AE. 1983. Arabinogalactan-proteins: structure, biosynthesis, and function. Annual Review of Plant Biology 34, 47–70. [Google Scholar]
- Gao M, Showalter AM. 1999. Yariv reagent treatment induces programmed cell death in arabidopsis cell cultures and implicates arabinogalactan protein involvement. The Plant Journal 19, 321–331. [DOI] [PubMed] [Google Scholar]
- Guan Y, Nothnagel EA. 2004. Binding of arabinogalactan proteins by Yariv phenylglycoside triggers wound-like responses in arabidopsis cell cultures. Plant Physiology 135, 1346–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauh GY, Lord EM. 1996. Localization of pectins and arabinogalactanproteins in lily (Lilium longiflorum L.) pollen tube and style, and their possible roles in pollination. Planta 199, 251–261. [Google Scholar]
- Johnson KL, Jones BJ, Bacic A, Schultz CJ. 2003. The fasciclin-like arabinogalactan proteins of arabidopsis. A multigene family of putative cell adhesion molecules. Plant Physiology 133, 1911–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox JP, Day S, Roberts K. 1989. A set of cell surface glycoproteins forms an early marker of cell position, bu not cell type, in the root apical meristem of Daucus carota L. Development 106, 47–56. [Google Scholar]
- Knox JP, Linstead PJ, Peart J, Cooper C, Roberts K. 1991. Developmentally regulated epitopes of cell surface arabinogalactan proteins and their relation to root tissue pattern formation. The Plant Journal 1, 317–326. [DOI] [PubMed] [Google Scholar]
- Kwasniewski M, Janiak A, Mueller-Roeber B, Szarejko I. 2010. Global analysis of the root hair morphogenesis transcriptome reveals new candidate genes involved in root hair formation in barley. Journal of Plant Physiology 167, 1076–1083. [DOI] [PubMed] [Google Scholar]
- Lamport DT, Várnai P. 2013. Periplasmic arabinogalactan glycoproteins act as a calcium capacitor that regulates plant growth and development. New Phytologist 197, 58–64. [DOI] [PubMed] [Google Scholar]
- Langan KJ, Nothnagel EA. 1997. Cell surface arabinogalactan-proteins and their relation to cell proliferation and viability. Protoplasma 196, 87–98. [Google Scholar]
- Lee KJ, Sakata Y, Mau SL, Pettolino F, Bacic A, Quatrano RS, Knight CD, Knox J.P. 2005. Arabinogalactan proteins are required for apical cell extension in the moss Physcomitrella patens . The Plant Cell 17, 3051–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon KA, Lord EM. 2000. In vivo pollen tube cell of Arabidopsis thaliana I: tube cell cytoplasm and wall. Protoplasma 214, 45–56. [Google Scholar]
- Lu H, Chen M, Showalter AM. 2001. Developmental expression and perturbation of arabinogalactan-proteins during seed germination and seedling growth in tomato. Physiologia Plantarum 112, 442–450. [DOI] [PubMed] [Google Scholar]
- Majewska-Sawka A, Nothnagel EA. 2000. The multiple roles of arabinogalactan proteins in plant development. Plant Physiology 122, 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzec M, Kurczynska EU. 2014. Importance of symplasmic communication in cell differentiation. Plant Signaling and Behavior 9, e27931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzec M, Melzer M, Szarejko I. 2013. Asymmetric growth of root epidermal cells is related to the differentiation of root hair cells in Hordeum vulgare (L.). Journal of Experimental Botany 64, 5145–5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzec M, Melzer M, Szarejko I. 2014a. The evolutionary context of root epidermis cell patterning in grasses (Poaceae). Plant Signaling and Behavior 9, e27972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzec M, Muszynska A, Melzer M, Sas-Nowosielska H, Kurczynska EU. 2014b. Increased symplasmic permeability in barley root epidermal cells correlates with defects in root hair development. Plant Biology 16, 476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller I, Marcus SE, Haeger A, Verhertbruggen Y, Verhoef R, Schols H, Ulvskov P, Mikkelsen JD, Knox JP, Willats W. 2008. High-throughput screening of monoclonal antibodies against plant cell wall glycans by hierarchical clustering of their carbohydrate microarray binding profiles. Glycoconjugate Journal 25, 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollet JC, Kim S, Jauh GY, Lord EM. 2002. Arabinogalactan proteins, pollen tube growth, and the reversible effects of Yariv phenylglycoside. Protoplasma 219, 89–98. [DOI] [PubMed] [Google Scholar]
- Moody SF, Clarke AE, Bacic A. 1988. Structural analysis of secreted slime from wheat and cowpea roots. Phytochemistry 27, 2857–2861. [Google Scholar]
- Nguema-Ona E, Bannigan A, Chevalier L, Baskin TI, Driouich A. 2007. Disruption of arabinogalactan-proteins disorganizes cortical microtubules in the root of Arabidopsis thaliana . The Plant Journal 52, 240–251. [DOI] [PubMed] [Google Scholar]
- Nguema-Ona E, Coimbra S, Vicré-Gibouin M, Mollet JC, Driouich A. 2012. Arabinogalactan proteins in root and pollen-tube cells: distribution and functional aspects. Annals of Botany 110, 383–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguema-Ona E, Vicré-Gibouin M, Cannesan MA, Driouich A. 2013. Arabinogalactan proteins in root–microbe interactions. Trends in Plant Science 18, 440–449. [DOI] [PubMed] [Google Scholar]
- Nothnagel E. 1997. Proteoglycans and related components in plant cells. International Review of Cytology 174, 195–191. [DOI] [PubMed] [Google Scholar]
- Park E, Nebenführ A. 2011. Cytoskeleton and root hair growth. In: Liu B. ed. The Plant Cytoskeleton. New York: Springer, 259–275. [Google Scholar]
- Paulsen BS, Craik DJ, Dunstan DE, Stone BA, Bacic A. 2014. The Yariv reagent: behavior in different solvents and interaction with a gum arabic arabinogalactanprotein. Carbohydrate Polymers j.carbpol.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Pennell RI, Janniche L, Kjellbom P, Scofield GN, Peart JM, Roberts K. 1991. Developmental regulation of a plasma membrane arabinogalactan protein epitope in oilseed rape flowers. The Plant Cell 3, 1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard BG, Fujiki M. 2005. Ca2+ pulsation in BY-2 cells and evidence for control of mechanosensory Ca2+ -selective channels by the plasmalemmal reticulum. Functional Plant Biology 32, 863–879. [DOI] [PubMed] [Google Scholar]
- Qin Y, Chen D, Zhao J. 2007. Localization of arabinogalactan proteins in anther, pollen, and pollen tube of Nicotiana tabacum L. Protoplasma 231, 43–53. [DOI] [PubMed] [Google Scholar]
- Roy SJ, Holdaway-Clarke TL, Hackett GR, Kunkel JG, Lord EM, Hepler PK. 1999. Uncoupling secretion and tip growth in lilly pollen tubes: evidence for the role of the calcium in exocytosis. The Plant Journal 19, 379–386. [DOI] [PubMed] [Google Scholar]
- Samaj J, Baluska F, Volkmann D. 1998. Cell-specific expression of two arabinogalactan protein epitopes recognized by monoclonal antobodies JIM8 and JIM13 in maize roots. Protoplasma 204, 1–12. [Google Scholar]
- Samaj J, Braun M, Baluška F, Ensikat HJ, Tsumuraya Y, Volkmann D. 1999. Specific localization of arabinogalactan-protein epitopes at the surface of maize root hairs. Plant Cell Physiology 40, 874–883. [Google Scholar]
- Samaj J, Samajova O, Peters M, Baluska F, Lichtscheidl I, Knox JP, Volkmann D. 2000. Immunolocalization of LM2 arabinogalactan protein epitope associated with endomembranes of plant cells. Protoplasma 212, 186–196. [Google Scholar]
- Schiefelbein JW, Shipley A, Rowse P. 1992. Calcium influx at the tip of growing root-hair cells of Arabidopsis thaliana . Planta 187, 455–459. [DOI] [PubMed] [Google Scholar]
- Seifert GJ, Xue H, Acet T. 2014. The Arabidopsis thaliana FASCICLIN LIKE ARABINOGALACTAN PROTEIN 4 gene acts synergistically with abscisic acid signalling to control root growth. Annals of Botany 10.1093/aob/mcu010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert GJ, Roberts K. 2007. The biology of arabinogalactan proteins. Annual Review of Plant Biology 58, 137–161. [DOI] [PubMed] [Google Scholar]
- Serpe MD, Nothnagel EA. 1999. Arabinogalactan-proteins in the multiple domains of the plant cell surface. Advances in Botanical Research 30, 207–289. [Google Scholar]
- Showalter AM. 2001. Arabinogalactan proteins: structure, expression and function. Cellular and Molecular Life Sciences 58, 1399–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood M, Beven A, Donovan N, Neill SJ, Peart J, Roberts K, Knox JP. 1994. Localization of cell wall proteins in relation to the developmental anatomy of the carrot root apex. The Plant Journal 5, 237–246. [Google Scholar]
- Speranza A, Taddei AR, Gambellini G, Ovidi E, Scoccianti V. 2009. The cell wall of kiwifruit pollen tubes is a target for chromium toxicity: alterations to morphology, callose pattern and arabinogalactan protein distribution. Plant Biology 11, 179–193. [DOI] [PubMed] [Google Scholar]
- Srivastava V, Schinkel H, Witzell J, Hertzberg M, Torp M, Srivastava MK, Melzer M, Wingsle G. 2007. Down-regulation of high isoelectric point extracellular superoxide dismutase mediates alterations in reactive oxygen species metabolism and developmental disturbance in hybrid aspen. The Plant Journal 49, 135–148. [DOI] [PubMed] [Google Scholar]
- Szarejko I, Janiak A, Chmielewska B, Nawrot M. 2005. Genetic analysis of several root hair mutants of barley. Barley Genetics Newsletter 35, 36–38. [Google Scholar]
- Talbot MJ, Offler CE, McCurdy DW. 2002. Transfer cell wall architecture: a contribution towards understanding localized wall deposition. Protoplasma 219, 197–209. [DOI] [PubMed] [Google Scholar]
- Teige M, Melzer M, Süss KH. 1998. Purification, properties and in situ localization of the amphibolic enzymes D-ribulose-5-phosphate 3-epimerase and transketolase from spinach chloroplasts. European Journal of Biochemistry 252, 237–244. [DOI] [PubMed] [Google Scholar]
- Tsumuraya Y, Ogura K, Hashimoto Y, Mukoyama H, Yamamoto S. 1988. Arabinogalactan-proteins from primary and mature roots of radish (Raphanus sativus L.). Plant Physiology 86, 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buuren ML, Maldonado-Mendoza IE, Trieu AT, Blaylock LA, Harrison MJ. 1999. Novel genes induced during an arbuscular mycorrhizal (AM) symbiosis formed between Medicago truncatula and Glomus versiforme . Molecular Plant-Microbe Interactions 12, 171–181. [DOI] [PubMed] [Google Scholar]
- van den Bosch KA, Bradley DJ, Knox JP, Perotto S, Butcher GW, Brewin NJ. 1989. Common components of the infection thread matrix and the intercellular space identified by immunocytochemical analysis of pea nodules and uninfected roots. The EMBO Journal 8, 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicre M, Santaella C, Blanchet S, Gateau A, Driouich A. 2005. Root border-like cells of Arabidopsis: microscopical characterization and role in the interaction with rhizobacteria. Plant Physiology 138, 998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willats GTW, Knox JP. 1996. A role for arabinogalactan-proteins in plant cell expansion: evidence from studies on the interaction of β-glucosyl Yariv reagent with seedlings of Arabidopsis thaliana . The Plant Journal 9, 919–925. [DOI] [PubMed] [Google Scholar]
- Wang HJ, Huang JC, Jauh GY. 2010. Pollen germination and tube growth. Advances in Botanical Research 54, 1–52. [Google Scholar]
- Xie D, Ma L, Šamaj J, Xu C. 2011. Immunohistochemical analysis of cell wall hydroxyproline-rich glycoproteins in the roots of resistant and susceptible wax gourd cultivars in response to Fusarium oxysporum f. sp. Benincasae infection and fusaric acid treatment. Plant Cell Reports 30, 1555–1569. [DOI] [PubMed] [Google Scholar]
- Yariv J, Rapport MM, Graf L. 1962. The interaction of glycosides and saccharides with antibody to the corresponding phenylazo glycosides. Biochemical Journal 85, 383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates EA, Knox JP. 1994. Investigations into the occurrence of plant cell surface epitopes in exudate gums. Carbohydrate Polymers 24, 281–286. [Google Scholar]
- Yates EA, Valdor JF, Haslam SM, Morris HR, Dell A, Mackie W, Knox JP. 1996. Characterization of carbohydrate structural features recognized by anti arabinogalactan-protein monoclonal antibodies. Glycobiology 6, 131–139. [DOI] [PubMed] [Google Scholar]
- Youl JJ, Bacic A, Oxley D. 1998. Arabinogalactan-proteins from Nicotiana alata and Pyrus communis contain glycosylphosphatidylinositol membrane anchors. Proceedings of the National Academy of Sciences, USA 95, 7921–7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


