Summary
Under well-watered conditions leaf hydraulic conductance increases with transpiration rate. This reduces the water potential gradient in leaves and potentially improves productivity under daily variation in evaporative demand.
Key words: Leaf hydraulic conductance, leaf water potential, stem water potential, stomatal conductance, transpiration, water relations.
Abstract
Leaf hydraulic conductance (k leaf) is a central element in the regulation of leaf water balance but the properties of k leaf remain uncertain. Here, the evidence for the following two models for k leaf in well-hydrated plants is evaluated: (i) k leaf is constant or (ii) k leaf increases as transpiration rate (E) increases. The difference between stem and leaf water potential (ΔΨstem–leaf), stomatal conductance (g s), k leaf, and E over a diurnal cycle for three angiosperm and gymnosperm tree species growing in a common garden, and for Helianthus annuus plants grown under sub-ambient, ambient, and elevated atmospheric CO2 concentration were evaluated. Results show that for well-watered plants k leaf is positively dependent on E. Here, this property is termed the dynamic conductance, k leaf(E), which incorporates the inherent k leaf at zero E, which is distinguished as the static conductance, k leaf(0). Growth under different CO2 concentrations maintained the same relationship between k leaf and E, resulting in similar k leaf(0), while operating along different regions of the curve owing to the influence of CO2 on g s. The positive relationship between k leaf and E minimized variation in ΔΨstem–leaf. This enables leaves to minimize variation in Ψleaf and maximize g s and CO2 assimilation rate over the diurnal course of evaporative demand.
Introduction
The co-variation between leaf water potential (Ψleaf), transpiration rate (E), stomatal conductance (g s), and CO2 assimilation rate (A) at any instant in time, or integrated over the life of a leaf, is considered to be strongly influenced by the water permeability of the cells that define the leaf hydraulic network (e.g. Sôber, 1997; Franks, 2006; Sack and Holbrook, 2006; Brodribb et al., 2007; Simonin et al., 2012). The cell types that comprise the leaf hydraulic network vary greatly in structure and function. At one extreme are the relatively rigid, dead, xylem cells comprising solely of cell wall, and at the other extreme are the live parenchyma cells of the extra-xylary tissue. At the whole-leaf level these two cell types in combination represent, on average, ~50% of the total liquid-phase conductance to water flow along the transpiration stream between the roots and sites of evaporation within the leaves of a plant (Nardini et al., 2005a ; Sack et al., 2005). Thus, in response to variation in water availability or demand, the conductance of both the leaf xylem and extra-xylary pathways strongly influence the changes in liquid flux.
Models used to describe changes in leaf hydraulic conductance (k leaf), in response to variation in water availability or demand, are often based on dynamics previously observed in stems by emphasizing xylem vulnerability to cavitation as Ψleaf decreases (e.g. Brodribb and Holbrook, 2006). According to this xylem-centric framework, k leaf is at a maximum when leaves are well hydrated (i.e. high Ψleaf). As E increases, k leaf may stay relatively constant, with Ψleaf decreasing until a threshold is reached that results in the formation of xylem emboli, followed by a rapid decline in k leaf with any further decrease in Ψleaf (e.g. Blackman et al., 2009; Johnson et al., 2009; Martorell et al., 2014). Under this scenario, increases in E would increase the driving gradient for water flow across a leaf. In other words, above the cavitation threshold a positive linear relationship is expected between the water potential difference between stem and leaf (ΔΨstem–leaf) and E (e.g. Fig. 1, solid black line).
Fig. 1.
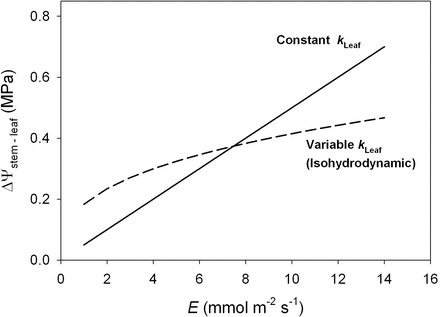
Two models describing the water potential drawdown from stem to leaf (ΔΨstem–leaf, MPa) in response to changes in transpiration rate (E, mmol m–2 s–1). The solid black line represents a model scenario when leaf hydraulic conductance (k leaf, mmol m–2 s–1 MPa–1) is constant, whereas the dashed line represents a model scenario when k leaf is positively dependent on E, commonly referred to as isohydrodynamic conditions.
Xylem-focused models have been extremely useful for characterizing the extent to which emboli induced by water stress begin to limit leaf gas exchange and primary productivity (e.g. Salleo et al., 2001; Sperry et al., 2002; Cochard et al., 2002; Johnson et al., 2012). However, it is becoming increasingly apparent that changes in how water is transported through the extra-xylary component of the transpiration stream can lead to large, sustained changes in k leaf before or even after the onset of cavitation (e.g. Matzner and Comstock, 2001; Lo Gullo et al., 2005; Nardini et al., 2005b ; Cochard et al., 2007; Sellin and Kupper, 2007; Sellin et al., 2008; Scoffoni et al., 2008; Pou et al., 2013; Shatil-Cohen et al., 2011). These observations suggest an additional mechanism driving the co-variation between Ψleaf, E, g s, and k leaf that cannot be explained by models that emphasize xylem vulnerability to cavitation. As the vast majority of carbon gain and water use occurs when leaf water potentials are above the water potential thresholds that lead to significant loss of hydraulic conductance via xylem embolism, there is a need for a greater understanding of the mechanisms governing the co-variation between Ψleaf, E, g s, k leaf, and ultimately A when leaves are well hydrated. A comprehensive quantification of the relationship between k leaf, g s, and E is crucial in vegetation models that incorporate hydraulic processes.
Although stomata and the leaf hydraulic network control two distinct phases of water transport across the soil–plant–atmosphere continuum (i.e. vapour and liquid flux), coordination between these two regulatory systems must exist if leaves are to maintain physiologically favourable water contents and avoid desiccation while maximizing carbon gain. If the goal is to maximize carbon gain, then leaves need to keep stomata open over a broad diurnal range of evaporative demand. The problem for any leaf with fixed k leaf, or one in which k leaf only declines with increasing E, is that the water potential drawdown (ΔΨstem–leaf) increases with E which, via the hydraulic feedback loop, tends to reduce g s and CO2 assimilation rate (Cowan, 1977; Buckley, 2005; Franks et al., 2007). One solution to this problem is for leaves to vary k leaf positively with E to minimize the change in ΔΨ stem–leaf, maintaining isohydrodynamic conditions. Leaves operating with this mechanism will show a nonlinear relationship between ΔΨstem–leaf and E where the ratio of E to ΔΨstem–leaf increases as E increases (Fig. 1, dashed line). In other words, a positive correlation between k leaf and E would reduce daytime depressions in Ψleaf and increase the maximum potential g s and A for a given leaf to air vapour pressure difference (VPD).
Isohydrodynamic behaviour has been shown for whole-plant hydraulic conductance (Franks et al., 2007). Reports of relatively constant ΔΨstem–leaf (Black, 1979; Brodribb and Holbrook, 2003) suggest that the same mechanism may operate at the leaf level. Here the hypothesis that variation in g s and k leaf are connected through optimization of the water potential drawdown across a leaf is tested. Specifically, it is predicted that variation in k leaf occurs to maximize leaf gas exchange while minimizing variation in ΔΨstem–leaf. Using in situ measurements of k leaf taken on species grown under well-watered conditions in a common garden and on plants grown under three different atmospheric CO2 concentrations, two possible models describing the mechanistic links between Ψleaf, E, g s, and k leaf in well-hydrated plants are tested: (i) constant k leaf, versus (ii) increasing k leaf as E increases.
Materials and methods
Common garden
Four deciduous and two evergreen temperate forest tree species were evaluated; three angiosperms, Acer macrophyllum Pursh (Aceraceae), Populus fremontii Watson (Salicaceae), and Quercus kelloggii Newberry (Fagaceae), and three gymnosperms, Metasequoia glyptosrtoboides Hu and Cheng (Cupressaceae), Pinus ponderosa P. Laws (Pinaceae), and Sequoia sempervirens D. Don (Cupressaceae). Four or five saplings of each species were grown from seed in 40 l pots and transferred to a single site (common garden), in full sun, on a ridge top at the University of California Botanical Garden (32°52′N 122°14′W, ~256 m elevation) between 1–7 March 2007. Individual saplings from each species were randomized spatially throughout the common garden. Saplings ranged from ~1.5–2.5 m in height. Plants were kept well watered using drip irrigation. Data were collected when leaves were fully expanded, ~1 month after the start of leaf emergence, which occurred in late May/early June of 2008 and 2009 for A. macrophyllum, M. glyptostroboides, P. fremontii, and Q. kelloggii and mid to late July for P. ponderosa, and S. sempervirens. Air temperature and relative humidity were measured with a Li-1600 steady-state porometer (Licor Inc., Lincoln NE, USA) in close proximity to the leaves in which gas exchange and water potential were measured. Photosynthetically active radiation intercepted by the adaxial surface of the leaf was measured with a quantum sensor (Model Li-190SB, Licor Inc., Lincoln NE, USA).
Diurnal variation in leaf hydraulic conductance (k leaf; mmol m–2 s–1 MPa–1) for sun-exposed leaves was measured on four to five individuals of each species using the in situ evaporative flux method (in situ EFM), with k leaf calculated as (Brodribb and Holbrook, 2003):
| (1) |
where E is the transpiration rate (mmol m–2 s–1), and ΔΨstem–leaf is the difference between stem xylem water potential (Ψstem; MPa) and leaf water potential (Ψleaf; MPa). This in situ technique required sampling two adjacent leaves, one of which was used to measure Ψstem whereas the adjacent leaf was sampled for E and Ψleaf. Leaves used as an assay for Ψstem were covered in plastic film and aluminium foil on the evening before the measurement period to ensure equilibration between the covered Ψleaf and Ψstem. Transpiration rate (E) was measured with the Li-1600 porometer. Owing to the open crown structure of the saplings, the wide spacing between trees, and the windy ridge top exposure of the common garden, it was assumed that leaf boundary layer conductance (g b) was much greater than g s. Additionally, during each measurement of E, leaf orientation, ambient humidity, and radiation interception was conserved. Therefore, E measured by the Li-1600 was likely to be similar to the actual E immediately before the measurement. While measuring E, the water potential of the adjacent covered leaf was sampled as a proxy for Ψstem. Immediately following determination of E, the uncovered leaf was excised, wrapped in plastic, and placed in a Scholander-type pressure chamber for determination of Ψleaf (Soil Moisture Equipment Corp., Santa Barbara CA, USA). Balancing pressure was recorded when xylem sap reached the cut stem surface, as verified by a dissecting scope at ×25 magnification. E, Ψstem, and Ψleaf were measured every ~2.5h over the course of a 14–18h period, beginning at pre-dawn (0400–0500h). Regression analysis was used to evaluate the co-variation between ΔΨstem–leaf, E, g s, and k leaf over a diurnal cycle of evaporative demand. Regression analyses were performed using SigmaPlot (Version 11; Systat Software Inc., San Jose, CA, USA).
Growth chamber experiment
ΔΨstem–leaf, E, g s, A, and k leaf were measured at two light levels (509±11.5 and 1310±26.4 µmol m–2 s–1) for Helianthus annuus plants grown under sub-ambient (194±35 ppm), ambient (450±46 ppm), and elevated CO2 (1027±74 ppm). H. annuus plants were grown in growth chambers located in the Controlled Environment Facility at the Center for Carbon, Water, and Food at the University of Sydney. Ten plants were grown under each CO2 concentration at 900±50 µmol m–2 s–1 of photosynthetically active radiation (PAR). Ambient CO2 concentrations were monitored using an isotope ratio infrared spectrometer (G1101-i, Picarro, CA, USA) that cycled through each room every 10min. Using the method outlined above, g s and E were measured with a portable photosynthesis system fitted with a large leaf cuvette that enclosed the entire leaf (Walz-USA, Pepperell MA, USA). Water potential was measured using a Scholander-style pressure chamber (Soil Moisture Equipment Co., Santa Barbara CA, USA) and k leaf was calculated from Eqn 1. Measurements were taken after the leaves were in the cuvette for ~40min during stable g s, E, A, and T leaf. After the 40min period leaves were cut from the stem, removed from the cuvette, and covered in plastic for water potential measurements with the Scholander-style pressure chamber as described above. Projected leaf area was measured digitally using the software program Image J (US National Institutes of Health, Bethesda, Md). Two leaves were used as an assay for Ψstem using the method outlined above. Leaves directly above and below the leaf in the cuvette were selected and the average of the two water potential measurements was taken as Ψstem. In all cases the distal leaf showed a slightly more negative Ψstem, ~0.02–0.04MPa. Measurements of Ψleaf, E, g s, A, and k leaf were made at 450 ppm CO2 inside the cuvette at two light levels, ~500 and 1300 µmol m–2 s–1 PAR, and a relatively constant leaf temperature of 24.7±0.2 °C by varying air temperature and ambient humidity inside the cuvette. In total, five leaves from each CO2 growth environment were measured at each light level (~500 and 1300 µmol m–2 s–1 PAR), all at 450 ppm CO2. All statistical analyses were performed using SigmaPlot and JMP (v.4.0.4; SAS Institute, Cary, NC, USA). ANCOVA was used to test for main and interactive effects of growth CO2 (low, medium, and high), light (low, high), and VPD on gs and A. An ANCOVA was also used to test for main and interactive effects of CO2 (low, medium and high), light (low, high), and E (covariate) on k leaf. Because measurements within the light treatment were done on the same plant, plants nested within the light treatment were used as a random factor (Quinn and Keough, 2002). Assumptions of normality were met.
Estimation of k leaf when E=0
As shown by Eqn 1, using the in situ EFM technique to measure k leaf requires an evaporative flux and a water potential drawdown which in turn prevents direct calculation of k leaf when E=0. However, theory predicts that when E=0, k leaf will have some finite value. By measuring k leaf across a broad range of E a linear model can be used to extrapolate to k leaf at zero E (i.e. the static conductance, k leaf(0)). Linear regression was used to estimate k leaf(0) for both gymnosperm and angiosperm trees from the common garden and H. annuus plants from the growth chamber experiment.
Results
Common garden
A strong non-linear relationship was observed between ΔΨstem–leaf and E for both the gymnosperm (Fig. 2) and angiosperm species (Fig. 3) from the common garden. Although the maximum transpiration rates for the gymnosperm species were lower than the angiosperm species (Table 1), on average, the co-variation between ΔΨstem–leaf and E was similar between species (gymnosperms, y=0.18x 0.35, r2=0.44, P<0.001; angiosperms, y=0.14x 0.48, r2=0.69, P<0.001), with angiosperms attaining higher overall ΔΨstem–leaf and E (Fig. 4). Assuming liquid fluxes into the leaf and vapour phase fluxes from the leaf were in steady-state, the observed correlation between Ψstem–leaf and E was the result of a strong coupling between k leaf and E (Table 1). A significant positive relationship was observed between k leaf and E across the gymnosperm and angiosperm species from the common garden (Figs 2, 3, 5). For each species the y-intercept of the linear model describing the co-variation between k leaf and E was greater than 0 (Figs 2, 3; Table 1). This static leaf hydraulic conductance when E=0 (k leaf(0)), varied greatly between angiosperm and gymnosperm species (Figs 2, 3; Table 1), with angiosperms having a higher k leaf(0) (Fig. 5; Table 1). For the plants growing in the common garden, a higher k leaf(0) was associated with greater maximum daytime g s, k leaf, and lower dk leaf/dE (Fig. 6; Table 1). The angiosperm species from the common garden operated at a higher day time maximum g s and ΔΨstem–leaf than the gymnosperm species (Figs 4, 6; Table 1).
Fig. 2.
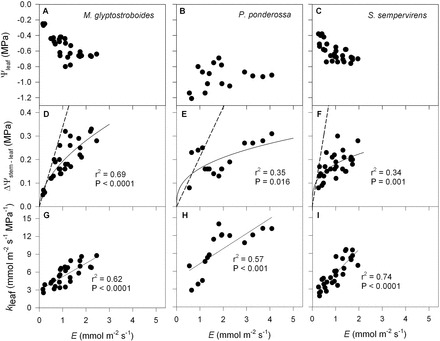
Variation in leaf water potential (Ψleaf, MPa), the difference between stem and leaf water potential (ΔΨstem–leaf, MPa), and leaf hydraulic conductance (k leaf, mmol m–2 s–1 MPa–1) as a function of transpiration rate (E, mmol m–2 s–1) for the three gymnosperm species from the common garden: M. glyptostroboides (A, D, G); P. ponderosa (B, E, H); and S. sempervirens (C, F, I). The solid black line in each panel represent the best-fit model describing the coordination between Ψstem–leaf and k leaf with variation in E. The dashed lines in panels D, E, and F represent the predicted changes in ΔΨstem–leaf for a leaf that possesses the average static leaf hydraulic conductance (i.e. k leaf(0)) for each individual species.. The coefficient of determination (r2) and significance (P) in each panel refer to the solid lines.
Fig. 3.
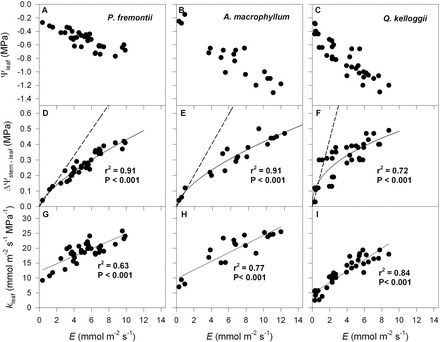
Variation in leaf water potential (Ψleaf, MPa), the difference between stem and leaf water potential (ΔΨstem–leaf, MPa), and leaf hydraulic conductance (k leaf, mmol m–2 s–1 MPa–1) as a function of transpiration rate (E, mmol m–2 s–1) for the three angiosperm species from the common garden: P. fremontii (A, D, G); A. macrophyllum (B, E, H); and Q. kelloggii (C, F, I). Solid and dashed lines, as well as r2 and P values are as for Fig. 2.
Table 1.
Daytime maximum stomatal conducatance (gs) and transpiration (E)±1 standard deviation for study species from the common garden
The r 2 for the linear model describing the co-variation between k leaf and E including the y-intercept (k leaf(0)) and gain (dk leaf/dE); for every species (P<0.001, for all species).
| Species | g s,max (mol m–2 s–1) | E max (mmol m–2 s–1) | k leaf(0) (mmol m–2 s–1 MPa–1) | k leaf(E) (mmol m–2 s–1 MPa–1) | dk leaf/dE (MPa–1) | k leaf vs E | |
|---|---|---|---|---|---|---|---|
| Angiosperms | Acer macrophyllum | 0.47±0.13 | 11.2±1.55 | 9.77±1.43 | 25±0.48 | 1.45±0.19 | r2=0.77 |
| Populus fremontii | 0.54±0.07 | 9.38±2.75 | 12.23±0.93 | 25.39±4.85 | 1.26±0.16 | r2=0.63 | |
| Quercus kelloggii | 0.27±0.07 | 5.18±1.15 | 4.66±0.65 | 17.83±1.41 | 1.91±0.14 | r2=0.84 | |
| Gymnosperms | Metasequoia glyptostroboides | 0.12±0.05 | 1.93±0.42 | 2.95±0.44 | 8.01±1.20 | 2.26±0.35 | r2=0.62 |
| Pinus ponderosa | 0.19±0.05 | 2.79±0.44 | 4.73±1.28 | 14.61±3.38 | 2.53±0.58 | r2=0.57 | |
| Sequoia sempervirens | 0.15±0.03 | 1.53±0.47 | 1.63±0.55 | 9.38±1.79 | 4.15±0.48 | r2=0.74 |
Fig. 4.
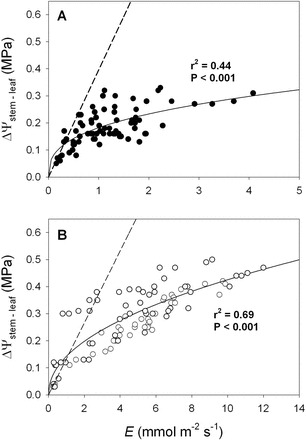
The difference between stem and leaf water potential (ΔΨstem–leaf, MPa) as a function of transpiration rate (E, mmol m–2 s–1) for: (A) gymnosperm and (B) angiosperm tree species from the common garden. Solid lines, as well as r 2 and P values are as for Fig. 2. The dashed lines represent the predicted changes in ΔΨstem–leaf for a leaf that possesses the average static leaf hydraulic conductance (i.e. k leaf(0)) for the (A) gymnosperm and (B) angiosperm species.
Fig. 5.
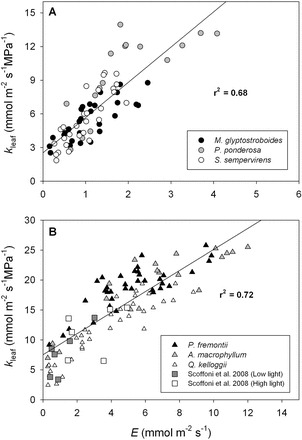
Leaf hydraulic conductance (k leaf, mmol m–2 s–1 MPa–1) as a function of transpiration rate (E, mmol m–2 s–1) for: (A) gymnosperm and (B) angiosperm tree species from the common garden. Previously published data taken from Table 2 in Scoffoni et al. (2008) is also shown as filled and open squares in B. Filled squares represent the k leaf values taken when leaves were exposed to low light (<10 µmol m–2 s–1), whereas the open squares represent the k leaf values when leaves were exposed to high light (>1000 µmol m–2 s–1; See Scoffoni et al. (2008) for further details). The solid line in each panel was fitted by linear regression through all the data, excluding the data by Scoffoni et al. (2008).
Fig. 6.
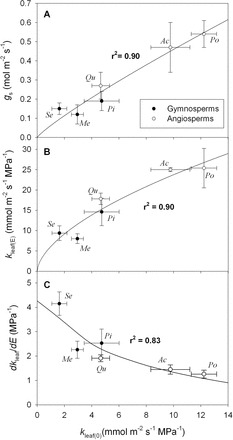
The influence of static leaf hydraulic conductance (k leaf(0), mmol m–2 s–1 MPa–1) on (A) maximum daytime stomatal conductance (g s, mol m–2 s–1); (B) maximum daytime leaf hydraulic conductance (k leaf(E), mmol m–2 s–1 MPa–1), and (C) the slope of the linear relationship between k leaf(E) and E (i.e. the hydraulic gain) for three gymnosperm and three angiosperm species. Se, Me, Pi, Qu, Ac and Po are Sequoia sempervirens, Metasequoia glyptostroboides, Pinus ponderosa, Quercus kelloggii, Acer macrophyllum, and Populus fremontii, respectively. Solid lines represent the best-fit model describing the coordination between g s, k leaf(E) and dk leaf/dE with variation in k leaf (0).
Qualitatively similar diurnal trends in g s, E, Ψleaf, Ψstem, and k leaf were observed for the angiosperm and gymnosperm species grown in the common garden. In general, from dawn to midday, Ψleaf and Ψstem decreased while g s, E, and k leaf increased (Supplementary Figs S1 and S2). After midday, Ψleaf and Ψstem increased slightly, whereas g s, E, and k leaf decreased (Supplementary Figs S1 and S2). Overall, plants in the common garden were well hydrated, with Ψstem and Ψleaf greater than –0.8 and –1.2MPa, respectively. The diurnal changes in Ψleaf and Ψstem followed a similar pattern such that ΔΨstem-leaf changed very little over the course of a day (Supplementary Figs S1 and S2). On average the angiosperm species showed greater diurnal variation in g s, E, and k leaf compared with the gymnosperm species (Supplementary Figs S1 and S2).
Growth chamber
Across all three CO2 treatments a similar range of E, VPD, A, T leaf, and g s, occurred at each light level (Fig. 7A–E). Overall, PAR had the greatest significant effect on g s (F=31.85, P<0.001) followed by VPD (F=8.55, P=0.012) and CO2 (F=7.7281, P=0.0129) with no significant interaction between PAR, CO2, and VPD. Across CO2 treatments, increases in PAR were associated with greater g s (t=5.64, P<0.001; Fig. 7E). On average, plants grown under sub-ambient CO2 operated at higher g s than plants from elevated CO2 (t=3.95, P=0.002) but were not statistically different from plants grown under ambient CO2 (t=1.89, P=0.08). Although VPD showed a significant main effect on g s, correlations between g s and VPD within each CO2 treatment were not significantly different, which was probably due to the small range of VPD and limited number of measurements at different VPD (n=5) at a given light level. Both PAR (F=31.49, P<0.001) and CO2 (F=9.76, P<0.002) significantly influenced variation in A. Across treatments, increasing PAR had a positive effect on A (t=5.612, P<0.001). Similar to g s, when measured at the same atmospheric CO2 concentration (450 ppm), plants from the sub-ambient CO2 treatment showed higher A than plants from the elevated CO2 treatment (t=4.24, P=0.001) but were not statistically different from the ambient CO2 treatment (t=0.89, P=0.39). Using an ANCOVA to test for main and interactive effects of CO2, light, and E on k leaf, it was found that E had the only significant effect on k leaf (F=16.1945, P=0.0027).
Fig. 7.
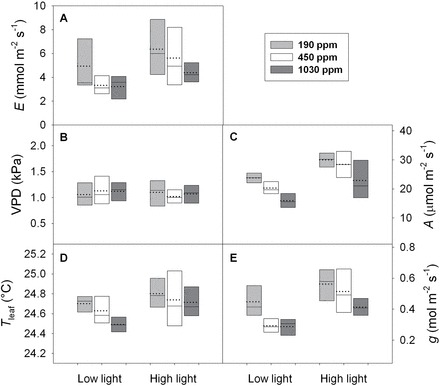
The range (indicted by boxes), median (horizontal line within boxes), and mean (dotted line within boxes) of: (A) transpiration rate (E, mmol m–2 s–1), (B) leaf-to-air vapour pressure difference (VPD, kPa), (C) CO2 assimilation rate (A, µmol m–2 s–1), (D) leaf temperature (T leaf, °C), and (E) leaf surface conductance, comprising the sum of stomatal and boundary layer conductances g s and g b (g, mol m–2 s–1) during measurements of leaf hydraulic conductance (k leaf) at both low (500 µmol m–2 s–1 PAR) and high light (1300 µmol m–2 s–1 PAR), for H. annus plants grown under ~190 (grey boxplot), 450 (open boxplot), and 1030 ppm CO2 (dark grey boxplot). Note that here, g b is considered sufficiently high such that g ≈g s.
Across all CO2 treatments an increase in E was associated with a decrease in Ψleaf, although the decreases in Ψleaf were relatively minor despite relatively large variation in E (Fig. 8A). Similar to plants from the common garden, a strong non-linear relationship between ΔΨstem–leaf and E was observed across all three growth CO2 treatments (y=0.106×E 0.507, r 2=0.77, P<0.001; Fig. 8B; assuming that the when E=0, ΔΨstem–leaf=0). This was the result of a significant positive relationship between k leaf and E across all three CO2 treatments (y=9.70 + 2.36×E, r 2=0.72; Fig. 9B). The lack of interactive effects between E and CO2 on k leaf suggests that k leaf(0) was not significantly influenced by growth CO2 (Table 2). Similarly, k leaf per unit g s and A were relatively unaffected by PAR, but were significantly, positively correlated with VPD (k leaf/g vs. VPD: y=0.0281×e (0.5355×VPD), r 2=0.23, P=0.009; k leaf/A vs. VPD: y=0.4837×e (0.4634×VPD), r 2=0.30, P=0.002).
Fig. 8.
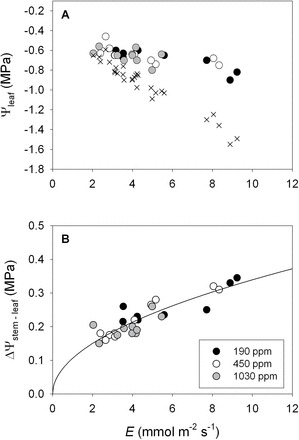
The influence of variation in transpiration rate (E, mmol m–2 s–1) on (A) leaf water potential and (B) the difference between stem and leaf water potential (ΔΨstem–leaf, MPa) for: H. annus plants grown under ~190 (filled circles), 450 (open circles), and 1030 ppm CO2 (grey circles). The crosses in A represent the predicted leaf water potentials for a leaf that possesses the average static leaf hydraulic conductance (i.e. k leaf(0)=9.08 mmol m–2 s–1 MPa–1) across all three CO2 treatments. The solid black line in panel (B) represents the best-fit model describing the coordination between ΔΨstem–leaf and E.
Fig. 9.
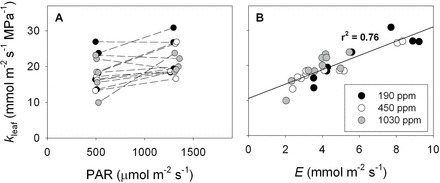
The influence of variation in: (A) photosynthetically active radiation (PAR, µmol m–2 s–1) and (B) transpiration rate (E, mmol m–2 s–1) on leaf hydraulic conductance (k leaf, mmol m–2 s–1 MPa–1). The dotted lines in A show the ‘light–g-effect’ on k leaf for leaves at a common leaf to air vapour pressure difference (VPD). In all cases where greater light interception resulted in an increase in k leaf, at a common VPD, there was a light induced increase in g and by extension E.
Table 2.
Mean stomatal conducatance (g s) and transpiration (E)±1 standard deviation for H. annus plants grown under ~190, 450, and 1030 ppm CO2. Also shown are the hydraulic gain (dkleaf/dE) and the y-intercept (kleaf(0)) and r2 for the linear model describing the co-variation between kleaf and E (kleaf vs E)*P < 0.001; **P < 0.01.
| Growth CO2 (ppm) | Mean g s (mol m–2 s–1) | Mean E (mmol m–2 s–1) | k leaf(0) (mmol m–2 s–1 MPa–1) | dk leaf/dE (MPa–1) | k leaf vs E |
|---|---|---|---|---|---|
| 190±35 | 0.50±0.12 | 5.57±2.41 | 9.75±2.44 | 2.12±0.41 | r2=0.80* |
| 450±46 | 0.40±0.15 | 0.54±0.07 | 9.38±2.75 | 12.23±0.93 | r2=0.91* |
| 1030±74 | 0.35±0.09 | 0.27±0.07 | 5.18±1.15 | 4.66±0.65 | r2=0.70** |
Discussion
The data presented here do not support a constant k leaf model, under well-watered conditions, and instead are consistent with a hydraulic mechanism whereby k leaf increases with E. Additionally, here it is shown that a positive dependence of k leaf on E results in a dynamic coupling between k leaf and g s that ultimately minimizes the water potential drawdown across the leaf (i.e. ΔΨstem–leaf) which, via the hydraulic feedback loop, increases the maximum potential g s and A for a given VPD (Fig. 10).
Static vs dynamic leaf hydraulic conductance
At steady-state, the coupling between liquid and vapour-phase water flux can be described as:
| (2) |
where g is the sum of stomatal (g s) and boundary layer conductance (g b) to water vapour in series, w i and w a are the mole fractions of water vapour (mol mol–1) inside the leaf and of the ambient atmosphere, e i and e a are the vapour pressures of water inside the leaf and in ambient air, and P is atmospheric pressure. The term (e i–e a)/P is commonly referred to as ‘VPD’. According to this steady-state description, when g s<<g b, up- or down-regulation of E via feedback responses of stomata to VPD can occur via changes in ΔΨstem–leaf, k leaf, or both.
If k leaf is static or only decreases as E increases then changes in g s and E, before water-stressed-induced xylem embolism, would require large changes in ΔΨstem–leaf and ultimately a positive linear correlation between ΔΨstem–leaf and g(w i – w a) or E (Fig. 1). From Eqn 2, when k leaf is static, the ratio g(w i–w a)/ΔΨleaf remains constant. Eventually, increases in g(w i–w a) may lower leaf water potential sufficiently to induce cavitation and embolisms, reducing k leaf and resulting in a positive feedback on ΔΨstem–leaf (Sperry, 2000). In this scenario g(w i–w a)/ΔΨleaf is negatively correlated with E. Because increasing ΔΨstem–leaf reduces maximum potential g and by extension CO2 assimilation rate (Buckley, 2005; Franks et al., 2007), a control system that relies solely on a constant or decreasing k leaf constrains carbon gain to occur within a relatively narrow range of low evaporative demand and high water availability. One way to avoid large drops in Ψleaf and g over a broad range of evaporative demand is to vary k leaf positively with E. This dynamic coupling between E and k leaf is represented here by the term k leaf(E), which is the leaf hydraulic conductance for a given magnitude of E. Here, k leaf(E) represents the dynamic hydraulic conductance.
Under relatively well-watered conditions no support was found for the hypothesis that g(w i–w a)/ΔΨleaf is constant or decreases with E. In fact the opposite relationship was observed: as E increased g(w i–w a)/ΔΨleaf increased (e.g. Figs 2, 3 and 8). As shown by Eqn 2, a positive dependence of k leaf on E can lead to increasing g(w i–w a)/ΔΨleaf as E increases. Therefore, these results provide strong evidence that the relationship between k leaf, g s, and CO2 assimilation rate, in response to short-term changes in evaporative demand (VPD), is the result of a positive dependence of k leaf on E (Fig. 10).
Co-variation between ΔΨstem–leaf, E, g s, and k leaf over a diurnal cycle of evaporative demand
Previous research has provided evidence that diurnal variation in k leaf can be partially attributed to circadian regulation (Nardini et al., 2005b ; Lo Gullo et al., 2005). Here evidence is provided that a positive correlation between k leaf and E is another factor influencing the coupling between k leaf and g s over a diurnal cycle of evaporative demand. Despite relatively large diurnal variation in g s and E for the gymnosperm and angiosperm species growing in a common garden, only minor variation was observed in ΔΨstem–leaf across a large range in E. Until now, isohydrodynamic behaviour, or a relatively constant water potential gradient, has only been explained by a mechanism occurring at the whole plant level, from root to leaf, over seasonal changes in soil water availability (Franks et al., 2007). The data presented here suggest that, under well-watered conditions, isohydrodynamic behaviour is common at the leaf level (e.g. Figs 2, 3, 8b). As described by Eqn 1 and 2, a minor variation in ΔΨstem–leaf over a large diurnal range in E and g s, can occur if k leaf is positively dependent on E.
Similar to an electrical circuit that maintains an electrical conductance even when there is no current, if a hydraulic connection exists between plants and the atmosphere then leaves will maintain the capacity to transport water, even when E=0. In other words, whether k leaf is dynamically coupled to E (k leaf(E)) or static, it has a finite value when E=0, i.e. k leaf(0) (see Methods). Here it is shown that, for the well-watered gymnosperm and angiosperm tree species in the common garden, this inherent capacity to transport water is greater for the angiosperm species than the gymnosperms (Fig. 6), and positively correlated with daytime maximum stomatal conductance (Fig. 6A). These patterns are consistent with the well-documented ‘coordination’ of hydraulic and gas exchange capacity across species (e.g. Meinzer and Grantz, 1990; Meinzer and Grantz, 1991; Sperry and Pockman, 1993; Winkel and Rambal, 1993; Meinzer et al., 1995; Andrade et al., 1998; Maherali et al., 1997; Mencuccini and Comstock, 1999; Mencuccini, 2003; Brodribb et al., 2005).
Influence of atmospheric CO2 on the co-variation between ΔΨstem–leaf, E, g s, A, and k leaf
Across all three growth CO2 treatments a strong non-linear relationship was observed between ΔΨstem–leaf and E where g(w i–w a)/ΔΨleaf increased as E increased. As with plants from the common garden experiment, this trend can be attributed to a positive correlation between k leaf and E which was relatively decoupled from variation in PAR (Fig. 9). Recent research relying on the evaporative flux method provides further evidence that k leaf can be up-regulated as transpiration increases (e.g. Scoffoni et al., 2008; Guyot et al., 2011). In this previous work, unlike the present evaluation, increases in transpiration rate were driven by a g s light response. As reported here, a positive dependence of k leaf on E can occur independent of variation in light availability. This suggests that an alternative mechanism is necessary to describe the coordination between k leaf and light. For example, an isohydroynamic model predicts greater k leaf as light interception increases if increased energy absorption results in greater E (see Figs 9 and 10).
Fig. 10.
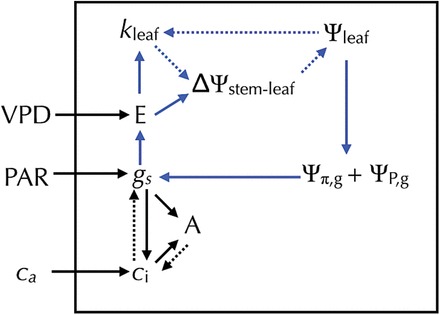
Model diagram showing the coordination between transpiration rate (E), the difference between stem and leaf water potential (ΔΨstem–leaf), leaf hydraulic conductance (k leaf), stomatal conductance (g s, where boundary layer conductance g b is sufficiently high for g s to dominate), and CO2 assimilation rate (A). The blue lines represent the hydraulic feedback loop between g s and E. Solid lines represent a positive relationship between parameters and dotted lines represent a negative relationship. The positive relationship between E and k leaf is the predicted relationship based on data gathered from H. annuus plants grown under low, medium, and high CO2 concentrations, and three gymnosperm and angiosperm tree species growing in a common garden (see Methods for more detail). The black box indicates the boundary between leaf processes and external environmental variables. (VPD, leaf-to-air vapour pressure difference; PAR, photosynthetically active radiation; C a, atmospheric CO2 concentration, C i, leaf internal CO2 concentration, Ψπ,g, is the guard cell osmotic pressure, and ΨP,g, guard cell turgor pressure).
The positive linear model describing the relationship between k leaf and E was similar across the CO2 treatments such that k leaf(0) was relatively conserved between CO2 treatments (Fig. 9B, Table 2). However, variation in growth CO2 influenced where plants operated along the linear model describing the co-variation between k leaf and E, with a lower maximum k leaf and E at high ambient CO2. Corresponding with this, average g s was lower in the elevated CO2 treatment relative to the sub-ambient CO2 treatment, consistent with many studies on plants growing under different atmospheric CO2 concentrations (e.g. Morison and Gifford, 1983; Cure and Acock, 1986; Tolley and Strain, 1985; Morison and Lawlor, 1999).
The relative stability of k leaf(0) across sub-ambient, ambient, and elevated CO2 treatments despite a significant decrease in g s between sub-ambient and elevated CO2 is consistent with previous research. Across species, the sensitivity of maximum stomatal conductance (g s(max)) to variation in atmospheric CO2 (c a) seems to be strongly non-linear whereby the sensitivity of g s(max) to changes in c a increases at low c a (Beerling and Woodward, 1997; Franks and Beerling, 2009; Franks et al., 2012). Recent research also suggests that the relative differences in g s between plants grown under sub-ambient, ambient, and elevated CO2 is less for plant species that possess an inherently high g s (Franks et al., 2012). Additionally, across species, there is a strong non-linear relationship between g s and k leaf, when measured at a common VPD, whereby dg s/dk leaf increases as g s increases (Franks, 2006). Taken together, this previous research suggests that plants with an inherently high g s and k leaf will show relatively minor adjustments in g s(max) and k leaf(0) when exposed to elevated CO2, as shown here with the H. annuus plants. Similarly, recent research on soybean suggests that k leaf is relatively insensitive to elevated CO2 (700 ppm) despite decreases in g s at elevated CO2 (Locke et al., 2013). Further research, including more species and greater ranges of CO2, is necessary to better understand the influence of elevated atmospheric CO2 on the coordination between E, k leaf, gs, and A.
The hydraulic gain, dk leaf/dE, and the sensitivity of g s to E
Previous research has clearly demonstrated a hydromechanical basis for stomatal movement whereby changes in the maximum potential aperture of stomata and by extension g s are strongly influenced by changes in bulk leaf water status i.e. Ψleaf (see reviews by Franks, 2004; Buckley, 2005). This hydraulic coupling between maximum potential g s and Ψleaf results in a hydromechanical control system that is strongly influenced by both E and k leaf. For example, a hydromechanical stomatal control system that includes a positive dependence of k leaf on E will reduce the sensitivity of Ψleaf to variation in E, when compared with a constant k leaf (e.g. Fig. 8A) This can be shown mathematically by: for a dynamic k leaf model compared with for a static k leaf model. This damping of variation in Ψleaf as E changes is expected to reduce the sensitivity of g s to changes in VPD via the hydraulic feedback loop (Fig. 10). In other words, the rate and direction of change in the ratio of k leaf on E (here this is termed the hydraulic gain; dk leaf/dE) is a good index of the sensitivity of leaf water status (i.e. Ψleaf) and g s to a change in E.
Using a hydromechanical model of g s, originally developed by Franks and Farquhar (1999) and further modified by Franks et al. (2007) to accommodate isohydrodynamic behaviour (i.e. decrease in ΔΨstem–leaf/E, as E increases), the impact of a dynamic conductance (k leaf(E)), as compared with a static k leaf, on the sensitivity of g s to changes in VPD was evaluated. The model output suggests that, for well-hydrated plants with a fixed Ψstem, a positive dependence of k leaf on E reduces the sensitivity of g s to variation in VPD when compared with a constant k leaf (Fig. 11). Previous research has provided strong empirical evidence that stomatal sensitivity to VPD is positively correlated with the daytime operating g s under well watered conditions at low VPD, i.e.<1kPa (e.g. Oren et al., 1999). Similarly, across species from the common garden a strong negative correlation was observed between dk leaf/dE and k leaf(0), with k leaf(0) positively correlated with daytime maximum g s (Fig. 6A, C). Taken together, the steady-state stomatal feedback control model proposed by Franks et al. (2007) and the negative correlation between dk leaf/dE and k leaf(0) observed here provide a mechanistic framework for evaluating empirical correlations between stomatal sensitivity to VPD and daytime operating g s under well-watered conditions at low VPD. Further research is needed to better characterize the coordination between k leaf(0), dk leaf/dE, maximum g s, and stomatal sensitivity to VPD across plants spanning a wide range in maximum potential g s.
Fig. 11.
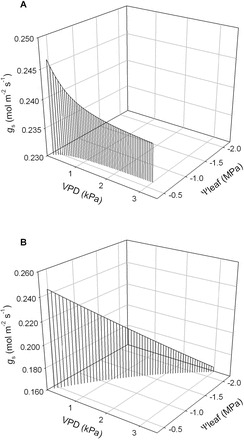
Model simulations comparing the relationship between leaf water potential (Ψleaf), leaf to air vapour pressure difference (VPD), and stomatal conductance (g s) when: (A) leaf hydraulic conductance (k leaf) is positively dependent on transpiration rate (E), based on the empirical relationship observed across the gymnosperm species in the common garden experiment (see Fig. 5) where k leaf=2.5+3.2×E, and (B) a constant k leaf based on the y-intercept of the combined gymnosperm data (i.e. k leaf(0)=2.5). Note that in A, Ψleaf remains high as VPD increases, whereas in (B) Ψleaf declines substantially with increasing VPD.
Possible processes underlying the variable k leaf mechanism
It is now well recognized that k leaf and leaf gas exchange are strongly influenced by variation in leaf vein traits (e.g. Brodribb et al., 2007; Blonder et al., 2011; Sack and Scoffoni, 2013). Although changes in xylem structure will directly impact the inherent hydraulic capacity of a leaf (e.g. k leaf(0)) it is unlikely that short-term increases in E and k leaf, when leaves are well-hydrated, are driven by up-regulation of xylem hydraulic conductance alone. Changes in the ion concentrations of stem xylem sap have been shown to significantly influence stem hydraulic conductance (e.g. Zwieniecki et al., 2001; Nardini et al., 2011). Yet, to date, this ion effect has not been found in leaves (Sack et al., 2004). Instead, leaf xylem hydraulic conductance, prior to any form of xylem dysfunction, is relatively constant and independent of short-term variation in evaporative demand, excluding any temperature effects on viscosity.
In contrast to that of the leaf xylem, there is growing evidence that the hydraulic conductance of the extra-xylem pathway can ramp up or down over relatively short time scales in response to changes in a particular environmental cue, such as light, temperature or water availability (e.g. Kikuta et al., 1997; Sôber, 1997; Matzner and Comstock, 2001; Lo Gullo et al., 2005; Sellin and Kupper, 2005a,b ; Cochard et al., 2007; Sellin and Kupper, 2007; Scoffoni et al., 2008; Sellin et al., 2008; Johnson et al., 2009; Pantin et al., 2012). However, as changes in light and temperature can have direct effects on E, the data presented here suggests that a similar range of E should be maintained when testing for light and temperature effects on the hydraulic conductance of the extra-xylem pathway independent of variation in E. To date, the relative contribution of symplastic, transcellular, and apoplastic water transport through the extra-xylary tissues is still under debate (e.g. Tyree and Cheung, 1977; Tyree et al., 1981; Johansson et al., 1996; Morillon and Chrispeels, 2001; Aasamaa et al., 2005; Aroca et al., 2006; Cochard et al., 2004; Cochard et al., 2007; Kim and Steudle, 2007; Sellin et al., 2008; Baaziz et al., 2012; Rockwell et al., 2014), as is the extent to which the different live tissues of a leaf (e.g. palisade and spongy mesophyll, bundle sheath, epidermis) participate in the transpiration stream (e.g. Zwieniecki et al., 2007; Canny et al., 2012).
Clearly one of the missing pieces of the puzzle is the location of the site(s) of evaporation inside the leaf (Pieruschka et al., 2010; Peak and Mott, 2011). It is difficult to evaluate water transport through the extra-xylary pathways in the leaf if there is no clear understanding of where the liquid flow path ends and whether or not the locations of these evaporation sites vary with changes in the absolute rate of leaf water loss (i.e. E). For example, recent research suggests that vapour transport through the intercellular air spaces can account for a substantial amount of water transport between mesophyll cells and thus the hydraulic conductance of the extra-xylary component (Rockwell et al., 2014; Buckley, 2014). Additionally, changes in E may shift the depth of the evaporation front within leaves (Rockwell et al., 2014; Buckley, 2014) and alter the relative contribution of liquid and vapour transport through these parallel pathways within the mesophyll. Characterizing where the evaporation sites occur in the leaf is needed to fully understand how water transport through the mesophyll is partitioned between these parallels pathways (i.e. liquid–apoplastic, symplastic, transcellular; vapour–intercellular air spaces).
Conclusions
The results presented here suggest that when plants are well-hydrated, k leaf does not remain fixed or decrease as E increases, but rather increases with E. Here, this dynamic k leaf is referred to as k leaf(E), which incorporates the inherent k leaf at zero E, k leaf(0). This positive dependence of k leaf on E tends to minimize or reduce water potential gradients along the soil–plant–atmosphere continuum. Minimizing variation in ΔΨstem–leaf over a broad range of E (i.e. maintaining isohydrodynamic conditions), and therefore maximizing leaf water content (LWC), has many potential implications for whole plant carbon balance. It is well recognized that decreases in Ψleaf and LWC can increase stomatal and biochemical limitations to CO2 assimilation rate (A) and thus decrease potential A for given environmental conditions (Lawlor, 2002; Lawlor and Cornic, 2002). A positive dependence of k leaf on E will ultimately increase the range of stem water potentials where leaves can maintain water potential above the turgor loss point, supporting high LWC, g s, and A. This mechanism will dominate only while the xylem remains hydraulically intact, i.e. in well-hydrated leaves. As Ψleaf falls below the cavitation threshold the subsequent drop in k xylem will dominate and the leaf will exhibit the classical pattern of falling E with declining k leaf. Minimizing ΔΨstem–leaf avoids other negative consequences of excessive water potential gradients such as reduced rates of export of photoassimilates from leaves (Nikinmaa et al., 2013; Turgeon, 2010; Hölttä et al., 2009; Hölttä et al. 2006; Thompson and Holbrook, 2004). The dynamic nature of k leaf is therefore integral to many aspects of plant water use and productivity and should be considered in mechanistic vegetation models.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Diurnal variation in photosynthetically active radiation (PAR, µmol m–2 s–1), stomatal conductance (gs, mol m–2 s–1), stem and leaf water potential (Ψstem and Ψleaf, MPa), leaf hydraulic conductance (kleaf, mmol m–2 s–1) and transpiration rate (E, mmol m–2 s–1) for three angiosperm species growing in a common garden.
Figure S2. Diurnal variation in photosynthetically active radiation (PAR, µmol m–2 s–1), stomatal conducatance (gs, mol m–2 s–1), stem and leaf water potential (Ψstem and Ψleaf, MPa), leaf hydraulic conductance (kleaf, mmol m–2 s–1), and transpiration rate (E, mmol m–2 s–1) for three gymnosperm species growing in a common garden.
Acknowledgements
We thank UC Berkeley Botanical Garden for providing the space necessary to conduct the field experiments. We thank Greg Goldsmith, Adam Roddy and Michal Shuldman for their help with measurements at the Berkeley Botanical Garden and Feike Dijkstra for comments that greatly improved the manuscript. We also thank the anonymous reviewers for the valuable feedback. Financial support was provided by a UC Berkeley BASC grant (UC Berkeley) awarded to KS.
References
- Aasamaa K, Niinemets Ü, Sõber A. 2005. Leaf hydraulic conductance in relation to anatomical and functional traits during Populous tremula leaf ontogeny. Tree Physiology 25, 1409–1418. [DOI] [PubMed] [Google Scholar]
- Andrade JL, Meinzer FC, Goldstein G, Holbrook NM, Cavelier J, Jackson P, Silvera K. 1998. Regulation of water flux through trunks, branches and leaves in tress of a lowland tropical forest. Oecologia 115, 463–471. [DOI] [PubMed] [Google Scholar]
- Aroca R, Ferrante A, Vernieri P, Chrispeels MJ. 2006. Drought, abscisic acid, and transpiration rate effects on the regulation of PIP aquaporin gene expression and abundance in Phaseolus vulgaris plants. Annals of Botany 98, 1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baaziz KB, Lopez D, Rabot A, Combes D, Gousset A, Bouzid S, Cochard H, Sakr S, Venisse J-S. 2012. Light mediated K leaf induction and contribution of both the PIP1s and PIP2s aquaporins in five tree species: walnut (Juglans regia) case study. Tree Physiology 32, 423–434. [DOI] [PubMed] [Google Scholar]
- Beerling DJ, Woodward FI. 1997. Changes in land plant function over the Phanerozoic: reconstructions based on the fossil record. Botonical Journal of the Linnean Society 124, 137–153. [Google Scholar]
- Black CR. 1979. The relationship between transpiration rate, water potential, and resistances to water movement in Sunflower (Helianthus annuus L.) Journal of Experimental Botany 30, 235–243. [Google Scholar]
- Blackman CJ, Brodribb TJ, Jordan GJ. 2009. Leaf hydraulics and drought stress: response, recovery and survivorship in four woody temperate plant species. Plant, Cell and Environment 32, 1584–1595. [DOI] [PubMed] [Google Scholar]
- Blonder B, Violle C, Bentley LP, Enquist BJ. 2011. Venation networks and the origin of the leaf economics spectrum. Ecology Letters 14, 91–100. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Feild TS, Jordan GJ. 2007. Leaf maximum phosotynthetic rate and venation are linked by hydraulics. Plant Physiology 144, 1890–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Holbrook NM. 2003. Changes in leaf hydraulic conductance during leaf shedding in seasonally dry tropical forest. New Phytologist 158, 295–303. [Google Scholar]
- Brodribb TJ, Holbrook NM. 2006. Declining hydraulic efficiency as transpiring leaves desiccate: two types of response. Plant, Cell and Environment 29, 2205–2215. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Holbrook NM, Zwieniecki MA, Palma B. 2005. Leaf hydraulic capacity in ferns, conifers and angiosperms: impacts on photosynthetic maxima. New Phytologist 165, 839–846. [DOI] [PubMed] [Google Scholar]
- Buckley TN. 2005. The control of stomata by water balance. New Phytologist 168, 275–292. [DOI] [PubMed] [Google Scholar]
- Buckley TN. 2014. The contributions of apoplastic, symplastic and gas phase pathways for water transport outside the bundle sheath in leaves. Plant, Cell and Environment. 10.1111/pce.12372 [DOI] [PubMed] [Google Scholar]
- Canny M, Wong SC, Huang C, Miller C. 2012. Differential shrinkage of mesophyll cells in transpiring cotton leaves: implications for static and dynamic pools of water, and for water transport pathways. Functional Plant Biology 39, 91–102. [DOI] [PubMed] [Google Scholar]
- Cochard H, Coll L, Le Roux X, Améglio T. 2002. Unraveling the effects of plant hydraulics on stomatal closure during water stress in Walnut. Plant Physiology 128, 282–290. [PMC free article] [PubMed] [Google Scholar]
- Cochard H, Nardini A, Coll L. 2004. Hydraulic architecture of leaf blades: where is the main resistance? Plant, Cell and Environment 27, 1257–1267. [Google Scholar]
- Cochard H, Venisse J-S, Barigah TS, Brunel N, Harbette S, Duilliot A, Tyree MT, Sakr S. 2007. Putative role of aquaporins in variable hydraulic conductance of leaves in response to light. Plant Phyisology 143, 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan IR. 1977. Stomatal behaviour and environment. Advances in Botanical Research 4, 117–227. [Google Scholar]
- Cure JD, Acock B. 1986. Crop responses to carbon dioxide doubling: A literature survey. Agricultural and Forest Meteorology 38, 127–145. [Google Scholar]
- Franks PJ. 2004. Stomatal control and hydraulic conductance, with special reference to tall trees. Tree Physiology 24, 865–878. [DOI] [PubMed] [Google Scholar]
- Franks PJ. 2006. Higher rates of leaf gas exchange are associated with higher leaf hydrodynamic pressure gradients. Plant, Cell and Environment 29, 584–592. [DOI] [PubMed] [Google Scholar]
- Franks PJ, Beerling DJ. 2009. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proceedings of the National Academy of Sciences, USA 106, 10343–10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks PJ, Drake PL, Froend RH. 2007. Anisohydric but isohydrodynamic: seasonally constant plant water potential gradient explained by a stomatal control mechanism incorporating variable plant hydraulic conductance. Plant, Cell and Environment 30, 19–30. [DOI] [PubMed] [Google Scholar]
- Franks PJ, Farquhar GD. 1999. A relationship between humidity response, growth form and photosynthetic operating point in C3 plants. Plant, Cell and Environment 22, 94–100. [Google Scholar]
- Franks PJ, Leitch IJ, Ruszala EM, Hetherington AM, Beerling DJ. 2012. Physiological framework for adaptation of stomata to CO2 from glacial to future concentrations. Philosophical Transactions of the Royal Society B–Biological Sciences 367, 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyot G, Scoffoni C, Sack L. 2011. Combined impacts of irradiance and dehydration on leaf hydraulic conductance: insights into vulnerability and stomatal control. Plant, Cell and Environment 35, 857–871. [DOI] [PubMed] [Google Scholar]
- Hölttä T, Vesala T, Sevanto S, Perämäki M, Nikinmaa E. 2006. Modeling xylem and phloem water flows in trees according to cohesion theory and Münch hypothesis. Trees–Structure and Function 20, 67–78. [Google Scholar]
- Hölttä T, Mencuccini M, Nikinmaa E. 2009. Linking phloem function to structure: analysis with a coupled xylem–phloem transport model. Journal of Theoretical Biology 259, 325–337. [DOI] [PubMed] [Google Scholar]
- Johansson I, Larsson C, Ek B, Kjelibom 1996. The major integral proteins of spinach leaf plasma membranes are putative aquaporins and are phosphorylated in response to Ca2+ apoplastic water potential. The Plant Cell 8, 1181–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DM, McCulloh KA, Woodruff DR, Meinzer FC. 2012. Evidence for xylem embolism as a primary factor in dehydration-induced declines in leaf hydraulic conductance. Plant, Cell and Environment 35, 760–769. [DOI] [PubMed] [Google Scholar]
- Johnson DM, Meinzer FC, Woodruff DR, McCulloh KA. 2009. Leaf xylem embolism, detected acoustically and by cryo-SEM, corresponds to decreases in leaf hydraulic conductance in four evergreen species. Plant, Cell and Environment 32, 828–836. [DOI] [PubMed] [Google Scholar]
- Kikuta SB, Lo Gullo MA, Nardini A, Richter H, Salleo S. 1997. Ultrasound acoustic emissions from dehydrating leaves of deciduous and evergreen trees. Plant, Cell and Environment 20, 1381–1390. [Google Scholar]
- Kim YX, Steudle E. 2007. Light and turgor affect the water permeability (aquaporins) of parenchyma cells in the midrib of leaves of Zea mays . Journal of Experimental Botany 58, 4119–4129. [DOI] [PubMed] [Google Scholar]
- Lawlor DW. 2002. Limitation to photosynthesis in water-stressed leaves: stomatal vs. metabolism and the role of ATP. Annals of Botany 89, 871–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DW, Cornic G. 2002. Photosynthetic carbon assimilation and associated metabolism in realtin to water deficits in higher plants. Plant, Cell and Environment 25, 275–294. [DOI] [PubMed] [Google Scholar]
- Locke AM, Sack L, Bernacchi CJ, Ort DR. 2013. Soybean leaf hydraulic conductance does not acclimate to growth at elevated [CO2] or temperature in growth chambers or in the field. Annals of Botany 112, 911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Gullo MA, Nardini A, Trifilò P, Salleo S. 2005. Diurnal and seasonal variations in leaf hydraulic conductance in evergreen and deciduous trees. Tree Physiology 25, 505–512. [DOI] [PubMed] [Google Scholar]
- Maherali H, DeLucia EH, Sipe TW. 1997. Hydraulic adjustment of maple saplings to canopy gap formation. Oecologia 112, 472–480. [DOI] [PubMed] [Google Scholar]
- Martorell S, Diaz-Espejo A, Medrano H, Ball MC, Choat B. 2014. Rapid hydraulic recovery in Eucalyptus pauciflora after drought: linkages between stem hydraulics and leaf gas exchange. Plant, Cell and Environment 37, 617 – –626. [DOI] [PubMed] [Google Scholar]
- Matzner S, Comstock J. 2001. The temperature dependence of shoot hydraulic resistance: implications for stomatal behavior and hydraulic limitation. Plant, Cell and Environment 24, 1299–1307. [Google Scholar]
- Meinzer FC, Goldstein G, Jackson P, Holbrook NM, Guitérrez MV, Cavelier J. 1995. Environmental and physiological regulation of transpiration in tropical forest gap species: the influence of boundary layer and hydraulic properties. Oecologia 101, 514–522. [DOI] [PubMed] [Google Scholar]
- Meinzer FC, Grantz DA. 1990. Stomatal and hydraulic conductance in growing sugar cane: stomatal adjustment to water transport capacity. Plant, Cell and Environment 13, 383–388. [Google Scholar]
- Meinzer FC, Grantz DA. 1991. Coordination of stomatal, hydraulic, and canopy boundary layer properties: Do stomata balance conductances by measuring transpiration? Physiologia Plantarum 83, 324–329. [Google Scholar]
- Mencuccini M. 2003. The ecological significance of long-distance water transport: short-term regulation, long-term acclimation and the hydraulic costs of stature across plant life forms. Plant, Cell and Envrionment 26, 163–182. [Google Scholar]
- Mencuccini M, Comstock J. 1999. Variability in hydraulic architecture and gas exchange of common bean (Phaseolus vulgarism) cultivars under well-watered conditions: interactions with leaf size. Australian Journal of Plant Physiology 26, 115–124. [Google Scholar]
- Morillon R, Chrispeels MJ. 2001. The role of ABA and the transpiration stream in the regulation of the osmotic water permeability of leaf cells. Proceedings of the National Academy of Sciences, USA 98, 14138–14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morison JIL, Gifford RM. 1983. Stomatal sensitivity to carbon dioxide and humidity a comparison of two C3 and C4 grass species. Plant Physiology 71, 789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morison JIL, Lawlor DW. 1999. Interactions between increasing CO2 concentration and temperature on plant growth. Plant, Cell and Environment 32, 659–682. [Google Scholar]
- Nardini A, Gortan E, Salleo S. 2005a. Hydraulic efficiency of the leaf venation system in sun- and shade-adapted species. Functional Plant Biology 32, 953–961. [DOI] [PubMed] [Google Scholar]
- Nardini A, Salleo S, Andri S. 2005b. Circadian regulation of leaf hydraulic conductance in sunflower (Helianthus annuus L. cv Margot). Plant, Cell and Environment 28, 750–759. [Google Scholar]
- Nardini A, Salleo S, Jansen S. 2011. More than just a vulnerable pipeline: xylem physiology in the light of ion-mediated regulation of plant water transport. Journal of Experimental Botany 62, 4701–4718. [DOI] [PubMed] [Google Scholar]
- Nikinmaa E, Hölttä T, Hari P, Kolari P, Mäkelä A, Sevanto S, Vesala T. 2013. Assimilate transport in phloem sets conditions for leaf gas exchange. Plant, Cell and Environment 36, 655–669. [DOI] [PubMed] [Google Scholar]
- Oren R, Sperry JS, Katul GG, Pataki DE, Ewers BE, Phillips N, Schäfer KVR. 1999. Survey and synthesis of intra- and interspecific variation in stomatal sensitivity to vapour pressure deficit. Plant, Cell and Environment 22, 1515–1526. [Google Scholar]
- Pantin F, Monner F, Jannoud D, Costa J M, Renaud J, Muller B, Simonneau T, Genty B. 2012. The dual effect of abscisic acid on stomata. New Phytologist 197, 65–72. [DOI] [PubMed] [Google Scholar]
- Peak D, Mott KA. 2011. A new, vapour-phase mechanism for stomatal responses to humidity and temperature. Plant, Cell and Environment 34, 162–178 [DOI] [PubMed] [Google Scholar]
- Pieruschka R, Huber G, Berry JA. 2010. Control of transpiration by radiation. Proceedings of the National Academy of Sciences, USA 107, 13372–13377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pou A, Medrano H, Flexas J, Tyerman S. 2013. A putative role for TIP and PIP aquaporins in dynamics of leaf hydraulic and stomatal conductances in grapevine under water stress and re-watering. Plant, Cell and Environment 36, 828–843. [DOI] [PubMed] [Google Scholar]
- Quinn JF, Dunham AE. 1983. On hypothesis testing in ecology and evolution. The American Naturalist 122, 602–617. [Google Scholar]
- Rockwell F, Holbrook NM, Stroock AD. 2014. The competition between liquid and vapor transport in transpiring leaves. Plant Physiology doi: 10.1104/pp.114.236323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack L, Holbrook NM. 2006. Leaf hydraulics. Annual Review of Plant Biology 57, 361–381. [DOI] [PubMed] [Google Scholar]
- Sack L, Scoffoni C. 2013. Leaf venation: structure, function, development, evolution, ecology and applications in the past, present and future. New Phytologist 198, 983–1000. [DOI] [PubMed] [Google Scholar]
- Sack L, Streeter CM, Holbrook NM. 2004. Hydraulic analysis of water flow through leaves of sugar maple and red oak. Plant Physiology 134, 1824–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack L, Tyree MT, Holbrook NM. 2005. Leaf hydraulic architecture correlates with regeneration irradiance in tropical rainforest trees. New Phytologist 167, 403–413. [DOI] [PubMed] [Google Scholar]
- Salleo S, Lo Gullo MA, Raimondo F, Nardini A. 2001. Vulnerability to cavitation of leaf minor veins: any impact on leaf gas exchange? Plant, Cell and Environment 24, 851–859. [Google Scholar]
- Scoffoni C, Pou A, Aasamaa K, Sack L. 2008. The rapid light response of leaf hydraulic conductance: new evidence from two experimental methods. Plant, Cell and Environment 31, 1803–1812. [DOI] [PubMed] [Google Scholar]
- Sellin A, Kupper P. 2005a. Within-crown variation in leaf conductance of Norway spruce: effects of irradiance, vapour pressure deficit, leaf water status and plant hydraulic constraints. Annals of Forest Science : 61, 419–429. [Google Scholar]
- Sellin A, Kupper P. 2005b. Variation in leaf conductance of silver birch: effects of irradiance, vapour pressure deficit, leaf water status and position within a crown. Forest Ecology and Management 206, 153–166. [Google Scholar]
- Sellin A, Kupper P. 2007. Temperature, light and leaf hydraulic conductance of little-leaf linden (Tilia cordata) in a mixed forest canopy. Tree Physiology 27, 679–688. [DOI] [PubMed] [Google Scholar]
- Sellin A, Õunapuu E, Kupper P. 2008. Effects of light intensity and duration on leaf hydraulic conductance and distribution of resistance in shoots of silver birch (Betula pendula). Physiologia Plantarum 134, 412–420. [DOI] [PubMed] [Google Scholar]
- Shatil-Cohen A, Attia Z, Moshelion M. 2011. Bundle-sheath cell regulation of xylem–mesophyll water transport via qauaporins under drought stress: a target of xylem-borne ABA? The Plant Journal 67, 72–80. [DOI] [PubMed] [Google Scholar]
- Simonin KA, Limm EB, Dawson TE. 2012. Hydraulic conductance of leaves correlates with leaf lifespan: implications for lifetime carbon gain. New Phytologist 193, 939–947. [DOI] [PubMed] [Google Scholar]
- Sôber A. 1997. Hydraulic conductance, stomatal conductance, and maximal phosotynthetic rate in bean leaves. Photosynthetica 34, 599–603. [Google Scholar]
- Sperry JS. 2000. Hydraulic constraints on plant gas exchange. Agricultural and Forest Meteorology 104, 13–23. [Google Scholar]
- Sperry JS, Pockman WT. 1993. Limitation of transpiration by hydraulic conductance and xylem cavitation in Betula occidentalis . Plant, Cell and Environment 16, 279–287. [Google Scholar]
- Sperry JS, Hacke UG, Oren R, Comstock JP. 2002. Water deficits and hydraulic limits to leaf water supply. Plant, Cell and Environment 25, 251–263. [DOI] [PubMed] [Google Scholar]
- Thompson MV, Holbrook NM. 2004. Scaling phloem transport: information transmission. Plant, Cell and Environment 27, 509–519. [Google Scholar]
- Tolley LC, Strain BR. 1985. Effects of CO2 enrichment and water stress on gas exchange of Liquidambar styraciflua and Pinus taeda seedlings grown under different irradiance levels. Oecologia 65, 166–172. [DOI] [PubMed] [Google Scholar]
- Turgeon R. 2010. The puzzle of phloem pressure. Plant Physiology 154, 578–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Cheung YNS. 1977. Resistance to water flow in Fagus grandifolia leaves. Canadian Journal of Botany 55, 2591–2599. [Google Scholar]
- Tyree MT, Cruiziat P, Benis M, LoGullo MA, Salleo S. 1981. The kinetics of rehydration of detached sunflower leaves from different initial water deficits. Plant, Cell and Environment 4, 309–317. [Google Scholar]
- Winkel T, Rambal S. 1993. Influence of water stress on grapevines growing in the field: from leaf to whole-plant response. Australian Journal of Plant Physiology 20, 143–157. [Google Scholar]
- Zwieniecki MA, Brodribb TJ, Holbrook NM. 2007. Hydarulic design of leaves: insights from rehydration kinetics. Plant, Cell and Environment 30, 910–921. [DOI] [PubMed] [Google Scholar]
- Zwieniecki MA, Melcher PJ, Holbrook NM. 2001. Hydrogel control of xylem hydraulic resistance in plants. Science 291, 1059–1062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


